Dietary Additive Combination for Dairy Calves After Weaning Has a Modulating Effect on the Profile of Short-Chain Fatty Acids in the Rumen and Fecal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additive
2.2. Installation and Animals
2.3. Experimental Design and Diet
2.4. Sample and Data Collection
2.5. Laboratory Analysis
2.5.1. Feed Analysis
2.5.2. Hemogram
2.5.3. Seric Biochemistry
2.5.4. Protein Profile by Electrophoresis
2.5.5. Oxidative and Antioxidants Response
2.5.6. Ruminal Fluid: VFA Levels
2.5.7. Gut Microbiota (Feces)
2.6. Statistical Analysis
3. Results
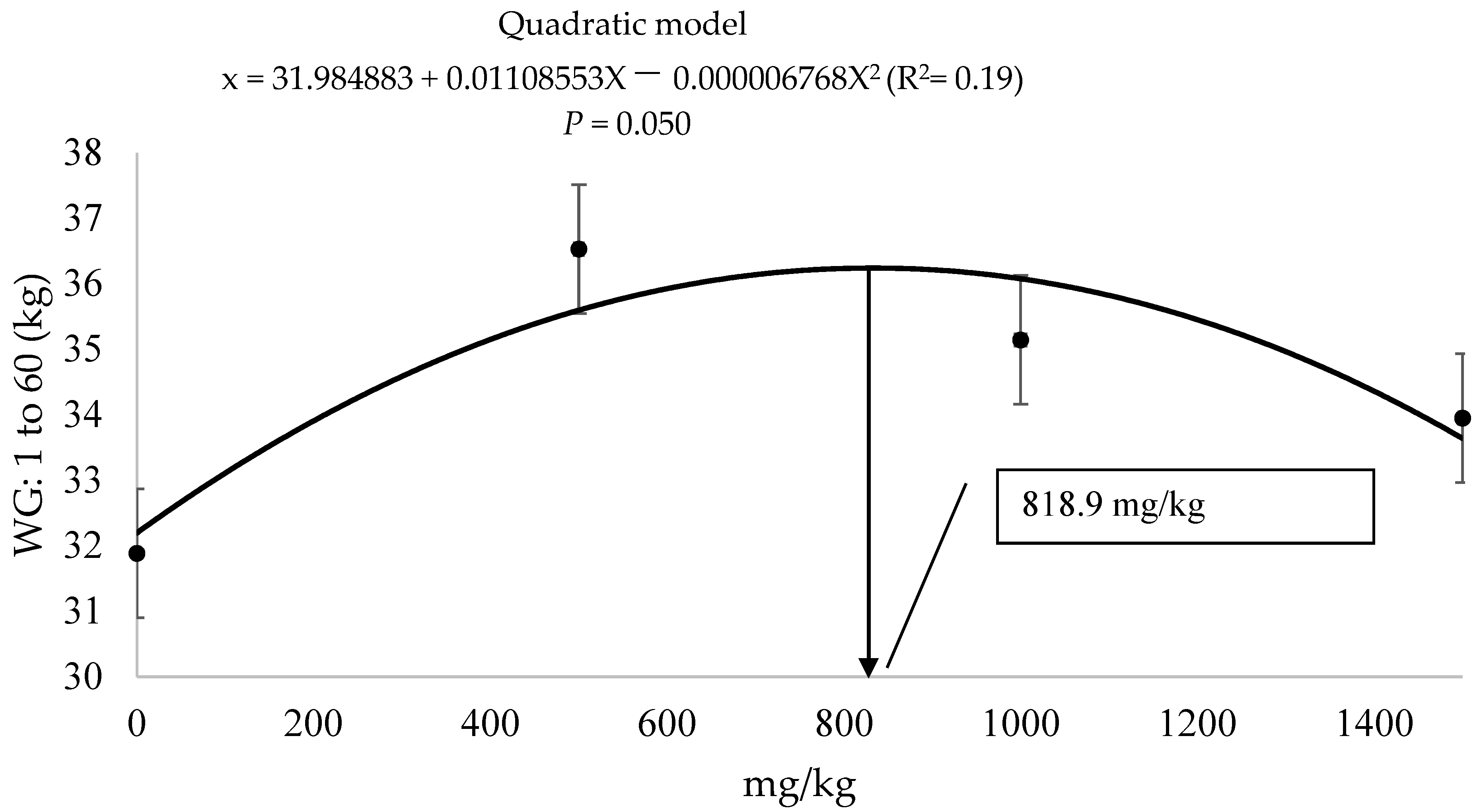
3.1. Performance
3.2. Hematology and Serum Biochemistry
3.3. Oxidative Status
3.4. VFA Levels in Ruminal Liquid
3.5. Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, D.T.; Carvalho, G.R.; Resende, J.C. Cadeia produtiva do leite no Brasil: Produção primária. Embrapa 2020, 1, 16. [Google Scholar]
- Curtis, G.; McGregor Argo, C.; Jones, D.; Grove-White, D. The impact of early life nutrition and housing on growth and reproduction in dairy cattle. PLoS ONE 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. J. Anim. Sci. 2005, 83, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Liu, T.; Cairang, D.; Cheng, S.; Hu, J.; Shi, B.; Zhu, H.; Chen, H.; Zhang, T.; Yi, X. Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves. Animals 2024, 14, 820. [Google Scholar] [CrossRef]
- Shu, C.; Ge, L.; Li, Z.; Chen, B.; Liao, S.; Lu, L.; Wu, Q.; Jiang, X.; An, Y.; Wang, Z.; et al. Antibacterial activity of cinnamon essential oil and its main component of cinnamaldehyde and the underlying mechanism. Front. Pharmacol. 2024, 15, 14. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.; Wu, Z.; Li, S.; Wang, Y.; Cao, Z. Review: Utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Hu, M.; Hou, J.; Du, Y.; Si, W.; Yang, L.; Xu, L.; Xu, Q. Modulating gastrointestinal microbiota to alleviate diarrhea in calves. Front. Microbiol. 2023, 14, 1181545. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Zhang, H.T. Effects of supplementation of Bacillus subtilis natto Na and N1 strains on rumen development in dairy calves. Anim. Feed Sci. Technol. 2011, 164, 154–160. [Google Scholar] [CrossRef]
- Cappellozza, B.I.; Copani, G.; Boll, E.J.; Queiroz, O. Supplementation of the direct-fed microbial Enterococcus faecium 669 impacts performance of pre-weaning dairy calves. JDS Commun. 2023, 4, 284–287. [Google Scholar] [CrossRef]
- Lucey, P.M.; Lean, I.J.; Aly, S.S.; Golder, H.M.; Block, E.; Thompson, J.S.; Rossow, H.A. Effects of mannan-oligosaccharide and Bacillus subtilis supplementation to preweaning Holstein dairy heifers on body weight gain, diarrhea, and shedding of fecal pathogens. J. Dairy Sci. 2021, 104, 4290–4302. [Google Scholar] [CrossRef]
