Production, Characterization, and Activation of Biochars from a Mixture of Waste Insulation Electric Cables (WIEC) and Waste Lignocellulosic Biomass (WLB)
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Carbonization Experiments
2.3. Biochar Washing Process
2.4. Biochar Activation Process
2.5. Biochar Characterization
2.5.1. Elemental Analysis
2.5.2. Thermogravimetric Analysis
2.5.3. High Heating Value and Low Heating Value
2.5.4. Chlorine Content and Mineral Composition
2.5.5. Ash Content
2.5.6. Apparent Density
2.5.7. Fourier-Transform Infrared
2.5.8. Nitrogen Adsorption at 77 K
3. Results and Discussion
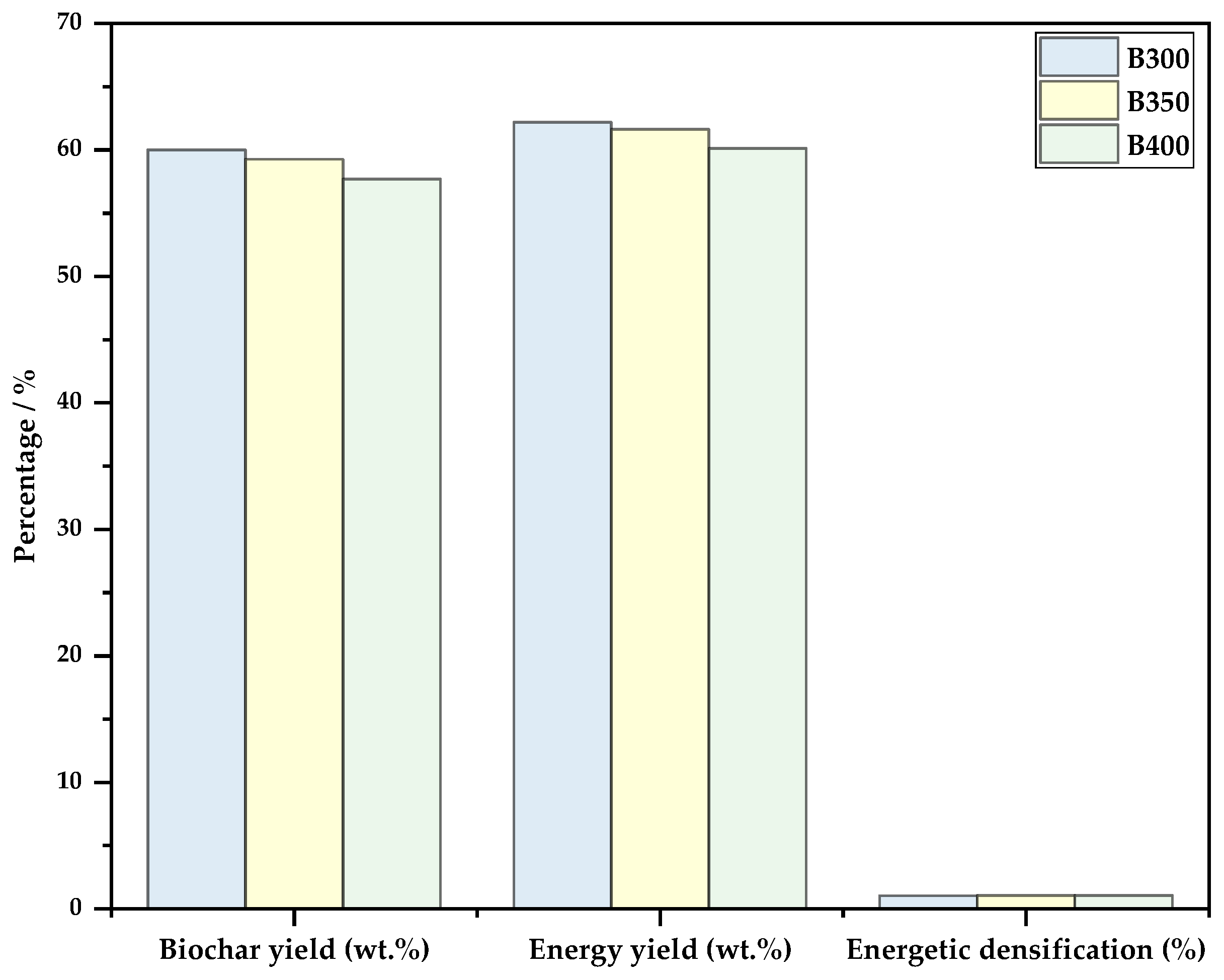
3.1. Biochar Yield, Enery Yield and Energetic Densification
3.2. Biochar Characterization
3.2.1. Elemental Analysis and Heating Value
