Neutral Red Film Augments Extracellular Electron Transfer Performed by Clostridium pasteurianum DSM 525
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cell Growth and Culture Medium
2.2. Glycerol-Fed C. pasteurianum DSM 525-Based MFC Setup (MFCDSM)
2.3. Electrochemical Measurements in MFCDSM
2.4. NR or MV as Mediator
2.5. FNR or FMV Deposition on the MFCDSM Anode
2.6. Electrochemical Setup and Measurements Obtained by Using C. pasteurianum DSM 525 Alone or in the Presence of NR, MV, FNR, or FMV
3. Results and Discussion
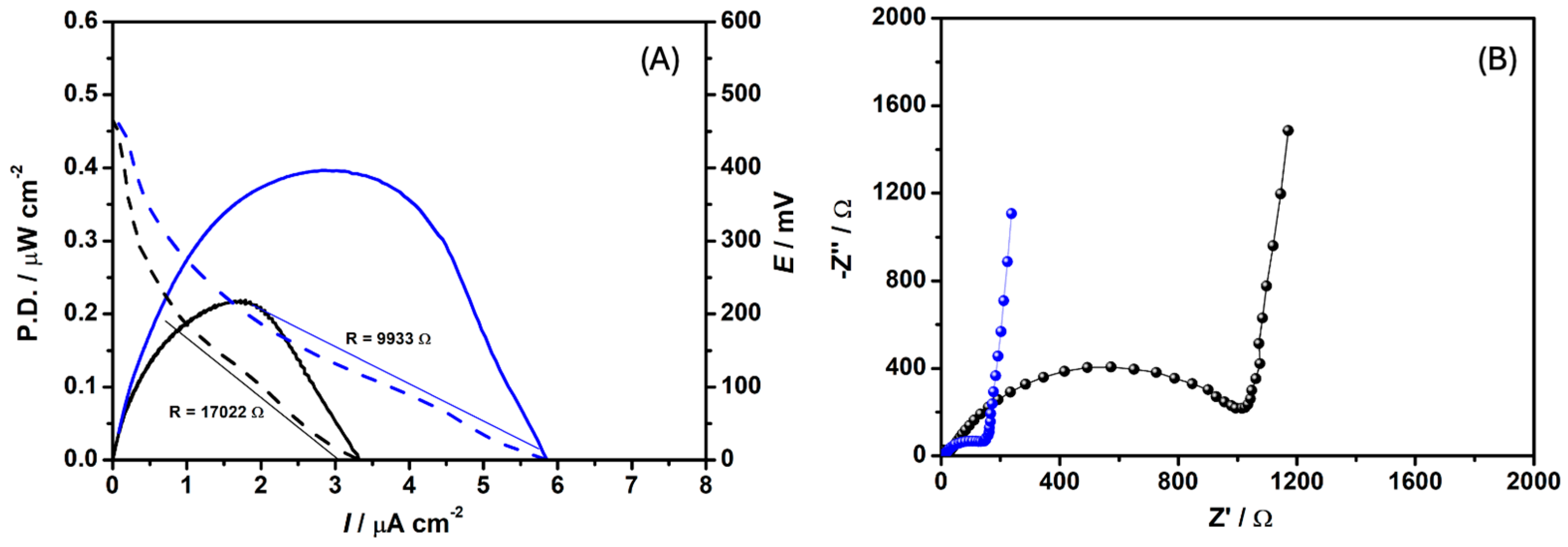
3.1. C. pasteurianum Electroactivity in MFCDSM
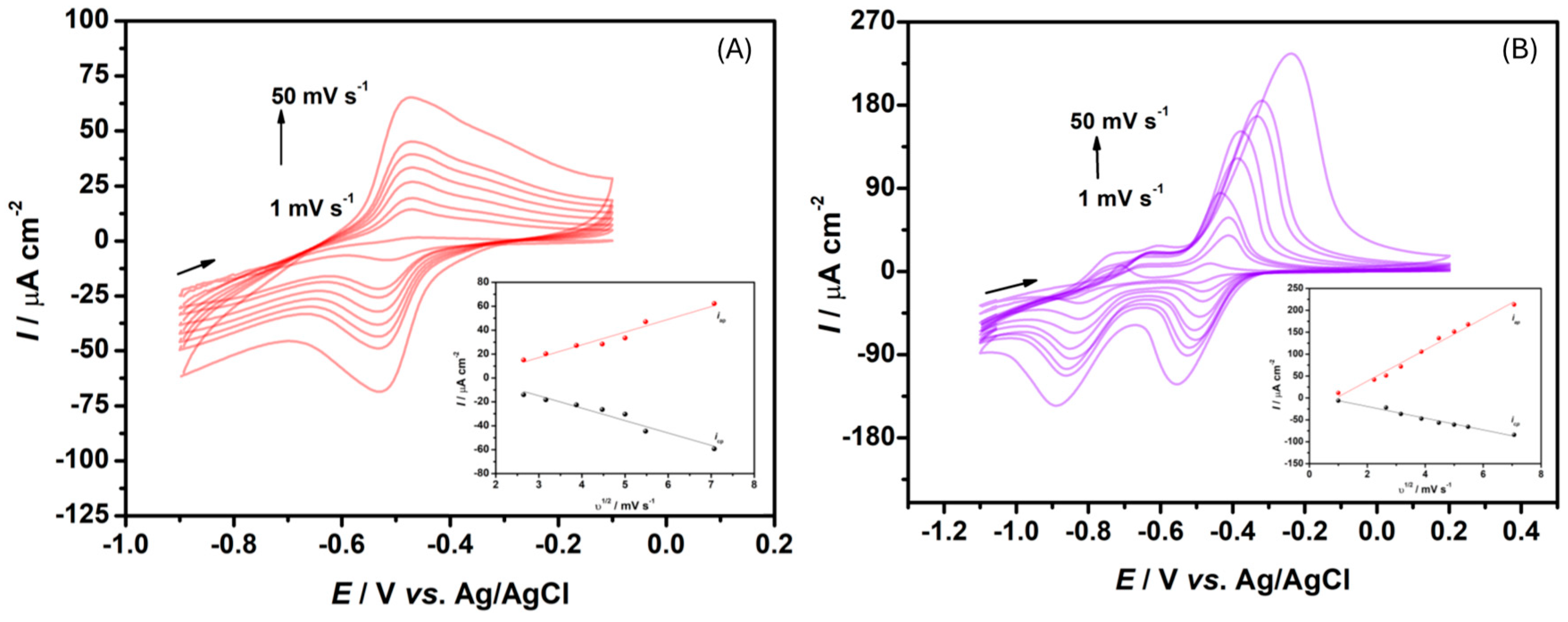
3.2. NR and MV Electrochemical Characterization
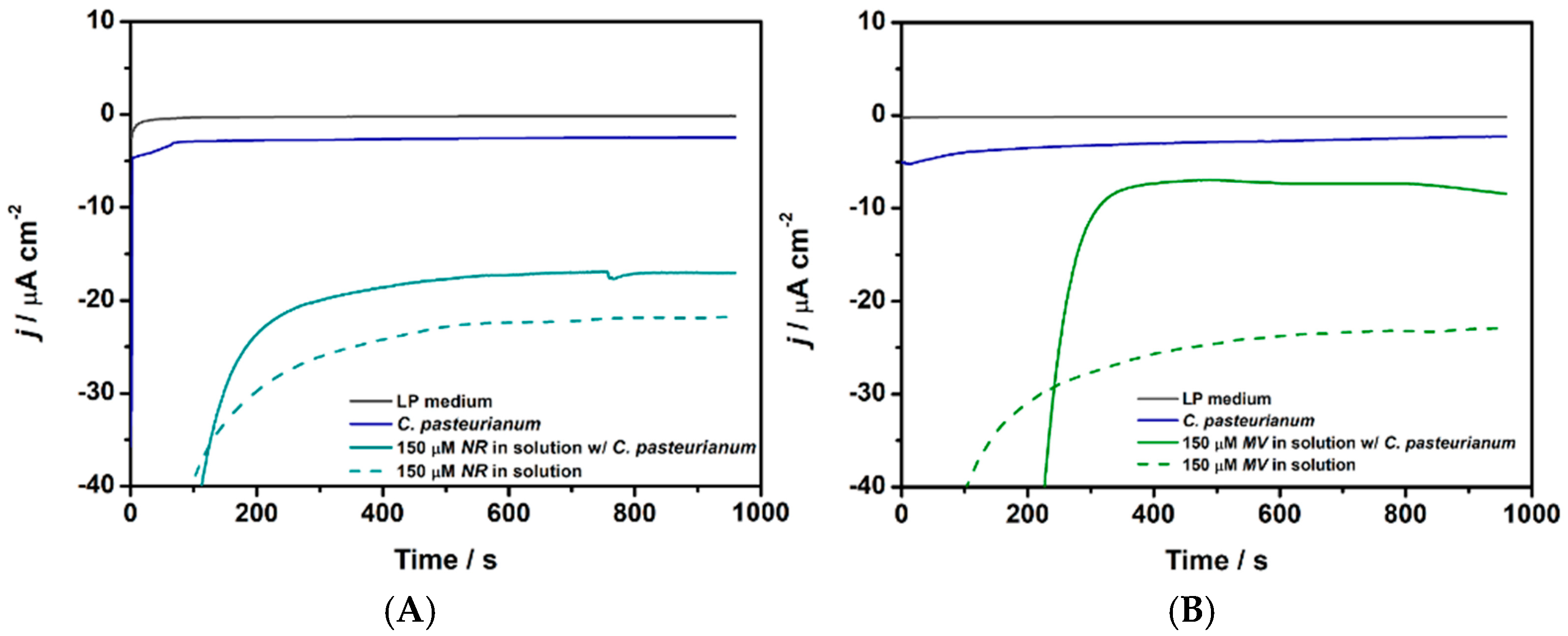
3.3. NR and MV as Redox Mediators for C. pasteurianum DSM 525
3.4. FNR or FMV Deposited on the MFCDSM Anode as Mediator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simoska, O.; Gaffney, E.M.; Lim, K.; Beaver, K.; Minteer, S.D. Understanding the Properties of Phenazine Mediators that Promote Extracellular Electron Transfer in Escherichia coli. J. Electrochem. Soc. 2021, 168, 25503. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; Chen, H.; Ye, J.; Cheng, Z.; Chen, J. Gaseous toluene, ethylbenzene, and xylene mixture removal in a microbial fuel cell: Performance, biofilm characteristics, and mechanisms. Chem. Eng. J. 2020, 386, 123916. [Google Scholar] [CrossRef]
- Lim, C.E.; Chew, C.L.; Pan, G.-T.; Chong, S.; Arumugasamy, S.K.; Lim, J.W.; Al-Kahtani, A.A.; Ng, H.-S.; Abdurrahman, M. Predicting microbial fuel cell biofilm communities and power generation from wastewaters with artificial neural network. Int. J. Hydrogen Energy 2024, 52, 1052–1064. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Paquete, C.M. Electroactivity across the cell wall of Gram-positive bacteria. Comput. Struct. Biotechnol. J. 2020, 18, 3796–3802. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.T.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 2001, 7, 297–306. [Google Scholar] [CrossRef]
