Microbial Electrosynthesis Using 3D Bioprinting of Sporomusa ovata on Copper, Stainless-Steel, and Titanium Cathodes for CO2 Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation and Enrichment of S. ovata
2.2. MES Reactor Construction and Operation
2.3. Bio-Printing of Synthetic Biofilms
2.4. Chemical Analyses
2.5. Calculations
2.6. Scanning Electron Microscopy (SEM)
3. Results
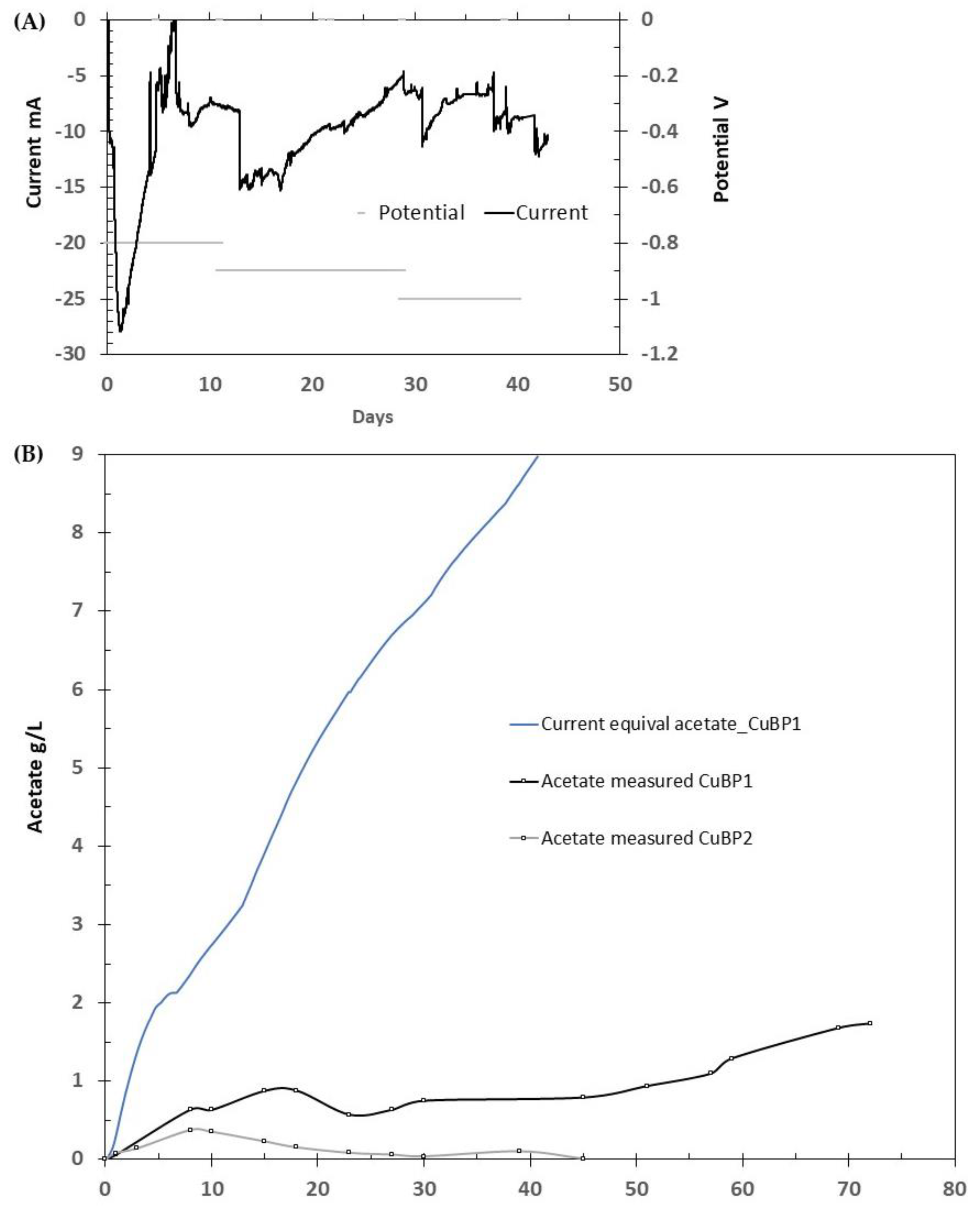
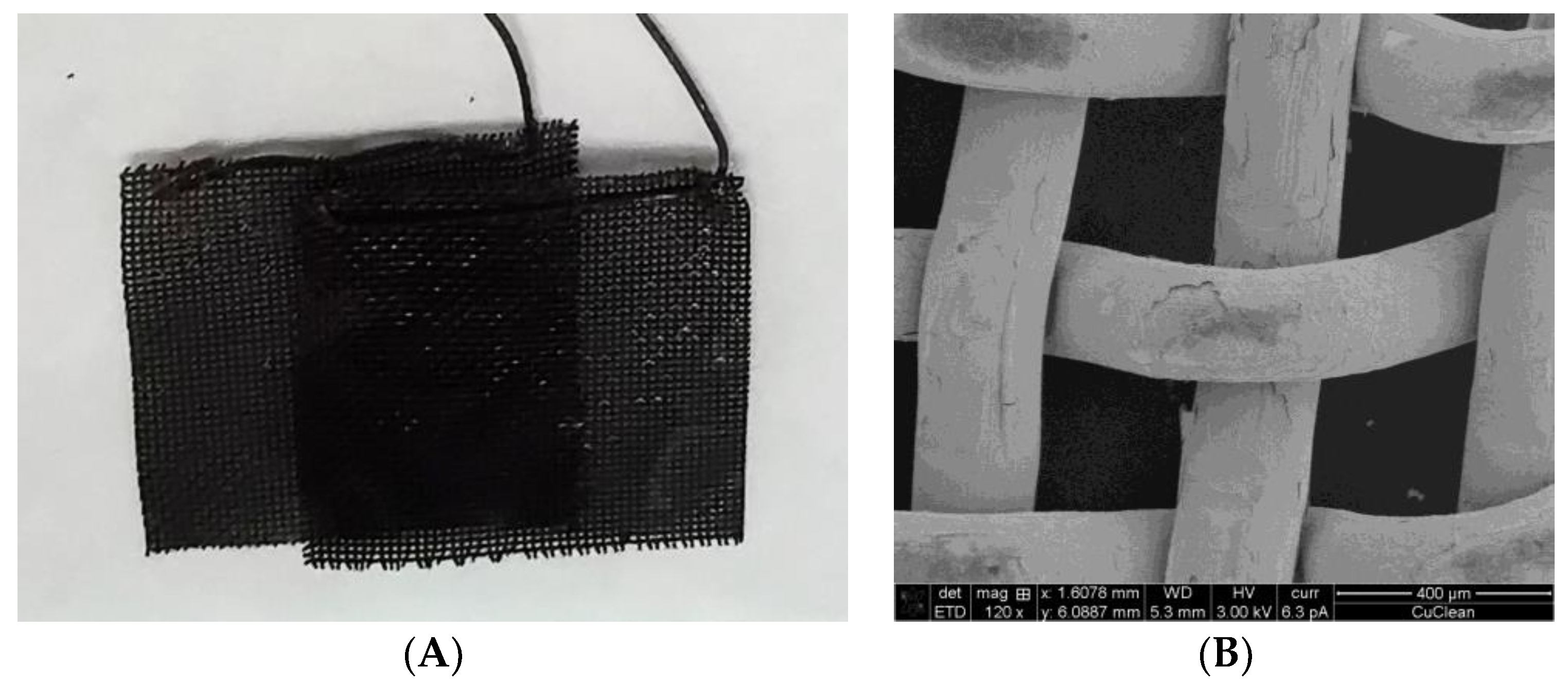
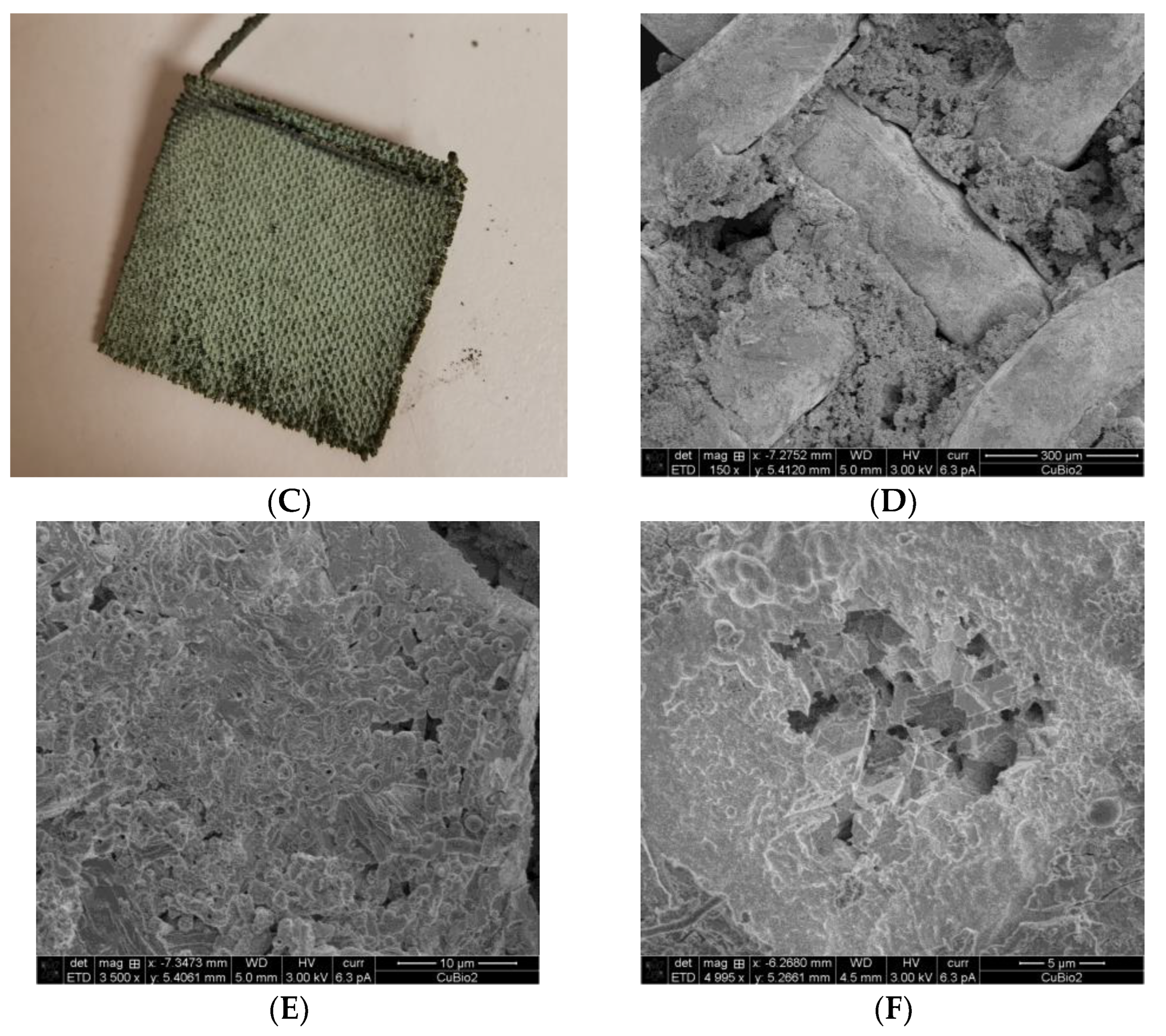
3.1. MES Performance of Bioprinted Cu Mesh
3.2. MES Performance of Bioprinted SS Mesh
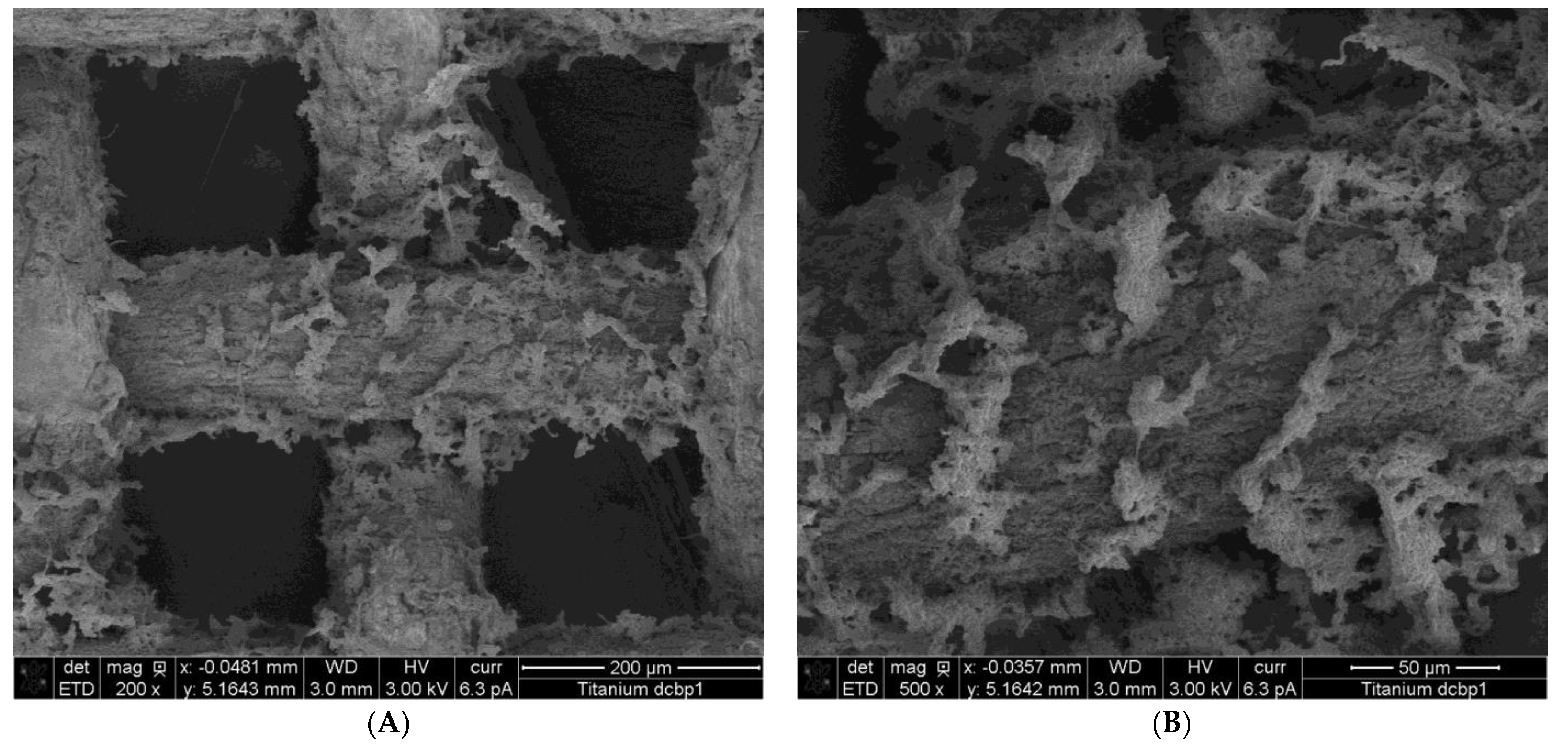
3.3. MES Performance of Bioprinted Ti Mesh
4. Discussion
4.1. Bioprinting of S. ovata Relatively Increased Acetate Production from CO2 Reduction
4.2. Metal Degradation in MES and Its Effect on MES Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity to Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic. MBio 2010, 1, e00103-10. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Prévoteau, A.; Carvajal-Arroyo, J.M.; Ganigué, R.; Rabaey, K. Microbial electrosynthesis from CO2: Forever a promise? Curr. Opin. Biotechnol. 