Optimizing the Brewing Process, Metabolomics Analysis, and Antioxidant Activity Analysis of Complexed Hongqu Rice Wine with Kiwiberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Preparation of Complexed Hongqu Rice Wine
2.2.1. Single-Factor Experimental Design
2.2.2. Response Surface Experimental Design
2.3. Alcohol Content
2.4. Sensory Score
2.5. Analysis of Major Nutritional Components
2.6. Metabolic Analysis
2.6.1. Sample Treatment
2.6.2. Sample Identification and Quantification
2.7. In Vitro Antioxidant Activity Assays
2.7.1. Determination of DPPH Radical Scavenging Activity
2.7.2. Determination of ABTS Radical Scavenging Activity
2.7.3. Determination of Hydroxyl Radical (OH) Scavenging Activity
2.7.4. Determination of Fe3+ Reducing Power (TCA)
2.8. In Vitro Assay for the Inhibition of Key Enzymes Involved in Glucose and Lipid Metabolism
2.8.1. Determination of α-Amylase Inhibition Rate
2.8.2. Determination of α-Glucosidase Inhibition Rate
2.9. Bile Acid Binding Capacity
2.10. Data Statistics and Analysis
3. Results
3.1. Process Optimization
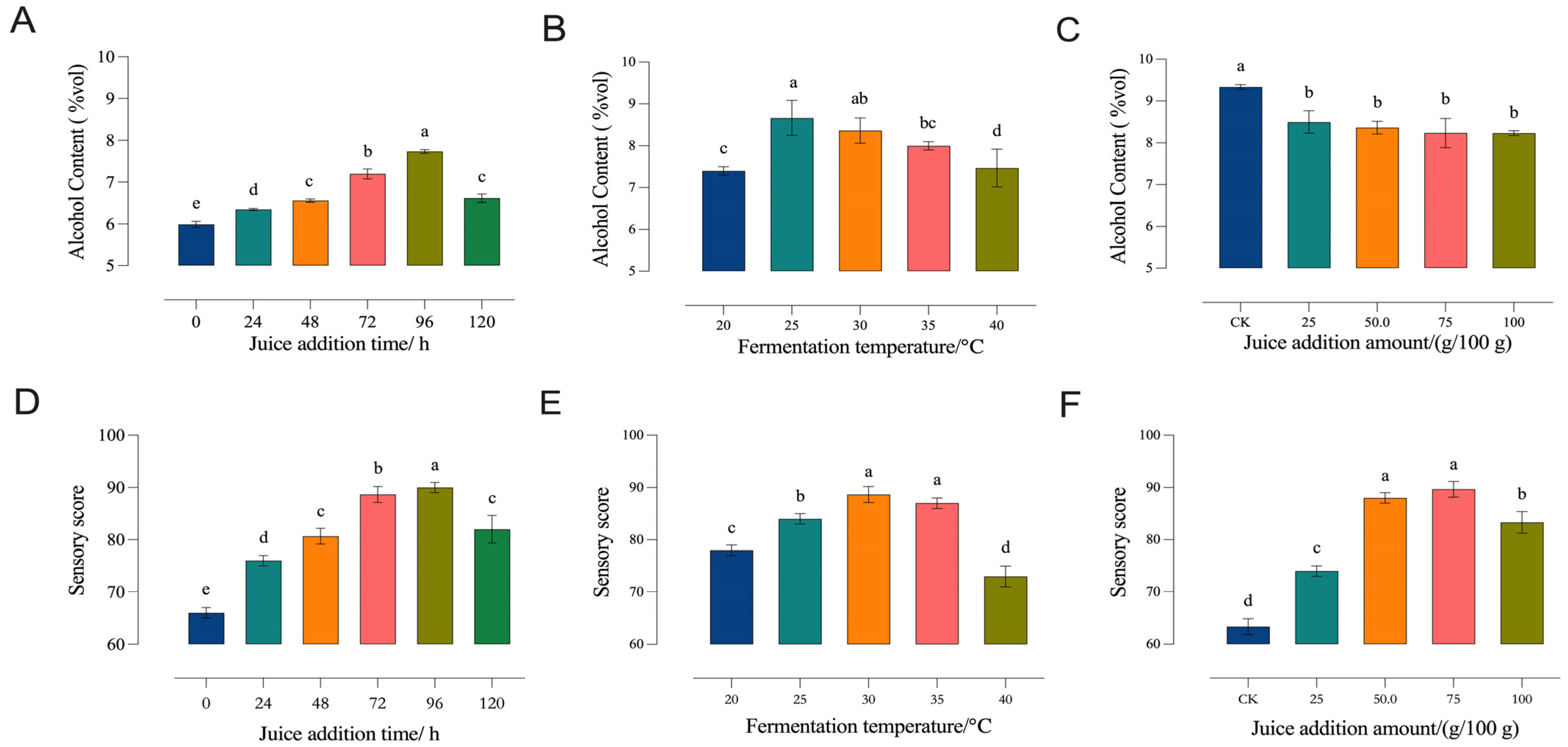
3.1.1. Single-Factor Experiments
- (a)
- Juice Addition Time
- (b)
- Fermentation Temperature
- (c)
- Juice Addition Amount
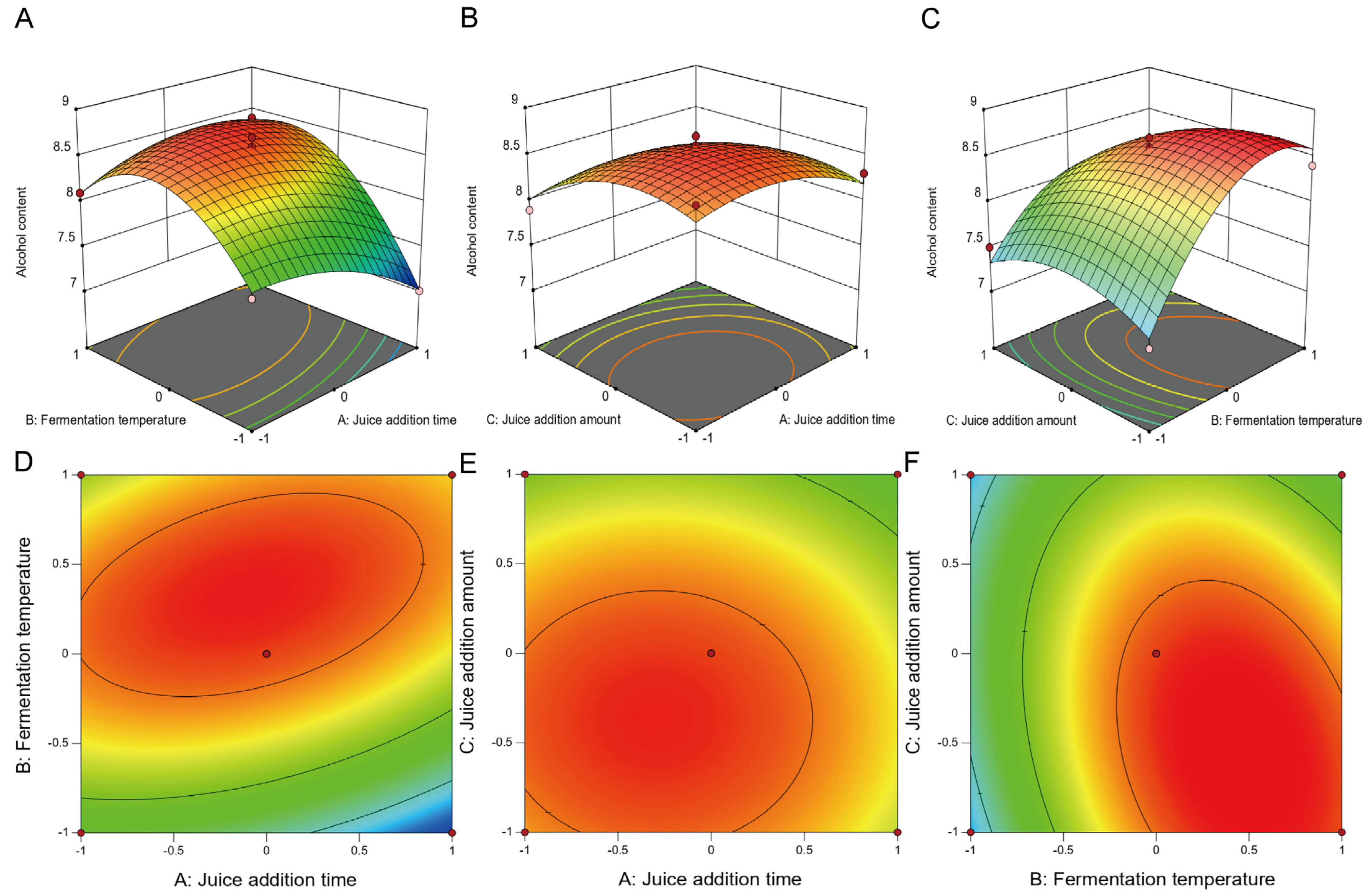
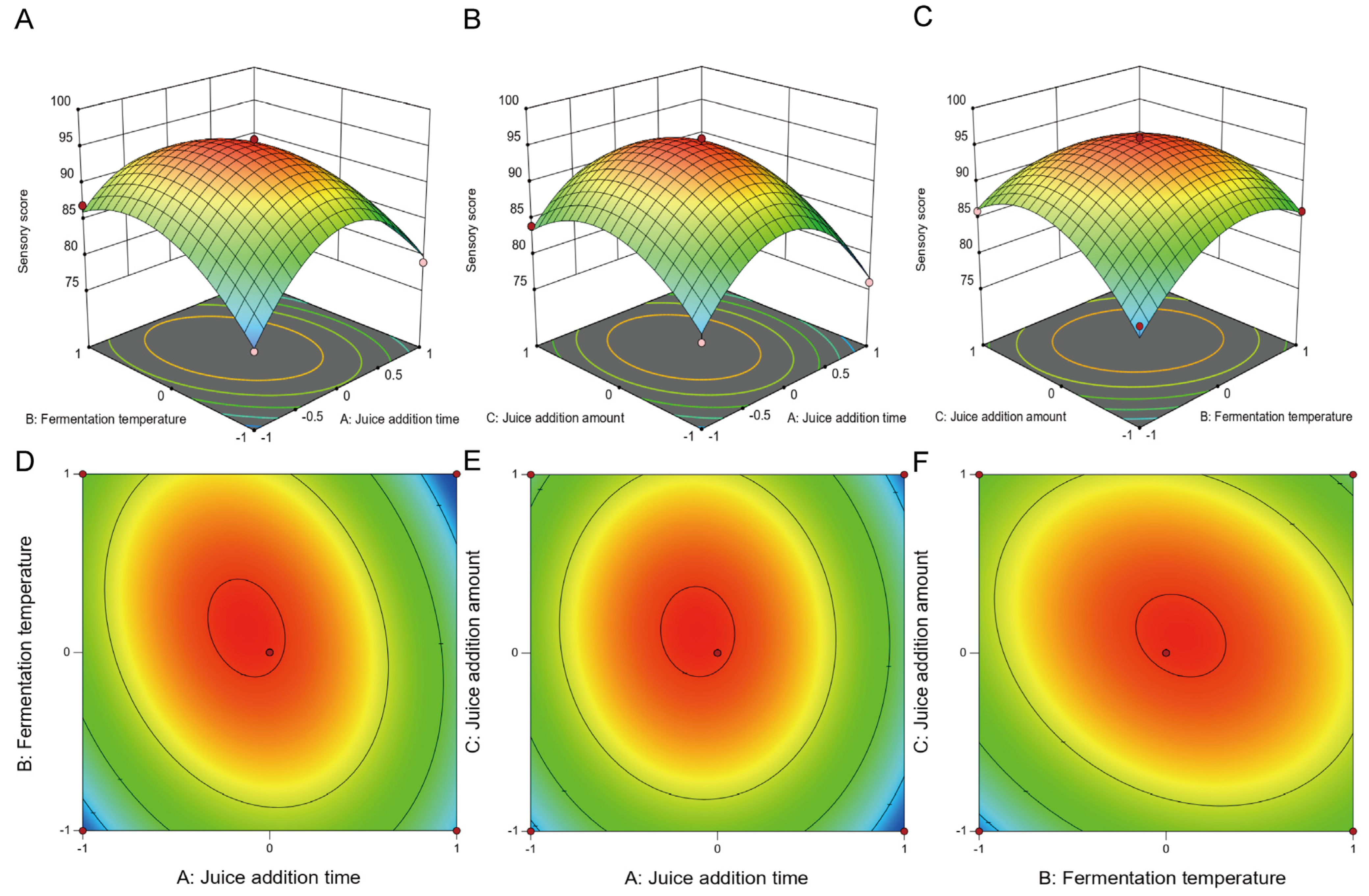
3.1.2. Fitting the Response Surface Models
- (a)
- RSM Model for Alcohol Content
- (b)
- RSM model for Sensory Score
3.1.3. Validation and Verification of the Optimized Conditions
3.2. Analysis of Main Functional Components in Complexed Hongqu Rice Wines
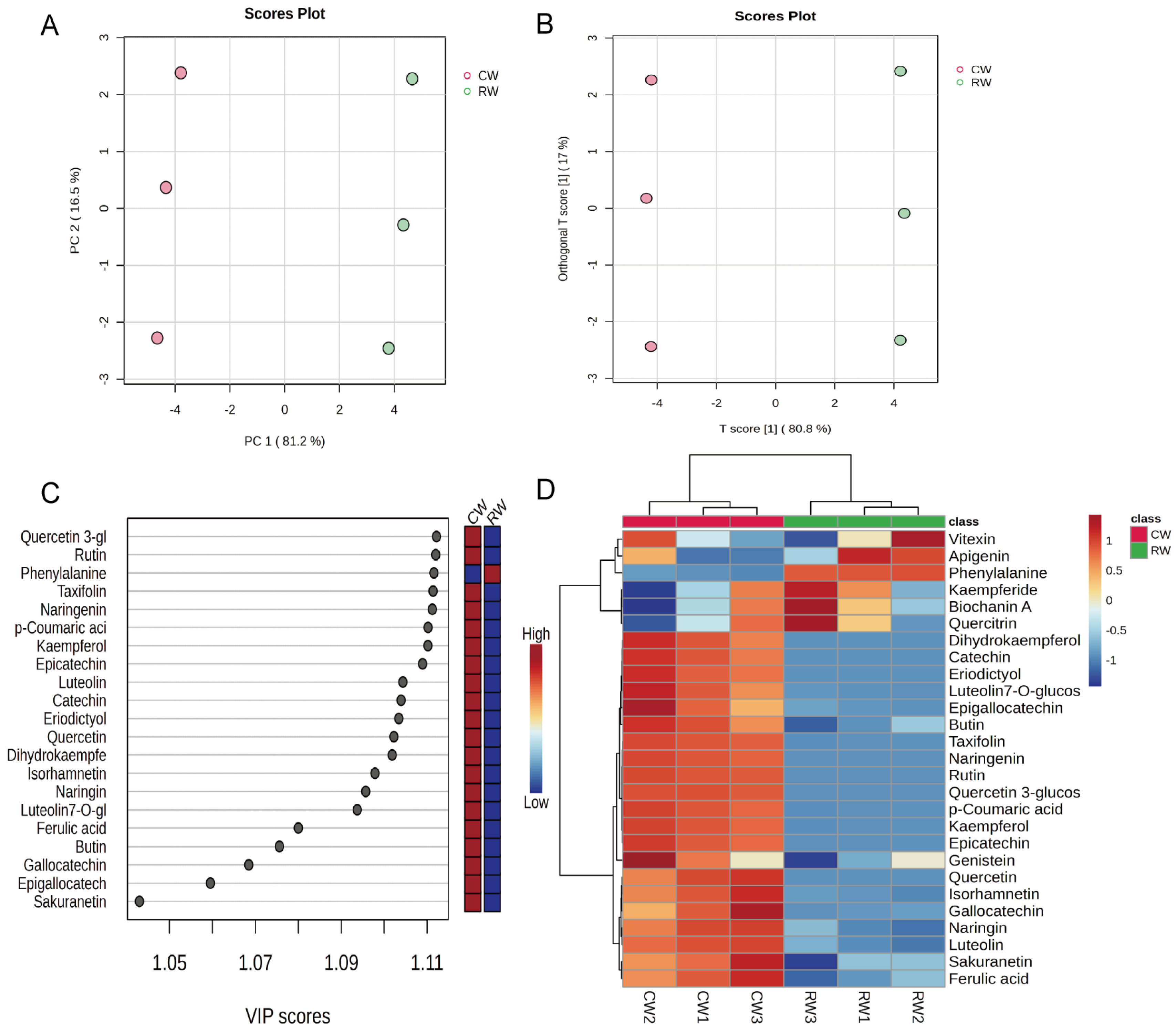
3.3. Analysis of Metabolites in Complexed Hongqu Rice Wine Using Metabolomics Approach
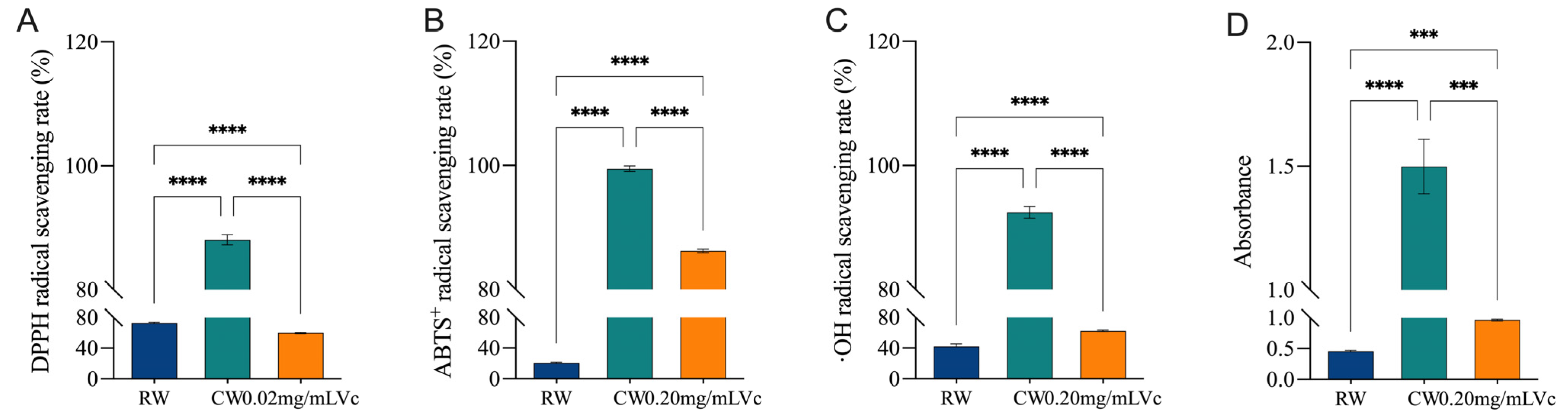
3.4. In Vitro Antioxidant Capacity of Complexed Hongqu Rice Wine
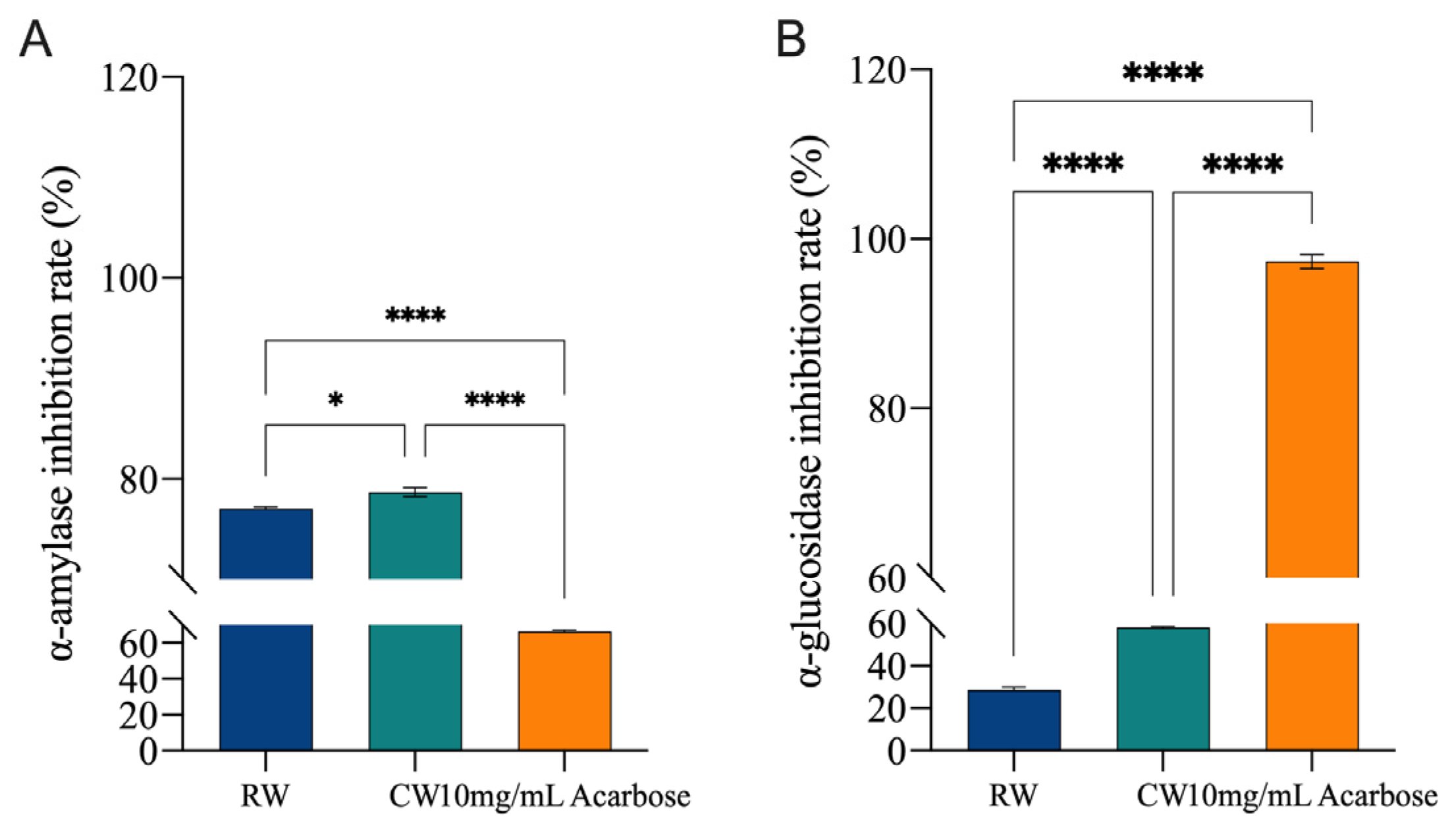