- Reis, M.E.; de Toledo, A.F.; da Silva, A.P.; Poczynek, M.; Cantor, M.C.; Virgínío, J.G.F.; Greco, L.; Bittar, C.M.M. Effect of supplementation with algae β-glucans on performance, health, and blood metabolites of Holstein dairy calves. J. Dairy Sci. 2022, 10, 7998–8007. [Google Scholar] [CrossRef]
- Guo, C.; Wang, X.; Dai, D.; Kong, F.; Wang, S.; Sun, X.; Li, S.; Xu, X.; Zhang, L. Effects of alkaline mineral complex supplementation on production performance, serum variables, and liver transcriptome in calves. Vet. Sci. 2023, 10, 11. [Google Scholar] [CrossRef]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Mousavi-Haghshenas, M.A.; Hashemzadeh, F.; Ghorbani, G.R. Trace minerals source in calf starters interacts with birth weights to affect growth performance. Sci. Rep. 2022, 12, 13. [Google Scholar] [CrossRef]
- Silva, D.J.; Queiroz, A.C. Análises de alimentos (métodos químicos e biológicos). Ed. UFV 2002, 3, 235. [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. III Study of effects of heating and drying on yield of fiber and lignin in forages. J. Assoc. Off. Agric. Chem. 1965, 48, 758–790. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Determination of lignin and cellulose in acid detergent fiber with permanganate. J. Assoc. Agric. Chem. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Feldman, B.F.; Sink, C.A. Urinálise e hematologia-laboratorial para o clínico de pequenos animais. Eduardo Roca 2006, 1, 128. [Google Scholar]
- Tomasi, T.; Volpato, A.; Pereira, W.A.B.; Debastiani, L.H.; Bottari, N.B.; Morsch, V.M.; Schetinger, M.R.C.; Leal, M.L.R.; Machado, G.; Da Silva, A.S. Metaphylactic effect of minerals on the immune response, biochemical variables and antioxidant status of newborn calves. J. Anim. Physiol. Anim. Nutr. 2018, 102, 819–824. [Google Scholar] [CrossRef]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- Tatsch, E.; Bochi, G.V.; Pereira, R.S.; Kober, H.; Agertt, V.A.; Bollick, Y.S.; Moresco, R.N. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin. Chim. Acta 2011, 412, 909–911. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Souza, L.C.; Barbosa, K.B.; Oliveira, L.L.; Magalhães, M.; Ferreira, C.F.; Huguenin, G.V. Selenium supplementation and exercise: Glutathione peroxidase activity and its relationship with oxidative stress and thyroid hormones. J. Trace Elem. Med. Biol. 2018, 47, 23–29. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Nelson, D.P.; Kiesow, L.A. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 1972, 49, 474–478. [Google Scholar] [CrossRef]
