Abstract
The objective of this study was to evaluate the effect of anaerobic fermentation on the in vitro ruminal production of total gas (TG), methane (CH4), carbon monoxide (CO) and hydrogen sulfide (H2S), as well as on the characteristics of ruminal fermentation and CH4 conversion efficiency of whole-plant maize (WPM) from four native maize genotypes (Amarillo, Olotillo, Tampiqueño and Tuxpeño) from Mexico, and a commercial hybrid was used as a control. In all genotypes, the fermented WPM produced the lowest amounts (p ≤ 0.0236) of TG and CH4 from degraded dry matter (DM), and Tampiqueño and Tuxpeño presented the highest production of these gases. In addition, Tuxpeño also presented the highest proportion of CH4 (mL 100 mL−1 TG), and Olotillo presented the lowest proportion of both gases. Something similar occurred in H2S, where the fermented WPM produced the lowest (p ≤ 0.0077) amount per DM degraded, and Amarillo and Tampiqueño presented the highest and lowest production, respectively. However, the fermented WPM presented the highest (p = 0.0128) CO production from degraded DM, and Tuxpeño and Olotillo presented the highest and lowest production, while the rumen pH was lower (p < 0.0001) in the fermented WPM, and Tuxpeño and Olotillo presented the highest and lowest pH, respectively. Furthermore, the fermented plant presented the greatest (p ≤ 0.0055) DM degradation, and the Amarillo and hybrid genotypes presented the highest percentages, while Olotillo presented the lowest. The short-chain fatty acid (SCFA) content and metabolizable energy (ME) did not differ (p ≥ 0.0899) between genotypes and were higher (p = 0.0009) in the fresh WPM. Despite the above, the fermented WPM was more efficient (p ≤ 0.0249), and the Amarillo and hybrid genotypes produced less CH4 per unit of SCFAs, ME and organic matter. In conclusion, the Amarillo genotype equaled the hybrid one, and although the production of CO increased, anaerobic fermentation showed the potential to reduce the rumen production of TG, CH4 and H2S, as well as to improve DM degradability and CH4 conversion efficiency.
1. Introduction
In 2015, all the member states of the United Nations, including Mexico, approved the 2030 Agenda, an action plan that includes 17 Sustainable Development Goals (SDGs), and among the most important is SDG 13, which consists of adopting urgent measures against climate change and its effects [1]. For this reason, these states in the same year committed to implementing the Paris Agreement, which establishes actions to keep the increase in global temperature below 2 °C and, if possible, try to reach 1.5 °C [1,2]. These global commitments have generated concern among specialists in ruminant livestock nutrition, since, during the digestion of feed, these animals produce organic acids and gases that, when expelled, accumulate in the atmosphere and generate the greenhouse effect [3], which favors global warming. Approximately 87% of these gases are produced in the rumen and are mainly composed of carbon dioxide (CO2) and methane (CH4), as well as, to a lesser extent, carbon monoxide (CO) and hydrogen sulfide (H2S) [4,5,6]. Of all these gases, CH4 is the most harmful to the environment as its global warming potential is up to 28 times greater than that of CO2 and its production during ruminal digestion represents an energy loss that ranges between 2 and 12 % of the gross energy ingested by animals [7,8]. However, the production of CH4 is the main metabolic pathway for the elimination of H2, so the amount of CH4 depends on the availability of H2 in the rumen, and both depend on the fibrosity of the feed, since fibrous carbohydrates increase the production of ruminal gases, including H2 [6]. Faced with this situation, there has been a constant search for strategies that allow for reducing greenhouse gas (GHG) emissions, especially those of CH4, and, above all, that do not compromise ruminal function and feed conversion efficiency.
A GHG mitigation strategy that has generated favorable results is the use of concentrates in livestock feeding, as they are primarily composed of non-fibrous carbohydrates that promote propionate formation in the rumen and therefore provide an alternate sink for H2 removal [9]. However, the high cost involved in this strategy and the economic inequalities among producers are factors that limit its implementation and, consequently, producers with limited resources turn to native maize (Zea mays L.) to produce forage, since it is cheaper compared to improved maize and is freely pollinated, which allows it to produce and conserve its own seed. In Mexico, native maize is made up of 64 breeds, which is equivalent to 29% of the 220 breeds recognized in Latin America [10] and for which Mexico is considered one of the centers of origin, domestication and diversification of this species. These breeds are made up of varieties, known as “native varieties” or “landraces”, that have adapted to the agroecological conditions of their local environment through evolutionary processes, driven by agronomic management practices and selective pressures imposed by farmers [11,12], which shows that they have co-evolved in accordance with the needs of human beings. Therefore, landraces play an important role for the resilience of the agricultural sector in the face of climate change and constitute a valuable plant genetic resource, not only for grain production but also for forage production [13]. However, although there are outstanding landraces that can respond the same or better than the improved varieties in terms of forage production [14], maize from warm regions has a higher concentration of fibrous carbohydrates (cellulose and hemicellulose) and lignin compared to maize from cold regions [15], which reduces digestibility. Faced with this situation, ensiling, which is actually a lactic acid fermentation process under anaerobic conditions, has traditionally been used for the conservation of forage with a high moisture content [16], but it can also be useful as a pre-ingestive treatment to improve the digestion of the forage fibers. This is because the organic acids (lactic, acetic, propionic and butyric acids) produced during fermentation trigger partial hydrolysis and the breaking of bonds between fibrous carbohydrates and lignin [17], which allows greater adherence and colonization by rumen microorganisms. In addition, the microbiological and biochemical processes that occur during fermentation can not only improve the nutritional content and digestibility of native maize forage but also improve rumen fermentation and reduce the impact of livestock on the environment, although due to the diversity that exists in maize, it is possible that the genotype is a determining factor in obtaining these benefits. Therefore, the objective of this study was to evaluate the effect of anaerobic fermentation on the in vitro ruminal production of total gas (TG), CH4, CO and H2S, as well as on the characteristics of ruminal fermentation and the CH4 conversion efficiency of whole-plant maize from four native maize genotypes from Mexico and a commercial hybrid.
