The Antarctic Yeast Sporobolomyces roseus AL103 as a Promising Source of Health-Promoting Biologically Active Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Media and Growth Conditions
2.3. Biomass Production and Extracts Preparation
2.4. Gas Chromatography/Mass Spectrometry (GC/MS) Profiling
2.5. Cell Lines and Culture Maintenance
2.6. MTT Assay
2.7. Bacterial Strains and Growth Conditions
2.8. Broth Microdilution Test and Redox Activity
3. Results
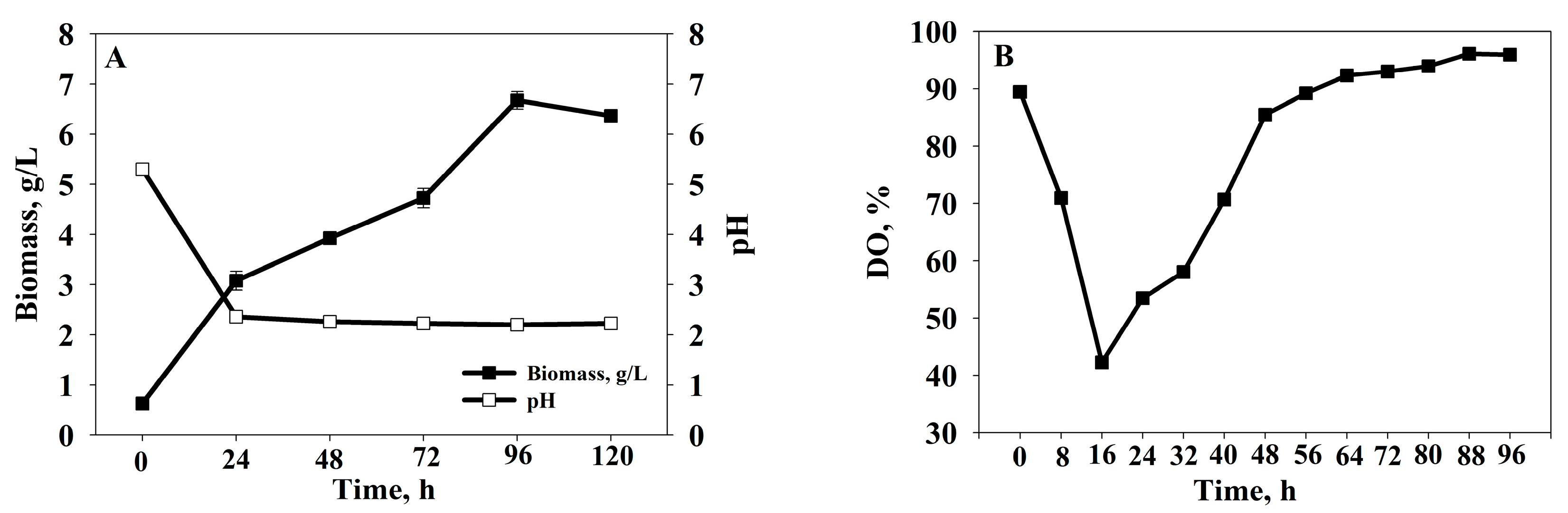
3.1. Submerged Cultivation of Sporobolomyces roseus AL103
3.2. Bioreactor Cultivation of Sporobolomyces roseus AL103
3.3. GC/MS Profiling
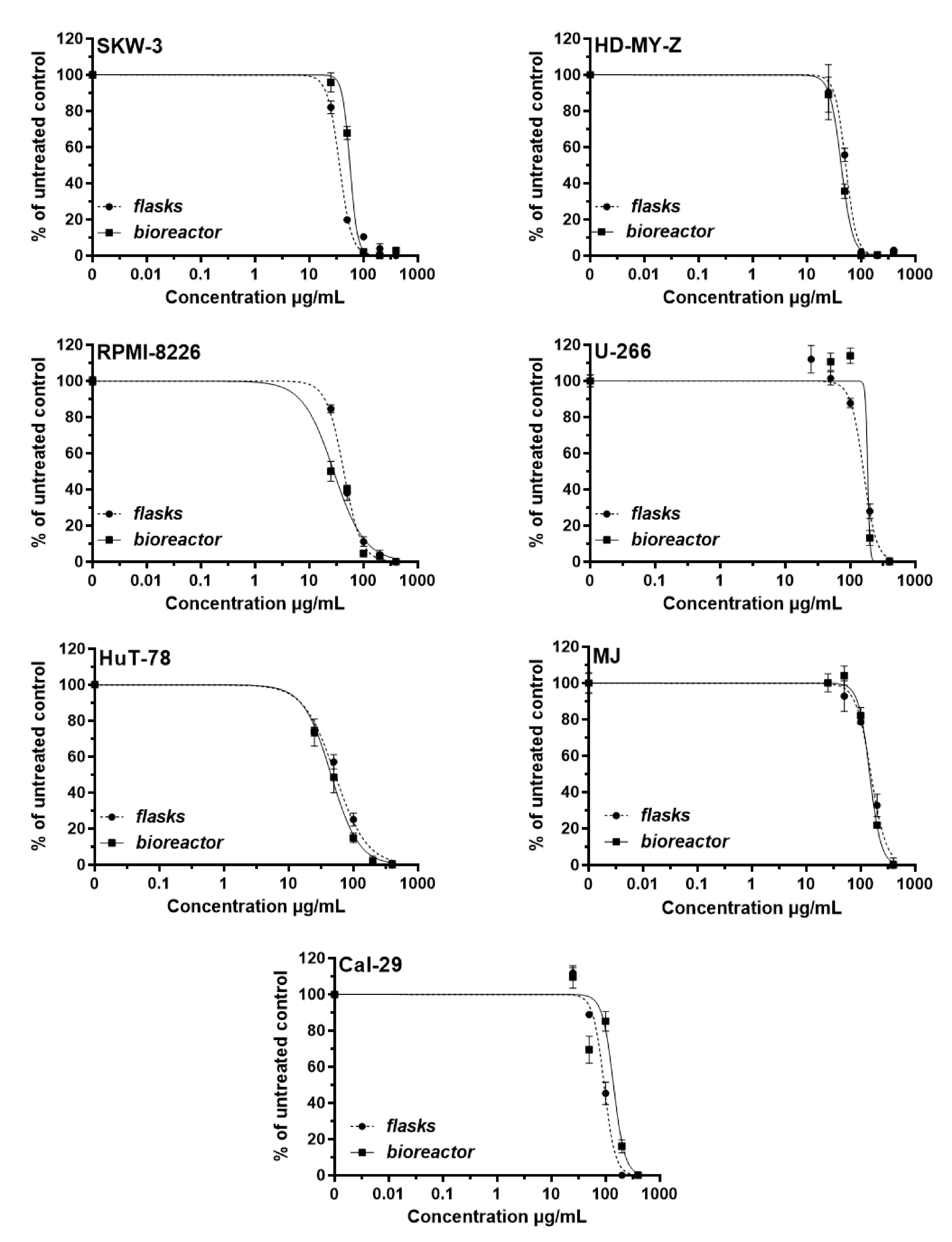
3.4. Antiproliferative Activity of Yeast Extracts on Malignant and Non-Tumorigenic Cell Lines
3.5. Minimal Inhibitory Concentrations (MICs)
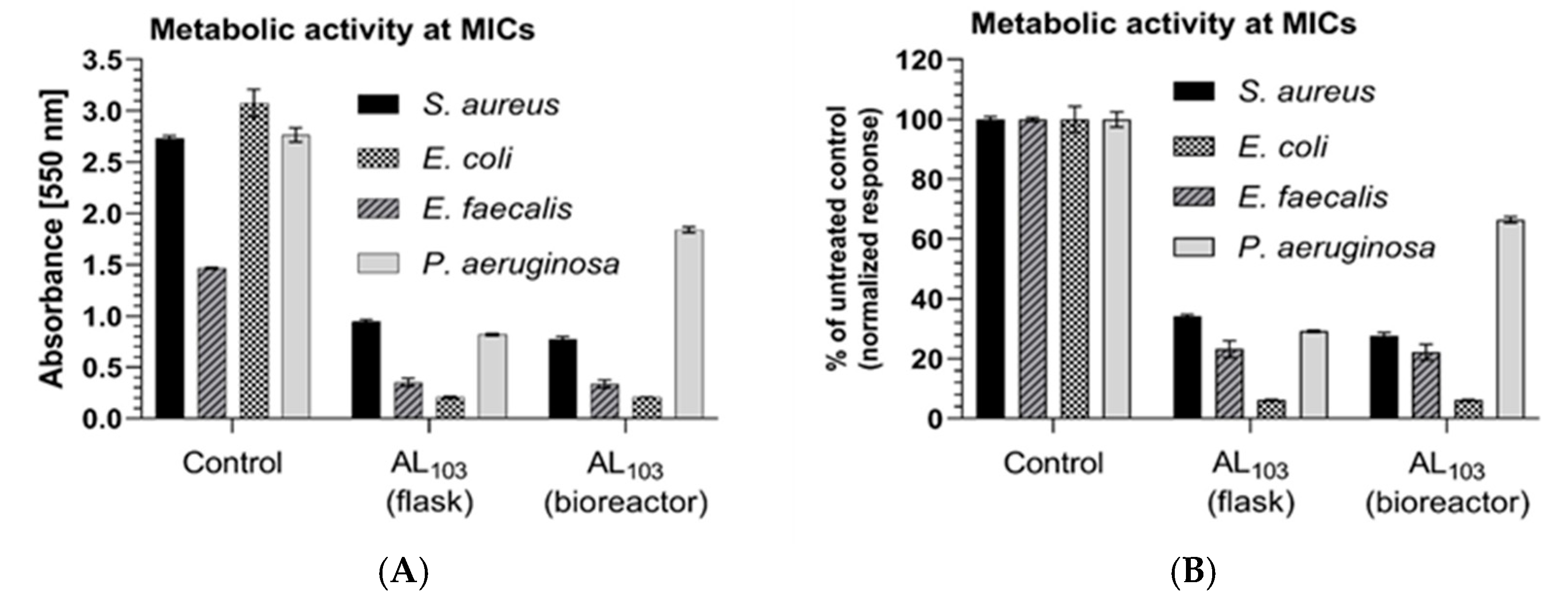
3.6. Inhibition of the Bacterial Metabolic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buzzini, P.; Margesin, R. (Eds.) Cold-adapted yeasts: A lesson from the cold and a challenge for the XXI century. In Cold-Adapted Yeasts; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. [Google Scholar]
