Designing the Properties of Probiotic Kefir with Increased Whey Protein Content
Abstract
1. Introduction
2. Materials and Methods
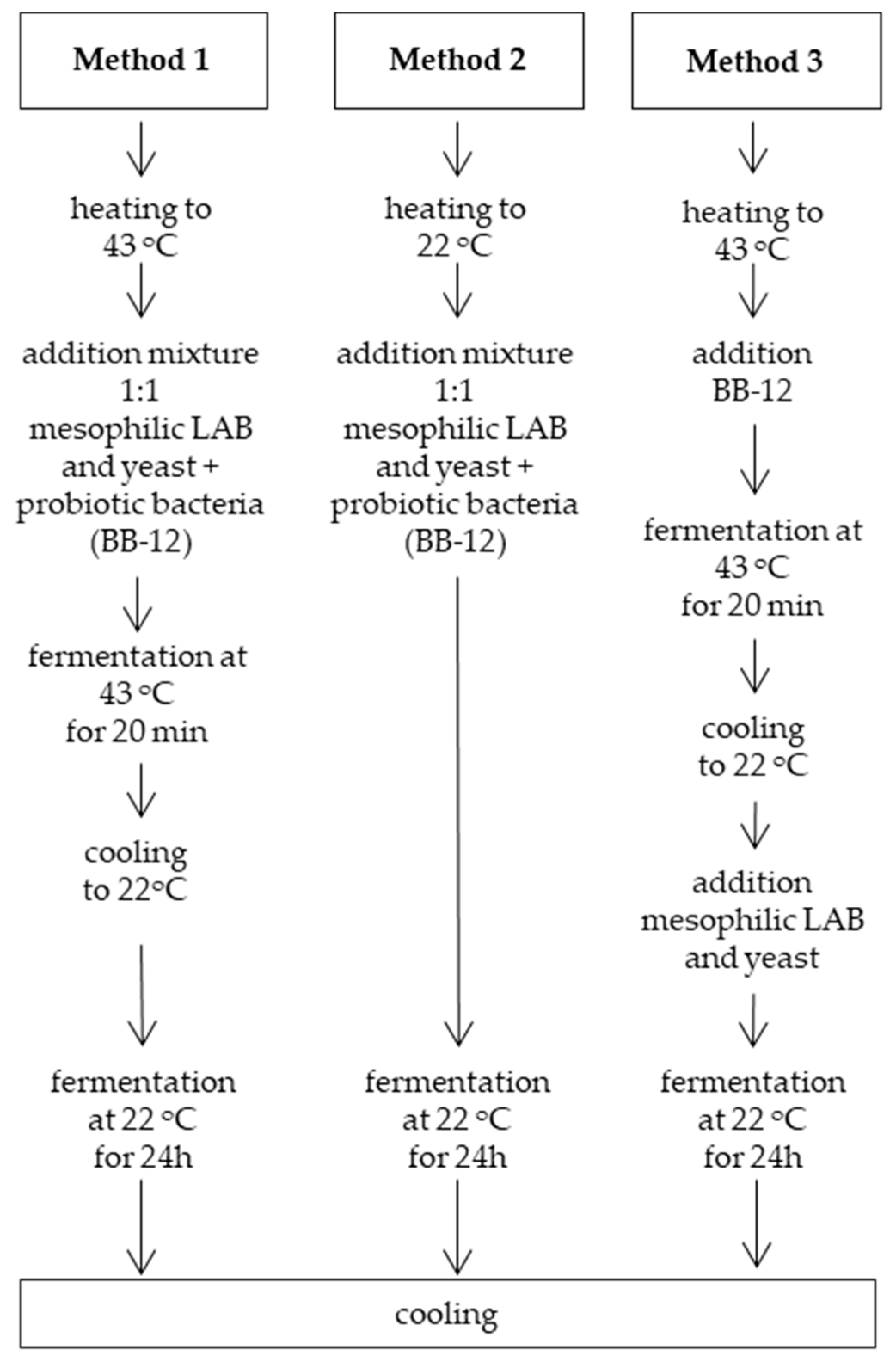
2.1. Preparation of Probiotic Kefir Samples
2.2. Proportion of L(+)-Lactic Acid
2.3. Lactase Activity
2.4. Determination of the Lactic Acid and Ethanol Content
2.5. Determination of the Number of Mesophilic Lactic Acid Bacteria, Yeast, and Probiotic Bacteria
2.6. Texture Analysis
2.7. Color Measurements
2.8. Water Activity
2.9. Statistical Evaluation
3. Results and Discussion
3.1. Number of Bacteria in Probiotic Kefir with Whey Protein Addition
3.2. Lactase Enzymatic Activity and Changes in Lactic Acid and Ethanol Contents
3.3. Texture and Color of Probiotic Kefir with Whey Protein Addition
3.4. Water Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, K.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Hussain, M.; Raza, A.; Rasheed, A.; Asghar, A.; Asif Shah, M. Nutritional and functional properties of kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir Micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammadi, A.R.; Ibrahim, R.A.; Moustafa, A.H.; Ismaiel, A.A.; Abou Zeid, A.; Enan, G. Chemical constitution and antimicrobial activity of kefir fermented beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, G.; Ke, C.; Fan, W.; Li, C.; Chen, Y.; Dixon, W.; Song, M.; Cao, Y.; Xiao, H. Inhibitory effects of a novel antimicrobial peptide from kefir against Escherichia coli. Food Control 2016, 65, 63–72. [Google Scholar] [CrossRef]
- da Silva Ghizi, A.C.; de Almeida Silva, M.; de Andrade Moraes, F.S.; da Silva, C.L.; Endringer, D.C.; Scherer, R.; Lenz, D.; de Lima, E.M.; Brasil, G.A.; Maia, J.F.; et al. Kefir improves blood parameters and reduces cardiovascular risks in patients with metabolic syndrome. PharmaNutrition 2021, 16, 100266. [Google Scholar] [CrossRef]
- da Conceição Teixeira de Moura, A.; Dornelas de Oliveira Martins, A.; Furtado Martins, E.M.; Olmi Silva, V.R.; Ribeiro da Silva, R.; Fagner da Cruz, W. In vitro resistance of lactic acid bacteria carried in kefir appetizer enriched with agroindustrial by-products. LWT 2021, 139, 110519. [Google Scholar] [CrossRef]
- Farag, M.; Jomaa, S.; El-Wahed, A.A.; El-Seedi, H.R. The many faces of kefir fermented dairy products: Quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef]
- Barukčić, I.; Gracin, L.; Jambrak, A.R.; Božanić, R. Comparison of chemical, rheological and sensory properties of kefir produced by kefir grains and commercial kefir starter. Mljekarstvo 2017, 67, 169–176. [Google Scholar] [CrossRef]
- Guangsen, T.; Xiang, L.; Jiahu, G. Microbial diversity and volatile metabolites of kefir prepared by different milk types. CyTA—J. Food 2021, 19, 399–407. [Google Scholar] [CrossRef]
