Fermentation, Identification, and Antioxidant Activity of Saponins Produced by a Wild Ginseng Endophytic Fungus Umbelopsis dimorpha Strain NSJG
Abstract
1. Introduction
2. Results
2.1. Total Saponin Concentration under Different Fermentation Conditions
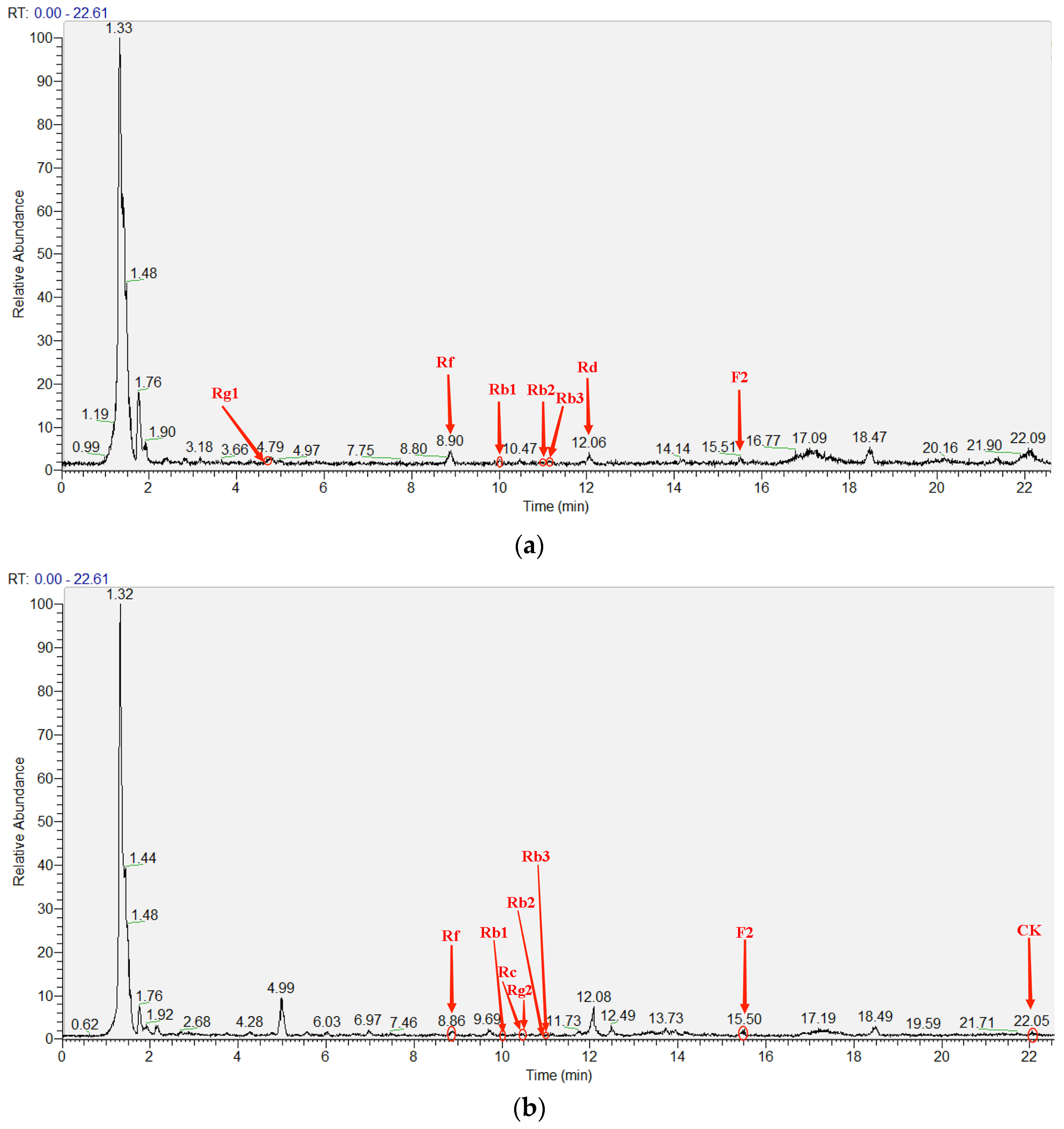
2.2. Identification and Analysis of Saponin Types of Fermentation Extracts
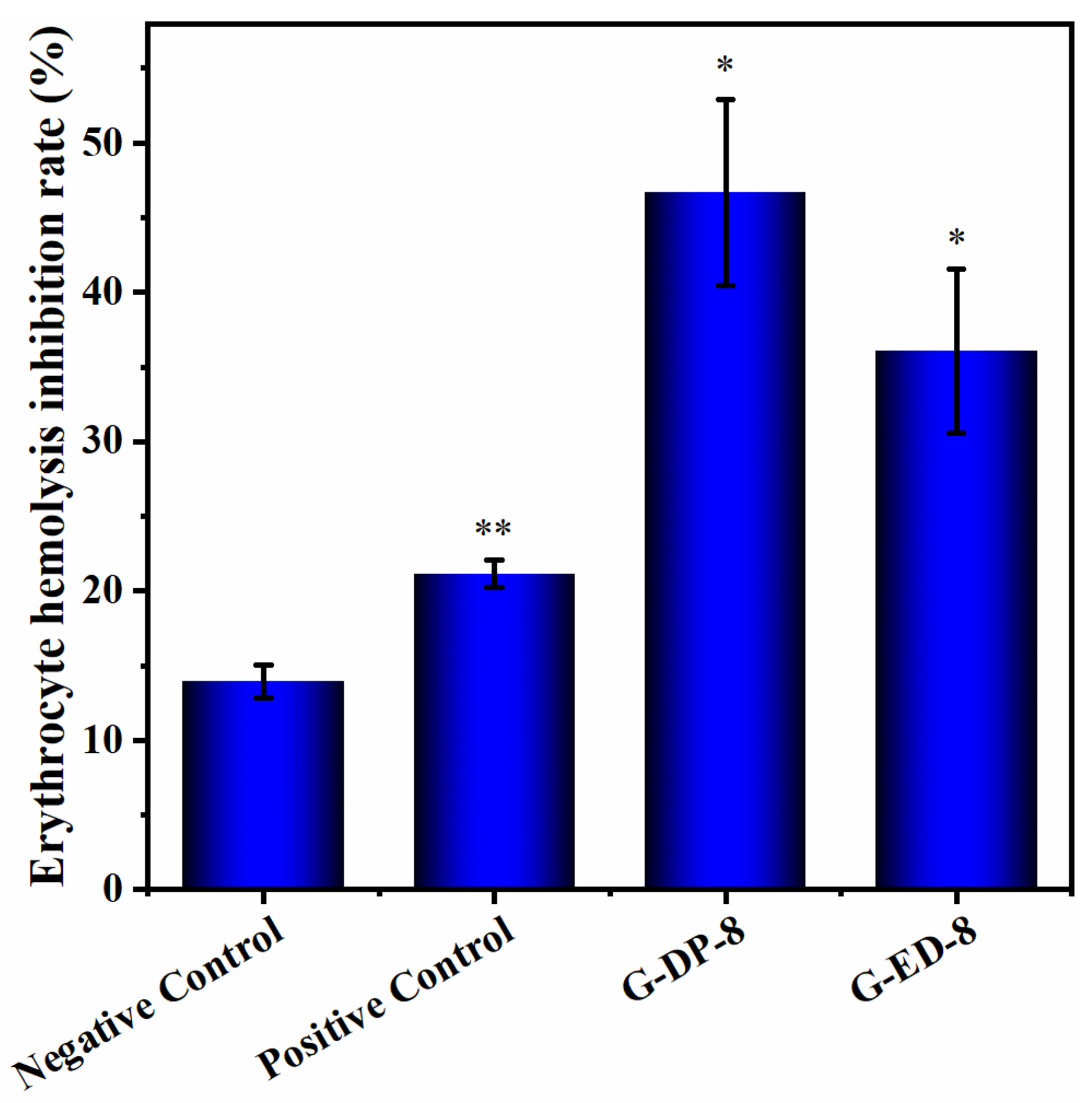
2.3. Determination of Antioxidant Activity of Saponin Extracts
3. Discussion
4. Materials and Methods
4.1. Strains and Chemicals
4.2. Liquid Fermentation of an Endophytic Fungus under Different Fermentation Conditions
4.3. Extraction of Total Saponins
4.4. Determination of Total Saponins
4.5. Identification and Analysis of Saponin Extracts
4.6. Determination of Erythrocyte Hemolysis Inhibition Rate
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Liu, J.; Zuo, T.T.; Hu, Y.; Li, Z.; Wang, H.D.; Xu, X.Y.; Yang, W.Z.; Guo, D.A. Advances and challenges in ginseng research from 2011 to 2020: The phytochemistry, quality control, metabolism, and biosynthesis. Nat. Prod. Rep. 2022, 39, 875–909. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Cardiovascular Diseases and Panax ginseng: A Review on Molecular Mechanisms and Medical Applications. J. Ginseng Res. 2012, 36, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Chaudhary, N.; Seo, H.J.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Immunomodulatory effect of tea saponin in immune T-cells and T-lymphoma cells via regulation of Th1, Th2 immune response and MAPK/ERK2 signaling pathway. Immunopharmacol. Immunotoxicol. 2014, 36, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Seo, J.Y.; Lee, S.K.; Oh, J.; Kim, J.S. Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism. J. Ginseng Res. 2021, 45, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.I.; Kang, M.Y.; Lee, S.C. In Vitro and In Vivo Antioxidant Activity of Aged Ginseng (Panax ginseng). Prev. Nutr. Food Sci. 2016, 21, 24–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar]
- Wang, K.; Zhang, Z.; Li, S.; Hu, J.; Liu, T.; Jiang, Y.; Wu, J.; Lu, M.; Zhao, M.; Li, L.; et al. Transcriptome-Wide Analysis for Ginsenoside Rb3 Synthesis-Related Genes and Study on the Expression of Methyl Jasmonate Treatment in Panax ginseng. Life 2021, 11, 387. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef]