- Hjelmsø, M.H.; Hansen, L.H.; Holm, P.E.; Jensen, L.E.; Brandt, K.K. Evaluation of the V3–V4 region of the 16S rRNA gene for bacterial community profiling in soil ecosystems. FEMS Microbiol. Ecol. 2014, 87, 415–426. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 23, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Rognes, T. Vsearch: A versatile open source tool for metagenomics. Peer J. 2016, 4, 22. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, 11. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, 9. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 21. [Google Scholar] [CrossRef]
- Silva, G.G.; Takiya, C.S.; Del Valle, T.A.; Jesus, E.F.; Grigoletto, N.T.; Nakadonari, B.; Cortinhas, C.S.; Acedo, T.S.; Rennó, F.P. Nutrient digestibility, ruminal fermentation, and milk yield in dairy cows fed a blend of essential oils and amylase. J. Dairy Sci. 2018, 101, 9815–9826. [Google Scholar] [CrossRef]
- Ferro, M.M.; De Moura, D.C.; Geron, L.J.V. Óleos essenciais em dietas para bovinos. Rev. Ciências Agroambientais 2016, 14, 2. [Google Scholar]
- Favaretto, J.A.; Alba, D.F.; Marchiori, M.S.; Marcon, H.J.; Souza, C.F.; Baldissera, M.D.; Bianchi, A.E.; Zanluchi, M.; Klein, B.; Wagner, R.; et al. Supplementation with a blend based on micro-encapsulated carvacrol, thymol, and cinnamaldehyde in lambs feed inhibits immune cells and improves growth performance. Livest. Sci. 2020, 240, 30. [Google Scholar] [CrossRef]
- Dantas, C.C.O.; Negrão, F.M. Funções e sintomas de deficiência dos minerais essenciais utilizados para suplementação dos bovinos de corte. UNI Ciências 2010, 14, 25. [Google Scholar]
- Maamouri, O.; Ben, S.M. The effect of live yeast Saccharomyces cerevisiae as probiotic supply on growth performance, feed intake, ruminal pH and fermentation in fattening calves. Vet. Med. Sci. 2022, 1, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Goes, R.H.T.B.; Mello, R.; Brabes, K.C.S.; Andrade, S.J.T. Uso de leveduras na alimentação de ruminantes. Rev. Bras. Zootec. 2005, 34, 225–231. [Google Scholar]
- Ramos, M.M.; Berber, R.C.A.; Moreira, P.S.A. Productive performance of Nellore steers in pasture with association of flavomycin and monensin. Núcleo 2019, 12, 54–58. [Google Scholar] [CrossRef]
- Reis, J.H.; Gebert, R.R.; Barreta, M.; Baldissera, M.D.; Dos Santos, I.D.; Roger, W.R.; Campigotto, G.; Jaguezeski, A.M.; Gris, A.; De Lima, J.L.F.; et al. Efeitos do aditivo fitogênico para rações à base de timol, carvacrol e aldeído cinâmico no peso corporal, parâmetros sanguíneos e bactérias ambientais em frangos de corte. Patogênese Microbiana 2018, 125, 168–176. [Google Scholar]
- Chagas, L.J. Desempenho, Metabolismo e Emissão de Metano de Bovinos Nelore em Terminação Recebendo Óleos Funcionais em Substituição ou Combinação com Monensina Sódica na Dieta. Ph.D. Dissertation, Escola Superior de Agricultura Luiz de Queiroz, Piracicaba, Brazil, 2015. [Google Scholar]
- Bortoli, A. Influência de um Aditivo Fitogênico nos Níveis Sanguíneos de Novilhas Jersey; UFSM Centro de Ciências Rurais: Santa Maria, Brazil, 2007; Volume 1, p. 68. [Google Scholar]
