An Update on Microbial Biosynthesis of β-Caryophyllene, a Sesquiterpene with Multi-Pharmacological Properties
Abstract
1. Introduction
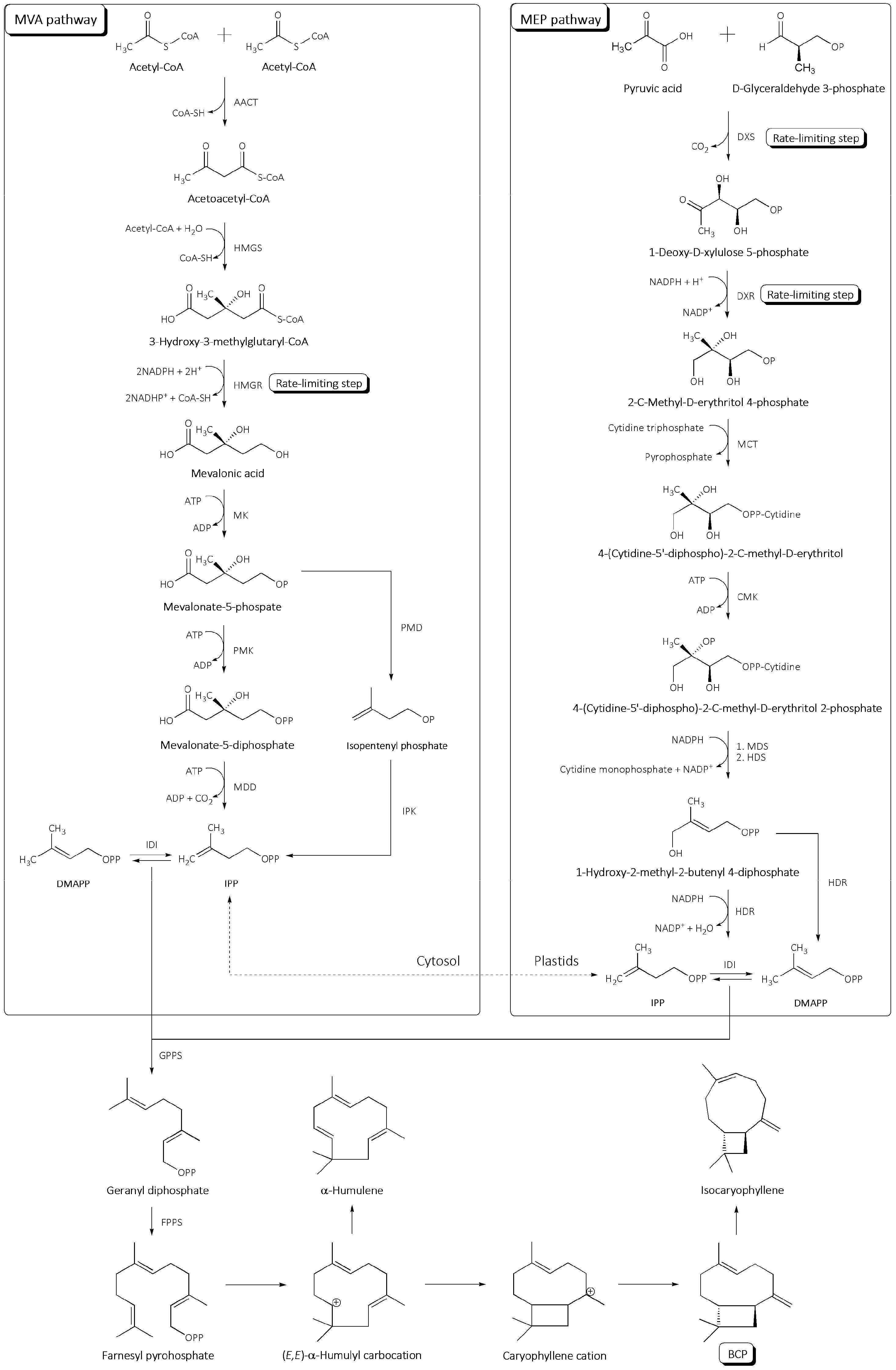
2. Biosynthesis of BCP in Plants
2.1. Biosynthesis of IPP (Upstream Module)
2.2. BCP Synthesis (Downstream Module)
3. Microbial Biosynthesis of BCP
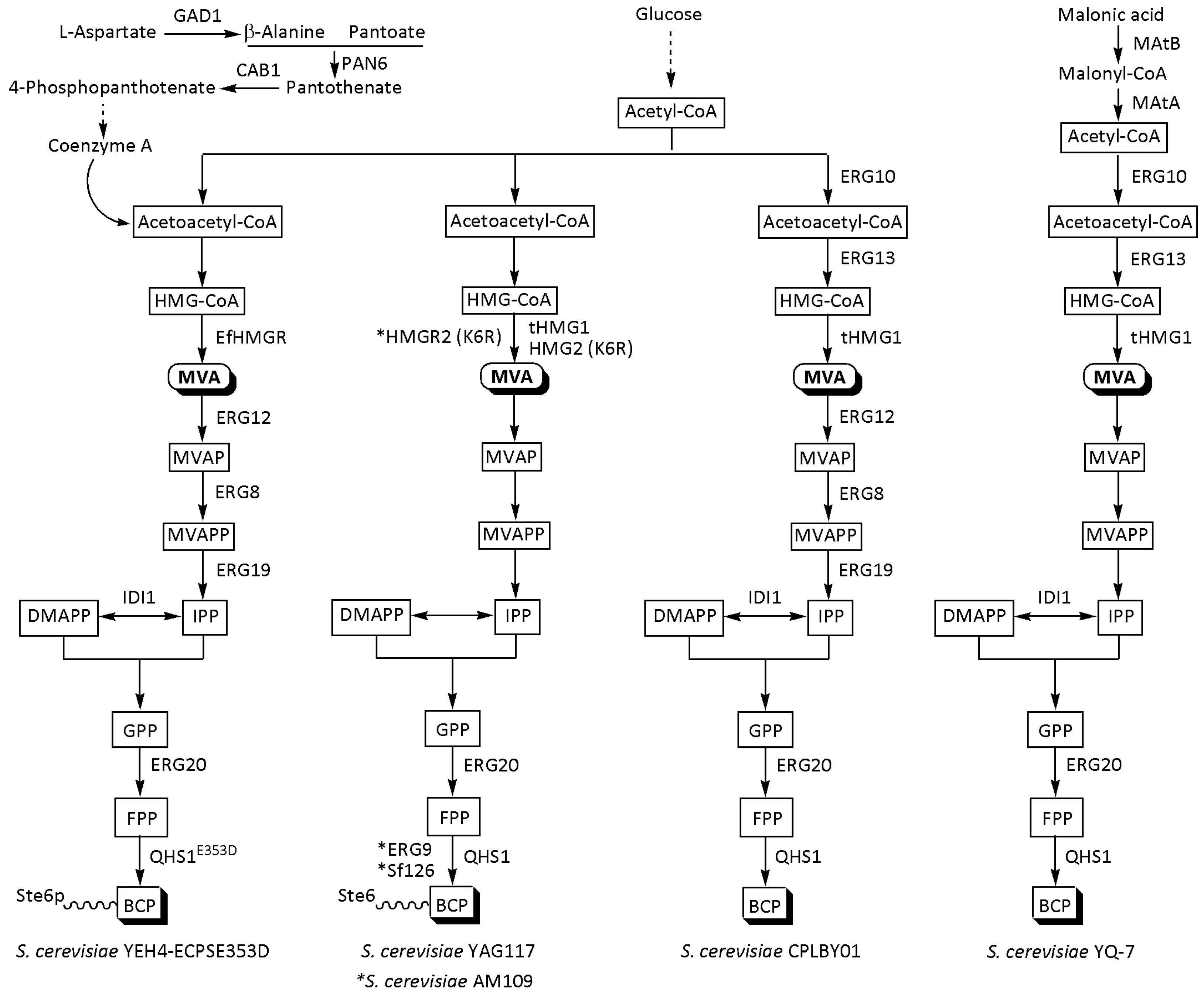
3.1. S. cerevisiae
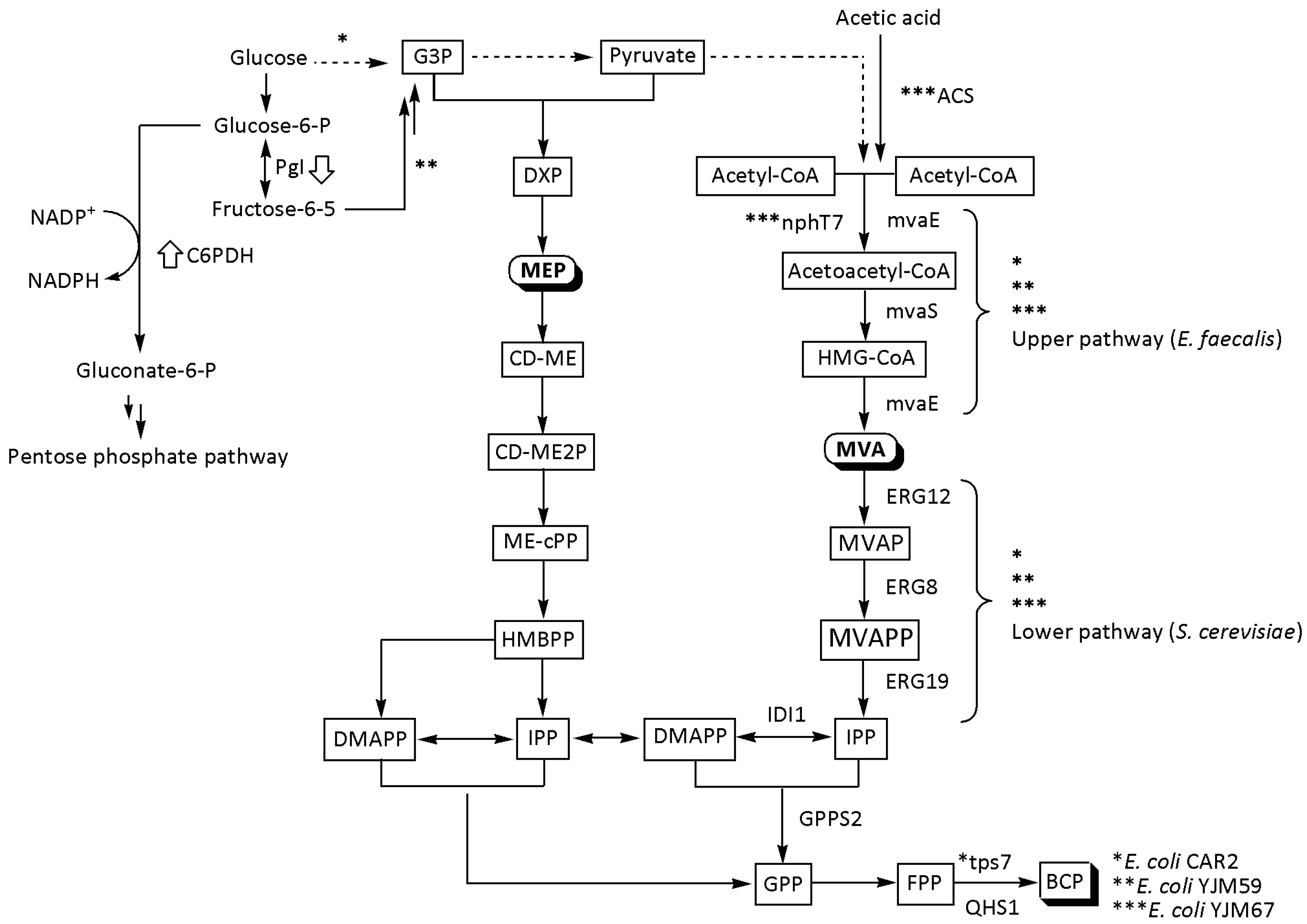
3.2. E. coli
3.3. R. capsulatus
3.4. Synechocystis sp.
3.5. S. elongatus
4. A Summary of Findings
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CFR—Code of Federal Regulations Title 21. 2023. Volume 3. 21CFR172.515. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=caryophyllene (accessed on 20 September 2023).
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Lacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Caryophyllene Market Analysis—Industry Size, Share, Research Report, Insights, COVID-19 Impact, Statistics, Trends, Growth and Forecast 2023–2030. Available online: https://markwideresearch.com/caryophyllene-market/ (accessed on 21 September 2023).
- Caryophyllene Market by Purity, Application, End Use and Region. Forecast 2023 to 2033. Available online: https://www.futuremarketinsights.com/reports/caryophyllene-market (accessed on 20 September 2023).