- dos Passos, V.F.; Marcilio, R.; Aquino-Neto, S.; Santana, F.B.; Dias, A.C.F.; Andreote, F.D.; de Andrade, A.R.; Reginatto, V. Hydrogen and electrical energy co-generation by a cooperative fermentation system comprising Clostridium and microbial fuel cell inoculated with port drainage sediment. Bioresour. Technol. 2019, 277, 94–103. [Google Scholar] [CrossRef]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef]
- Beaver, K.; Dantanarayana, A.; Zani, A.B.; Lehto, D.L.; Minteer, S.D. Nitric Oxide as a Signaling Molecule for Biofilm Formation and Dispersal in Mediated Electron Transfer Microbial Electrochemical Systems. J. Electrochem. Soc. 2023, 170, 045503. [Google Scholar] [CrossRef]
- Hassan, R.Y.A.; Febbraio, F.; Andreescu, S. Microbial Electrochemical Systems: Principles, Construction and Biosensing Applications. Sensors 2021, 21, 1279. [Google Scholar] [CrossRef]
- Zani, A.C.B.; de Almeida, É.J.R.; Furlan, J.P.R.; Pedrino, M.; Guazzaroni, M.-E.; Stehling, E.G.; de Andrade, A.R.; Reginatto, V. Electrobiochemical skills of Pseudomonas aeruginosa species that produce pyocyanin or pyoverdine for glycerol oxidation in a microbial fuel cell. Chemosphere 2023, 335, 139073. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Grattieri, M.; Rhodes, Z.; Hickey, D.P.; Beaver, K.; Minteer, S.D. Understanding Biophotocurrent Generation in Photosynthetic Purple Bacteria. ACS Catal. 2019, 9, 867–873. [Google Scholar] [CrossRef]
- de Lacey, A.L.; Bes, M.T.; Gómez-Moreno, C.; Fernández, V.M. Amperometric enzyme electrode for NADP+ based on a ferrodoxin-NADP+ reductase and viologen-modified glassy carbon electrode. J. Electroanal. Chem. 1995, 390, 69–76. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel Mode of Microbial Energy Metabolism: Organic Carbon Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese. Appl. Environ. Microbiol. 1998, 54, 1472–1480. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Minteer, S.D. Complete Oxidation of Glycerol in an Enzymatic Biofuel Cell. Fuel Cells 2009, 9, 63–69. [Google Scholar] [CrossRef]