2. Materials and Methods
2.1. Crop Management and Anaerobic Fermentation of Whole-Plant Maize
The maize was grown in the city of Aldama, Tamaulipas, Mexico (22°59′09″ N and 98°10′25″ W, at 190 m asl), where the predominant climate is type Aw0, which corresponds to the driest of the warm subhumid climates [18]. The maize genotypes evaluated were four natives from Mexico (Amarillo, Olotillo, Tampiqueño and Tuxpeño), and a commercial hybrid was used as a control. All genotypes were sown in triplicate in 12 × 10 m plots at a density of 62,500 plants ha−1 and grown under rainfed conditions during the autumn/winter 2021 agricultural cycle. The harvest was carried out at a height of 10 cm above ground level once the grain reached the milky-dough state, which was when the plants were 90 ± 5 days old and had a height of 2.5 ± 0.5 m, and for anaerobic fermentation, whole-plant maize (WPM: leaf, stem and cob) was used and chopped into 2–3 cm in a hammer-and-blade mill. The WPM was fermented in triplicate in black polyethylene bags (30 × 50 cm, diameter and height; 500 caliber), placing 5 kg of fresh chopped WPM and vacuum-sealing the bags with a household vacuum cleaner and a heat-sealing machine. During fermentation, the bags were stored at room temperature in a place free from direct solar radiation and humidity, and after 120 d, they were opened.
2.2. Chemical Composition
At the time of harvesting the fresh WPM and opening the bags of the fermented WPM, three samples were obtained, one from each plot and from each bag, and in both cases, the pH was determined. All samples were dehydrated at 60 °C for 72 h and ground in a hammer mill (Thomas Wiley® Laboratory Mill model 4, Thomas Scientific™, Swedesboro, NJ, USA) with a 1 mm screen. The contents (g kg−1 DM) of dry matter (DM; method ID 934.01), ash (method ID 942.05), nitrogen (N; method ID 954.01) and ether extract (EE; method ID 920.39) were determined according to the methods of the Association of Official Analytical Chemists [19]. The neutral detergent fiber (NDF) and acid detergent (ADF) fiber were analyzed in the ANKOM200 Fiber Analyzer Unit (ANKOM Technology Corp., Macedonia, NY, USA) [20], while the lignin (method ID 973.18) content was measured according to the AOAC, [19]. Sodium sulfite and thermostable α-amylase were used in the NDF analysis, and the NDF and ADF values were expressed without residual ash. The organic matter (OM), crude protein (CP) and non-fibrous carbohydrate (NFC) contents were calculated in g kg−1 DM as follows: OM = 1000 − ash, CP = (N × 6.25) × 10 and NFC = 1000 − (CP + NDF + EE + ash). The contents of starch, water-soluble carbohydrates, ammoniacal nitrogen and organic acids (lactic, acetic and butyric acids) were estimated by near-infrared reflectance spectroscopy (NIRS) [21]. The results of the analysis of the chemical composition of the fresh and fermented WPM of each genotype are shown in Table 1.

Table 1.
pH, DM and chemical composition of the fresh and fermented whole-plant of different genotypes of maize (Zea mays L.). Adapted from Alvarado-Ramírez et al. [22].
2.3. In Vitro Rumen Incubation
The nutritive medium was prepared following the method of Goering and Van Soest [23], and the ruminal liquid was obtained from the rumen content of four steers (450 ± 25 kg LW) slaughtered in the municipal slaughterhouse of Toluca, State of Mexico, Mexico, which is regulated by the Mexican Official Standard NOM-033-SAG/ZOO-2014, which establishes the methods to kill domestic and wild animals. Before slaughter, these animals were fed with hay and commercial concentrate (Purina®, Toluca, State of Mexico, Mexico) in a ratio of 50:50 and with a constant supply of fresh water. The rumen content was transferred to the laboratory in hermetic thermos and filtered with four layers of gauze to eliminate solid particles and obtain only the rumen liquid. The incubation was carried out in vials with a capacity of 160 mL, and 500 mg of fresh or fermented WPM (depending on the treatment) were added to each one, as well as 50 mL of a solution made up of 40 mL of nutrient medium and 10 mL of rumen liquid. The vials were sealed with butyl rubber stoppers and aluminum seals and incubated in an incubator at 39 °C for 48 h. In total, three incubation cycles were carried out, and in each one, 33 vials were incubated, since in all cases it was incubated in triplicate, and 3 vials were added that were used as blanks (without WPM).
2.4. Gas Measurement
The total gas (TG) production was measured at 2, 4, 6, 24, 26, 28, 30 and 48 h of incubation using a digital manometer (Manometer model 407910, Extech® Instruments, Nashue, NH, USA), as indicated by the methodology proposed by Theodorou et al. [24]. The methane (CH4), carbon monoxide (CO) and hydrogen sulfide (H2S) contents were estimated using a portable gas detector (Dräger X-am®, model 2500, Dräger, Lübeck, SH, Germany) connected to an external pump (Dräger X-am®, Dräger, Lübeck, SH, Germany), by which a known quantity of gas was injected, and the detector indicated the concentration of each gas. After the measurements, the gas that had accumulated in the empty space of the vials was released to avoid partial dissolution of the gases and erroneous estimates.
2.5. pH and Dry Matter Degradation
After 48 h of incubation, the contents of the vials were filtered following the methodology of Alvarado-Ramírez et al. [25], which consists of retaining the residual substrate (in this case, fresh or fermented WPM) in bags with a porosity of 25 µm (Filter bags F57, ANKOM Technology Corp., Macedon, NY, USA) and collecting the liquid in beakers using a vacuum pump. In the collected liquid, the pH was measured using a potentiometer with a glass electrode (pH wireless electrode, HALO® model HI11102, Hanna® Instruments, Woonsocket, RI, USA), while the residual substrate was dehydrated at 105 °C for 48 h to calculate the percentage of dry matter degradation.