- Maffei, M.E. Plant natural sources of the endocannabinoid (E)-β-caryophyllene: A systematic quantitative analysis of published literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of terpenoid compounds in food and pharmaceutical products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Blake, K. Beta-Caryophyllene: A review of current research. Altern. Complement. Ther. 2021, 27, 222–226. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Kaabi, J.A.; Ojha, S. A focused review on CB2 receptor-selective pharmacological properties and therapeutic potential of β-caryophyllene, a dietary cannabinoid. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Guo, L.; Du, J.; Bae, H.J. Biosynthesis of β-caryophyllene, a novel terpene-based high-density biofuel precursor, using engineered Escherichia coli. Renew. Energy 2016, 99, 216–223. [Google Scholar] [CrossRef]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Czaczyk, K. β-Caryophyllene-rich pepper essential oils suppress spoilage activity of Pseudomonas fluorescens KM06 in fresh-cut lettuce. LWT Food Sci. Technol. 2017, 83, 118–126. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM fragrance ingredient safety assessment. β-Caryophyllene, CAS Registry Number 87-44-5. Food Chem. Toxicol. 2022, 159 (Suppl. 1), 112707. [Google Scholar] [CrossRef]
- Hancock, E.N.; Wahl, J.M.; Brown, M.K. Recent advances in the synthesis of gem-dimethylcyclobutane natural products. Nat. Prod. Rep. 2019, 36, 1383–1393. [Google Scholar] [CrossRef]
- Navale, G.R.; Dharne, M.S.; Shinde, S.S. Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 457–475. [Google Scholar] [CrossRef]
- Liu, C.L.; Xue, K.; Yang, Y.; Liu, X.; Li, Y.; Lee, T.S.; Bai, Z.; Tan, T. Metabolic engineering strategies for sesquiterpene production in microorganism. Crit. Rev. Biotechnol. 2022, 42, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Klaus, O.; Hilgers, F.; Nakielski, A.; Hasenklever, D.; Jaeger, K.E.; Axmann, I.M.; Drepper, T. Engineering phototrophic bacteria for the production of terpenoids. Curr. Opin. Biotechnol. 2022, 77, 102764. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic engineering of yeast, filamentous fungi and bacteria for terpene production and applications in food industry. Food Res. Int. 2021, 147, 110487. [Google Scholar] [CrossRef] [PubMed]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, K.; Guo, J.; Yang, Q.; Li, Y.; Xian, M.; Zhang, R. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnol. Biofuels Bioprod. 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, F.; Habash, S.S.; Loeschcke, A.; Ackermann, Y.S.; Neumann, S.; Heck, A.; Klaus, O.; Hage-Hülsmann, J.; Grundler, F.M.W.; Jaeger, K.E.; et al. Heterologous production of β-caryophyllene and evaluation of its activity against plant pathogenic fungi. Microorganisms 2021, 9, 168. [Google Scholar] [CrossRef]
- Reinsvold, R.E.; Jinkerson, R.E.; Radakovits, R.; Posewitz, M.C.; Basu, C. The production of the sesquiterpene β-caryophyllene in a transgenic strain of the cyanobacterium Synechocystis. J. Plant Physiol. 2011, 168, 848–852. [Google Scholar] [CrossRef]
- Lei, C.; Shubin, L.; Sun, T.; Zhang, W. Synechococcus Genetically Engineered Bacteria for Biosynthesizing Caryophyllene and Its Construction Methods and Applications. CN111394383A, 10 July 2020. [Google Scholar]
- Muthusamy, S.; Vetukuri, R.R.; Lundgren, A.; Ganji, S.; Zhu, L.H.; Brodelius, P.E.; Kanagarajan, S. Transient expression and purification of β-caryophyllene synthase in Nicotiana benthamiana to produce β-caryophyllene in vitro. PeerJ 2020, 8, e8904. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Wang, C.; Liwei, M.; Park, J.B.; Jeong, S.H.; Wei, G.; Wang, Y.; Kim, S.W. Microbial platform for terpenoid production: Escherichia coli and yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Durairaj, J.; Di Girolamo, A.; Bouwmeester, H.J.; de Ridder, D.; Beekwilder, J.; van Dijk, A.D. An analysis of characterized plant sesquiterpene synthases. Phytochemistry 2019, 158, 157–165. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive potential of caryophyllane sesquiterpenes: An overview of preliminary evidence. Cancers 2020, 12, 3034. [Google Scholar] [CrossRef]