2020, 62, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Sousa, J.; van Stralen, N.; Strik, D.P.B.T.B. Techno-economic assessment of microbial electrosynthesis from CO2 and/or organics: An interdisciplinary roadmap towards future research and application. Appl. Energy 2020, 279, 115775. [Google Scholar] [CrossRef]
- Wood, J.C.; Grové, J.; Marcellin, E.; Heffernan, J.K.; Hu, S.; Yuan, Z.; Virdis, B. Strategies to improve viability of a circular carbon bioeconomy—A techno-economic review of microbial electrosynthesis and gas fermentation. Water Res. 2021, 201, 117306. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.-L.; Höglund, D.; Koza, A.; Bonde, I.; Zhang, T. Adaptation of the autotrophic acetogen Sporomusa ovata to methanol accelerates the conversion of CO2 to organic products. Sci. Rep. 2015, 5, 16168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217. [Google Scholar] [CrossRef]
- Aryal, N.; Halder, A.; Zhang, M.; Whelan, P.R.; Tremblay, P.L.; Chi, Q.; Zhang, T. Freestanding and flexible graphene papers as bioelectrochemical cathode for selective and efficient CO2 conversion. Sci. Rep. 2017, 7, 9107. [Google Scholar] [CrossRef]
- Aryal, N.; Tremblay, P.-L.; Xu, M.; Daugaard, A.E.; Zhang, T. Highly Conductive Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate Polymer Coated Cathode for the Microbial Electrosynthesis of Acetate from Carbon Dioxide. Front. Energy Res. 2018, 6, 72. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef]
- Jourdin, L.; Freguia, S.; Donose, B.C.; Chen, J.; Wallace, G.G.; Keller, J.; Flexer, V. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J. Mater. Chem. A 2014, 2, 13093–13102. [Google Scholar] [CrossRef]
- Jourdin, L.; Winkelhorst, M.; Rawls, B.; Buisman, C.J.N.; Strik, D.P.B.T.B. Enhanced selectivity to butyrate and caproate above acetate in continuous bioelectrochemical chain elongation from CO2: Steering with CO2 loading rate and hydraulic retention time. Bioresour. Technol. Rep. 2019, 7, 100284. [Google Scholar] [CrossRef]
- Jourdin, L.; Raes, S.M.T.; Buisman, C.J.N.; Strik, D.P.B.T.B. Critical Biofilm Growth throughout Unmodified Carbon Felts Allows Continuous Bioelectrochemical Chain Elongation from CO2 up to Caproate at High Current Density. Front. Energy Res. 2018, 6, 7. [Google Scholar] [CrossRef]
- LaBelle, E.V.; May, H.D. Energy Efficiency and Productivity Enhancement of Microbial Electrosynthesis of Acetate. Front. Microbiol. 2017, 8, 756. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes. Faraday Discuss. 