2.6. Calculations
The asymptotic gas production, the gas production rate and the time of the lag phase before the production of TG, CH4, CO and H2S were estimated with the NLIN procedure of SAS [26] and using the model proposed by France et al. [27]:
where y is the production (mL g−1 DM) of TG, CH4, CO and H2S at time t (h); b is the asymptotic production (mL g−1 DM) of TG, CH4, CO and H2S; c is the production rate (mL h−1) of TG, CH4, CO and H2S and Lag is the lag phase (h) before the production of TG, CH4, CO and H2S.
y = b × [1 − e −c (t − Lag)]
The metabolizable energy (ME; MJ kg−1 DM) and the concentration of short-chain fatty acids (SCFA; mmol 200 mg−1 DM) were calculated with the equations proposed by Menke et al. [28] and Getachew et al. [29]:
where CP is the crude protein content (g kg−1 DM) and TGP is the net production (mL 200 mg−1 DM) of total gas at 24 h of incubation.
ME = 2.20 + (0.136 × TGP) + (0.057 × CP)
SCFA = (0.0222 × TGP) − 0.00425
Additionally, the CH4 conversion efficiency was estimated based on the CH4 production per unit of SCFA (CH4:SCFA, mmol:mmol−1), ME (CH4:ME, g:MJ−1) and OM (CH4:OM, mL:g−1).
2.7. Statistical Analysis
Before the statistical analysis, the repetitions of each treatment were averaged per incubation cycle, and the value obtained was considered as the average of the experimental unit. Data were analyzed as a factorial experiment (5 × 2) using the PROC GLM option of SAS version 9.2 [26] and according to the following statistical model:
where Y is the response variable; μ is the overall mean; G is the effect of the maize genotype; F is the effect of the anaerobic fermentation of WPM; (G × F) is the effect of the interaction between the maize genotype and the anaerobic fermentation of WPM and ε is the experimental error. Tukey’s test was used for the comparison of means, and they were considered significantly different when p < 0.05.
Yijk = µ + Gi + Fj + (G × F)ij + εijk
3. Results
3.1. In Vitro Rumen Total Gas Production
The genotype influenced (p = 0.0438) the parameters of total gas (TG) production, and the response increased (p = 0.0093) with anaerobic fermentation in all genotypes. The whole-plant maize (WPM) fermentation increased (p = 0.0005) the production of TG per DM incubated in the Amarillo and hybrid genotypes at 6 h, and it reduced it in Olotillo, Tampiqueño and Tuxpeño. However, at 24 h, the genotype did not influence it (p = 0.0996), and the TG production was higher (p = 0.0009) in the fermented WPM, and at 48 h, the genotype was affected, but the amount of TG continued to be higher (p ≤ 0.0428) in the fermented WPM. Despite the above, in all genotypes, the fermented WPM produced a lower (p ≤ 0.0236) amount of TG per degraded DM than the fresh WPM, and the fermented WPM of the Tampiqueño and Olotillo genotypes presented the highest and lowest production of TG per degraded DM at the end of incubation, respectively, (Table 2 and Figure 1).

Table 2.
Parameters and in vitro rumen total gas (TG) production of the fresh and fermented whole-plant (WPM) of different genotypes of maize (Zea mays L.), at 6, 24 and 48 h of incubation.
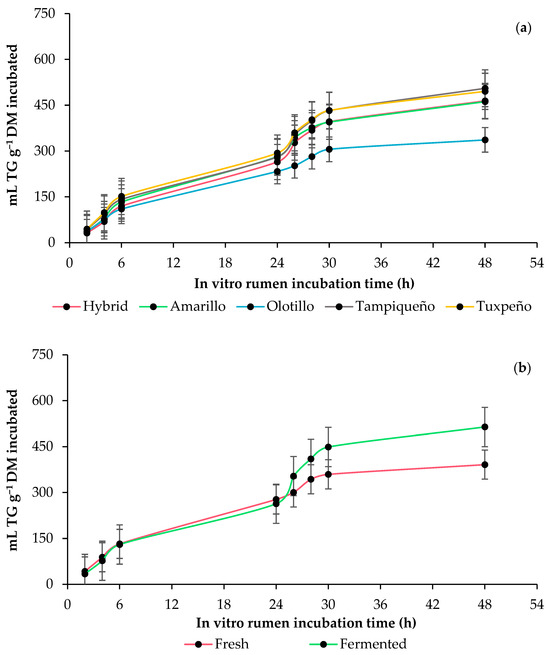
Figure 1.
In vitro rumen total gas (TG) production kinetics of whole-plant maize (Zea mays L.): (a) effect of genotype (Amarillo, Hybrid, Olotillo, Tampiqueño and Tuxpeño) and (b) anaerobic fermentation of the whole-plant maize (fresh or fermented). The bars at each point indicate the standard error of the mean.
3.2. In Vitro Rumen Methane Production
The asymptotic methane (CH4) production and time in the lag phase were similar (p ≥ 0.1288) between genotypes and with anaerobic fermentation, whereas the CH4 production rate was lower (p ≤ 0.0004) in the fresh WPM and higher in the fermented WPM. In terms of CH4 per incubated DM, the fermented WPM presented the lowest (p ≤ 0.0445) production at 6 and 24 h, while at 48 h, it presented the highest production. However, the fermented WPM presented the lowest (p ≤ 0.0049) production and proportion of CH4 (mL 100 mL−1 TG) per degraded DM during the entire incubation, and after 24 h, it presented the highest production (p ≤ 0.0445) of CH4 in g kg−1 DM. With anaerobic fermentation, the WPM of the Tuxpeño and Olotillo genotypes presented the highest and lowest values in the production and proportion of CH4 at 48 h of incubation (Table 3 and Table 4 and Figure 2).

Table 3.
Parameters and in vitro rumen methane (CH4) production of the fresh and fermented whole-plant (WPM) of different genotypes of maize (Zea mays L.), at 6, 24 and 48 h of incubation.

Table 4.
In vitro ruminal methane (CH4) production based on total gas (TG) and kg DM of the fresh and fermented whole-plant (WPM) from different genotypes of maize (Zea mays L.) at 6, 24 and 48 h of incubation.