- Li, M.; Ma, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; An, T.; Wang, G. Advances in the biosynthesis and metabolic engineering of rare ginsenosides. Appl. Microbiol. Biotechnol. 2023, 107, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F. Endophytes: The Second Layer of Plant Defense. Trends Plant Sci. 2020, 25, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.K.; Choi, M.S.; Eom, A.H. Diversity of endophytic fungi isolated from Korean ginseng leaves. Mycobiology 2014, 42, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, Y.; Mishra, R.C.; Bae, H. Fungal endophytes inhabiting mountain-cultivated ginseng (Panax ginseng Meyer): Diversity and biocontrol activity against ginseng pathogens. Sci. Rep. 2017, 7, 16221. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, H.; Yin, Z.; Lian, M.; Yin, C. Endophytic Bacteria Isolated from Panax ginseng Improves Ginsenoside Accumulation in Adventitious Ginseng Root Culture. Molecules 2017, 22, 837. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Hong, S.Y.; Lee, S.M.; Kim, Y.H.; Kahng, G.G.; Lim, Y.P.; Kim, H.; Yun, H.D. Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb. Ecol. 2007, 54, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Lee, S.W.; Jo, I.H. Diversity of bacterial endophytes in Panax ginseng and their protective effects against pathogens. 3 Biotech 2018, 8, 397. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Wei, G.; Chen, Z.; Wang, B.; Wei, F.; Zhang, G.; Wang, Y.; Zhu, G.; Zhou, Y.; Zhao, Q.; He, M.; et al. Endophytes isolated from Panax notoginseng converted ginsenosides. Microb. Biotechnol. 2021, 14, 1730–1746. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.Y.; You, X.L.; Li, Y.H. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. Springerplus 2013, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Dan, Y.; Xu, Z.; Xiang, L. Implications of Oxidative Stress in the Pathogenesis and Treatment of Hyperpigmentation Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 7881717. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Gao, X.; Wang, C.; Huo, X.; Liu, Z.; Sun, H.; Yang, X.; Sun, P.; Ma, X.; Meng, Q.; et al. Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Env. Toxicol. 2018, 33, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, W.; Lu, P.; Ma, Y.; Liang, X.; Liu, Y. Ginsenoside Rg1 attenuates the inflammation and oxidative stress induced by diabetic nephropathy through regulating the PI3K/AKT/FOXO3 pathway. Ann. Transl. Med. 2021, 9, 1789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Zhang, M.; Gao, Y.; Yan, Z. Protective Effects of Ginsenosides (20R)-Rg3 on H(2) O(2) -Induced Myocardial Cell Injury by Activating Keap-1/Nrf2/HO-1 Signaling Pathway. Chem. Biodivers. 2021, 18, e2001007. [Google Scholar] [CrossRef]
- He, B.; Chen, D.; Zhang, X.; Yang, R.; Yang, Y.; Chen, P.; Shen, Z. Oxidative Stress and Ginsenosides: An Update on the Molecular Mechanisms. Oxid. Med. Cell Longev. 2022, 2022, 9299574. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Luo, X.Y.; Liu, G.Z.; Chen, Y.P.; Wang, Z.C.; Sun, Y.X. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J. Agric. Food Chem. 2003, 51, 2555–2558. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Winston, G.W.; Regoli, F.; Dugas, A.J., Jr.; Fong, J.H.; Blanchard, K.A. A rapid gas chromatographic assay for determining oxyradical scavenging capacity of antioxidants and biological fluids. Free Radic. Biol. Med. 1998, 24, 480–493. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Gao, Y.; Wang, Z.; Gao, X.; Yan, P. Biotransformation of American Ginseng Stems and Leaves by an Endophytic Fungus Umbelopsis sp. and Its Effect on Alzheimer’s Disease Control. Nutrients 2023, 15, 4878. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.Y.; Murthy, H.N.; Hahn, E.J.; Zhong, J.J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv. Biochem. Eng. Biotechnol. 2009, 113, 151–176. [Google Scholar]
- Xu, W.; Lyu, W.; Duan, C.; Ma, F.; Li, X.; Li, D. Preparation and bioactivity of the rare ginsenosides Rg3 and Rh2: An updated review. Fitoterapia 2023, 167, 105514. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Gao, Y.; Gao, X.; Yan, P. Optimization of Fermentation Conditions and Product Identification of a Saponin-Producing Endophytic Fungus. Microorganisms 2023, 11, 2331. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Zhang, P.; Zhang, L.; Zhou, R.; Xu, Y.; Ding, H.; Wang, Q.; Wang, Z. Semi-synthesis of Twelve Known 20Z/E Pseudo-Ginsenosides and Their Comparative Study of Antioxidative Activity in Free Radical Induced Hemolysis of Rabbit Erythrocytes. Chem. Pharm. Bull. 2018, 66, 535–540. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, D.L.; Liu, G.H.; Fu, X.; Gu, Y.C.; Ding, L.S.; Zhou, Y. Antifungal Halogenated Cyclopentenones from the Endophytic Fungus Saccharicola bicolor of Bergenia purpurascens by the One Strain-Many Compounds Strategy. J. Agric. Food Chem. 2020, 68, 185–192. [Google Scholar] [CrossRef]
- Li, R.; Duan, W.; Ran, Z.; Chen, X.; Yu, H.; Fang, L.; Guo, L.; Zhou, J. Diversity and correlation analysis of endophytes and metabolites of Panax quinquefolius L. in various tissues. BMC Plant Biol. 2023, 23, 275. [Google Scholar]
- Yan, H.; Jin, H.; Fu, Y.; Yin, Z.; Yin, C. Production of Rare Ginsenosides Rg3 and Rh2 by Endophytic Bacteria from Panax ginseng. J. Agric. Food Chem. 2019, 67, 8493–8499. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef]
- Liu, G.M.; Lu, T.C.; Sun, M.L.; Jia, W.Y.; Ji, X.; Luo, Y.G. Ginsenoside Rd Inhibits Glioblastoma Cell Proliferation by Up-Regulating the Expression of miR-144-5p. Biol. Pharm. Bull. 2020, 43, 1534–1541. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Lee, M.; Sung, C. Ginsenoside Rd as a potential neuroprotective agent prevents trimethyltin injury. Biomed. Rep. 2017, 6, 435–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, N.; An, X.; Lang, P.; Wang, F.; Xie, Y. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed. Pharmacother. 2019, 109, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Li, J.; Guan, Y.; Shen, T.; Li, F.; Li, X.; Yang, X.; Hu, W. Ginsenoside F2 Suppresses Adipogenesis in 3T3-L1 Cells and Obesity in Mice via the AMPK Pathway. J. Agric. Food Chem. 2021, 69, 9299–9312. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Seo, W.; Eun, H.S.; Kim, S.Y.; Jo, E.; Kim, M.H.; Choi, W.M.; Lee, J.H.; Shim, Y.R.; Cui, C.H.; et al. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem. Biophys. Res. Commun. 