4.1. Gas Production, Greenhouse Gases, and Dry Matter Disappearance
Dry matter disappearance is an indication that the diet would be better utilized and release more nutrients to the animal [
29]. In this study, we did not observe any significant effect with the inclusion of Gos and Gosmos on the DMD of the substrate. The values for gas volume maintained a similar trend as the DMD values and corroborated the findings as gas production is an indication of microbial activity on the substrate and is a marker for digestibility. Recently, Alabi et al. [
16] reported similar weak effects of galactooligosaccharides and/or mannan-oligosaccharides on in vitro gas production and nutrient degradability (i.e., DM, NDF, ADF, and ADL degradabilities). Several forms of oligosaccharides are useful during digestion where they have positive influences and reduce the activities of unfavorable microbes in the rumen that could impede feed breakdown [
30]. One possible explanation for the weak effects of the oligosaccharides on DMD is that the inclusion level of the oligosaccharides was too low to elicit an effect and the low quantities may have simply been degraded by rumen microbes. This observation may also be corroborated by the trend observed in the abundance of the phyla in this study, which followed the same trend as the fermentation parameters, with the oligosaccharide treatments having statistically similar values as the control.
The inclusion of Eofumaric reduced the DMD and in vitro gas volume. However, Alabi et al. [
2] reported inconsistent results compared to the present study, where the administration of EOB and fumaric acid mixture at 10 µL EO/g feed and 3% fumaric into diets had no negative effects on in vitro gas production or DMD. The differences in results may be attributed to the higher dose of additives in the present study (200 μL EO/g feed and fumaric acid at 3%), as well as the variation in the experimental methods (RUSITEC vs. batch culture) and incubation period (9 days in the previous study vs. 24 h in the present study). The negative effects of EOBs and fumaric acids on DMD were previously observed [
5]. It was expected that mixing fumaric acid with EOBs would work synergistically to increase gas production and DMD; however, this effect was not observed, indicating that the antimicrobial effects of EOBs masked the positive effects of fumaric on ruminal microbes. The active biomolecules in the EOB in the Eofumaric treatment could potentially have diminished the microbial population involved in the degradation of feed. Essential oils comprise terpenes and terpenoids, and these compounds possess antimicrobial properties [
1,
31] against some ruminal microbes. These compounds in the essential oil blend may have caused the marked significant reduction in the phylum
Fibrobacterota in this study.
Fibrobacterota currently includes one officially recognized genus, which is
Fibrobacter. One common species found in cattle notable for degradation of fibrous materials is
Fibrobacter succinogenes [
32]. Kobayashi et al. [
33] reported that
Fibrobacter succinogenes is the primary fibrolytic species crucial for rumen fiber digestion. Fibrolytic bacteria could be adversely affected by elevated levels of phenolic compounds found in EOBs, potentially leading to a reduction in fiber disappearance when these EOB supplements are used [
1].
The inclusion of Eofumaric caused a reduction in greenhouse gasses. Consistent with these findings, Alabi et al. [
5] observed that the combination of EOBs and fumaric acid decreased CH
4 and CO
2 emissions during in vitro fermentation of a beef cow diet. However, Alabi [
2,
16] reported minimal effect with the administration of a mixture of EOBs at 3 µL or 10 µL/g and fumaric acid at 3% on CH
4, CO
2, NH
3, and H
2S production. As previously mentioned, the differences in experimental conditions could be the primary reason for inconsistency in the results. Methane is a byproduct of ruminal fermentation and is an indication of a loss of the total energy consumed by ruminants, accounting for 2–12% of the total energy [
34]. Fumaric acid has an important role in the succinate–propionate pathway and propionic acid precursor, which acts as an alternative H
2 sink to reduce enteric CH
4 production. Moreover, essential oils impact rumen fermentation by modifying microbial communities [
1,
30]. In their experiment, Kouazounde et al. [
35] reported rumen microbial communities were modified with the administration of essential oils, positively impacting rumen fermentation and reducing greenhouse gas emissions. Surprisingly, the abundance of total archaea was not significantly different between the treatments. However, there were some significant differences at the family/genus level. Overall, the pattern showed that the abundance of the members of
Euryarchaeota was higher with the inclusion of Eofumaric while members of
Thermoplasmatota were lower. It therefore seems logical that the abundance of archaea would exhibit the strongest correlation with methane emissions. However, studies have contradicted this assumption indicating that CH
4 production is not based solely on methanogen abundance but on the interaction between the community dynamics and abundance. For example, it has been shown that there is no significant relationship between the abundance of methanogens and CH
4 emissions in dairy cows [
36]. One implication could be that the metabolic potential of individual methanogens holds more credibility in explaining CH
4 output rather than the abundance.