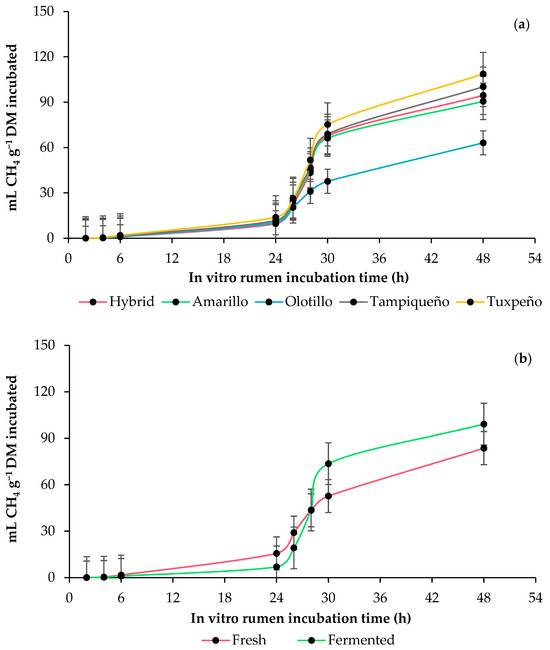
Figure 2.
In vitro rumen methane (CH4) production kinetics of whole-plant maize (Zea mays L.): (a) effect of genotype (Amarillo, Hybrid, Olotillo, Tampiqueño and Tuxpeño) and (b) anaerobic fermentation of the whole-plant maize (fresh or fermented). The bars at each point indicate the standard error of the mean.
3.3. In Vitro Rumen Carbon Monoxide Production
The carbon monoxide (CO) production parameters did not show an effect (p ≥ 0.1774) due to the genotype, anaerobic fermentation or the interaction of both. However, the CO production (mL g−1 incubated and degraded DM) was higher (p ≤ 0.0128) in the fermented WPM, and at 48 h of incubation, the fermented WPM of the Tuxpeño and Olotillo genotypes presented the highest and lowest (p ≤ 0.0128) CO production, respectively, (Table 5 and Figure 3).

Table 5.
Parameters and in vitro rumen carbon monoxide (CO) production of the fresh and fermented whole-plant (WPM) from different genotypes of maize (Zea mays L.) at 6, 24 and 48 h of incubation.
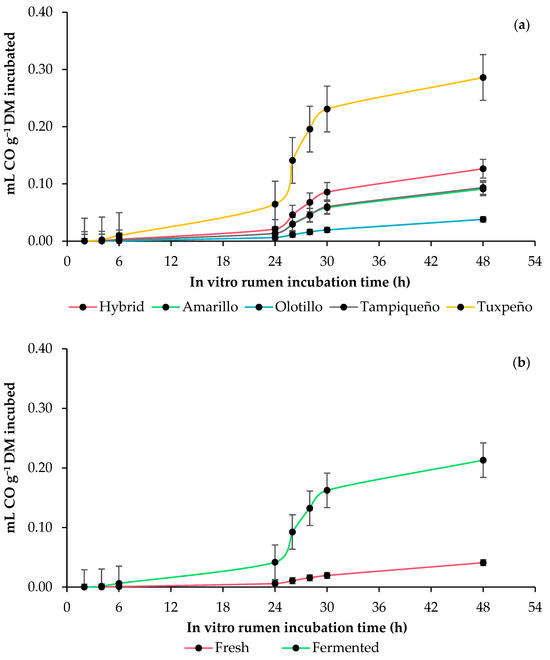
Figure 3.
In vitro rumen carbon monoxide (CO) production kinetics of whole-plant maize (Zea mays L.): (a) effect of genotype (Amarillo, Hybrid, Olotillo, Tampiqueño and Tuxpeño) and (b) anaerobic fermentation of the whole-plant maize (fresh or fermented). The bars at each point indicate the standard error of the mean.
3.4. In Vitro Rumen Hydrogen Sulfide Production
The asymptotic production of hydrogen sulfide (H2S) and time in the lag phase did not present any effects (p ≥ 0.1500), and the H2S production rate only varied (p = 0.0462) between genotypes. In all genotypes, WPM fermentation reduced (p ≤ 0.0251) H2S production with the incubated and degraded DM during the entire incubation. At 48 h, the fermented WPM of the Tuxpeño and Olotillo genotypes presented the highest and lowest H2S production rates with incubated DM, while with degraded DM, the highest and lowest H2S production rates were presented by the Tampiqueño and Amarillo genotypes, respectively (Table 6 and Figure 4).

Table 6.
Parameters and in vitro rumen hydrogen sulfide (H2S) production of the fresh and fermented whole-plant (WPM) from different genotypes of maize (Zea mays L.) at 6, 24 and 48 h of incubation.
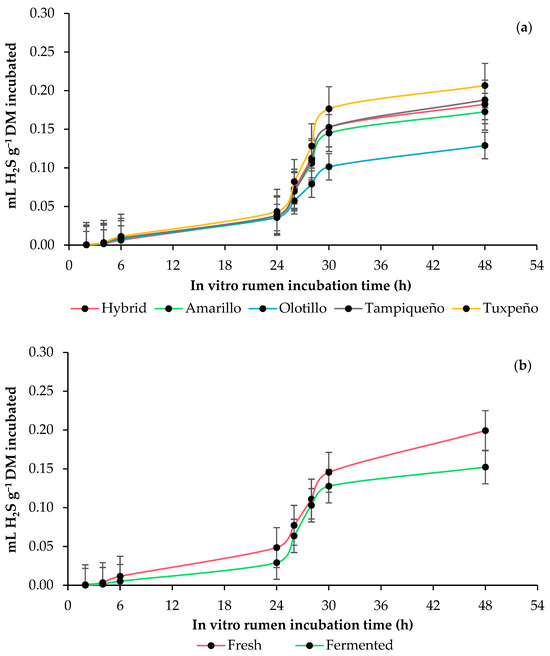
Figure 4.
In vitro rumen hydrogen sulfide (H2S) production kinetics whole-plant maize (Zea mays L.): (a) effect of genotype (Amarillo, Hybrid, Olotillo, Tampiqueño and Tuxpeño) and (b) anaerobic fermentation of the whole-plant maize (fresh or fermented). The bars at each point indicate the standard error of the mean.