- Ghica, M.; Brett, C. Glucose oxidase inhibition in poly(neutral red) mediated enzyme biosensors for heavy metal determination. Mikrochim. Acta 2008, 163, 185–193. [Google Scholar] [CrossRef]
- Kumar, S.S.; Malyan, S.K.; Basu, S.; Bishnoi, N.R. Syntrophic association and performance of Clostridium, Desulfovibrio, Aeromonas and Tetrathiobacter as anodic biocatalysts for bioelectricity generation in dual chamber microbial fuel cell. Environ. Sci. Pollut. Res. 2017, 24, 16019–16030. [Google Scholar] [CrossRef]
- Taufemback, W.F.; Hotza, D.; Recouvreux, D.D.O.S.; Calegari, P.C.; Pineda-Vásquez, T.G.; Antônio, R.V.; Watzko, E.S. Techniques for obtaining and mathematical modeling of polarization curves in microbial fuel cells. Mater. Chem. Phys. 2024, 315, 128998. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.; Machini, W.B.S.; Zanoni, M.V.B.; Oliveira-Brett, A.M. Human Hair Keratin Direct Electrochemistry and In Situ Interaction with p-Toluenediamine and p-Aminophenol Hair Dye Precursors using a Keratin Electrochemical Biosensor. ChemElectroChem 2020, 7, 1277–1285. [Google Scholar] [CrossRef]
- Rhodes, Z.; Simoska, O.; Dantanarayana, A.; Stevenson, K.J.; Minteer, S.D. Using structure-function relationships to understand the mechanism of phenazine-mediated extracellular electron transfer in Escherichia coli. iScience 2021, 24, 103033. [Google Scholar] [CrossRef] [PubMed]
- Pauliukaite, R.; Ghica, M.E.; Barsan, M.; Brett, C.M.A. Characterisation of poly(neutral red) modified carbon film electrodes; application as a redox mediator for biosensors. J. Solid State Electrochem. 2007, 11, 899–908. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Ghica, M.E.; Brett, C.M.A. A glucose biosensor using methyl viologen redox mediator on carbon film electrodes. Anal. Chim. Acta 2005, 532, 145–151. [Google Scholar] [CrossRef]
- Peguin, S.; Goma, G.; Delorme, P.; Soucaille, P. Metabolic flexibility of Clostridium acetobutylicum in response to methyl viologen addition. Appl. Microbiol. Biotechnol. 1994, 42, 611–616. [Google Scholar] [CrossRef]
- Shpilevaya, I.; Foord, J.S. Electrochemistry of Methyl Viologen and Anthraquinonedisulfonate at Diamond and Diamond Powder Electrodes: The Influence of Surface Chemistry. Electroanalysis 2014, 26, 2088–2099. [Google Scholar] [CrossRef]