3.5. In Vitro Ability of Complexed Hongqu Rice Wine to Inhibit Key Enzymes Involved in Glucose and Lipid Metabolism
3.6. Bile Acid-Binding Capacity of Complexed Hongqu Rice Wine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.-M.; Li, W.-L.; Tong, S.-G.; Qiu, Y.-T.; Han, J.-Z.; Lv, X.-C.; Ai, L.-Z.; Sun, J.-Y.; Sun, B.-G.; Ni, L. Effects of the microbial community on the formation of volatile compounds and biogenic amines during the traditional brewing of hongqu rice wine. Curr. Res. Food Sci. 2022, 5, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, H.; Jiang, L.; Zhang, Y.; Li, J.; Zhu, X. Analysis of the flavour components, total phenolic content and antioxidant capacity between rice wine from two raw rice and starters. Int. J. Food Sci. Technol. 2024, 59, 129–141. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Li, Y.; Lin, F.; Wu, L. Screening and identification of Monascus strains with high-yield monacolin K and undetectable citrinin by integration of HPLC analysis and pksCT and ctnA genes amplification. J. Appl. Microbiol. 2020, 129, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yuan, Y.; Tang, S.; Yang, Z.; Wu, Q.; Liang, Z.; Chen, S.; Li, W.; Lv, X.; Ni, L. Comparative analysis of microbial communities and volatile flavor components in the brewing of Hongqu rice wines fermented with different starters. Curr. Res. Food Sci. 2023, 7, 100628. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.C.; Pan, X. Antioxidant activities of five Chinese rice wines and the involvement of phenolic compounds. Food Res. Int. 2006, 39, 581–587. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.X.; Lu, W.P.; Ning, X.; Li, J.Y.; Wu, L. Study on Ploidy Variation and Fruit Evaluation of actinidia arguta (SIEB. & ZUCC) PLANCH. EX MIQ. Fresenius Environ. Bull. 2022, 31, 817–822. [Google Scholar]