In the present study,
Methanobrevibacter was the dominant methanogen, and its abundance was numerically higher than that of the control. Next to the
Methanobrevibacter group, members of the order
Methanomassiliicoccales were the most abundant in the rumen. Despite their lower abundance,
Methanomassiliicoccales are believed to be very efficient CH
4 producers due to their lower hydrogen threshold, which allows them to function at lower H concentrations [
37]. It is possible that the EOB in the Eofumaric treatment was able to inhibit the activities of this group of methanogens shown by their significantly lower abundance compared to the control and resulted in lower methane levels in this study. To buttress this, a 2.3 negative fold-change for an uncultured genus belonging to the family
Methanomethylophilaceae (order
Methanomassiliicoccales) was also observed in this study. High abundance of
Methanobrevibacter spp. has been reported to have a positive correlation with low CH
4, indicating a less contributory role of this group of methanogens to CH
4 emission [
36]. The trend observed in this study for CH
4 production and methanogen abundance is very similar to the findings of Liu et al. [
38] with 3NOP and fumarate where 3NOP in combination with fumarate increased the abundance of a given OTU and reduced the abundance of another, thereby buttressing the equal importance of archaea composition and structure in explaining ruminal CH
4 production dynamics. Overall, it is possible that the EOB used in this study functioned similarly to 3NOP in deactivating methyl-coenzyme M reductase but additionally directly inhibited the growth of the members of the
Methanomassiliicoccales order.
The reduced production of ruminal NH
3 with Eofumaric treatment indicates less dietary protein degradation and amino acid deamination. Essential oils have the ability to terminate the activity and growth of Gram-positive proteolytic bacteria [
1,
39]. Alabi et al. [
5] reported significant decreases in ruminal NH
3 production with an EOB and fumaric acid. Methane and CO
2 production in the present study showed that the Eofumaric inclusion in livestock diets could reduce energy loss and reduce greenhouse gas emissions from cattle that play an important role in global warming due to their harmful effects on the ozone layer [
40].
4.2. Volatile Fatty Acids
The inclusion of Eofumaric additives decreased total VFA, which may be due to their negative effects on DMD and other nutrient disappearance and the efficiency of microbial mass production. An inverse relationship between the concentration of total VFA and efficiency of microbial mass production has been reported previously [
41]. Similar findings were reported by Alabi et al. [
5], who observed that the combination of EOBs and fumaric acid decreased total VFA concentration during in vitro fermentation of a beef cow diet. The reduction in propionate observed in the present study was unexpected as this treatment had improved propionate in a previous study [
17]. Essential oils are touted to have more antimicrobial effects against Gram-positive bacteria than Gram-negative bacteria, which favors the growth of Gram-negative bacteria [
1,
39], but this was not generally observed in this study as the bacteroidota phylum, which are mostly Gram-negative, was significantly reduced. Gram-negative bacteria in the rumen are more often associated with the propionate pathway [
42]. A possible explanation may be due to the modified batch culture system used in this study where the rumen solid was included in the rumen content used for the experiment, which might have introduced an additional microbial consortium. Regular fermentation and digestion in the rumen include close interactions between the microbes that attach to the fibrous plant materials and those suspended in the rumen fluid and this interaction is facilitated by the contractions and subsequent mixing of digesta fractions, which leads to a closer relationship between the communities [
43]. Sbardellati et al. [
44] reported that there are variations in the microbial community among the different rumen geographic regions.
The major rumen fiber degraders are known to mostly attach to the feed materials. Overall, the lower total VFAs and propionate are a reflection of the lower digestibility values recorded for Eofumaric and might have been a result of the marked lower abundance of several taxa observed in this study, including
Prevotella,
Fibrobacter,
Succinimonas,
Treponema,
Selenomonas and
Ruminobacter.
Prevotella is among the most abundant rumen microbes and this taxon can degrade diverse polysaccharides and possess the ability to produce VFAs, with propionate being the major VFA, which serves as a crucial substrate for gluconeogenesis in the livers of ruminant animals [
45].