3.5. Rumen Fermentation Characteristics and Methane Conversion Efficiency
The fermented WPM presented the lowest pH (p < 0.0001) in all genotypes, and Tuxpeño and Olotillo obtained the highest and lowest pH, respectively, (Table 7). In addition, the fermented WPM obtained (p ≤ 0.0009) the highest dry matter degradation, and the hybrid and Olotillo genotypes presented the highest and lowest percentage. The short-chain fatty acid (SCFA) content and metabolizable energy (ME) did not show an effect (p ≥ 0.0899) of the genotype, and in all of them, the anaerobic fermentation reduced (p ≤ 0.0009) the content of SCFA and amount of ME. However, the fermented WPM was more efficient (p ≤ 0.0249) than the fresh WPM, and the hybrid, Amarillo and Tampiqueño genotypes produced the least amount of CH4 per unit of SCFAs, ME and OM (Table 7).

Table 7.
In vitro ruminal fermentation characteristics and CH4 conversion efficiency of the fresh and fermented whole-plant (WPM) from different genotypes of maize (Zea mays L.).
4. Discussion
4.1. In Vitro Rumen Total Gas Production
In all the genotypes, the shortest time in the lag phase occurred in the fresh whole-plant maize (WPM), and the longest occurred in the fermented WPM, which motivates us to assume that the pH of the fresh WPM favored the adherence of rumen microorganisms, since some ruminal fungi and protozoa are sensitive to acidic pH [30], and these microorganisms are the initiators of feed degradation [31,32]. However, due to the structural complexity of the fibrous carbohydrates of the fresh WPM, the rate of total gas (TG) production was lower compared to the fermented WPM. Other studies have indicated that during the aerobic fermentation of WPM, the complexity of fibrous carbohydrates decreases due to the changes that occur in their structure [33], which favors the adhesion and colonization of the feed by ruminal microbes [34] and allows us to assume that it was the reason why the rate of TG production was higher in the fermented WPM. In addition, the TG production also indirectly reflects the degradability of the feed and is dependent on the chemical composition, especially carbohydrates and proteins [35,36]. In the current study, the fermented WPM presented the highest TG production per incubated DM but the lowest per degraded DM, and the Tampiqueño and Tuxpeño genotypes presented the highest TG production, while Olotillo presented the lowest. It is important to note that, despite presenting the highest amount of non-fibrous carbohydrates (NFCs), the fermented WPM from the Amarillo and hybrid genotypes was not the one that produced the highest amount of TG. This shows that, although NFCs favor the increase in microbial enzymatic activity and ruminal microflora [37], they contribute less gas to the total production compared to fibrous carbohydrates, which is corroborated by the greater amount of neutral detergent fiber (NDF) and acid detergent (ADF) presented by the Tampiqueño and Tuxpeño genotypes (Table 1). Meanwhile, the low TG production in Olotillo in both the fresh and fermented WPM is attributed to the lignin structure, since its complexity can make it difficult for rumen microbes to degrade the feed and, consequently, there is a lower TG production [38,39]. Instead, in Tampiqueño, it is possibly not influenced by the content and structure of lignin, since despite having less lignin in the fresh and fermented WPM than Olotillo, it had a higher TG production than this genotype.
4.2. In Vitro Rumen Methane Production
The anaerobic fermentation increased the CH4 production rate of the WPM, and the Tuxpeño and Olotillo genotypes presented the highest and lowest CH4 production rates, both in the fresh and fermented WPM. Although the fermented WPM obtained the highest production of CH4 per incubated DM, when it was expressed in CH4 per degraded DM, it presented the lowest production, and the proportion of CH4 (mL 100 mL−1 TG) decreased up to 18.2% with the anaerobic fermentation, which indicates that the production of CH4 with incubated DM in the fermented WPM was the consequence of a higher degradability and TG production. In previous studies, it has been reported that reductions in the rate of production of CH4 are generally caused by changes in the short-chain fatty acid (SCFA) profile, especially in the proportion of acetic, propionic and butyric acids, which are produced from the fermentation of WPM, and its proportion depends on the relationship between fibrous carbohydrates and NFCs [40]. Considering that the ratio between WPM carbohydrates improved between 22.7 and 54.1% after anaerobic fermentation, it is possible to assume that the production of acetic acid and butyric acid decreased, and that that of propionic acid increased [41,42], which led to a lower acetate:propionate ratio and reduced the H2 available for CH4 production [43], mainly in the Amarillo and hybrid genotypes due to the amount of starch they contained. However, although starch contributes to the reduction in the rate of production of CH4 and maize is rich in this polysaccharide, it is covered by the zein protein, which acts as a physical barrier and limits its digestion due to its low digestibility [44], which is why the fresh WPM presented the highest rate of CH4 production in all the genotypes. Contrary to this, anaerobic fermentation of WPM contributes to the degradation of hydrophobic proteins attached to starch, including zein [45], which allows rumen bacteria to have greater access to it [41]. In addition, the greater availability of NFCs, including starch, decreases the rumen pH and fosters an inadequate environment for the growth of methanogens and protozoa [46], which consequently decreases the rate of production of CH4. However, the high production rate of CH4 in Tuxpeño is attributed to the low content of lactic acid and acetic acid and the greater amount of butyric acid, since the products of anaerobic fermentation of WPM also affect the production of CH4 [47], while in the case of Olotillo, the degradability and low NFC content influenced it.
4.3. In Vitro Rumen Carbon Monoxide Production
Carbon monoxide (CO) is an intermediate product of the degradation of the organic matter (OM) of the feed during ruminal fermentation [48] and has a low energy density, and although it is toxic for most living organisms [49], there are anaerobic microorganisms such as methanogenic, acetogenic and sulfate-reducing bacteria (SRB) that can metabolize it [50]. In this study, the fresh WPM presented the lowest CO production, and the fermented one presented the highest, and among the genotypes, Olotillo produced less CO and Tuxpeño produced more, which is possibly related to the CH4 production of both WPM states and maize genotypes, since in anaerobic conditions, CO production is higher when the CH4 concentration is low and vice versa, i.e., CO production is lower when the amount of CH4 is higher [48]. Therefore, the decrease in CO may be associated with the continued oxidation of this gas to CH4, since CO oxidizes on contact with water to H2 and carbon dioxide (CO2) [51], and these gases are used by methanogens for the formation of CH4. Instead, the increase in CO can be attributed to acetogenic bacteria using carbon monoxide dehydrogenase enzymes together with acetyl-CoA synthetase to catalyze the reduction of CO2 to CO via the carbonyl branch of the pathway acetyl-CoA following the metabolic pathway of Wood-Ljungdahl [52,53]. In the current study, this could have occurred in response to the low H2 availability for CH4 production in the fermented WPM.