- Segal-Kischinevsky, C.; Romero-Aguilar, L.; Alcaraz, L.; Lopez-Ortiz, G.; Martinez-Castillo, B.; Torres-Ramirez, N.; Sandoval, G.; Gonzalez, J. Yeasts inhabiting extreme environments and their biotechnological applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Kambourova, M.; Alipieva, K.; Nachkova, S.; Simova, S. Metabolic profiling of Antarctic yeasts by proton nuclear magnetic resonance-based spectroscopy. Biotechnol. Biotechnol. Eq. 2019, 1, 12–19. [Google Scholar] [CrossRef]
- Zlatanov, M.; Pavlova, K.; Antova, G.; Angelova-Romova, M.; Georgieva, K.; Rusinova-Videva, S. Biomass production by Antarctic yeast strains: An investigation on lipid composition. Biotechnol. Biotechnol. Eq. 2010, 24, 2096–2101. [Google Scholar] [CrossRef]
- Dimitrova, S.; Pavlova, K.; Lukanov, L.; Zagorchev, P. Synthesis of coenzyme Q10 and b-caroten by yeasts isolated from Antarctic soil and lichen in response to ultraviolet and visible radiations. Appl. Biochem. Biothechnol. 2010, 162, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Kieliszek, M.; Piwowarek, K.; Blazejak, S.; Mussagy, C. Sporobolomysec and Sporidiobolus-non-conventional yeasts for use in industries. Fungal Biol. Rev. 2021, 37, 41–58. [Google Scholar] [CrossRef]
- Barahona, S.; Yuivar, Y.; Socias, G.; Alcaino, J.; Cifuentes, V.; Baeza, M. Identification and characterization of yeast isolated from sedimentary rocks of Union Glacier at the Antarctica. Extremophiles 2016, 20, 479–491. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Hristova, D.; Konstantinov, S. Antineoplastic activity of extracts of the Antarctic yeast Sporobolomyces roseus AL103 on urinary bladder cancer cells. C. R. Acad. Bulg. Sci. 2023, 76, 778–788. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer Nov. 2021, 157, 308–347. [Google Scholar] [CrossRef]
- ESIC. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 7 January 2024).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Data and Statistics. 20 April 2020. 2020. Available online: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/data-and-statistics (accessed on 16 January 2024).