2017, 202, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Krige, A.; Rova, U.; Christakopoulos, P. 3D bioprinting on cathodes in microbial electrosynthesis for increased acetate production rate using Sporomusa ovata. J. Environ. Chem. Eng. 2021, 9, 106189. [Google Scholar] [CrossRef]
- Kong, F.; Ren, H.Y.; Liu, D.; Wang, Z.; Nan, J.; Ren, N.Q.; Fu, Q. Improved decolorization and mineralization of azo dye in an integrated system of anaerobic bioelectrochemical modules and aerobic moving bed biofilm reactor. Bioresour. Technol. 2022, 353, 127147. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cestellos-Blanco, S.; Kim, J.M.; Shen, Y.; Kong, Q.; Lu, D.; Liu, C.; Zhang, H.; Cao, Y.; Yang, P. Close-Packed Nanowire-Bacteria Hybrids for Efficient Solar-Driven CO2 Fixation. Joule 2020, 4, 800–811. [Google Scholar] [CrossRef]
- Nie, H.; Zhang, T.; Cui, M.; Lu, H.; Lovley, D.R.; Russell, T.P. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys. Chem. Chem. Phys. 2013, 15, 14290–14294. [Google Scholar] [CrossRef]
- Bian, B.; Alqahtani, M.F.; Katuri, K.P.; Liu, D.F.; Bajracharya, S.; Lai, Z.P.; Rabaey, K.; Saikaly, P.E. Porous Nickel Hollow Fiber Cathodes Coated with CNTs for Efficient Microbial Electrosynthesis of Acetate from CO2 using Sporomusa ovata. J. Mater. Chem. A 2018, 6, 17201–17211. [Google Scholar] [CrossRef]
- Bajracharya, S.; Krige, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Dual cathode configuration and headspace gas recirculation for enhancing microbial electrosynthesis using Sporomusa ovata. Chemosphere 2022, 287, 132188. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagiotou, K.; Soekhoe, V.; Jourdin, L.; Buisman, C.J.N.; Bitter, J.H.; Strik, D.P.B.T.B. Catalytic Cooperation between a Copper Oxide Electrocatalyst and a Microbial Community for Microbial Electrosynthesis. Chempluschem 2021, 86, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Madjarov, J.; Soares, R.; Paquete, C.M.; Louro, R.O. Sporomusa ovata as Catalyst for Bioelectrochemical Carbon Dioxide Reduction: A Review across Disciplines from Microbiology to Process Engineering. Front. Microbiol. 2022, 13, 2159. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.A.; Berger, C.; Schmitz, S.; Uhlig, R. Microbial electrosynthesis I: Pure and defined mixed culture engineering. In Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2019; Volume 167, pp. 181–202. Available online: https://link.springer.com/chapter/10.1007/10_2017_17 (accessed on 1 October 2023).