4.4. In Vitro Rumen Hydrogen Sulfide Production
In the rumen, the production of hydrogen sulfide (H2S) depends on the concentration of sulfur (S) in the feed and its availability [54], and it is metabolized by SRB [55]. Like other cereals, maize also has sulfur amino acids such as cysteine and methionine [56], and they are found mainly in the endosperm of the grain [57], so it can be asserted that the proportion of the grain influenced the production of H2S of each genotype. In addition, although the WPM presented changes in the concentration of protein and micro- and macrominerals during anaerobic fermentation, the S content remained unchanged [58], which allows us to assume that the variations between fresh and fermented WPM were due to the content of sulfur amino acids and S in mineral form in each genotype. Considering the above and that H2S did not present a positive correlation with CO in the current study as that reported in other studies [50], it is also possible that the higher production in the fresh WPM occurred due to a higher H2 availability caused by limited starch digestion, while in the fermented WPM, there was a higher H2 demand for propionic acid formation due to the higher starch availability, and this decreased the amount of H2 available for H2S production.
4.5. Rumen Fermentation Characteristics and Methane Conversion Efficiency
The pH is an important factor that affects the populations of the rumen microbial community and its activities, and the variations that the pH presents are generally associated with the chemical composition of the feed [59]. In this regard, it has been reported that the adequate pH range is between 5.5 and 7.0 [60] and that it can occasionally increase up to 7.5 when the feed is very fibrous [61], since it is positively related to the contents of NDF and ADF [62]. This was corroborated in this study because the pH of the fresh WPM was higher than that obtained in the fermented WPM, and this coincided with the higher content of fibrous carbohydrates in the fresh WPM. However, the fresh WPM presented the lowest dry matter degradation (DMD), and the fermented one presented the highest, and of all the genotypes, the hybrid and Amarillo genotypes presented the highest degradation, which indicates that the DMD was not only influenced by the fibrous carbohydrate content but also by the content of NFCs. Despite the above, the SCFA content and metabolizable energy (ME) did not increase in the fermented WPM, which is contrary to what was reported in other studies [30]. A possible explanation for this is that, unlike the fermented WPM, the fresh WPM possibly contained a higher availability of substrates for the rumen microbes to produce more SCFAs and, consequently, more energy availability. This is supported by the fact that organic acids are produced during the anaerobic fermentation of WPM [63], which implies a consumption of nutrients, as is believed to have occurred in the fermented WPM. On the other hand, the CH4 conversion efficiency is an indicator of the CH4 produced per unit of rumen fermentation product, and the results revealed that the anaerobic fermentation improved the WPM efficiency, since it reduced the production of CH4 by up to 60.3, 64.5 and 67.6% per unit of SCFAs, ME and OM, respectively. This is associated with the SCFA profile, in particular with the ratio of acetic, propionic and butyric acids, since, as mentioned above, propionate reduces the availability of H2 for CH4 formation [32].
5. Conclusions
This study demonstrated that anaerobic fermentation has the potential to reduce the production of TG, CH4 and H2S from WPM, given that during in vitro ruminal fermentation, it reduced the production of these gases per degraded DM by up to 56.4, 60.0 and 78.6%, and although it increased the CO production by up to five times, it did not compromise the production of the other gases, including CH4. Likewise, it also increased the DMD by up to 17.3% and improved the CH4 conversion efficiency, in the latter case, in terms of CH4 per unit of SFCAs, ME and OM, since it decreased it by up to 60.3, 63.8 and 66.0%, respectively. In addition, of the native maizes, the Amarillo genotype was the one that equaled the hybrid, and the WPM of both presented the best values with anaerobic fermentation, so the use of these genotypes and anaerobic fermentation can contribute to reducing the greenhouse gases emissions derived from ruminant production, which is important from an environmental point of view, since it allows for the development of cleaner and more sustainable livestock production.
Author Contributions
E.R.A.-R.: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing. M.M.M.Y.E.: Methodology, Data Curation, Formal Analysis. M.A.R.-J., P.E.H.-R. and E.B.F.-P.: Conceptualization, Methodology, Visualization, Writing—Review and Editing. S.C., A.V. and M.I.C.: Funding Acquisition, Supervision, Validation, Writing—Review and Editing. A.Z.M.S.: Conceptualization, Methodology, Formal Analysis, Resources, Supervision, Project Administration, Data Curation, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
As indicated in the Section 2, the ruminal liquid was obtained from animals that were slaughtered at the municipal slaughterhouse of Toluca, State of Mexico, Mexico, so no ethical review or approval from any Educational Institution was required. At the slaughterhouse, the animals were slaughtered based on the guidelines established by the official Mexican standard NOM-033-SAG/ZOO-2014, which establishes methods for the humane slaughter of domestic and wild animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UN (United Nations). The Sustainable Development Agenda. Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 15 November 2023).