Selenomonas enhances fiber digestion when it is cultured alongside
R. flavefaciens and
Fibrobacter succinogenes by converting succinate, a metabolite produced by
R. flavefaciens and
Fibrobacter succinogenes, into propionate [
46].
Succinimonas is also a succinate producer and contributes to the succinate pool in the rumen [
47]. At the phylum level, the marked decrease in
Bacteroidota and
Proteobacteria phyla, both of which participate in the succinate pathway, might have contributed to the lower propionate value of the Eofumaric treatment [
48]. Ranilla et al. [
49] reported propionate molar fractions of 13.6–15.0 and 12.0–13.7, which are lower than the values recorded in this current study. It had been reported that the inclusion of dietary oligosaccharides could regulate the composition of ruminal microbiota and alter rumen fermentation [
50] but this was not exactly the case in this study as the oligosaccharides used in this study recorded statistically similar total and individual VFA molar fractions as the control.
4.3. Ruminal Bacteria
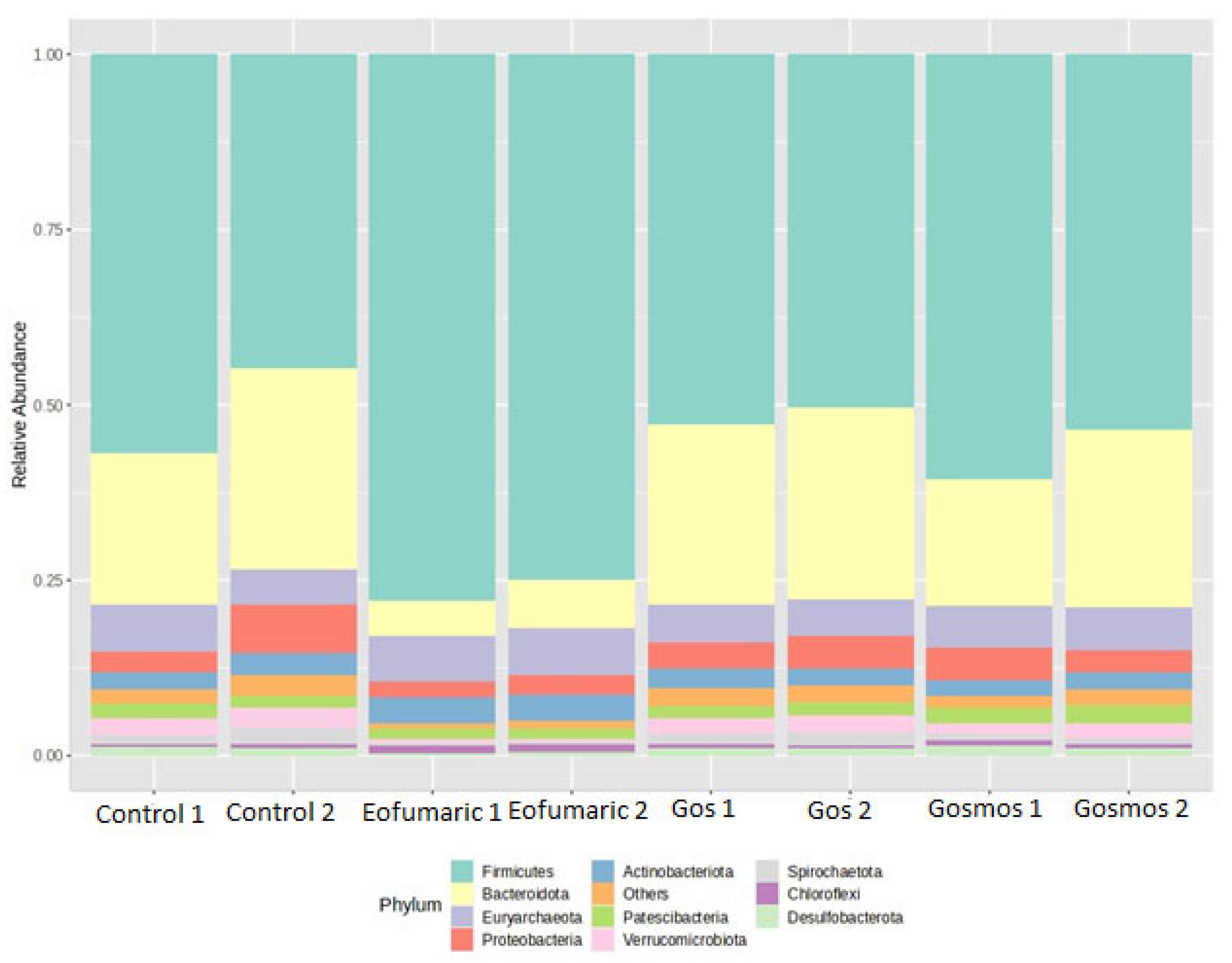
There were no significant differences in the bacteria and archaea domains observed in this study. According to Thomas et al. [
51], the use of additives at higher taxonomic levels such as the phylum level may not bring about a significant effect. Results in the present study showed that
Firmicutes was the dominant phylum. This is in contrast to the results of Deusch et al. [
52] where
Bacteroidetes was the most abundant phyla in a corn silage-based diet. Animal differences may account for the differences as that study used lactating Jersey cows. The inclusion of Eofumaric significantly increased the abundance of
Firmicutes by 30% and
Bacteroidota by 77% compared to the control. Essential oils are known to exhibit strong antibacterial properties with more antimicrobial activity against Gram-positive bacteria compared to Gram-negative bacteria [
53]. This, however, negates the trend observed in the current study. Though most rumen microbes occur in both the rumen liquid and solid fractions, it is noteworthy that
Bacteroidota (Gram-negative) are more established in the rumen fluid while firmicutes (Gram-positive) more often adhere to the plant fiber. The in vitro batch culture set up used in this study is such that the essential oils have greater access and surface area to interact with the planktonic microbes compared to the solids and this may have caused the marked reduction in
Bacteroidota. Tavares et al. [
39] reported that cinnamon leaf essential oil inhibited both Gram-positive and Gram-negative bacteria even at low concentrations. Eofumaric also significantly reduced the abundance of
Spirochaetota,
Fibrobacterota,
Verrucomicrobiota, and
Desulfobacterota compared to the control based on the Z-score differential abundance. These are known to be fiber-adherent taxa [
54] but have less ruminal presence compared to firmicutes. It is also possible that the reduction in the abundance of these fewer taxa created a competitive advantage for the
Firmicutes to proliferate, hence the increase in abundance of this phylum. Microbes belonging to the