- Chen, L.; Tremblay, P.-L.; Mohanty, S.; Xu, K.; Zhang, T. Electrosynthesis of acetate from CO2 by a highly structured biofilm assembled with reduced graphene oxide–tetraethylene pentamine. J. Mater. Chem. A 2016, 4, 8395–8401. [Google Scholar] [CrossRef]
- Lee, C.R.; Kim, C.; Song, Y.E.; Im, H.; Oh, Y.K.; Park, S.; Kim, J.R. Co-culture-based biological carbon monoxide conversion by Citrobacter amalonaticus Y19 and Sporomusa ovata via a reducing-equivalent transfer mediator. Bioresour. Technol. 2018, 259, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.; Halder, A.; Tremblay, P.L.; Chi, Q.; Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 2016, 217, 117–122. [Google Scholar] [CrossRef]
- Knoll, M.T.; Fuderer, E.; Gescher, J. Sprayable biofilm—Agarose hydrogels as 3D matrix for enhanced productivity in bioelectrochemical systems. Biofilm 2022, 4, 100077. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production.pdf. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Faraghiparapari, N.; Zhang, T. Accelerated H2 Evolution during Microbial Electrosynthesis with Sporomusa ovata. Catalysts 2019, 9, 166. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Grote, J.-P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Aryal, N.; Wan, L.; Overgaard, M.H.; Stoot, A.C.; Chen, Y.; Tremblay, P.L.; Zhang, T. Increased carbon dioxide reduction to acetate in a microbial electrosynthesis reactor with a reduced graphene oxide-coated copper foam composite cathode. Bioelectrochemistry 2019, 128, 83–93. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagiotou, K.; Jourdin, L.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Bitter, J.H. CO2 Conversion by Combining a Copper Electrocatalyst and Wild-type Microorganisms. ChemCatChem 2020, 12, 3900–3912. [Google Scholar] [CrossRef]
- Chatzipanagiotou, K.R.; Jourdin, L.; Bitter, J.H.; Strik, D.P.B.T.B. Concentration-dependent effects of nickel doping on activated carbon biocathodes. Catal. Sci. Technol. 2022, 12, 2500–2518. [Google Scholar] [CrossRef]
- Desloover, J.; Arends, J.B.A.; Hennebel, T.; Rabaey, K. Operational and technical considerations for microbial electrosynthesis. Biochem. Soc. Trans. 2012, 40, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Shakeel, S.; Khan, M.Z. Enhanced production and utilization of biosynthesized acetate using a packed-fluidized bed cathode based MES system. J. Environ. Chem. Eng. 2022, 10, 108067. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Zhang, D.; Dong, W.; Jiang, T.; Zhou, H.; Li, L.; Mao, B. Industrial stainless steel meshes for efficient electrocatalytic hydrogen evolution. J. Energy Storage 2021, 41, 102844. [Google Scholar] [CrossRef]
- Munoz, L.D.; Erable, B.; Etcheverry, L.; Riess, J.; Basséguy, R.; Bergel, A.; De Silva Munoz, L.; Erable, B.; Etcheverry, L.; Riess, J.; et al. Combining phosphate species and stainless steel cathode to enhance hydrogen evolution in microbial electrolysis cell (MEC). Electrochem. Commun. 2010, 12, 183–186. [Google Scholar] [CrossRef]
- Khalil, M.W.; Abdel Rahim, M.A. Hydrogen evolution reaction on Titanium and oxide-covered titanium electrodes. Materwiss. Werksttech. 1991, 22, 390–395. [Google Scholar] [CrossRef]
- Wang, L.; He, Z.; Guo, Z.; Sangeetha, T.; Yang, C.; Gao, L.; Wang, A.; Liu, W. Microbial community development on different cathode metals in a bioelectrolysis enhanced methane production system. J. Power Sources 2019, 444, 227306. [Google Scholar] [CrossRef]