- Volpato, A.; Crecencio, R.B.; Tomasi, T.; Galli, G.M.; Griss, L.G.; Silva, A.D.; Schetinger, M.R.C.; Schogor, A.L.B.; Baldissera, M.D.; Stefani, L.M.; et al. Phytogenic as feed additive for suckling dairy calves has a beneficial effect on animal health and performance. An. Acad. Bras. Ciências 2019, 91, 10. [Google Scholar] [CrossRef]
- Odongo, N. Long-term effects of feeding monensin on methane production in lactating dairy cows. J. Dairy Sci. 2007, 90, 1781–1788. [Google Scholar] [CrossRef]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 1. Metabolic effects. J. Dairy Sci. 2008, 91, 1334–1346. [Google Scholar] [CrossRef]
- González, F.H.D. Ferramentas de diagnóstico e monitoramento das doenças metabólicas. Ciência Anim. Bras. 2009, 1, 22. [Google Scholar]
- Joseph, J. Harnessing Nasal Immunity with IgA to Prevent Respiratory Infections. Immuno 2022, 2, 571–583. [Google Scholar] [CrossRef]
- Hanthorn, C.J.; Dewell, G.A.; Dewell, R.D. Serum concentrations of haptoglobin and haptoglobin-matrix metalloproteinase 9 (Hp-MMP 9) complexes of bovine calves in a bacterial respiratory challenge model. BMC Vet. Res. 2014, 10, 285. [Google Scholar] [CrossRef]
- Bozukluhan, K.; Oguz, M. Clinical significance of some acute phase proteins in cattle. Vet. Med. Sci. 2023, 16, 134. [Google Scholar]
- Camaschella, C. Iron Deficiency. Am. Soc. Hematol. 2024, 1, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Prevedello, M.T.; Comachio, G. Antioxidants and their relationship with free radicals, and chronic non communicable diseases. Braz. J. Dev. 2021, 7, 55244–55285. [Google Scholar] [CrossRef]
- Huber, P.C.; Almeida, W.P.; Fátima, Â. Glutationa e enzimas relacionadas: Papel biológico e importância em processos patológicos. Química Nova 2008, 31, 1170–1179. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 12. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Lay, J.; Andries, K. Effects of live yeast on differential genetic and functional attributes of rumen microbiota in beef cattle. J. Anim. Sci. Biotechnol. 2019, 10, 68. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Z.; Yang, H.; Wang, Y.; Wang, W.; Li, S. Analysis of serum antioxidant capacity and gut microbiota in calves at different growth stages in Tibet. Front. Microbiol. 2023, 13, 11. [Google Scholar] [CrossRef]
- Grondin, J.A.; Jamal, A.; Mowna, S.; Seto, T.; Khan, W.I. Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence. Pathogens 2024, 13, 608. [Google Scholar] [CrossRef]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef]