- Dimitreli, G.; Gregoriou, E.A.; Kalantzidis, G.; Antoniou, K.D. Rheological properties of kefir as affected by heat treatment and whey protein addition. J. Texture Stud. 2013, 44, 418–423. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Wójtowski, J.; Pikul, J. Rheological, texture and sensory properties of kefir from mare’s milk and its mixtures with goat and sheep milk. Mljekarstvo 2016, 66, 272–281. [Google Scholar] [CrossRef]
- Sarkar, S. Biotechnological Innovations in kefir production: A Review. Br. Food J. 2008, 110, 283–295. [Google Scholar] [CrossRef]
- Travičić, V.; Šovljanski, O.; Tomić, A.; Perović, M.; Milošević, M.; Ćetković, N.; Antov, M. Augmenting functional and sensorial quality attributes of kefir through fortification with encapsulated blackberry juice. Foods 2023, 12, 4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Wang, M.; Guo, M. Chemical, physiochemical, and microstructural properties, and probiotic survivability of fermented goat milk using polymerized whey protein and starter culture kefir Mild 01. J. Food Sci. 2017, 82, 2650–2658. [Google Scholar] [CrossRef]
- Yiğit, A.; Bielska, P.; Cais-Sokolińska, D.; Samur, G. Whey proteins as a functional food: Health effects, functional properties, and applications in food. J. Am. Nutr. Assoc. 2023, 42, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Minj, S.; Anand, S. Whey proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Gantumur, M.A.; Sukhbaatar, N.; Jiang, Q.; Enkhtuya, E.; Hu, J.; Gao, C.; Jiang, Z.; Li, A. Effect of modified fermented whey protein fortification on the functional, physical, microstructural, and sensory properties of low-fat yogurt. Food Control 2024, 155, 110032. [Google Scholar] [CrossRef]
- Berber, M.; González-Quijano, G.K.; Alvarez, V.B. Whey protein concentrate as a substitute for non-fat dry milk in yogurt. J. Food Process Technol. 2015, 6, 530. [Google Scholar] [CrossRef]
- Sabokbar, N.; Khodaiyan, F. Characterization of pomegranate juice and whey based novel beverage fermented by kefir grains. J. Food Sci. Technol. 2015, 52, 3711–3718. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. CODEX Standard for Fermented Milk. Codex Stan 2003, 243–2003. Available online: https://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 23 June 2022).
- Gawehn, K. D-(-)-Lactate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers: Cambridge, UK, 1988; pp. 588–592. [Google Scholar]
- Noll, F. L-(+)-Lacate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers: Cambridge, UK, 1988; pp. 582–588. [Google Scholar]
- Passerat, B.; Desmaison, A.M.; Pharmaceutiques, D.S.; de Conférences, M. Lactase Activity of Bifidobacterium bifidum. Nutr. Res. 1995, 15, 1287–1295. [Google Scholar] [CrossRef]