- Lymphoma Coalition Europe. Lymphoma Incidence, Mortality and Survival Data for Europe. 2020. Available online: https://www.lymphomacoalition.org/europe (accessed on 20 January 2024).
- American Institute for Cancer Research. Global Cancer Data by Country. 20 April 2020. 2020. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/data-cancer-frequency-country (accessed on 10 January 2024).
- Shweta, S.; Gurumurthy, B.; Ravikanth, G.; Ramanan, U.; Shivanna, M. Endophytic fungi from Miquelia dentate Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomed 2013, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Himaya, S.; Dewapriya, P.; Zhang, C.; Kim, S. Fumigaclavine C from a ma-rine-derived fungus Aspergillus fumigates induces apoptosis in MCF -7 breast cancer cells. Mar. Drugs 2013, 11, 5063–5086. [Google Scholar] [CrossRef] [PubMed]
- Türker, M. Yeast Biotechnology: Diversity and Applications. In Proceedings of the 27th VH Yeast Conference, Advances in Science and Industrial Production of Baker’s Yeast, Istanbul, Turkey, 14–15 April 2014; pp. 1–26. [Google Scholar]
- Saber, A.; Alipou, B.; Faghfoor, Z.; Moisavijam, A.; Khosroushahi, A. Secretion metabo-lites of probiotic yeast, Pichiakudravzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nut. Res. 2017, 41, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Khosroushahi, A. Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different hu-man cancer cell lines and exhibit anti-pathogenic effects. J. Fun. Foods 2017, 34, 408–421. [Google Scholar] [CrossRef]
- Sarkate, A.; Banerjee, S.; Iqbal Mir, J.; Roy, P. Antioxidant and cytotoxic activity of bio-active phenolic metabolites isolated from the yeast-extract treated cell culture of apple. PCTOC 2017, 130, 641–649. [Google Scholar] [CrossRef]
- Andrade, R.; Lima, R.; Ribeaux, D.; Araújo, H.; Franco, L.; Pessoa-Júnior, A.; Cam-pos-Takaki, G. Production of β-carotene by a newly isolated Rhodotorula glutinis UCP1555 strain and cytotoxic effect evaluation. J. Chem. Eng. 2016, 10, 212–220. [Google Scholar] [CrossRef][Green Version]
- McCormack, P.; Wildman, H.; Jeffries, P. Production of antibacterial compounds by phylloplane inhabiting yeasts and yeastlike fungi. Appl. Environ. Microbiol. 1994, 60, 927–931. [Google Scholar] [CrossRef]
- Manimala, M.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Natur. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Ognyanov, M.; Georgiev, Y.; Petrova, A.; Dimitrova, P.; Kambourova, M. Chemical characterization and biological effect of exopolysaccharides synthesized by Antarctic yeasts Cystobasidium ongulense AL101 and Leucosporidium yakuticum AL102 on murine innate immune cells. World J. Microbiol. Biotechnol. 2023, 39, 211–231. [Google Scholar] [CrossRef]
- Dagnon, S.; Novakova, Z.; Bojilov, D.; Nedialkov., P.; Kouassi, C. Development of surro-gate standards approach for the determination of polyphenols in Vernonia amygdalina Del. J. Food Compos. Anal. 2019, 82, 103231. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and application of curcumin from plant materials—A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO20776/1-2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. Technical Committee CEN/TC 140, Technical Committee ISO/TC 21. European Committee for Standardization (CEN): Brussels, Belgium, 2006; p. 19.