- Brooke, C.G.; Najafi, N.; Dykier, K.C.; Hess, M. Prevotella copri, a potential indicator for high feed efficiency in western steers. Anim. Sci. J. 2019, 90, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.L.; Indugu, N.; Vecchiarelli, B.; Bender, J.; Pappalardo, C.; Leibstein, M. Temporal changes in the fecal bacterial community in Holstein dairy calves from birth through the transition to a solid diet. PLoS ONE 2020, 15, e0238882. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, M.; Arvind, K.; Saxena, R.; Pulikkan, J.; Sharma, V.K.; Grace, T. Exploring variation in the fecal microbial communities of Kasaragod Dwarf and Holstein crossbred cattle. Antonie Van Leeuwenhoek 2023, 116, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sanabria, E.; Goonewardene, L.A.; Wang, Z.; Durunna, O.N.; Moore, S.S.; Guan, L.L. Impact of feed efciency and diet on adaptive variations in the bacterial community in the rumen fuid of cattle. Appl. Environ. Microbiol. 2012, 78, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Stolze, Y.; Zakrzewski, M.; Maus, I.; Eikmeyer, F.; Jaenicke, S.; Rottmann, N.; Siebner, C.; Pühler, A.; Schlüter, A. Comparative metagenomics of biogas-producing microbial communities from production-scale biogas plants operating under wet or dry fermentation conditions. Biotechnol Biofuels 2015, 8, 14. [Google Scholar] [CrossRef]
- Foditsch, C.; Pereira, R.V.V.; Ganda, E.K.; Gomez, M.S.; Marques, E.C.; Santin, T. Oral Administration of Faecalibacterium prausnitzii Decreased the Incidence of Severe Diarrhea and Related Mortality Rate and Increased Weight Gain in Preweaned Dairy Heifers. PLoS ONE 2015, 10, e0145485. [Google Scholar] [CrossRef]
- Miquel, S.; Leclerc, M.; Martin, R.; Chain, F.; Lenoir, M.; Raguideau, S. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. MBio 2015, 6, 10. [Google Scholar] [CrossRef]
- Li, J.; Lian, H.; Zheng, A.; Zhang, J.; Dai, P.; Niu, Y.; Gao, T.; Li, M.; Zhang, L.; Fu, T. Effects of Different Roughages on Growth Performance, Nutrient Digestibility, Ruminal Fermentation, and Microbial Community in Weaned Holstein Calves. Front. Vet. Sci. 2022, 9, 12. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, C.; Diao, Q.; Tu, Y. Effect of Dietary and Age Changes on Ruminal Microbial Diversity in Holstein Calves. Microorganisms 2024, 12, 12. [Google Scholar] [CrossRef]
- O’Hara, E.; Kelly, A.; McCabe, M.S. Effect of a butyrate-fortified milk replacer on gastrointestinal microbiota and products of fermentation in artificially reared dairy calves at weaning. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Pant, S.D.; Zhao, G.; Alkhalil, S.S.; Abdulfattah, A.M.; Alotibi, I.; Al-Zahrani, M.; Alshehri, W.A.; Aloufi, B.H.; Lei, H.; et al. Impact of wheat processing on growth, serum biochemistry, and ruminal microbiota in sheep (Ovis aries). Microb. Pathog. 2024, 195, 106887. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Composition (%) | ||
|---|---|---|---|
| Corn silage | 13.4 | ||
| Hay: Tifton 85 | 35.9 | ||
| Concentrate 1 | 50.7 | ||
| Chemical composition 2 (%) | Corn silage | Hay | Concentrate a |