- Indyk, H.; Edwards, M.; Woollard, D.C. High performance liquid chromatographic analysis of lactose-hydrolysed milk. Food Chem. 1996, 57, 575–580. [Google Scholar] [CrossRef]
- Bylund, D.; Norström, S.; Essén, S.A.; Lundström, U.S. Analysis of low molecular mass organic acids in natural waters by ion exclusion chromatography tandem mass spectrometry. J. Chromatogr. 2007, 1176, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cais-Sokolińska, D.; Wójtowski, J.; Pikul, J.; Danków, R.; Majcher, M.; Teichert, J.; Bagnicka, E. Formation of volatile compounds in kefir made of goat and sheep milk with high polyunsaturated fatty acid content. J. Dairy Sci. 2015, 98, 6692–6705. [Google Scholar] [CrossRef]
- Mainville, I.; Montpetit, D.; Durand, N.; Farnworth, E.R. Deactivating the bacteria and yeast in kefir using heat treatment, irradiation and high pressure. Int. Dairy J. 2001, 11, 45–49. [Google Scholar] [CrossRef]
- Markiewicz, L.; Biedrzycka, E. Identification of Lactobacillus and Bifidobacterium species with PCR applied to quality control of fermented dairy beverages. Pol. J. Food Nutr. Sci. 2005, 14, 359–365. [Google Scholar]
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A. Enumeration and identification of yeasts isolated from Zimbabwean traditional fermented milk. Int. Dairy J. 2000, 10, 459–466. [Google Scholar] [CrossRef]
- Simova, E.; Simov, Z.; Beshkova, D.; Frengova, G.; Dimitrov, Z.; Spasov, Z. Amino acid profiles of lactic acid bacteria, isolated from kefir grains and kefir starter made from them. Int. J. Food Microbiol. 2006, 107, 112–123. [Google Scholar] [CrossRef]
- Kim, D.H.; Chon, J.W.; Kim, H.; Kim, H.S.; Choi, D.; Hwang, D.G.; Seo, K.H. Detection and enumeration of Lactic Acid Bacteria, Acetic Acid Bacteria and Yeast in kefir grain and milk using quantitative Real-Time PCR. J. Food Saf. 2015, 35, 102–107. [Google Scholar] [CrossRef]
- Arslan, S. A Review: Chemical, microbiological and nutritional characteristics of kefir. CyTA—J. Food 2015, 13, 340–345. [Google Scholar] [CrossRef]
- Witthuhn, R.C.; Schoeman, T.; Britz, T.J. Isolation and characterization of the microbial population of different South African Kefir grains. Int. J. Dairy Technol. 2004, 57, 33–37. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Corbu, V.M.; Petrut, S.; Pelinescu, D. Environmental Stress Responses in Yeasts and Lactic Acid Bacteria Strains Isolated from Dairy Traditional Romanian Fermented Products. Rom. Biotechnol. Lett. 2021, 26, 2548–2559. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Kamal, M.; Avery, A. Influence of temperature rise on kinetic parameters during batch propagation of Kluyveromyces Fragilis in cheese whey under ambient conditions. World J. Microbiol. Biotechnol. 2003, 19, 741–749. [Google Scholar] [CrossRef]