- Owens, F.N.; Basalan, M. Ruminal Fermentation. In Rumenology; Millen, D., de Beni, A.M., Lauritano, P.R., Eds.; Springer: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar] [CrossRef]
- Davison, T.M.; Black, J.L.; Moss, J.F. Red meat—An essential partner to reduce global greenhouse gas emissions. Anim. Front. 2020, 10, 14–21. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Obaisi, A.I.; Elghandour, M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Camacho-Díaz, L.M. Sustainable agriculture options for production, greenhouse gasses and pollution alleviation, and nutrient recycling in emerging and transitional nations—An overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Hernández, R.P.E.; Mellado, M.; Adegbeye, M.J.; Salem, A.Z.M.; Covarrubias, J.L.P.; Elghandour, M.M.M.Y.; Omotoso, O.B. Effects of long-term supplementation of Caesalpinia coriaria fruit extract on ruminal methane, carbon monoxide, and hydrogen sulfide production in sheep. Biomass Conv. Bioref. 2022, 1–14. [Google Scholar] [CrossRef]
- Pereira, A.M.; de Lurdes, N.E.D.M.; Borba, A.E.S. Alternative pathways for hydrogen sink originated from the ruminal fermentation of carbohydrates: Which microorganisms are involved in lowering methane emission? Anim. Microbiome 2022, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. ISBN 978-92-9169-143-2. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiong, B.; Zhao, X. Could propionate formation be used to reduce enteric methane emission in ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Serna-Saldívar, S.O. Corn history and culture. In Corn Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Bellon, M.R.; Dulloo, E.; Sardos, J.; Thormann, I.; Burdon, J.J. In situ conservation-harnessing natural and human-derived evolutionary forces to ensure future crop adaptation. Evol. Appl. 2017, 10, 965–977. [Google Scholar] [CrossRef]
- Janzen, G.M.; Aguilar-Rangel, M.R.; Cíntora-Martínez, C.; Blöcher-Juárez, K.A.; González-Segovia, E.; Studer, A.J.; Runcie, D.E.; Flint-Garcia, S.A.; Rellán-Álvarez, R.; Sawers, R.J.H.; et al. Demonstration of local adaptation in maize landraces by reciprocal transplantation. Evol. Appl. 2022, 15, 817–837. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Rivas-Jacobo, M.A.; Mendoza, P.S.I.; Sangerman-Jarquín, D.M.; Sánchez, H.M.Á.; Herrera, C.C.A.; Rojas, G.A.R. Forage evaluation of maize from various origins of Mexico in the semi-arid region. Rev. Mex. Cienc. Agríc. 2020, 11, 93–104. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Daniel, J.L.P.; Adesogan, A.T.; McAllister, T.A.; Drouin, P.; Nussio, L.G.; Cai, Y. Silage review: Unique challenges of silages made in hot and cold regions. J. Dairy Sci. 2018, 101, 4001–4019. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Muck, R.E. Ensiling in 2050: Some challenges and opportunities. Grass Forage Sci. 2019, 74, 178–187. [Google Scholar] [CrossRef]
- Tišma, M.; Planinić, M.; Bucić-Kojić, A.; Panjičko, M.; Zupančič, G.D.; Zelić, B. Corn silage fungal-based solid-state pretreatment for enhanced biogas production in anaerobic co-digestion with cow manure. Bioresour. Technol. 2018, 253, 220–226. [Google Scholar] [CrossRef]
- Vargas, T.V.; Hernández, R.M.E.; Gutiérrez, L.J.; Plácido, C.J.M.; Jiménez, C.A. Clasificación climática del estado de Tamaulipas, México. CienciaUAT 2007, 2, 15–19. [Google Scholar]
- AOAC. Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 1997. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zicarelli, F.; Sarubbi, F.; Iommelli, P.; Grossi, M.; Lotito, D.; Tudisco, R.; Infascelli, F.; Musco, N.; Lombardi, P. Nutritional Characteristics of Corn Silage Produced in Campania Region Estimated by Near Infrared Spectroscopy (NIRS). Agronomy 2023, 13, 634. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, E.R.; Ballesteros-Rodea, G.; Salem, A.Z.M.; Reyes-Hernández, J.; Herrera-Corredor, C.A.; Hernández-Meléndez, J.; Limas-Martínez, A.G.; López-Aguirre, D.; Rivas-Jacobo, M.A. The Impact of Genotype on Chemical Composition, Feeding Value and In Vitro Rumen Degradability of Fresh and Ensiled Forage of Native Maize (Zea mays L.) from Mexico. Agriculture 2023, 13, 2161. [Google Scholar] [CrossRef]
- Goering, M.K.; Van Soest, P.J. Forage Fibre Analysis (Apparatus, Reagents, Procedures and Some Applications); Agricultural Research Service USDA: Washington, DC, USA, 1970; pp. 1–24.
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, E.R.; Maggiolino, A.; Elghandour, M.M.M.Y.; Rivas-Jacobo, M.A.; Ballesteros-Rodea, G.; Palo, P.D.; Salem, A.Z.M. Impact of co-ensiling of maize with Moringa oleifera on the production of greenhouse gases and the characteristics of fermentation in ruminants. Animals 2023, 13, 764. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System) Institute. User’s Guide: Statistics, Version 9.0; SAS Institute: Cary, NC, USA, 2002.
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Getachew, G.; Makkar, H.P.S.; Becker, K. Tropical browses: Contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J. Agric. Sci. 2002, 139, 341–352. [Google Scholar] [CrossRef]
- Moraïs, S.; Mizrahi, I. Islands in the stream: From individual to communal fiber degradation in the rumen ecosystem. FEMS Microbiol. Rev. 2019, 43, 362–379. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Nguyen, T.T.; Reid, I.D.; Yanke, J.L.; Wang, P.; Abbott, D.W.; Tsang, A.; McAllister, T. Application of transcriptomics to compare the carbohydrate active enzymes that are expressed by diverse genera of anaerobic fungi to degrade plant cell wall carbohydrates. Front. Microbiol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Noel, S.J.; Lund, P.; Larsen, M.; Weisbjerg, M.R.; Børsting, C.F. Feeding up to 91% concentrate to Holstein and Jersey dairy cows: Effects on enteric methane emission, rumen fermentation and bacterial community, digestibility, production, and feeding behavior. J. Dairy Sci. 2022, 105, 9523–9541. [Google Scholar] [CrossRef]
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of microorganisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef]