- He, S.M.; Wang, X.; Yang, S.C.; Dong, Y.; Zhao, Q.M.; Yang, J.L.; Cong, K.; Zhang, J.J.; Zhang, G.H.; Wang, Y.; et al. De novo transcriptome characterization of Rhodomyrtus tomentosa leaves and identification of genes involved in α/β-pinene and β-caryophyllene biosynthesis. Front. Plant Sci. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Cai, Y.; Jia, J.W.; Crock, J.; Lin, Z.X.; Chen, X.Y.; Croteau, R. A cDNA clone for β-caryophyllene synthase from Artemisia annua. Phytochemistry 2002, 61, 523–529. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, J.; Han, S.; Chong, S.L.; Meng, G.; Song, M.; Wang, Y.; Zhou, S.; Liu, C.; Lou, L.; et al. The chromosome-scale genome of Phoebe bournei reveals contrasting fates of terpene synthase (TPS)-a and TPS-b subfamilies. Plant Commun. 2022, 3, 100410. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Sun, X.; Li, D.; Wei, G.; Liu, L.; Chen, F.; Cai, Y.; Fan, H. Terpenoid biosynthesis in Dendrobium officinale: Identification of (E)-β-caryophyllene synthase and the regulatory MYB genes. Ind. Crops Prod. 2022, 182, 114875. [Google Scholar] [CrossRef]
- Feng, K.; Kan, X.Y.; Yan, Y.J.; Wang, Y.; Sun, N.; Yang, Z.Y.; Zhao, S.P.; Wu, P.; Li, L.J. Identification and characterization of terpene synthase OjTPS1 involved in β-caryophyllene biosynthesis in Oenanthe javanica (Blume) DC. Ind. Crops Prod. 2023, 192, 115998. [Google Scholar] [CrossRef]
- Kollner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.J.; Gershenzon, J.; Degenhardta, J. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef]
- Wang, L.; Xu, G.; Li, L.; Ruan, M.; Bennion, A.; Wang, G.L.; Li, R.; Qu, S. The OsBDR1-MPK3 module negatively regulates blast resistance by suppressing the jasmonate signaling and terpenoid biosynthesis pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2211102120. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, G.; Huang, X.; Guo, H.; Su, X.; Han, L.; Zhang, Y.; Qi, Z.; Xiao, Y.; Cheng, H. Overexpression of the caryophyllene synthase gene GhTPS1 in cotton negatively affects multiple pests while attracting parasitoids. Pest Manag. Sci. 2020, 76, 1722–1730. [Google Scholar] [CrossRef]
- Yahyaa, M.; Tholl, D.; Cormier, G.; Jensen, R.; Simon, P.W.; Ibdah, M. Identification and characterization of terpene synthases potentially involved in the formation of volatile terpenes in carrot (Daucus carota L.) roots. J. Agric. Food Chem. 2015, 63, 4870–4878. [Google Scholar] [CrossRef]
- Nawade, B.; Shaltiel-Harpaz, L.; Yahyaa, M.; Kabaha, A.; Kedoshim, R.; Bosamia, T.C.; Ibdah, M. Characterization of terpene synthase genes potentially involved in black fig fly (Silba adipata) interactions with Ficus carica. Plant Sci. 2020, 298, 110549. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Wenig, M.; Ghirardo, A.; van der Krol, A.; Vlot, A.C.; Schnitzler, J.P.; Rosenkranz, M. Isoprene and β-caryophyllene confer plant resistance via different plant internal signaling pathways. Plant Cell Environ. 2021, 44, 1151–1164. [Google Scholar] [CrossRef]
- Huang, A.C.; Osbourn, A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019, 75, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Cvetkovic, I.; Loupassaki, S.; Kefalas, P.; Johnson, C.B.; Kampranis, S.C.; Makris, A.M. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb. Cell Fact. 2011, 10, 4. [Google Scholar] [CrossRef]
- Ignea, C.; Trikka, F.A.; Kourtzelis, I.; Argiriou, A.; Kanellis, A.K.; Kampranis, S.C.; Makris, A.M. Positive genetic interactors of HMG2 identify a new set of genetic perturbations for improving sesquiterpene production in Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Nong, F.T.; Wang, Y.Z.; Yan, C.X.; Gu, Y.; Song, P.; Sun, X.M. Strategies for efficient production of recombinant proteins in Escherichia coli: Alleviating the host burden and enhancing protein activity. Microb. Cell Fact. 2022, 21, 191. [Google Scholar] [CrossRef]