- Magalhães, K.T.; Pereira, G.V.D.M.; Campos, C.R.; Dragone, G.; Schwan, R.F. Brazilian Kefir: Structure, microbial communities and chemical composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef]
- Ziarno, M.; Hasalliu, R.; Cwalina, A. Effect of the addition of milk protein preparations on selected quality parameters and nutritional characteristics of kefir. Appl. Sci. 2021, 11, 966. [Google Scholar] [CrossRef]
- Kök-Taş, T.; Seydim, A.C.; Özer, B.; Guzel-Seydim, Z.B. Effects of different fermentation parameters on quality characteristics of kefir. J. Dairy Sci. 2013, 96, 780–789. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ghaly, M.F.; El-Naggar, A.K. Some physicochemical analyses of kefir produced under different fermentation conditions. J. Sci. Ind. Res. 2011, 70, 365–372. [Google Scholar]
- Popescu, L.; Bulgaru, V.; Siminiuc, R. Effect of temperature, pH and amount of enzyme used in the lactose hydrolysis of milk. Food Nutr. Sci. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]
- Putri, Y.D.; Setiani, N.A.; Warya, S. The Effect of temperature, incubation and storage time on lactic acid content, pH and viscosity of goat milk kefir. Curr. Res. Biosci. Biotechnol. 2020, 2, 101–104. [Google Scholar] [CrossRef]
- Tratnik, L.; Božanić, R.; Herceg, Z.; Drgalić, I. The quality of plain and supplemented kefir from goat’s and cow’s milk. Int. J. Dairy Technol. 2006, 59, 40–46. [Google Scholar] [CrossRef]
- Seiler, H. A Review: Yeasts in kefir and kumiss. Milchwissenschaft 2003, 58, 392–396. [Google Scholar]
- Jeong, D.; Kim, D.-H.; Chon, J.-W.; Kim, H.; Lee, S.-K.; Kim, H.-S.; Song, K.-Y.; Kang, I.-B.; Kim, Y.-J.; Park, J.-H.; et al. Evaluation and determination of lactase activity on various Lactic Acid Bacteria isolated from kefir by using HPLC. J. Milk Sci. Biotechnol. 2016, 34, 231–237. [Google Scholar] [CrossRef]
- Bielska, P.; Cais-Sokolińska, D.; Teichert, J.; Biegalski, J.; Kaczyński, Ł.K.; Chudy, S. Effect of honeydew honey addition on the water activity and water holding capacity of kefir in the context of its sensory acceptability. Sci. Rep. 2021, 11, 22956. [Google Scholar] [CrossRef] [PubMed]
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage Stability of texture and sensory properties of yogurt with the addition of polymerized whey proteins. Foods 2019, 8, 548. [Google Scholar] [CrossRef]
- Bierzuńska, P.; Kaczyński, Ł.K.; Cais-Sokolińska, D.; Kulczyński, B. Texture profile of kefir and yogurt with modified configuration of proteins. Nauk. Przyr. Technol. 2017, 11, 10. [Google Scholar]