- Chen, C.; Xin, Y.; Li, X.; Ni, H.; Zeng, T.; Du, Z.; Guan, H.; Wu, Y.; Yang, W.; Cai, Y.; et al. Effects of Acremonium cellulase and heat-resistant lactic acid bacteria on lignocellulose degradation, fermentation quality, and microbial community structure of hybrid elephant grass silage in humid and hot areas. Front. Microbiol. 2022, 13, 1066753. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Wang, S.R.; Guo, G.; Shao, T. Effects of applying lactic acid bacteria and propionic acid on fermentation quality, aerobic stability, and in vitro gas production of forage-based total mixed ration silage in Tibet. Anim. Prod. Sci. 2019, 59, 376–383. [Google Scholar] [CrossRef]
- Wang, S.R.; Zhao, J.; Yu, C.Q.; Li, J.F.; Tao, X.X.; Chen, S.F.; Shao, T. Nutritional evaluation of wet brewers’ grains as substitute for common vetch in ensiled total mixed ration. Ital. J. Anim. Sci. 2020, 19, 1015–1025. [Google Scholar] [CrossRef]
- Jančík, F.; Kubelková, P.; Loučka, R.; Jambor, V.; Kumprechtová, D.; Homolka, P.; Koukolová, V.; Tyrolová, Y.; Výborná, A. Shredlage processing affects the digestibility of maize silage. Agronomy 2022, 12, 1164. [Google Scholar] [CrossRef]
- He, Y.; Mouthier, T.M.; Kabel, M.A.; Dijkstra, J.; Hendriks, W.H.; Struik, P.C.; Cone, J.W. Lignin composition is more important than content for maize stem cell wall degradation. J. Sci. Food Agric. 2018, 98, 384–390. [Google Scholar] [CrossRef]
- Yan-Lu, W.; Wei-Kang, W.; Qi-Chao, W.; Fan, Z.; Wen-Juan, L.; Sheng-Li, L.; Wei, W.; Zhi-Jun, C.; Hong-Jian, Y. In situ rumen degradation characteristics and bacterial colonization of corn silages differing in ferulic and p-coumaric acid contents. Microorganisms 2022, 10, 2269. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Xie, T.Y.; Janssen, P.H.; Sun, X.Z.; Beauchemin, K.A.; Tan, Z.L.; Gao, M. Shifts in rumen fermentation and microbiota are associated with dissolved ruminal hydrogen concentrations in lactating dairy cows fed different types of carbohydrates. J. Nutr. 2016, 146, 1714–1721. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shi, W.; Huang, L.T.; Ding, C.L.; Dai, C.C. The effect of lactic acid bacterial starter culture and chemical additives on wilted rice straw silage. Anim. Sci. J. 2016, 87, 525–535. [Google Scholar] [CrossRef]
- Sun, H.; Cui, X.; Li, R.; Guo, J.; Dong, R. Ensiling process for efficient biogas production from lignocellulosic substrates: Methods, mechanisms, and measures. Bioresour. Technol. 2021, 342, 125928. [Google Scholar] [CrossRef]
- Rooke, J.A.; Wallace, R.J.; Duthie, C.A.; McKain, N.; de Souza, S.M.; Hyslop, J.J.; Ross, D.W.; Waterhouse, T.; Roehe, R. Hydrogen and methane emissions from beef cattle and their rumen microbial community vary with diet, time after feeding and genotype. Br. J. Nutr. 2014, 112, 398–407. [Google Scholar] [CrossRef]
- Duvnjak, M.; Butorac, A.; Kljak, K.; Nišavić, M.; Cindrić, M.; Grbeša, D. The evaluation of γ-zein reduction using mass spectrometry-the influence of proteolysis type in relation to starch degradability in silages. Fermentation 2022, 8, 551. [Google Scholar] [CrossRef]
- Junges, D.; Morais, G.; Spoto, M.H.F.; Santos, P.S.; Adesogan, A.T.; Nussio, L.G.; Daniel, J.L.P. Influence of various proteolytic sources during fermentation of reconstituted corn grain silages. J. Dairy Sci. 2017, 100, 9048–9051. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Esen, S.; Cabi, E.; Koç, F. Effect of freeze-dried kefir culture inoculation on nutritional quality, in vitro digestibility, mineral concentrations, and fatty acid composition of white clover silages. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Haarstad, K.; Bergersen, O.; Sørheim, R. Occurrence of carbon monoxide during organic waste degradation. J. Air Waste Manag. Assoc. 2006, 56, 575–580. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lens, P.N.; Veiga, M.C.; Kennes, C. Selective butanol production from carbon monoxide by an enriched anaerobic culture. Sci. Total Environ. 2022, 806, 150579. [Google Scholar] [CrossRef] [PubMed]
- Techtmann, S.M.; Colman, A.S.; Robb, F.T. ‘That which does not kill us only makes us stronger’: The role of carbon monoxide in thermophilic microbial consortia. Environ. Microbiol. 2009, 11, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 2015, 6, 1275. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Naveira, Á.; Veiga, M.C.; Kennes, C. H-B-E (hexanol-butanol-ethanol) fermentation for the production of higher alcohols from syngas/waste gas. J. Chem. Technol. Biotechnol. 2017, 92, 712–731. [Google Scholar] [CrossRef]
- Shah, A.M.; Ma, J.; Wang, Z.; Hu, R.; Wang, X.; Peng, Q.; Amevor, F.K.; Goswami, N. Production of hydrogen sulfide by fermentation in rumen and its impact on health and production of animals. Processes 2020, 8, 1169. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Meng, Q.; Zhou, Z. Effect of high sulfur diet on rumen fermentation, microflora, and epithelial barrier function in steers. Animals 2021, 11, 2545. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Rebellato, A.P.; Pallone, J.A.L.; Ferrari, R.A.; Clerici, M.T.P.S. Kernel characterization and starch morphology in five varieties of Peruvian Andean maize. Food Res. Int. 2021, 140, 110044. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.R.A.A.; Gul, K.; Dar, B.N. Wholegrains: A review on the amino acid profile, mineral content, physicochemical, bioactive composition, and health benefits. Int. J. Food Sci. Technol. 2022, 57, 1849–1865. [Google Scholar] [CrossRef]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Changes in macro-and micromineral concentrations in herbage during the harvesting and conservation processes. Grass Forage Sci. 2018, 73, 918–925. [Google Scholar] [CrossRef]
- Jenkins, T. The link between endotoxins and mycotoxins. Sci. Solut. 2018, 53, 8–10. [Google Scholar]
- Lyle, R.R.; Johnson, R.R.; Wilhite, J.V. Rumen characteristics in steers as affected by adaptation from forage to all concentrate diets. J. Anim. Sci. 1981, 53, 1383–1390. [Google Scholar] [CrossRef][Green Version]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing sub-acute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Kolver, E.S.; De Veth, M.J. Prediction of ruminal pH from pasture-based diets. J. Dairy Sci. 2002, 85, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Q.; Wang, C.; Song, C.; Lyu, Y.; Li, J.; Shan, A. The interaction between temperature and citric acid treatment in the anaerobic fermentation of Chinese cabbage waste. J. Clean. Prod. 2023, 383, 135502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).