- Bureau, J.A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yang, Q.; Zhang, J.; Zhao, Y.; Zheng, Y.; Yang, J. Recent advances in the biosynthesis of isoprenoids in engineered Saccharomyces cerevisiae. Adv. Appl. Microbiol. 2021, 114, 1–35. [Google Scholar] [CrossRef]
- Daletos, G.; Katsimpouras, C.; Stephanopoulos, G. Novel strategies and platforms for industrial isoprenoid engineering. Trends Biotechnol. 2020, 38, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.; Wang, P.; Yan, X.; Zhou, Z. Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae. Synth. Biol. 2021, 2, 792–803. [Google Scholar] [CrossRef]
- Elison, G.L.; Xue, Y.; Song, R.; Acar, M. Insights into bidirectional gene expression control using the canonical GAL1/GAL10 promoter. Cell Rep. 2018, 25, 737–748.e4. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, C.; Guo, X.; Du, Z.; Cheng, Y.; Wang, Z.; He, X. Enhancing fluxes through the mevalonate pathway in Saccharomyces cerevisiae by engineering the HMGR and β-alanine metabolism. Microb. Biotechnol. 2022, 15, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Deng, H.; Zhou, C.; Du, Z.; Guo, X.; Cheng, Y.; He, X. Enhancement of β-caryophyllene biosynthesis in Saccharomyces cerevisiae via synergistic evolution of β-caryophyllene synthase and engineering the chassis. ACS Synth. Biol. 2023, 12, 1696–1707. [Google Scholar] [CrossRef]
- Godara, A.; Kao, K.C. Adaptive laboratory evolution of β-caryophyllene producing Saccharomyces cerevisiae. Microb. Cell Fact. 2021, 20, 106. [Google Scholar] [CrossRef]
- Hirasawa, T.; Maeda, T. Adaptive laboratory evolution of microorganisms: Methodology and application for bioproduction. Microorganisms 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Liang, B.; Yang, Q.; Zhang, X.; Zhao, Y.; Liu, Y.; Yang, J.; Wang, Z. Switching carbon metabolic flux for enhancing the production of sesquiterpene-based high-density biofuel precursor in Saccharomyces cerevisiae. Biotechnol. Biofuels Bioprod. 2023, 16, 124. [Google Scholar] [CrossRef]
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the production of isoprenoids. FEMS Microbiol. Lett. 2018, 365, fny079. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Q. Engineering Escherichia coli to convert acetic acid to β-caryophyllene. Microb. Cell Fact. 2016, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Kutscha, R.; Pflügl, S. Microbial upgrading of acetate into value-added products-examining microbial diversity, bioenergetic constraints and metabolic engineering approaches. Int. J. Mol. Sci. 2020, 21, 8777. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lama, S.; Agrawal, D.; Kumar, V.; Park, S. Acetate as a potential feedstock for the production of value-added chemicals: Metabolism and applications. Biotechnol. Adv. 2021, 49, 107736. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Davis, R. One-pot bioconversion of algae biomass into terpenes for advanced biofuels and bioproducts. Algal Res. 2016, 17, 316–320. [Google Scholar] [CrossRef]
- Wu, W.; Liu, F.; Davis, R.W. Engineering Escherichia coli for the production of terpene mixture enriched in caryophyllene and caryophyllene alcohol as potential aviation fuel compounds. Metab. Eng. Commun. 2018, 6, 13–21. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Yi, X.; Nie, Q. A Method of Synthesizing Beta-Caryophyllene by Microbial Catalysis and a Reconstituted Cell Capable of Synthesizing the Beta-Caryophyllene. CN104120141A, 29 October 2014. [Google Scholar]
- Zhang, R.; Li, Y.X.; Zhang, K.; Yang, A.; Wang, Y. Engineering Bacterium for Producing β-Caryophyllene and Construction Method and Application Thereof. CN111004763A, 14 April 2020. [Google Scholar]
- Grozdev, L.; Kaiser, J.; Berensmeier, S. One-step purification of microbially produced hydrophobic terpenes via process chromatography. Front. Bioeng. Biotechnol. 2019, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Troost, K.; Loeschcke, A.; Hilgers, F.; Özgür, A.Y.; Weber, T.M.; Santiago-Schübel, B.; Svensson, V.; Hage-Hülsmann, J.; Habash, S.S.; Grundler, F.M.W.; et al. Engineered Rhodobacter capsulatus as a phototrophic platform organism for the synthesis of plant sesquiterpenoids. Front. Microbiol. 2019, 10, 1998. [Google Scholar] [CrossRef]