- He, A.-Y.; Yin, C.-Y.; Xu, H.; Kong, X.-P.; Xue, J.-W.; Zhu, J.; Jiang, M.; Wu, H. Enhanced butanol production in a microbial electrolysis cell by Clostridium beijerinckii IB4. Bioproc. Biosyst. Eng. 2016, 39, 245–254. [Google Scholar] [CrossRef]
- Martínez-Ruano, J.; Suazo, A.; Véliz, F.; Otálora, F.; Conejeros, R.; González, E.; Aroca, G. Electro-fermentation with Clostridium autoethanogenum: Effect of pH and neutral red addition. Environ. Technol. Innov. 2023, 31, 103183. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Zhang, X.; Ran, J.; Han, X.; Yang, Z.; Xu, T. Degradation of electrochemical active compounds in aqueous organic redox flow batteries. Curr. Opin. Electrochem. 2022, 32, 100895. [Google Scholar] [CrossRef]
- Chen, S.-M.; Lin, K.-C. The electrocatalytic properties of polymerized neutral red film modified electrodes. J. Electroanal. Chem. 2001, 511, 101–114. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zani, A.C.B.; de Souza, J.C.; de Andrade, A.R.; Reginatto, V. Neutral Red Film Augments Extracellular Electron Transfer Performed by Clostridium pasteurianum DSM 525. Fermentation 2024, 10, 497. https://doi.org/10.3390/fermentation10100497
Zani ACB, de Souza JC, de Andrade AR, Reginatto V. Neutral Red Film Augments Extracellular Electron Transfer Performed by Clostridium pasteurianum DSM 525. Fermentation. 2024; 10(10):497. https://doi.org/10.3390/fermentation10100497
Chicago/Turabian StyleZani, Ana Clara Bonizol, João Carlos de Souza, Adalgisa Rodrigues de Andrade, and Valeria Reginatto. 2024. "Neutral Red Film Augments Extracellular Electron Transfer Performed by Clostridium pasteurianum DSM 525" Fermentation 10, no. 10: 497. https://doi.org/10.3390/fermentation10100497
APA StyleZani, A. C. B., de Souza, J. C., de Andrade, A. R., & Reginatto, V. (2024). Neutral Red Film Augments Extracellular Electron Transfer Performed by Clostridium pasteurianum DSM 525. Fermentation, 10(10), 497. https://doi.org/10.3390/fermentation10100497