- Wang, H.; Cheng, H.; Wang, F.; Wei, D.; Wang, X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J. Microbiol. Methods 2010, 82, 330–333. [Google Scholar] [CrossRef]
- Fazenda, L.; Harvey, L.; McNeil, B. Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum. J. Microbiol. Biotechnol. 2010, 20, 844–851. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Pavlova, K.; Georgieva, K. Effect of different carbon sources on bio-synthesis of exopolysaccharide from Antarctic strain Cryptococcus laurentii AL62. Biotechnol. Biotechnol. Eq. 2011, 25, 80–84. [Google Scholar] [CrossRef][Green Version]
- Rusinova-Videva, S.; Nachkova, S.; Adamov, A.; Dimitrova-Dyulgerova, I. Antarctic yeast Cryptococcus laurentii (AL65): Biomass and exopolysaccharide production and biosorp-tion of metals. J. Chem. Technol. Biotechnol. 2020, 95, 1372–1379. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncagila, L.; De Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Factories 2010, 9, 73. [Google Scholar] [CrossRef]
- Alchihab, M.; Destain, J.; Aguedo, M.; Majad, L.; Ghalfi, H.; Wathelet, J.-P.; Thonart, P. Pro-duction of γ–decalactone by a psychrophilic and a mesophilic strain of the yeast Rhodotorula aurantiaca. Appl. Biochem. Biotechnol. 2009, 158, 41–50. [Google Scholar] [CrossRef]
- Dalmazzo, L.; Santana-Lemos, B.; Jacomo, R.; Garcia, A.; Rego, E.; Da Fonseca, L.; Falcao, R. Antibody-targeted horseradish peroxidase associated with indole-3-acetic acid induces apoptosis in vitro in hematological malignancies. Leukemia Res. 2011, 35, 657–662. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, S.; Park, K. Oxidation of Indol-3-acetic acid by horseradish peroxidase iduses apoptoses in G361 human melanoma cells. Cell. Sign 2003, 16, 81–88. [Google Scholar] [CrossRef]
- Kalawaj, K.; Slawinska-Brych, A.; Mizerska-Kowalska, M.; Zurek, A.; Bojarska-Junak, A.; Kandefer-Szerszen, M.; Zdzisinska, B. Alpha- ketoglutarate exerts in vitro an-ti-osteosarcoma effect through inhibition of cell proliferation, induction of apoptosis via the JNK and Caspase 9-dependent mechanism, and suppression of TGF-β and VEGF production and metastatic potential of cells. Int. J. Mol. Sci. 2020, 21, 9406. [Google Scholar] [CrossRef]