- Zheng, R.-L.; Ren, T.; Niu, C.-T.; Zheng, F.-Y.; Wang, J.-J.; Liu, C.-F.; Li, Q. Anthocyanins composition and antioxidant activity of purple rice and color degradation under sunlight exposure of purple rice wine. J. Food Meas. Charact. 2022, 16, 1889–1900. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Z.; Feng, X.; Pan, B.; Irfan, M.; Liu, C. Transcriptomic and metabolomics of flavonoid compounds in actinidia arguta var. arguta. J. King Saud Univ. Sci. 2021, 33, 101605. [Google Scholar] [CrossRef]
- Sawicki, T.; Blaszczak, W.; Latocha, P. In vitro anticholinergic and antiglycaemic properties of frost-hardy Actinidia fruit extracts and their polyphenol profile, L-ascorbic acid content and antioxidant capacity. Food Res. Int. 2023, 173, 113324. [Google Scholar] [CrossRef]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; de la Luz Cádiz-Gurrea, M.; Fernández-Ochoa, Á.; Segura-Carretero, A.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Insights into the polyphenols extraction from Actinidia arguta fruit (kiwiberry): A source of pro-healthy compounds. Sci. Hortic. 2023, 313, 111910. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, S.; Yu, Z.; Ge, H.; Qi, S.; Zhang, X.; Long, L.; Xiong, X.; Chu, D.; Ma, X.; et al. The dietary freeze-dried fruit powder of Actinidia arguta ameliorates dextran sulphate sodium-induced ulcerative colitis in mice by inhibiting the activation of MAPKs. Food Funct. 2019, 10, 5768–5778. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Ryu, S.H.; Yeon, S.W.; Lee, S.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Phenyldilactones from the leaves of hardy kiwifruit (Actinidia arguta). Biochem. Syst. Ecol. 2023, 108, 104636. [Google Scholar] [CrossRef]