2016, 478, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.H.; Kim, K.H.; Lee, K.A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules 2021, 26, 4102. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Z.K.; Li, C.Y.; Liang, Y.Q.; Yang, F. Anticancer properties and pharmaceutical applications of ginsenoside compound K: A review. Chem. Biol. Drug Des. 2022, 99, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Evaluation of glucosidases of Aspergillus niger strain comparing with other glucosidases in transformation of ginsenoside Rb1 to ginsenosides Rg3. J. Ginseng Res. 2014, 38, 47–51. [Google Scholar] [CrossRef]
- Gum, S.I.; Rahman, M.K.; Won, J.S.; Cho, M.K. A Distinctive Pattern of Beauveria bassiana-biotransformed Ginsenoside Products Triggers Mitochondria/FasL-mediated Apoptosis in Colon Cancer Cells. Phytother. Res. 2016, 30, 136–143. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Wang, C.; Liu, L.; Zhang, D.; Wang, Z.; Li, W.; Zheng, Y.; Sung, C. Microbial transformation of ginsenoside Rg3 to ginsenoside Rh2 by Esteya vermicola CNU 120806. World J. Microbiol. Biotechnol. 2012, 28, 1807–1811. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Alalikhan, A.; Aghaee-Bakhtiari, S.H.; Hashemy, S.I. The redox modulatory effects of SP/NK1R system: Implications for oxidative stress-associated disorders. Life Sci. 2022, 296, 120448. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Miki, M.; Mino, M.; Takahashi, M.; Niki, E. Synergistic inhibition of oxidation in dispersed phosphatidylcholine liposomes by a combination of vitamin E and cysteine. Arch. Biochem. Biophys. 1989, 270, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Nuruki, Y.; Matsumoto, H.; Tsukada, M.; Tsukahara, H.; Takajo, T.; Tsuchida, K.; Anzai, K. Method to Improve Azo-Compound (AAPH)-Induced Hemolysis of Erythrocytes for Assessing Antioxidant Activity of Lipophilic Compounds. Chem. Pharm. Bull. 2021, 69, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Niki, E.; Kamiya, Y.; Miki, M.; Tamai, H.; Mino, M. Free radical chain oxidation and hemolysis of erythrocytes by molecular oxygen and their inhibition by vitamin E. J. Nutr. Sci. Vitaminol. 