- Brodziak, A.; Król, J.; Barłowska, J.; Teter, A.; Florek, M. Changes in the physicochemical parameters of yoghurts with added whey protein in relation to the starter bacteria strains and storage time. Animals 2020, 10, 1350. [Google Scholar] [CrossRef]
- Buran, İ.; Akal, C.; Ozturkoglu-Budak, S.; Yetisemiyen, A. Rheological, sensorial and volatile profiles of synbiotic kefirs produced from cow and goat milk containing varied probiotics in combination with fructooligosaccharide. LWT 2021, 148, 111591. [Google Scholar] [CrossRef]
- İnce-Coşkun, A.E.; Özdestan-Ocak, Ö. Effect of whey protein isolate microparticle addition on some physical and chemical properties of kefir. Chem. Pap. 2022, 76, 3109–3118. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I.; Mortas, M.; Dervisoglu, M. Rheological, textural, colour and sensorial properties of kefir produced with buffalo milk using kefir grains and starter culture: A comparison with cows’ milk kefir. Int. J. Dairy Technol. 2018, 71, 73–80. [Google Scholar] [CrossRef]
- Setyawardani, T.; Sumarmono, J. Chemical and microbiological characteristics of goat milk kefir during storage under different temperatures. J. Indones. Trop. Anim. Agric. 2015, 40, 2460–6278. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Water in milk and dairy products. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 299–320. [Google Scholar] [CrossRef]
| Yeast (log cfu/mL) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 4.23 ± 0.57 aA | 3.70 ± 0.03 abA | 2.87 ± 0.38 bA |
| K_5 | 4.28 ± 0.47 aA | 3.62 ± 0.30 abA | 3.00 ± 0.32 bA |
| Bacteria (log cfu/mL) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 7.08 ± 0,65 aA | 6.92 ± 0.12 aA | 6.38 ± 0.56 aA |
| K_5 | 6.80 ± 1.01 aA | 7.00 ± 0.43 aA | 6.30 ± 0.41 aA |
| Probiotic bacteria (log cfu/mL) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 6.12 ± 0.02 aA | 4.86 ± 0.02 bA | 3.68 ± 0.02 cA |
| K_5 | 5.60 ± 0.17 aB | 5.52 ± 0.02 aB | 4.01 ± 0.01 bB |
| Lactase enzymatic activity (µkat/100 g) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 0.37± 0.06 aA | 0.70 ± 0.07 bA | 0.49 ± 0.05 abA |
| K_5 | 0.43 ± 0.14 aA | 0.68 ± 0.15 bA | 0.47 ± 0.04 abA |
| Changes in lactic acid content (g/L) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 8.10 ± 0,03 aA | 11.20 ± 0.40 bA | 11.98 ± 0.33 bA |
| K_5 | 9.08 ± 0.05 aA | 9.68 ± 0.23 aB | 11.61 ± 0.73 bA |
| Proportion of L(+) form in total content (%) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 81.60 ± 0.06 aA | 92.16 ± 0.06 bA | 52.30 ± 0.30 cA |
| K_5 | 85.43 ± 0.25 aB | 96.33 ± 0.29 bB | 58.86 ± 0.06 cB |
| Changes in ethanol content (g/L) | |||
| Method 1 | Method 2 | Method 3 | |
| K_0 | 0.74 ± 0.01 aA | 0.96 ± 0.01 bA | 0.37 ± 0.01 cA |
| K_5 | 0.61 ± 0.01 aB | 0.81 ± 0.02 bB | 0.32 ± 0.01 cB |
| Day | K_0 | K_1 | K_3 | K_5 | |