- Ha, J.S.; Kang, J.Y.; Kang, J.E.; Park, S.K.; Kim, J.M.; Kim, C.-W.; Oh, S.-I.; Lee, U.; Kim, D.-O.; Heo, H.J. Pentacyclic triterpenoid-rich fraction of the hardy kiwi (Actinidia arguta) improves brain dysfunction in high fat diet-induced obese mice. Sci. Rep. 2020, 10, 5788. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Dong, K.; Yin, X.; Hao, C.; Zhang, W.; Irfan, M.; Chen, L.; Wang, Y. Metabolomic and transcriptomic exploration of the uric acid-reducing flavonoids biosynthetic pathways in the fruit of Actinidia arguta Sieb. Zucc. Front. Plant Sci. 2022, 13, 1025317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, J.; Gao, N.; Gong, E.S.; Xin, G.; Liu, C.; Si, X.; Sun, X.; Li, B. Assessment of the phytochemical profile and antioxidant activities of eight kiwiberry (Actinidia arguta (Siebold & Zuccarini) Miguel) varieties in China. Food Sci. Nutr. 2021, 9, 5616–5625. [Google Scholar] [PubMed]
- Błaszczak, W.; Latocha, P.; Jeż, M.; Wiczkowski, W. The impact of high-pressure processing on the polyphenol profile and anti-glycaemic, anti-hypertensive and anti-cholinergic activities of extracts obtained from kiwiberry (Actinidia arguta) fruits. Food Chem. 2021, 343, 128421. [Google Scholar] [CrossRef]
- Han, N.; Park, H.; Kim, C.-W.; Kim, M.-S.; Lee, U. Physicochemical quality of hardy kiwifruit (Actinidia arguta L. cv. Cheongsan) during ripening is influenced by harvest maturity. For. Sci. Technol. 2019, 15, 187–191. [Google Scholar]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. Standardization Technical Committee of the National Food Industry Brewing Technical Committee: Beijing, China, 2006.
- Pashazadeh, H.; Ozdemir, N.; Zannou, O.; Koca, I. Antioxidant capacity, phytochemical compounds, and volatile compounds related to aromatic property of vinegar produced from black rosehip (rosa pimpinellifolia L.) juice. Food Biosci. 2021, 44, 101318. [Google Scholar] [CrossRef]