Verrucomicrobiota phylum exhibit extensive activity in breaking down lignocellulose and utilizing sugars, producing an array of VFAs, indicating their potential to play a substantial role in the functioning of the rumen [
55]. Rubino et al. [
56] indicated that the rumen microbiome is in constant competition for environmental conditions and resources. On the contrary,
Patescibacteria,
Synergistota,
Chloroflexi,
Actinobacteriota,
Firmicutes,
and Euryarchaeota were differentially higher than the control based on the Z-score heatmap.
Patescibacteria was recently found to be a dominant phylum in Holstein dairy cow for the first time [
57]. They indicated a notable finding that total protein was significantly positively correlated with the abundance of
Patescibacteria, indicating that this phylum may indirectly facilitate the absorption of total protein.
The inclusion of Eofumaric resulted in a decrease in alpha and beta diversity indices measured in the present study. This was expected due to the significant reduction in the abundance of several taxa. This is similar to the findings of Thomas et al. [
51] where the inclusion of antibiotic feed additives had an inverse relationship with Shannon and Simpson indices, which had lower values for the antibiotic treatments. In another study, monensin also reduced bacteria diversity [
58]. The Shannon Diversity Index serves as a measure of biodiversity or species diversity within a specific ecosystem or community and is popularly used to assess the diversity and distribution of species in a given area [
59]. By providing a single numerical value, the Shannon Diversity Index effectively encapsulates the complexity and diversity of a community, taking into consideration both the total number of individual species present in a given microbial community, which is the species richness, and the relative abundance or proportion of each species, known as the species evenness [
60]. Higher values of the Shannon index indicate greater species diversity, implying that more species are present in relatively equal abundance. On the contrary, lower values indicate less diversity, either due to fewer species present where one or a few species dominate the community. This trend was observed in the present study with Eofumaric treatment being dominated by firmicutes. Faith’s Phylogenetic Diversity assesses the evolutionary relatedness of species within a population or ecosystem and while valuable, this measurement does not consider features crucial to ecosystem stability and functional diversity [
61]. Hence, the variety of functions carried out by species could be missed and therefore, it is often paired with other metrics to gain a comprehensive understanding of biodiversity. The higher the phylogenetic diversity value, the greater the diversity of the features observed in the community. A single organism is incapable of fully metabolizing all the organic material ingested by host animals. Instead, this process relies on the functional capabilities and cooperative interactions of diverse microorganisms working sequentially. Bacterial populations work together to produce a spectrum of fibrolytic enzymes, which are subsequently metabolized into volatile fatty acids and microbial proteins, and therefore a more diverse microbial community is generally preferred [
62].