- Orsi, E.; Folch, P.L.; Monje-López, V.T.; Fernhout, B.M.; Turcato, A.; Kengen, S.W.M.; Eggink, G.; Weusthuis, R.A. Characterization of heterotrophic growth and sesquiterpene production by Rhodobacter sphaeroides on a defined medium. J. Ind. Microbiol. Biotechnol. 2019, 46, 1179–1190. [Google Scholar] [CrossRef]
- Satta, A.; Esquirol, L.; Ebert, B.E. Current metabolic engineering strategies for photosynthetic bioproduction in cyanobacteria. Microorganisms 2023, 11, 455. [Google Scholar] [CrossRef]
- Herold, R.A.; Bryan, S.J. Engineered terpenoid production in Synechocystis sp. PCC 6803 under different growth conditions. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Lindberg, P. Metabolic engineering of Synechocystis sp. PCC 6803 for improved bisabolene production. Metab. Eng. Commun. 2021, 12, e00159. [Google Scholar] [CrossRef] [PubMed]
- Dietsch, M.; Behle, A.; Westhoff, P.; Axmann, I.M. Metabolic engineering of Synechocystis sp. PCC 6803 for the photoproduction of the sesquiterpene valencene. Metab. Eng. Commun. 2021, 13, e00178. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Vincent, M.; Sassi, J.F.; Cassier-Chauvat, C.; Chauvat, F. Exploring the potential of the model cyanobacterium Synechocystis PCC 6803 for the photosynthetic production of various high-value terpenes. Biotechnol. Biofuels Bioprod. 2022, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, X.; Wu, Y.; Sun, H.; Luan, G.; Lu, X. Conversion of carbon dioxide into valencene and other sesquiterpenes with metabolic engineered Synechocystis sp. PCC 6803 cell factories. GCB Bioenergy 2023, 15, 1154–1165. [Google Scholar] [CrossRef]
- Lei, C.; Shubin, L.; Sun, T.; Zhang, W. Synechococcus Genetic Engineering Bacteria for Biosynthesizing Caryophyllene, and Construction Method and Application Thereof. CN111394383B, 4 October 2020. [Google Scholar]
- Rinaldi, M.A.; Ferraz, C.A.; Scrutton, N. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Nat. Prod. Rep. 2022, 39, 90–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.H.; Kaufmann-Malaga, B.B.; Lerman, J.A.; Dougherty, D.P.; Zhang, Y.; Kilbo, A.L.; Wilson, E.H.; Ng, C.Y.; Erbilgin, O.; Curran, K.C.; et al. An Automated scientist to design and optimize microbial strains for the industrial production of small molecules. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gurdo, N.; Volke, D.C.; McCloskey, D.; Nikel, P.I. Automating the design-build-test-learn cycle towards next-generation bacterial cell factories. New Biotechnol. 2023, 74, 1–15. [Google Scholar] [CrossRef]
- Kwak, S.; Crook, N.; Yoneda, A.; Ahn, N.; Ning, J.; Cheng, J.; Dantas, G. Functional mining of novel terpene synthases from metagenomes. Biotechnol. Biofuels 2022, 15, 104. [Google Scholar] [CrossRef]
- Jin, K.; Xia, H.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Compartmentalization and transporter engineering strategies for terpenoid synthesis. Microb. Cell Fact. 2022, 21, 92. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Chen, X.; Liu, L. Engineering microbial membranes to increase stress tolerance of industrial strains. Metab. Eng. 2019, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Lozada, N.J.H.; Pfleger, B.F. Efflux systems in bacteria and their metabolic engineering applications. Appl. Microbiol. Biotechnol. 2015, 99, 9381–9393. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.G.; Geiger, C.J.; Corzett, C.H.; Kram, K.E.; Finkel, S.E. Removal of toxic volatile compounds in batch culture prolongs stationary phase and delays death of Escherichia coli. Appl. Environ. Microbiol. 2021, 87, e0186021. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.L.; Li, J.; Nong, F.T.; Yan, C.X.; Ma, W.; Zhu, X.F.; Zhang, L.; Sun, X.M. Application of adaptive laboratory evolution in lipid and terpenoid production in yeast and microalgae. ACS Synth. Biol. 2023, 12, 1396–1407. [Google Scholar] [CrossRef]