- Pisani, M.; Astolfi, P.; Sabbatini, S.; Carloni, P. Antioxidant activity level, bioactive compounds, colour and spectroscopic analysis (UV-vis and FT-IR) of flavoured drinks made with wine and sour cherries (prunus cerasus var. austera). Foods 2021, 10, 1953. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Yan, X.; Leng, T.; Xie, J.; Yu, Q.; Chen, Y. Metabolomics combined with correlation analysis revealed the differences in antioxidant activities of lotus seeds with varied cultivars. Foods 2024, 13, 1084. [Google Scholar] [CrossRef]
- Ren, N.; Gong, W.; Zhao, Y.; Zhao, D.; Xu, Y. Innovation in sweet rice wine with high antioxidant activity: Eucommia ulmoides leaf sweet rice wine. Front. Nutr. 2023, 9, 1108843. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qin, C.; He, X.; Chen, B.; Tang, J.; Liu, G.; Li, L.; Yang, Y.; Ye, D.; Li, J.; et al. Development of green banana fruit wines: Chemical compositions and in vitro antioxidative activities. Antioxidants 2023, 12, 93. [Google Scholar] [CrossRef]
- Yang, P.; Huang, K.; Zhang, Y.; Li, S.; Cao, H.; Song, H.; Zhang, Y.; Guan, X. Biotransformation of quinoa phenolic compounds with Monascus anka to enhance the antioxidant capacity and digestive enzyme inhibitory activity. Food Biosci. 2022, 46, 101568. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Li, S. Enhancement in the physicochemical properties, antioxidant activity, volatile compounds, and non-volatile compounds of watermelon juices through lactobacillus plantarum JHT78 fermentation. Food Chem. 2023, 420, 136146. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Wang, T.; Wang, Q.; Feng, L.; Su, R.; Zhang, M.; Xu, B.; Chen, F.; Li, P. Optimization of main ingredient ratio, metabolomics analysis, and antioxidant activity analysis of lycopene-enriched compound fruit wine. Fermentation 2023, 9, 591. [Google Scholar] [CrossRef]