- Zdzisinska, B.; Zurek, A.; Kandefer-Szerszen, M. Alpha-ketoglutarate as a molecule with pleiotropic activity: Well-know and novel possibilities of therapeutic use. Arch. Immunol. Ther. Ex. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Abla, H.; Sollazzo, M.; Gasparre, G.; Iommarini, L.; Porcelli, A. The multifaceted con-tribution of α–ketoglutarate to tumor progression: An opportunity to exploit. Sem. Cell Dev. Biol. 2020, 98, 26–33. [Google Scholar] [CrossRef]
- To, N.; Nguyen, Y.; Moon, J.; Ediriweera, M.; Cho, S. Pentadecanoic acid, an odd-chain fat-ty acid, suppresses the stemness of MCF-7/SC Human Breast cancer stem-like cells through JAK2/STAT3 signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Phonnok, S.; Uthaisang-Tanechpongtamb, W.; Wongsatayanon, T. Anticancer and apoptosis-inducing activities of microbial metabolites. Electron. J. Biotechnol. 2010, 13, 1–2. [Google Scholar] [CrossRef][Green Version]
- Thomas, T.; Rao, J.; Subrahmanyam, M.; Chandrashekhar, H.; Maliyakkal, N.; Kisan, K.; Joseph, A.; Udupa, N. In vitro anticancer activity of microbial isolates from diverse habi-tats. Braz J. Pharm. Sci. 2011, 47, 279–287. [Google Scholar] [CrossRef]
- Heydari, H.; Koc, A.; Simsek, D.; Gozcelioglu, B.; Altanlar, N.; Konuklugil, B. Isolation, identification and bioactivity screening of Turkish marine-derived fungi. Farmacia 2019, 67, 780–788. [Google Scholar] [CrossRef]
- Sanzani, S.; Sgaramella, M.; Mosca, S.; Solfrozzo, M.; Ippolito, A. Control of Penicillium expansum by an Epiphytic basidiomycetous yeast. Horticulturae 2021, 7, 473. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Zhou, J.; Velliou, E.; Hong, S.H. Investigating the effects of nisin and free fatty acid combined treatment on Listeria monocytogenes inactivation. LWT 2020, 133, 110115. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.; Kaleva, M.; Georgieva, A.; et al. Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept. Molecules 2022, 27, 519. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Again. Microb. Path. 2011, 1, 61–71. [Google Scholar] [CrossRef]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef]

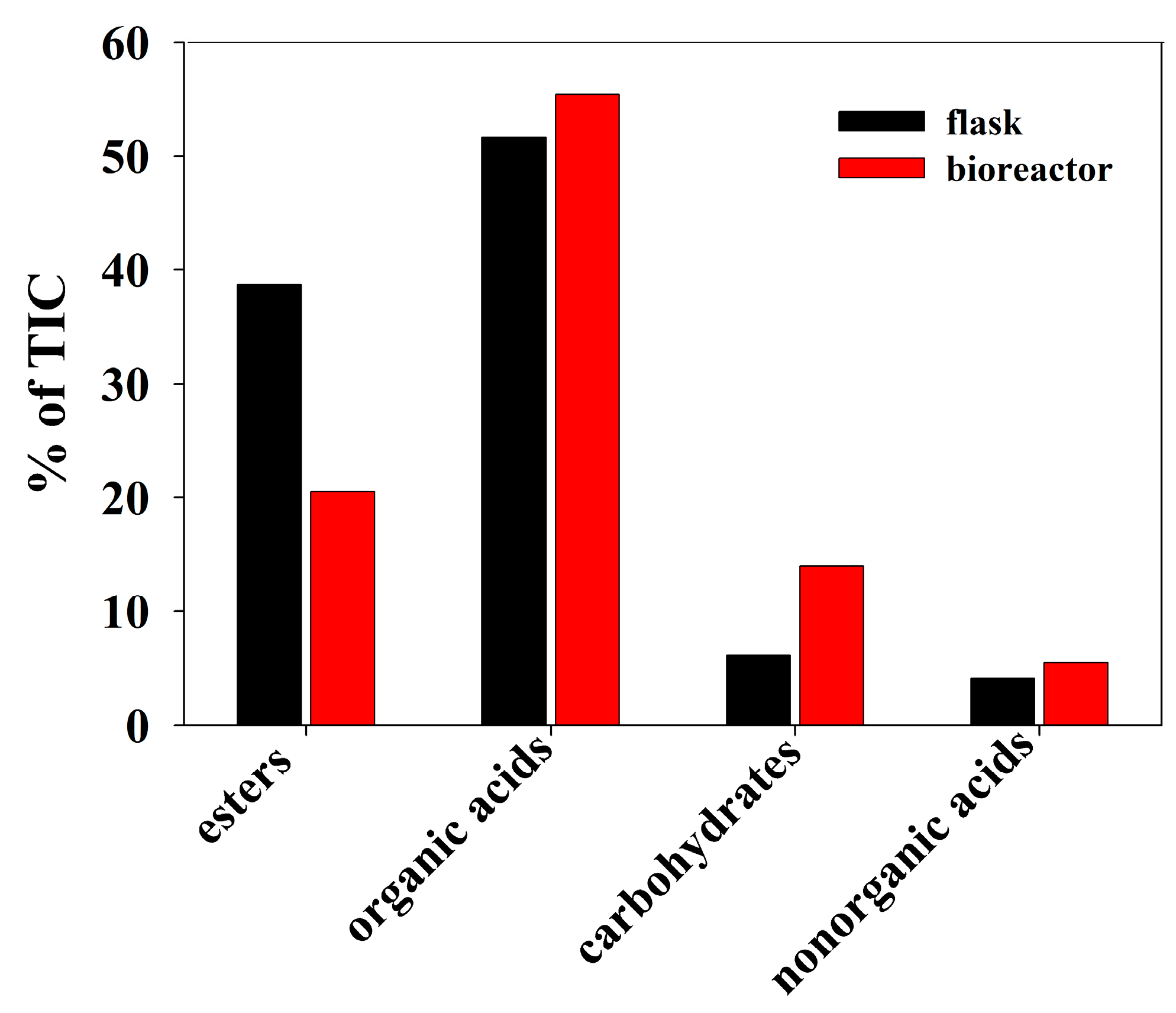