The trend for beta diversity, visualized using the Bray–Curtis 2D plot, showed that Eofumaric and the control were closely aligned with each other and clearly separated from the other samples, which were more clustered together. This means that the species composition when Eofumaric was added was more dissimilar than the other treatments. In a Bray–Curtis 2D plot, which is a Principal Coordinate Analysis (PCoA) plot, both axes explain an aspect of variation among the samples with the horizontal axis, often referred to as the first principal component explaining the largest amount of the variation [
63]. Every point on the x-axis represents a distinct sample from the dataset. The species composition of samples is more similar when they are closer together along the x-axis, while samples that are farther away show less similarity. PCoA is a useful tool for exploring and visualizing relationships and clusters between samples based on their pairwise dissimilarities [
64].
The Random Forest Classification showed that five phyla (
Fibrobacterota,
Spirochaetota,
Firmicutes,
Elusimicrobiota,
Chloroflexi) were above 0.01 mean decrease accuracy. Out of these, Eofumaric showed lower relative abundances for
Fibrobacterota,
Spirochaetota, and
Elusimicrobiota and higher relative abundances for
Firmicutes and
Chloroflexi, indicating that these features had more importance in explaining the observations in this study, therefore corroborating the previously observed trends so far.
Spirochaetota primarily engages in the breakdown of complex polysaccharides found in plant cell walls, proteins, and the synthesis of B vitamins within the rumen [
65].
Treponema, which is a genus in the
Spirochaetota phylum, produces propionate as the major VFA [
66]. Generally, B vitamins obtained from the TMR and synthesized by the rumen microflora of lactating dairy cows are often sufficient to meet the nutritional needs of the animal [
67]. The Random Forest Classification plot is a visualization tool used to understand the importance of different features in classifying sample groups. The Mean Decrease Accuracy values on the x-axis measures the importance of each feature, with higher values indicating that a feature contributes more to the accuracy of the classification model and is considered more important for distinguishing between sample groups [
68].
The observations from the Z-score-based top twenty differentially abundant genera based on adjusted
p value between the control and Eofumaric samples further corroborate the findings from the present study.
Streptococcus,
Coprococcus,
Vibrio, and
Catenibacterium were higher when Eofumaric was added to the diet while the rest of the observed taxa were differentially lower in abundance.
Coprococcus are Gram-positive bacteria involved in the breakdown of complex carbohydrates such as cellulose, hemicellulose, and pectin yielding butyric acid, followed by acetic and propionic acids in decreasing proportions [
69]. This observation may also explain the higher value of butyrate obtained in this study for Eofumaric. Butyric acid, a short-chain fatty acid, serves as a vital energy source for the epithelial cells lining the rumen and holds significant importance in rumen fermentation and overall digestive well-being in ruminant species [
70].
Catenibacterium is a Gram-positive that has shown a positive correlation with lactate and may utilize a wide range of substrates [
71]. Lactate and formate are substrates that compete for the same active site on methyl-coenzyme M reductase such that elevated lactate levels interfere with formate which is the preferred substrate in methanogenesis, reducing formate availability and hence the potential to inhibit methane production [
72].
Ruminobacter,
Fibrobacter,
Prevotella, and
Treponema were all differentially reduced with the inclusion of Eofumaric. These genera are involved actively in the propionate pathway encoding succinate CoA synthetase and propionyl CoA carboxylase, which are key enzymes involved in this pathway [
8,
66]. Ruminobacter is mainly known for amylolytic activities [
73]. Z-scores enable the normalization of abundance values across various features and sample groups, facilitating the identification of features that consistently exhibit differential abundance across multiple comparisons [
74].