- Sylla, N.; Bouyahya, A.; Taha, D.; Dakka, N.; Elhajji, H. Study of the antioxidant and antidiabetic activity in vitro of free and encapsulated phenolic compounds of olive pomace. Biocatal. Agric. Biotechnol. 2021, 36, 102126. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Luo, Y.; Dong, G.-L.; Ren, Y.-Y.; Chen, L.-J.; Guo, M.-Z.; Wang, X.-T.; Yang, X.-Y.; Zhang, Y. Effects of the ultra-high pressure on structure and α-glucosidase inhibition of polysaccharide from Astragalus. Int. J. Biol. Macromol. 2016, 87, 570–576. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Dou, Z.-M.; Duan, Q.-F.; Chen, C.; Liu, R.-H.; Jiang, Y.-M.; Yang, B.; Fu, X. A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods: Antioxidant and bile acid-binding capacity. Food Sci. Hum. Wellness 2024, 13, 494–505. [Google Scholar] [CrossRef]


| Factor | Title | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Juice addition time (h) | 72 | 96 | 120 |
| B | Fermentation temperature (°C) | 25 | 30 | 35 |
| C | Juice addition amount (g/100 g) | 50 | 75 | 100 |
| Run | Dependent Variable | Response Value | |||
|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | |
| 1 | 0 | 0 | 0 | 8.7 | 94 |
| 2 | −1 | 0 | 1 | 7.9 | 84 |
| 3 | 0 | −1 | −1 | 7.2 | 80 |
| 4 | 1 | 0 | 1 | 7.6 | 79 |
| 5 | 0 | 0 | 0 | 8.6 | 96 |
| 6 | 0 | −1 | 1 | 7.5 | 86 |
| 7 | 0 | 0 | 0 | 8.7 | 95 |
| 8 | −1 | 0 | −1 | 8.6 | 78 |
| 9 | 0 | 1 | 1 | 7.8 | 82 |
| 10 | 0 | 0 | 0 | 8.5 | 96 |
| 11 | 0 | 0 | 0 | 8.5 | 95 |
| 12 | −1 | 1 | 0 | 8.1 | 87 |
| 13 | 1 | −1 | 0 | 7 | 79 |
| 14 | 1 | 1 | 0 | 8.4 | 76 |
| 15 | 0 | 1 | −1 | 8.4 | 86 |
| 16 | −1 | −1 | 0 | 7.7 | 77 |
| 17 | 1 | 0 | −1 | 8.3 | 76 |
| Source | SS | DF | MS | F Value | p-Value | SS | DF | MS | F Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol Content (Y1) (a) | Sensory Score (Y2) (b) | |||||||||
| Model | 4.47 | 9 | 0.4967 | 16.76 | 0.0006 | 898.39 | 9 | 99.82 | 55.68 | <0.0001 |
| A | 0.125 | 1 | 0.125 | 4.22 | 0.0791 | 32.00 | 1 | 32.00 | 17.85 | 0.0039 |
| B | 1.36 | 1 | 1.36 | 45.92 | 0.0003 | 10.13 | 1 | 10.13 | 5.65 | 0.0491 |
| C | 0.3613 | 1 | 0.3613 | 12.19 | 0.0101 | 15.13 | 1 | 15.13 | 8.44 | 0.0228 |