- Ting, W.W.; Ng, I.S. Adaptive laboratory evolution and metabolic regulation of genetic Escherichia coli W3110 toward low-carbon footprint production of 5-aminolevulinic acid. J. Taiwan Inst. Chem. Eng. 2022, 141, 104612. [Google Scholar] [CrossRef]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef]
| Strain | Main Characteristics | Fermentation Type | Titer (mg/L) | Time (h) | Reference |
|---|---|---|---|---|---|
| S. cerevisiae | MVA pathway | ||||
| YEH4-ECPSE353D | β-Alanine metabolism genes (GAD1, PAN6, and CAB1); MVA genes (EfHMGR, ERG12, ERG8, CUP1, ERG19, IDI1, and ERG20); improved transmembrane transport of BCP (STE6T1025N), and 353D mutant of QHS1 (A. annua) | Test tube | 70.45 | 48 | [59] |
| Fed-batch | 594.05 | 96 | |||
| YAG117 | Deleted cytosol catalase gene (CTT1) to reduce yeast resistance to hydrogen peroxide, overexpressed codon-optimized QHS1 (A. annua), tHMG1, HMG2 (K6R), UPC2-1, and ERG20; mutations in MST27/tR(UCU)G1 and STE6 | Test tube | 104.7 ± 6.2 | n.m. | [60] |
| AM109 | HMG2 (K6R), deleted (Ubc7p, Ssm4p, and Pho86p), ERG9, and Sf126 (S. fruticosa) | Shake flask | 125.0 | * n.m. | [50] |
| CPLBY01 | MVA genes (ERG10, ERG13, tHMG1, ERG12, ERG8; tHMG1, ERG19, IDI1, and ERG20), and codon-optimized QHS1 (A. annua) | Shake flask | 250.4 | n.m. | [56] |
| Fed-batch | 2949.1 | 140 | |||
| YQ-7 | MVA genes (QHS1 from A. annua, tHMG1, IDI1, ERG20, ERG13, and ERG10), and artificial synthetic malonic acid-acetoacetyl-CoA metabolic pathway (Mae I from Schizosaccharomyces pombe, MatB, and ACCS) | Shake flask | 328 ± 13.44 | 144 | [63] |
| E. coli | Hybrid MVA pathway | ||||
| YJM67 | From acetic acid; QHS1 (A. annua), mvA and mvS (E. faecalis), GPPS2 (A. grandis), ERG12, ERG8, ERG19, and IDI1 (S. cerevisiae), ACS (A. pasteurianus), and nphT7 (Streptomyces sp.) | Shake flask | 22 ± 1.8 | 24 | [65] |
| Fed-batch | 1050 | 72 | |||
| CAR2 | mvaE and mvaS (E. faecalis), ERG12, ERG8, ERG19, and IDI1 (S. cerevisiae), tps7 (N. tabacum), and T7 promoter, Cm R | Shake flask | 100.3 | 24 | [19] |
| Fed-batch | 4319 | 60 | |||
| In situ fed-batch | 5142 | 64 | |||
| YJM59 | QHS1 (A. annua), mvA, and mvaS (E. faecalis), GPPS2 (A. grandis), C6PDH (E. coli), ERG12, ERG8, ERG19, and IDI1 (S. cerevisiae), and deleted pgi gene | Shake flask | 220 ± 6 | n.m. | [9] |
| Fed-batch | 1520.0 | 60 | |||
| R. capsulatus | Heterologous MVA pathway | ||||
| SB1003-MVA | MVA genes (P. zeaxanthinifaciens), QHS1 (A. annua), and ispA | Gas-tight hangate tubes, UV | 90 ± 19 | 72 | [20] |
| Screw-neck vials, IR | 139 ± 31 | 72 | |||
| Synechocystis sp. | MEP pathway | ||||
| PCC6803 | Stabilized QSH1 (A. annua) | Shake flask | 46.4 × 10−5 ± 2.9 | Week | [21] |
| S. elongatus | MEP pathway | ||||
| NSI-gpps-ispA-idi1sc-II-idi1sc-III-tps21 | gpps-ispA-idi1sc-II-idi1sc-III-tps21, where tps21 encodes TPS21 (A. thaliana) | Photobioreactor | 212.37 × 10−3 | 96 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsigoriyna, L.; Sango, C.; Batovska, D. An Update on Microbial Biosynthesis of β-Caryophyllene, a Sesquiterpene with Multi-Pharmacological Properties. Fermentation 2024, 10, 60. https://doi.org/10.3390/fermentation10010060
Tsigoriyna L, Sango C, Batovska D. An Update on Microbial Biosynthesis of β-Caryophyllene, a Sesquiterpene with Multi-Pharmacological Properties. Fermentation. 2024; 10(1):60. https://doi.org/10.3390/fermentation10010060
Chicago/Turabian StyleTsigoriyna, Lidia, Chakarvati Sango, and Daniela Batovska. 2024. "An Update on Microbial Biosynthesis of β-Caryophyllene, a Sesquiterpene with Multi-Pharmacological Properties" Fermentation 10, no. 1: 60. https://doi.org/10.3390/fermentation10010060
APA StyleTsigoriyna, L., Sango, C., & Batovska, D. (2024). An Update on Microbial Biosynthesis of β-Caryophyllene, a Sesquiterpene with Multi-Pharmacological Properties. Fermentation, 10(1), 60. https://doi.org/10.3390/fermentation10010060