| DM | 26.1 | 89.8 | 89.1 |
| Ash | 3.63 | 8.76 | 6.35 |
| CP | 5.68 | 12.3 | 20.9 |
| NDF | 44.9 | 69.9 | 19.0 |
| ADF | 23.6 | 31.5 | 8.43 |
| Variables | T-CON 1 | T-0 1 | T-500 1 | T-1000 1 | T-1500 1 | SEM | p-Valor |
|---|---|---|---|---|---|---|---|
| Body weight, kg | |||||||
| Initial | 68.2 | 68.2 | 68.5 | 67.9 | 69.6 | 1.94 | 0.97 |
| Final | 105.5 a | 95.5 a | 105.1 a | 102.7 a | 90.1 b | 2.04 | 0.05 |
| Weight gain, kg | |||||||
| Day 1 to 60 | 37.2 a | 32.2 bc | 36.5 a | 35.1 a | 29.9 c | 1.43 | 0.03 |
| ADG, kg 2 | 0.620 a | 0.536 bc | 0.608 a | 0.585 a | 0.498 c | 0.06 | 0.03 |
| DMI kg/day 2 | 6.12 | 6.06 | 6.05 | 6.11 | 6.01 | 0.12 | 0.95 |
| FC, kg/kg 2 | 9.87 b | 11.3 a | 9.95 b | 10.4 ab | 12.0 b | 0.04 | 0.01 |
| FE, kg/kg 2 | 0.101 a | 0.088 bc | 0.100 a | 0.095 ab | 0.082 c | 0.06 | 0.01 |
| Variables | T-CON | T-0 | T-500 | T-1000 | T-1500 | SEM | P: Treat | P: Treat × Day |
|---|---|---|---|---|---|---|---|---|
| Erythrocytes (×106/µL) | 0.01 | 0.01 | ||||||
| d1 | 8.75 | 8.90 | 9.59 | 9.03 | 9.47 | 0.69 | ||
| d15 | 9.18 | 9.92 | 10.4 | 9.97 | 10.5 | 0.72 | ||
| d30 | 8.41 B | 8.31 B | 10.3 A | 8.91 AB | 8.34 B | 0.72 | ||
| d45 | 8.63 B | 8.43 B | 10.6 A | 8.90 AB | 8.68 B | 0.66 | ||
| d60 | 8.49 B | 8.65 B | 10.7 A | 9.21 AB | 8.56 B | 0.52 | ||
| Hemoglobin (mg/dL) | 0.04 | 0.01 | ||||||
| d1 | 8.63 | 8.41 | 9.29 | 8.94 | 9.11 | 0.16 | ||
| d15 | 9.53 | 10.2 | 10.7 | 10.6 | 11.3 | 0.18 | ||
| d30 | 12.1 B | 11.2 B | 14.7 A | 12.9 AB | 11.3 B | 0.17 | ||
| d45 | 12.7 B | 11.9 B | 15.9 A | 12.9 AB | 12.2 B | 0.15 | ||
| d60 | 13.0 AB | 11.5 B | 15.9 A | 14.5 AB | 11.9 A | 0.16 | ||
| Hematocrit (%) | 0.03 | 0.01 | ||||||
| d1 | 39.1 | 38.2 | 42.2 | 40.1 | 41.4 | 0.65 | ||
| d15 | 43.2 | 42.4 | 46.0 | 45.5 | 45.8 | 0.62 | ||
| d30 | 40.8 B | 38.9 B | 50.1 A | 44.3 AB | 38.3 B | 0.64 | ||
| d45 | 43.2 B | 40.6 B | 53.9 A | 43.9 B | 41.8 B | 0.59 | ||
| d60 | 41.9 B | 40.1 B | 49.5 A | 46.1 AB | 40.2 B | 0.54 | ||
| Leukocytes (×103/µL) | 0.32 | 0.02 | ||||||
| d1 | 16.6 | 14.6 | 14.4 | 14.9 | 12.5 | 1.52 | ||
| d15 | 15.0 | 14.7 | 13.0 | 14.7 | 13.5 | 1.50 | ||
| d30 | 14.8 | 11.7 | 14.9 | 13.5 | 10.6 | 1.56 | ||
| d45 | 12.5 A | 12.4 A | 10.7 AB | 10.3 AB | 8.31 B | 1.48 | ||
| d60 | 11.3 AB | 12.8 A | 10.9 A | 10.2 BC | 9.41 B | 1.45 | ||
| Lymphocytes (×103/µL) | 0.25 | 0.01 | ||||||
| d1 | 9.14 | 8.56 | 8.36 | 8.67 | 8.13 | 1.14 | ||
| d15 | 8.89 | 8.34 | 8.41 | 8.25 | 7.99 | 1.21 | ||
| d30 | 7.76 | 7.25 | 7.94 | 8.10 | 7.08 | 1.15 | ||
| d45 | 7.41 A | 7.65 A | 6.58 AB | 6.39 AB | 5.24 B | 1.15 | ||
| d60 | 7.32 A | 7.58 A | 6.52 AB | 6.14 AB | 5.38 B | 1.12 | ||
| Neutrophilis (×103/µL) | 3.24 | 3.12 | 3.05 | 2.97 | 2.85 | 0.95 | 0.19 | 0.28 |
| Monocytes (×103/µL) | 0.81 | 0.74 | 0.72 | 0.68 | 0.54 | 0.50 | 0.51 | 0.69 |