| AB | 0.25 | 1 | 0.25 | 8.43 | 0.0229 | 42.25 | 1 | 42.25 | 23.57 | 0.0018 |
| AC | 0 | 1 | 0 | 0 | 1 | 2.25 | 1 | 2.25 | 1.25 | 0.2995 |
| BC | 0.2025 | 1 | 0.2025 | 6.83 | 0.0347 | 25.00 | 1 | 25.00 | 13.94 | 0.0073 |
| A2 | 0.1901 | 1 | 0.1901 | 6.41 | 0.0391 | 408.52 | 1 | 408.52 | 227.86 | <0.0001 |
| B2 | 1.45 | 1 | 1.45 | 49.03 | 0.0002 | 132.04 | 1 | 132.04 | 73.65 | <0.0001 |
| C2 | 0.348 | 1 | 0.348 | 11.74 | 0.011 | 156.67 | 1 | 156.67 | 87.39 | <0.0001 |
| Residual | 0.2075 | 7 | 0.0296 | 12.55 | 7 | 1.79 | ||||
| Lack of fit | 0.1675 | 3 | 0.0558 | 5.58 | 0.065 | 9.75 | 3 | 3.25 | 4.64 | 0.0861 |
| Pure error | 0.04 | 4 | 0.01 | 2.80 | 4 | 0.70 | ||||
| Total | 4.68 | 16 | 910.94 | 16 | ||||||
| Run | Metabolite Name | CW | RW | p-Value | Fold Change (FC) |
|---|---|---|---|---|---|
| 1 | Apigenin | 0.99 ± 0.1 | 1.11 ± 0.1 | Not | 0.5 < FC < 2 |
| 2 | Biochanin A | 0.49 ± 0.04 | 0.52 ± 0.04 | Not | 0.5 < FC < 2 |
| 3 | Butin | 0.43 ± 0.03 | 0.23 ± 0.04 | <0.01 | 0.5 < FC < 2 |
| 4 | Catechin | 972.54 ± 104.12 | 10.52 ± 0.41 | <0.01 | >2 |
| 5 | Dihydrokaempferol | 61.24 ± 7.34 | 0.65 ± 0.09 | <0.01 | >2 |
| 6 | Epicatechin | 938.67 ± 62.42 | 12.42 ± 2.6 | <0.01 | >2 |
| 7 | Epigallocatechin | 29.53 ± 4.3 | 13.96 ± 0.42 | <0.01 | >2 |
| 8 | Eriodictyol | 430.36 ± 46.76 | 1.21 ± 0.16 | <0.01 | >2 |
| 9 | Ferulic acid | 18.44 ± 0.86 | 12.66 ± 0.88 | <0.01 | 0.5 < FC < 2 |
| 10 | Gallocatechin | 25.89 ± 5.44 | 4.08 ± 0.51 | <0.01 | >2 |
| 11 | Genistein | 4.1 ± 0.22 | 3.66 ± 0.2 | Not | 0.5 < FC < 2 |
| 12 | Isorhamnetin | 10.5 ± 0.62 | 5.97 ± 0.16 | <0.01 | 0.5 < FC < 2 |
| 13 | Vitexin | 1.82 ± 0.15 | 1.83 ± 0.21 | Not | 0.5 < FC < 2 |
| 14 | Kaempferide | 0.39 ± 0.1 | 0.46 ± 0.09 | Not | 0.5 < FC < 2 |
| 15 | Kaempferol | 22.41 ± 1.15 | 1.75 ± 0.13 | <0.01 | >2 |
| 16 | Luteolin | 29.32 ± 0.99 | 12.17 ± 1.53 | <0.01 | >2 |