1986, 32, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Tan, W.; Feng, X.; Feng, K.; Zhong, W.; Liao, C.; Liu, Y.; Li, S.; Hu, W. Protective Effect of Flavonoids from Mulberry Leaf on AAPH-Induced Oxidative Damage in Sheep Erythrocytes. Molecules 2022, 27, 7625. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Feng, J.; Yang, N.; Guo, Y.; Chen, C.; Qin, Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-kappaB pathway. Int. Immunopharmacol. 2021, 98, 107841. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Liu, P.; Wang, H.; Dong, M.; Li, G.; Li, Y. Ginsenoside Rb1 attenuates diabetic retinopathy in streptozotocin-induced diabetic rats1. Acta Cir. Bras. 2019, 34, e201900201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Guo, R.; Xiao, J.; Liu, X.; Dong, M.; Luan, X.; Ji, X.; Lu, H. Ginsenoside Rb1 Ameliorates Diabetic Arterial Stiffening via AMPK Pathway. Front. Pharmacol. 2021, 12, 753881. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Wang, C.Q.; Yi, L.W.; Zhao, L.; Zhou, Y.Z.; Guo, F.; Huo, Y.S.; Zhao, D.Q.; Xu, F.; Wang, X.; Cai, S.Q. 177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MS(n), and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng. Molecules 2021, 26, 3371. [Google Scholar] [CrossRef]

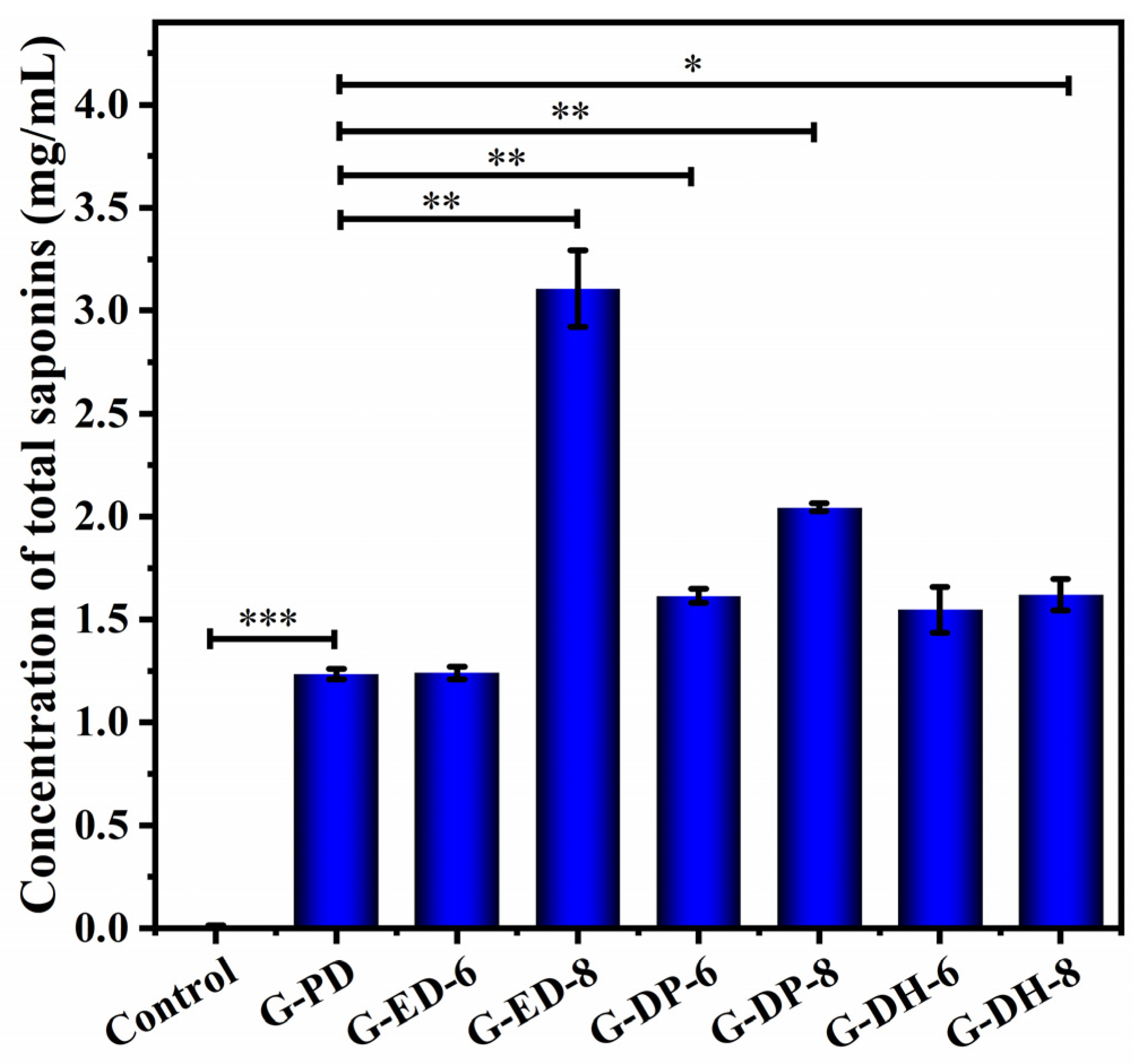
| Protocols | Potato Concentration (g/L) | Glucose Concentration (g/L) | Inoculation Volume (%) | pH | Incubation Temperature (°C) | Incubation Time (d) |
|---|---|---|---|---|---|---|
| G-PD | 200.0 | 20.0 | 10.0 | Natural | 26.0 | 7 |
| G-ED-6 | 104.5 | 37.5 | 9.4 | 2.1 | 28.5 | 6 |
| G-ED-8 | 104.5 | 37.5 | 9.4 | 2.1 | 28.5 | 8 |
| G-DP-6 | 325.9 | 35.3 | 9.3 | 4.5 | 21.0 | 6 |
| G-DP-8 | 325.9 | 35.3 | 9.3 | 4.5 | 21.0 | 8 |
| G-DH-6 | 98.3 | 36.1 | 2.1 | 4.2 | 31.0 | 6 |
| G-DH-8 | 98.3 | 36.1 | 2.1 | 4.2 | 31.0 | 8 |
| Saponin Type | Retention Time (RT) of Standards (min) [34] | RT of Sample (min) | Charge-to-Mass Ratio (M/Z, M + Na) | Calculate Mass Errors (ppm) | Peak Area | Concentration (mg/L) |
|---|---|---|---|---|---|---|
| Ginsenoside Rg1 | 4.78 | 4.69 | 823.4766 | 5.22 | 1,497,935 | 0.71 |
| Ginsenoside Rf | 8.90 | 8.90 | 823.4889 | −9.71 | 14,871,676 | 4.55 |
| Ginsenoside Rb1 | 9.93 | 10.02 | 1131.5887 | 2.56 | 4,720,040 | 65.74 |
| Ginsenoside Rb2 | 11.01 | 10.98 | 1101.5778 | 2.90 | 3,285,821 | 1.47 |