| Eosinophilis (×103/µL) | 0.52 | 0.45 | 0.36 | 0.48 | 0.51 | 0.84 | 0.63 | 0.77 |
| Variables | T-CON | T-0 | T-500 | T-1000 | T-1500 | SEM | P: Treat | P: Treat × Day |
|---|---|---|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 91.1 a | 77.7 ab | 80.7 ab | 76.2 ab | 68.0 b | 2.81 | 0.05 | 0.15 |
| Urea (mg/dL) | 32.8 | 26.1 | 29.7 | 30.2 | 25.2 | 2.09 | 0.45 | 0.26 |
| Glucose (mg/dL) | 108 | 105 | 94.5 | 98.1 | 104 | 4.12 | 0.65 | 0.54 |
| Total protein (g/dL) | 8.33 | 8.38 | 8.60 | 8.19 | 8.08 | 0.14 | 0.89 | 0.21 |
| Globulin (g/dL) | ||||||||
| d1 | 4.50 | 4.03 | 4.81 | 4.23 | 4.11 | 0.11 | 0.15 | 0.01 |
| d15 | 5.09 | 4.97 | 5.81 | 5.13 | 4.64 | 0.10 | ||
| d30 | 6.27 | 5.94 | 5.86 | 6.40 | 6.01 | 0.10 | ||
| d45 | 5.36 B | 6.20 A | 5.58 B | 5.47 B | 5.24 B | 0.11 | ||
| d60 | 5.42 B | 6.30 A | 5.51 B | 5.02 B | 5.02 B | 0.09 | ||
| Albumin (g/dL) | 2.80 | 2.64 | 2.65 | 2.69 | 2.85 | 0.06 | 0.82 | 0.73 |
| Ig- heavy-chain (g/dL) | 1.24 a | 1.03 b | 1.27 a | 1.11 b | 1.08 b | 0.05 | 0.02 | 0.11 |
| IgA | 0.95 b | 1.67 a | 0.89 b | 0.94 b | 1.02 b | 0.08 | 0.05 | 0.17 |
| Ceruloplasmin (g/dL) | 0.01 | 0.01 | ||||||
| d1 | 0.74 | 0.76 | 0.71 | 0.74 | 0.75 | 0.04 | ||
| d15 | 0.71 | 0.82 | 0.70 | 0.76 | 0.78 | 0.04 | ||
| d30 | 0.68 B | 0.97 A | 0.81 AB | 0.71 B | 0.67 B | 0.05 | ||
| d45 | 0.70 B | 0.92 A | 0.73 B | 0.66 B | 0.65 B | 0.05 | ||
| d60 | 0.69 B | 0.99 B | 0.75 B | 0.67 B | 0.62 B | 0.06 | ||
| Haptoglobin (g/dL) | 0.12 | 0.02 | ||||||
| d1 | 0.42 | 0.47 | 0.45 | 0.45 | 0.42 | 0.03 | ||
| d15 | 0.49 | 0.51 | 0.47 | 0.54 | 0.52 | 0.04 | ||
| d30 | 0.52 | 0.54 | 0.58 | 0.51 | 0.50 | 0.04 | ||
| d45 | 0.47 B | 0.59 A | 0.52 AB | 0.46 B | 0.42 B | 0.04 | ||
| d60 | 0.49 B | 0.64 A | 0.55 AB | 0.48 B | 0.45 B | 0.05 | ||
| Ferritin (g/dL) | 0.39 a | 0.41 a | 0.42 a | 0.37 ab | 0.32 b | 0.03 | 0.08 | 0.12 |
| Transferrin (g/dL) | 0.52 a | 0.35 b | 0.50 a | 0.57 a | 0.60 a | 0.04 | 0.03 | 0.25 |
| C-reactive protein (g/dL) | 0.28 | 0.32 | 0.27 | 0.28 | 0.27 | 0.02 | 0.11 | 0.20 |
| Variables | T-CON | T-0 | T-500 | T-1000 | T-1500 | SEM | P: Treat | P: Treat × Day |
|---|---|---|---|---|---|---|---|---|
| ROS (U DCF/mL) | 0.01 | 0.01 | ||||||
| d1 | 8.10 | 8.06 | 8.21 | 7.96 | 8.41 | 0.85 | ||
| d15 | 7.96 | 7.92 | 7.85 | 7.37 | 7.65 | 0.85 | ||
| d30 | 7.68 | 7.25 | 7.36 | 8.88 | 8.96 | 0.85 | ||
| d45 | 6.79 C | 8.54 BC | 7.61 C | 10.7 AB | 12.7 A | 1.05 | ||
| d60 | 6.82 C | 9.28 AB | 6.24 C | 7.68 BC | 11.0 A | 0.92 | ||
| SOD (U SOD/mg of protein) | 0.01 | 0.01 | ||||||
| d1 | 3.52 | 3.47 | 3.48 | 3.24 | 3.81 | 0.09 | ||
| d15 | 3.54 B | 3.74 B | 4.56 AB | 4.74 AB | 5.10 A | 0.09 | ||
| d30 | 3.08 C | 2.85 C | 4.44 B | 5.01 AB | 5.27 A | 0.12 | ||
| d45 | 2.57 C | 2.96 BC | 3.68 AB | 4.42 A | 4.76 A | 0.10 | ||