| 17 | Luteolin-7-O-glucoside | 37.96 ± 5.65 | 2.86 ± 0.43 | <0.01 | >2 |
| 18 | Naringenin | 215.35 ± 9.02 | 0.02 ± 0.002 | <0.01 | >2 |
| 19 | Naringin | 0.46 ± 0.01 | 0.41 ± 0.01 | <0.01 | 0.5 < FC < 2 |
| 20 | p-Coumaric acid | 27.38 ± 1.43 | 1.29 ± 0.19 | <0.01 | >2 |
| 21 | Phenylalanine | 7145.31 ± 213.95 | 14,966.61 ± 160.5 | <0.01 | <0.5 |
| 22 | Quercetin | 963.11 ± 113.56 | 18.86 ± 0.41 | <0.01 | >2 |
| 23 | Quercetin-3-glucoside | 11,917.27 ± 263.02 | 6.59 ± 0.2 | <0.01 | >2 |
| 24 | Quercitrin | 5.69 ± 0.46 | 5.92 ± 0.52 | Not | 0.5 < FC < 2 |
| 25 | Rutin | 8164.22 ± 207.76 | 28.39 ± 1.83 | <0.01 | >2 |
| 26 | Sakuranetin | 3.24 ± 0.01 | 3.17 ± 0.02 | <0.01 | 0.5 < FC < 2 |
| 27 | Taxifolin | 231.58 ± 7.99 | 20.66 ± 0.83 | <0.01 | >2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Liu, Y.; Guo, X.; Ding, Y.; Liu, D. Optimizing the Brewing Process, Metabolomics Analysis, and Antioxidant Activity Analysis of Complexed Hongqu Rice Wine with Kiwiberry. Fermentation 2024, 10, 494. https://doi.org/10.3390/fermentation10100494
Wang M, Liu Y, Guo X, Ding Y, Liu D. Optimizing the Brewing Process, Metabolomics Analysis, and Antioxidant Activity Analysis of Complexed Hongqu Rice Wine with Kiwiberry. Fermentation. 2024; 10(10):494. https://doi.org/10.3390/fermentation10100494
Chicago/Turabian StyleWang, Mengze, Yuxin Liu, Xing Guo, Yuping Ding, and Dejiang Liu. 2024. "Optimizing the Brewing Process, Metabolomics Analysis, and Antioxidant Activity Analysis of Complexed Hongqu Rice Wine with Kiwiberry" Fermentation 10, no. 10: 494. https://doi.org/10.3390/fermentation10100494
APA StyleWang, M., Liu, Y., Guo, X., Ding, Y., & Liu, D. (2024). Optimizing the Brewing Process, Metabolomics Analysis, and Antioxidant Activity Analysis of Complexed Hongqu Rice Wine with Kiwiberry. Fermentation, 10(10), 494. https://doi.org/10.3390/fermentation10100494