| Peak. | RT | RI | Name | % of TIC | |
|---|---|---|---|---|---|
| Flask | Bioreactor | ||||
| 1 | 4.605 | 1075 | Glycolic acid | 0.711 | 1.574 |
| 2 | 5.04 | 1157 | 3-Hydroxypropanoic acid | 0.332 | 0.074 |
| 3 | 5.309 | 1242 | 4-Hydroxybutanoic acid | 0.257 | 0.123 |
| 4 | 5.425 | 1266 | Phosphoric acid | 4.126 | 5.5022 |
| 5 | 7.053 | 1390 | 2-Methyl-2-butenedioic acid | 0.401 | 0.11 |
| 6 | 7.239 | 1414 | 2,4-Dihydroxybutanoic acid | 0.765 | 0.199 |
| 7 | 8.281 | 1580 | 2-Ketoglutaric acid | 0.594 | 0.373 |
| 8 | 9.187 | 1608 | 2,3-Dihydroxybutanedioic acid | 0.322 | 0.115 |
| 9 | 9.763 | 1631 | 2,4,5-Trihydroxypentanoic acid | 1.226 | 2.522 |
| 10 | 10.242 | 1660 | Arabinose | 1.27 | 0.58 |
| 11 | 10.388 | 1672 | Arabinose | 2.679 | 1.039 |
| 12 | 10.719 | 1720 | Phenylpyruvic acid | 2.508 | 4.017 |
| 13 | 10.852 | 1756 | cis-Aconitic acid | 3.538 | 2.766 |
| 14 | 11.159 | 1848 | 2-Keto-L-gulonic acid | 0.455 | 1.517 |
| 15 | 11.279 | 1870 | Fructose | 0.99 | 5.154 |
| 16 | 11.33 | 1881 | Fructose | 0.534 | 3.829 |
| 17 | 11.762 | 1897 | Glucose | 0.359 | 3.156 |
| 18 | 11.879 | 1905 | Glucose | 0.297 | 0.245 |
| 19 | 12.581 | 1946 | Pentadecanoic acid | 0.216 | 0.402 |
| 20 | 12.705 | 1958 | Methyl pentanoate | 2.773 | 0.187 |
| 21 | 13.024 | 1970 | Indole-3-acetic acid | 0.9729 | 1.531 |
| 22 | 13.58 | 1981 | Gluconic acid | 1.5088 | 0.133 |
| 23 | 13.832 | 1999 | Methyl palmitate | 0.12 | 0.736 |
| 24 | 14.903 | 2040 | Palmitic acid | 17.938 | 13.566 |
| 25 | 15.71 | 2068 | Methyl linoleate | 0.532 | 0.62 |
| 26 | 15.83 | 2079 | Methyl oleate | 0.155 | 0.113 |
| 27 | 16.709 | 2090 | Methyl 11-octadecenoate | 34.265 | 18.625 |
| 28 | 16.932 | 2135 | Methyl stearate | 0.837 | 0.246 |
| 29 | 17.635 | 2197 | Linoleic acid | 3.651 | 3.814 |
| 30 | 17.776 | 2202 | Oleic acid | 14.413 | 20.683 |
| 31 | 18.121 | 2236 | Stearic acid | 1.541 | 1.6216 |
| 32 | 22.02 | 2441 | Gluconic acid-6-phosphate | 0.3 | 0.245 |
| Cell Lines | Parameters Evaluated After Both Types of Cultivation | |||||
|---|---|---|---|---|---|---|
| Flasks | Bioreactor | |||||
| IC50 (µg/mL) | 95% CI | R | IC50 (µg/mL) | 95% CI | R | |
| SKW-3 (KE-37) | 35.3 | 33.15–37.59 | 0.96 | 55.9 | 51.69–60.45 | 0.98 |