- Sharma, M.; Bajracharya, S.; Gildemyn, S.; Patil, S.A.; Alvarez-Gallego, Y.; Pant, D.; Rabaey, K.; Dominguez-Benetton, X. A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim. Acta 2014, 140, 191–208. [Google Scholar] [CrossRef]
- Ohmi, T.; Nakagawa, Y.; Nakamura, M.; Ohki, A.; Koyama, T. Formation of chromium oxide on 316L austenitic stainless steel. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 14, 2505. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Petrović Mihajlović, M.B.; Radovanović, M.B.; Antonijević, M.M. New trends in corrosion protection of copper. Chem. Pap. 2019, 73, 2103–2132. [Google Scholar] [CrossRef]
- Baudler, A.A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U.; Schroder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Busalmen, J.P.; Vázquez, M.; De Sánchez, S.R. New evidences on the catalase mechanism of microbial corrosion. Electrochim. Acta 2002, 47, 1857–1865. [Google Scholar] [CrossRef]
- Jiang, Y.; Chu, N.; Zhang, W.; Ma, J.; Zhang, F.; Liang, P.; Zeng, R.J. Zinc: A promising material for electrocatalyst-assisted microbial electrosynthesis of carboxylic acids from carbon dioxide. Water Res. 2019, 159, 87–94. [Google Scholar] [CrossRef]
- Tashiro, Y.; Hirano, S.; Matson, M.M.; Atsumi, S.; Kondo, A. Electrical-biological hybrid system for CO2 reduction. Metab. Eng. 2018, 47, 211–218. [Google Scholar] [CrossRef]




| Study | Cathode | Cathode Potential | Volume-Based Productivity | Area-Based Productivity | CE for Acetate | Max Product Titer |
|---|---|---|---|---|---|---|
| [V vs. Ag/AgCl] | [g/L/d] | [g/m2/d] | [%] | [mM] | ||
| This study | Synthetic biofilm on Ti | −0.8 | 0.34 ± 0.12 | 53 ± 19 | 55 ± 20.1 | 157 |
| This study | Synthetic biofilm on SS | −0.8 to −1 | 0.1 ± 0.01 | 24.6 ± 8.8 | 51 | 43 |
| This study | Synthetic biofilm on Cu | −0.8 to −0.9 | 0.05 ± 0.01 | 23.05 ± 7.1 | 26 | 29.5 |
| Krige et al. [16] | Synthetic biofilm CC | −0.8 | 0.31 ± 0.03 | 47.3 ± 5.1 | 62.7 ± 15.4 | 60 |
| tSu et al. [18] | Si nanowire array | −1.4 | 0.30 | 44.3 | ~80 | - |
| Bian et al. [20] | Ni-PHF/CNT + direct CO2 supply | −0.6 | 0.02 | 1.85 | 83 | 2.7 |
| Aryal et al. [28] | 3D graphene-functionalized CF | −0.93 | 0.12 | 13.9 ± 0.4 | 86.5 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajracharya, S.; Krige, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Microbial Electrosynthesis Using 3D Bioprinting of Sporomusa ovata on Copper, Stainless-Steel, and Titanium Cathodes for CO2 Reduction. Fermentation 2024, 10, 34. https://doi.org/10.3390/fermentation10010034
Bajracharya S, Krige A, Matsakas L, Rova U, Christakopoulos P. Microbial Electrosynthesis Using 3D Bioprinting of Sporomusa ovata on Copper, Stainless-Steel, and Titanium Cathodes for CO2 Reduction. Fermentation. 2024; 10(1):34. https://doi.org/10.3390/fermentation10010034
Chicago/Turabian StyleBajracharya, Suman, Adolf Krige, Leonidas Matsakas, Ulrika Rova, and Paul Christakopoulos. 2024. "Microbial Electrosynthesis Using 3D Bioprinting of Sporomusa ovata on Copper, Stainless-Steel, and Titanium Cathodes for CO2 Reduction" Fermentation 10, no. 1: 34. https://doi.org/10.3390/fermentation10010034
APA StyleBajracharya, S., Krige, A., Matsakas, L., Rova, U., & Christakopoulos, P. (2024). Microbial Electrosynthesis Using 3D Bioprinting of Sporomusa ovata on Copper, Stainless-Steel, and Titanium Cathodes for CO2 Reduction. Fermentation, 10(1), 34. https://doi.org/10.3390/fermentation10010034