|---|---|---|---|---|---|
| Firmness (g) | 0 | 26.50 ± 1.50 aA | 18.62 ± 4.46 bA | 18.43 ± 1.82 bA | 25.00 ± 3.95 aA |
| 14 | 17.17 ± 0.60 aB | 16.67 ± 0.58 aA | 18.31 ± 1.46 aA | 16.40 ± 0.72 aB | |
| Consistency (g·s) | 0 | 598.30 ± 12.76 aA | 335.70± 146.91 bA | 354.90 ± 31.89 bA | 506.00 ± 68.99 aA |
| 14 | 357.50 ± 11.34 aB | 351.70 ± 12.61 aA | 381.80 ± 30.71 aA | 336.50 ± 6.68 aB | |
| Cohesiveness (g) | 0 | 50.64 ± 3.72 aA | 30.00 ± 10.68 bA | 31.24 ± 4.57 bA | 44.52 ± 9.82 abA |
| 14 | 12.98 ± 1.29 aB | 20.95 ± 4.80 abA | 34.74 ± 8.99 bA | 17.45 ±0.73 aB | |
| Viscosity (g·s) | 0 | 120.50 ± 38.70 aA | 52.49 ± 44.89 bA | 53.73 ± 18.90 bA | 105.60 ± 20.13 abA |
| 14 | 5.69 ± 4.63 aB | 23.55 ± 11.10 abA | 76.63 ± 35.06 bA | 14.11 ± 2.07 aB | |
| Apparent viscosity (mPa·s) | 0 | 3817 ± 51.32 aA | 5380 ± 222.70 bA | 7240 ± 79.37 cA | 7083 ± 125.00 cA |
| 14 | 2087 ± 40.41 aB | 3120 ± 20.00 bB | 4127 ± 287.30 cB | 3677 ± 172.40 dB |
| Day | K_0 | K_1 | K_3 | K_5 | |
|---|---|---|---|---|---|
| WI | 0 | 81.85 ± 0.33 aA | 76.38 ± 0.05 bA | 74.03 ± 0.17 cA | 72.68 ± 0.52 dA |
| 14 | 78.66 ± 0.04 aB | 81.36 ± 0.09 bB | 74.95 ± 0.32 cB | 78.03 ± 0.11 dB | |
| YI | 0 | 9.01 ± 0.60 aA | 10.15 ± 0.07 bA | 9.37 ± 0.68 abA | 10.04 ± 0.94 abA |
| 14 | 9.82 ± 0.03 aA | 9.79 ± 0.08 aA | 10.31 ± 0.10 aA | 10.86 ± 0.18 aA | |
| C | 0 | 5.52 ± 0.34 abA | 5.79 ± 0.04 aA | 5.15 ± 0.36 bA | 5.39 ± 0.45 abA |
| 14 | 5.73 ± 0.01 aA | 5.96 ± 0.06 aA | 5.73 ± 0.03 aB | 6.29 ± 0.09 aB |
| Day | K_0 | K_1 | K_3 | K_5 | |
|---|---|---|---|---|---|
| Water activity | 0 | 0.976 ± 0.005 aA | 0.981 ± 0.002 abA | 0.982 ± 0.001 bA | 0.982 ± 0.003 bA |
| 14 | 0.979 ± 0.000 aA | 0.979 ± 0.000 aA | 0.980 ± 0.001 aA | 0.983 ± 0.001 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiğit Ziolkowski, A.; Bielska, P.; Cais-Sokolińska, D.; Samur, G. Designing the Properties of Probiotic Kefir with Increased Whey Protein Content. Fermentation 2024, 10, 495. https://doi.org/10.3390/fermentation10100495
Yiğit Ziolkowski A, Bielska P, Cais-Sokolińska D, Samur G. Designing the Properties of Probiotic Kefir with Increased Whey Protein Content. Fermentation. 2024; 10(10):495. https://doi.org/10.3390/fermentation10100495
Chicago/Turabian StyleYiğit Ziolkowski, Aslı, Paulina Bielska, Dorota Cais-Sokolińska, and Gülhan Samur. 2024. "Designing the Properties of Probiotic Kefir with Increased Whey Protein Content" Fermentation 10, no. 10: 495. https://doi.org/10.3390/fermentation10100495
APA StyleYiğit Ziolkowski, A., Bielska, P., Cais-Sokolińska, D., & Samur, G. (2024). Designing the Properties of Probiotic Kefir with Increased Whey Protein Content. Fermentation, 10(10), 495. https://doi.org/10.3390/fermentation10100495