| Ginsenoside Rb3 | 11.11 | 11.13 | 1101.5779 | 2.81 | 4,875,101 | 2.52 |
| Ginsenoside Rd | 12.08 | 12.06 | 969.5361 | 0.72 | 4,234,268 | 2.68 |
| Ginsenoside F2 | 15.47 | 15.51 | 807.4835 | 2.97 | 6,833,629 | 3.69 |
| Saponin Type | RT of Standards (min) [34] | RT of Sample (min) | M/Z (M + Na) | Calculate Mass Errors (ppm) | Peak Area | Concentration (mg/L) |
|---|---|---|---|---|---|---|
| Ginsenoside Rf | 8.90 | 8.86 | 823.4791 | 2.19 | 12,663,803 | 3.88 |
| Ginsenoside Rb1 | 9.93 | 10.02 | 1131.5881 | −0.62 | 2,824,377 | 39.34 |
| Ginsenoside Rc | 10.52 | 10.44 | 1101.5786 | 2.18 | 646,020 | 1.15 |
| Ginsenoside Rg2 | 10.72 | 10.50 | 807.4838 | 2.60 | 2,149,977 | 5.47 |
| Ginsenoside Rb2 | 11.01 | 10.97 | 1101.5767 | 3.90 | 867,864 | 0.39 |
| Ginsenoside Rb3 | 11.11 | 11.11 | 1101.5780 | 2.72 | 4,500,360 | 2.37 |
| Ginsenoside F2 | 15.47 | 15.5 | 807.4840 | 2.35 | 8,396,911 | 4.53 |
| Ginsenoside CK | 22.07 | 22.05 | 645.4325 | 0.93 | 3,855,952 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, J.; Gao, Y.; Wang, Z.; Wang, D.; Gao, X.; Yan, P. Fermentation, Identification, and Antioxidant Activity of Saponins Produced by a Wild Ginseng Endophytic Fungus Umbelopsis dimorpha Strain NSJG. Fermentation 2024, 10, 9. https://doi.org/10.3390/fermentation10010009
Chen Q, Wang J, Gao Y, Wang Z, Wang D, Gao X, Yan P. Fermentation, Identification, and Antioxidant Activity of Saponins Produced by a Wild Ginseng Endophytic Fungus Umbelopsis dimorpha Strain NSJG. Fermentation. 2024; 10(1):9. https://doi.org/10.3390/fermentation10010009
Chicago/Turabian StyleChen, Qiqi, Jingying Wang, Yuhang Gao, Zixin Wang, Di Wang, Xiujun Gao, and Peisheng Yan. 2024. "Fermentation, Identification, and Antioxidant Activity of Saponins Produced by a Wild Ginseng Endophytic Fungus Umbelopsis dimorpha Strain NSJG" Fermentation 10, no. 1: 9. https://doi.org/10.3390/fermentation10010009
APA StyleChen, Q., Wang, J., Gao, Y., Wang, Z., Wang, D., Gao, X., & Yan, P. (2024). Fermentation, Identification, and Antioxidant Activity of Saponins Produced by a Wild Ginseng Endophytic Fungus Umbelopsis dimorpha Strain NSJG. Fermentation, 10(1), 9. https://doi.org/10.3390/fermentation10010009