| d60 | 2.81 B | 2.95 B | 4.25 A | 4.63 A | 5.04 A | 0.08 | ||
| TBARS (MDA/mL) | 14.1 ab | 21.7 a | 10.8 b | 18.9 ab | 22.1 a | 4.01 | 0.05 | 0.12 |
| NOx (µmol/L) | 52.4 | 57.1 | 50.6 | 60.2 | 57.6 | 2.94 | 0.22 | 0.15 |
| GST (U GST/mg of protein) | 241 b | 260 a | 245 b | 253 ab | 265 a | 7.10 | 0.04 | 0.35 |
| GPx (U GPx/mg of protein) | 6.85 | 5.96 | 5.71 | 6.25 | 5.87 | 0.52 | 0.46 | 0.23 |
| CAT (mmol/mg Hb) | 4.25 | 4.01 | 4.62 | 4.64 | 4.27 | 0.13 | 0.68 | 0.41 |
| Variables | T-CON | T-0 | T-500 | T-1000 | T-1500 | SEM | P: Treat |
|---|---|---|---|---|---|---|---|
| pH | 6.42 | 6.40 | 6.37 | 6.54 | 6.56 | 0.06 | 0.31 |
| MBRT (seconds) | 102 b | 125 a | 99.5 b | 88.2 bc | 122 a | 4.62 | 0.01 |
| SCFA (mmol/L) | 56.7 ab | 50.4 b | 58.4 ab | 61.6 a | 52.7 c | 2.57 | 0.04 |
| Acetic acid (mmol/L) | 37.9 bc | 32.7 c | 38.9 ab | 40.5 a | 34.1 c | 1.25 | 0.01 |
| Propionic acid (mmol/L) | 11.5 bc | 10.4 c | 12.2 ab | 12.6 a | 10.6 c | 0.28 | 0.03 |
| Butyric acid (mmol/L) | 5.86 b | 5.77 b | 5.73 b | 7.16 a | 6.21 ab | 0.11 | 0.05 |
| Isovaleric acid (mmol/L) | 0.92 | 0.90 | 0.83 | 0.96 | 0.98 | 0.07 | 0.45 |
| Valeric acid (mmol/L) | 0.52 | 0.63 | 0.74 | 0.70 | 0.81 | 0.18 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos, T.L.; Favaretto, J.A.R.; Brunetto, A.L.R.; Zatti, E.; Marchiori, M.S.; Pereira, W.A.B.; Bajay, M.M.; Da Silva, A.S. Dietary Additive Combination for Dairy Calves After Weaning Has a Modulating Effect on the Profile of Short-Chain Fatty Acids in the Rumen and Fecal Microbiota. Fermentation 2024, 10, 528. https://doi.org/10.3390/fermentation10100528
Dos Santos TL, Favaretto JAR, Brunetto ALR, Zatti E, Marchiori MS, Pereira WAB, Bajay MM, Da Silva AS. Dietary Additive Combination for Dairy Calves After Weaning Has a Modulating Effect on the Profile of Short-Chain Fatty Acids in the Rumen and Fecal Microbiota. Fermentation. 2024; 10(10):528. https://doi.org/10.3390/fermentation10100528
Chicago/Turabian StyleDos Santos, Tainara Leticia, Jorge Augusto Rosina Favaretto, Andrei Lucas Rebelatto Brunetto, Emerson Zatti, Maiara Sulzbach Marchiori, Wanderson Adriano Biscola Pereira, Miklos Maximiliano Bajay, and Aleksandro S. Da Silva. 2024. "Dietary Additive Combination for Dairy Calves After Weaning Has a Modulating Effect on the Profile of Short-Chain Fatty Acids in the Rumen and Fecal Microbiota" Fermentation 10, no. 10: 528. https://doi.org/10.3390/fermentation10100528
APA StyleDos Santos, T. L., Favaretto, J. A. R., Brunetto, A. L. R., Zatti, E., Marchiori, M. S., Pereira, W. A. B., Bajay, M. M., & Da Silva, A. S. (2024). Dietary Additive Combination for Dairy Calves After Weaning Has a Modulating Effect on the Profile of Short-Chain Fatty Acids in the Rumen and Fecal Microbiota. Fermentation, 10(10), 528. https://doi.org/10.3390/fermentation10100528