| HD-MY-Z | 52.10 | 48.97–55.42 | 0.97 | 43.01 | 40.94–45.18 | 0.99 |
| RPMI-8226 | 42.33 | 40.0–44.79 | 0.98 | 28.27 | 23.77–33.61 | 0.95 |
| U-266 | 163.0 | 147.7–179.9 | 0.96 | 185.5 | 160.5–210.5 | 0.95 |
| HuT-78 | 53.23 | 48.09–58.92 | 0.96 | 45.89 | 42.32–49.76 | 0.98 |
| MJ | 154.5 | 136.8–174.4 | 0.95 | 146.2 | 131.0–163.3 | 0.97 |
| CAL-29 | 94.18 | 87.81–101.0 | 0.98 | 140.2 | 115.7–169.9 | 0.9 |
| Bacterial Strain | MICs* [mg/mL] | |||
|---|---|---|---|---|
| S. roseus × Flask | S. roseus × Bioreactor | Referent Antibiotics | ||
| INN | Value | |||
| Staphylococcus aureus (ATCC 29213) | 2.5 | 5 | Gentamicin | 0.00025 |
| Enterococcus faecalis (ATCC 29212) | 5 | 5 | Penicillin | 0.0025 |
| Escherichia coli (25922) | 5 | 5 | Gentamicin | 0.002 |
| Pseudomonas aeruginosa (ATCC 27853) | 5 | >5 | Gentamicin | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinova-Videva, S.; Zaharieva, M.M.; Hristova, D.; Nachkova, S.; Kambourova, M.; Najdenski, H.; Konstantinov, S. The Antarctic Yeast Sporobolomyces roseus AL103 as a Promising Source of Health-Promoting Biologically Active Compounds. Fermentation 2024, 10, 508. https://doi.org/10.3390/fermentation10100508
Rusinova-Videva S, Zaharieva MM, Hristova D, Nachkova S, Kambourova M, Najdenski H, Konstantinov S. The Antarctic Yeast Sporobolomyces roseus AL103 as a Promising Source of Health-Promoting Biologically Active Compounds. Fermentation. 2024; 10(10):508. https://doi.org/10.3390/fermentation10100508
Chicago/Turabian StyleRusinova-Videva, Snezhana, Maya M. Zaharieva, Dilyana Hristova, Stefka Nachkova, Margarita Kambourova, Hristo Najdenski, and Spiro Konstantinov. 2024. "The Antarctic Yeast Sporobolomyces roseus AL103 as a Promising Source of Health-Promoting Biologically Active Compounds" Fermentation 10, no. 10: 508. https://doi.org/10.3390/fermentation10100508
APA StyleRusinova-Videva, S., Zaharieva, M. M., Hristova, D., Nachkova, S., Kambourova, M., Najdenski, H., & Konstantinov, S. (2024). The Antarctic Yeast Sporobolomyces roseus AL103 as a Promising Source of Health-Promoting Biologically Active Compounds. Fermentation, 10(10), 508. https://doi.org/10.3390/fermentation10100508