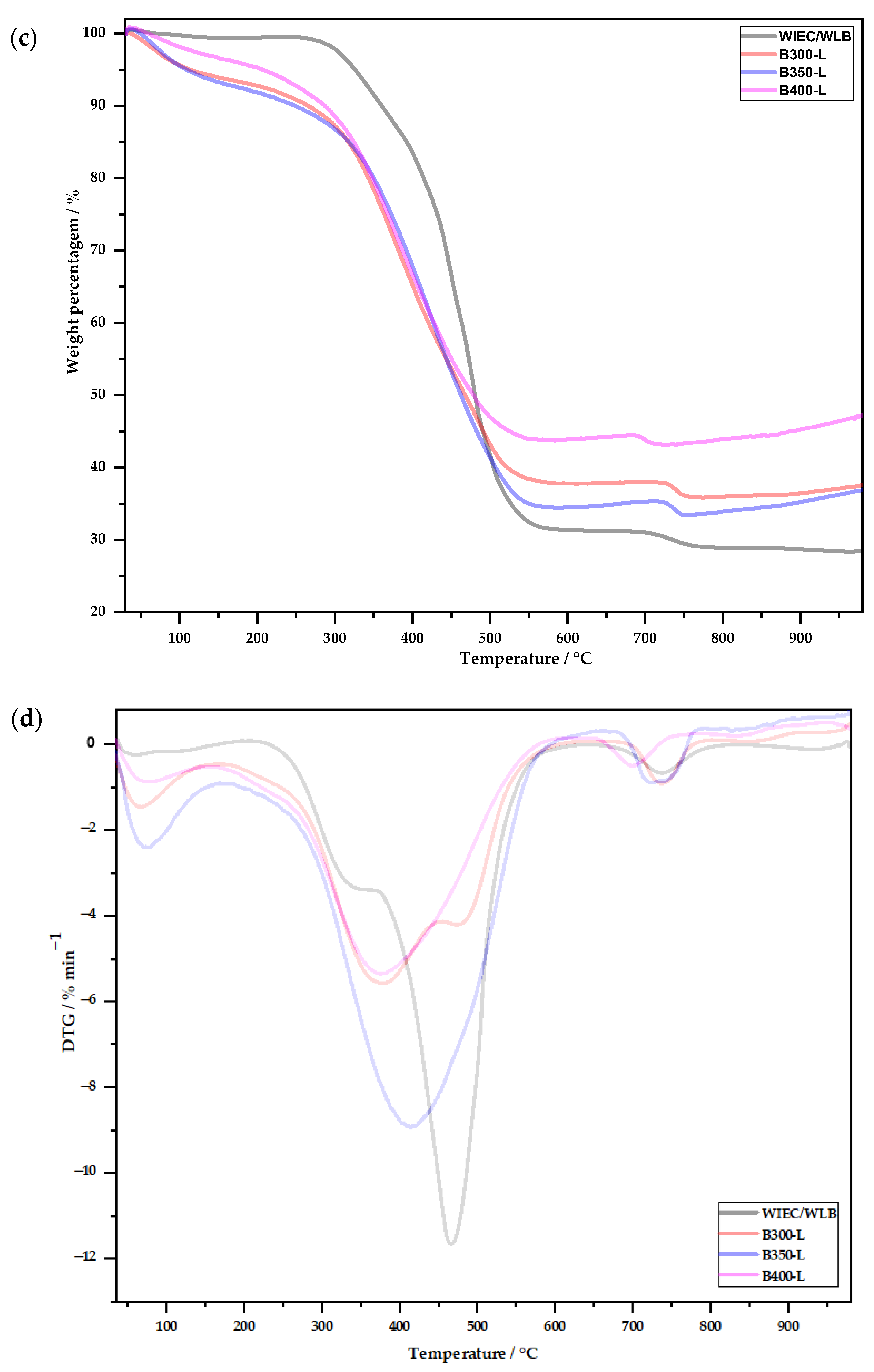
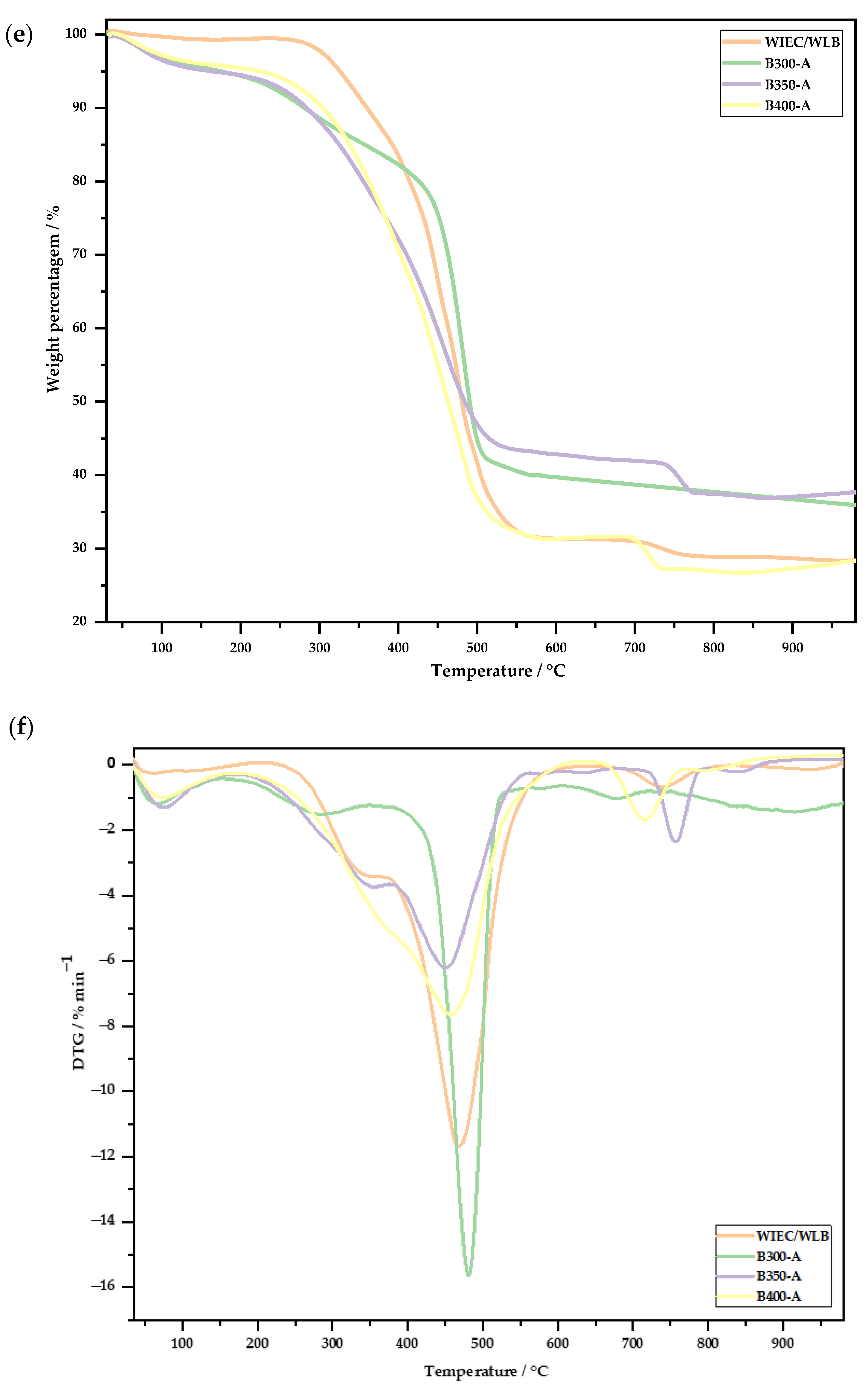
3.2.2. Thermogravimetric Analysis
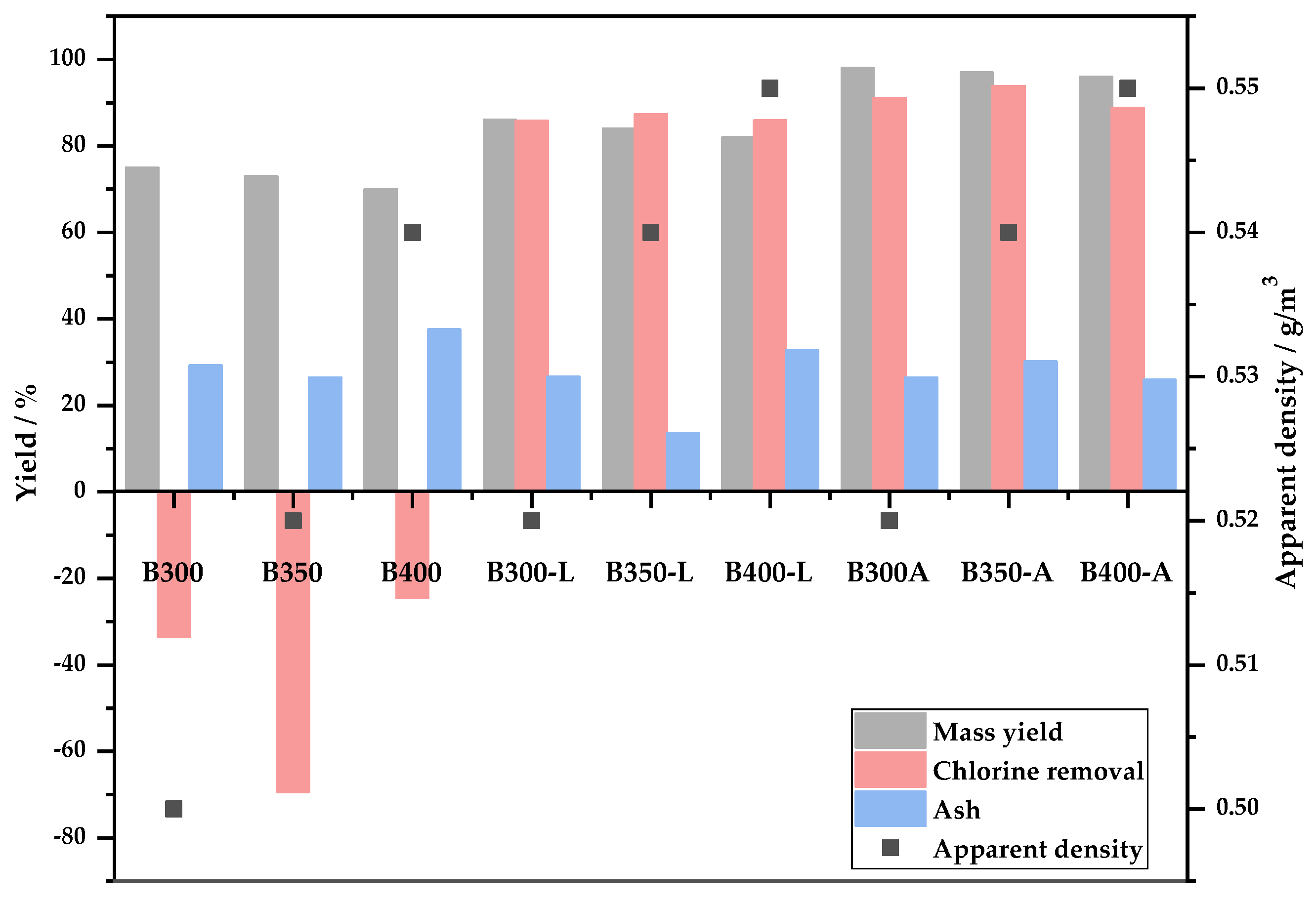
3.2.3. Mass Yield, Ash Content, Chlorine Removal Potential and Apparent Density of the Biochars
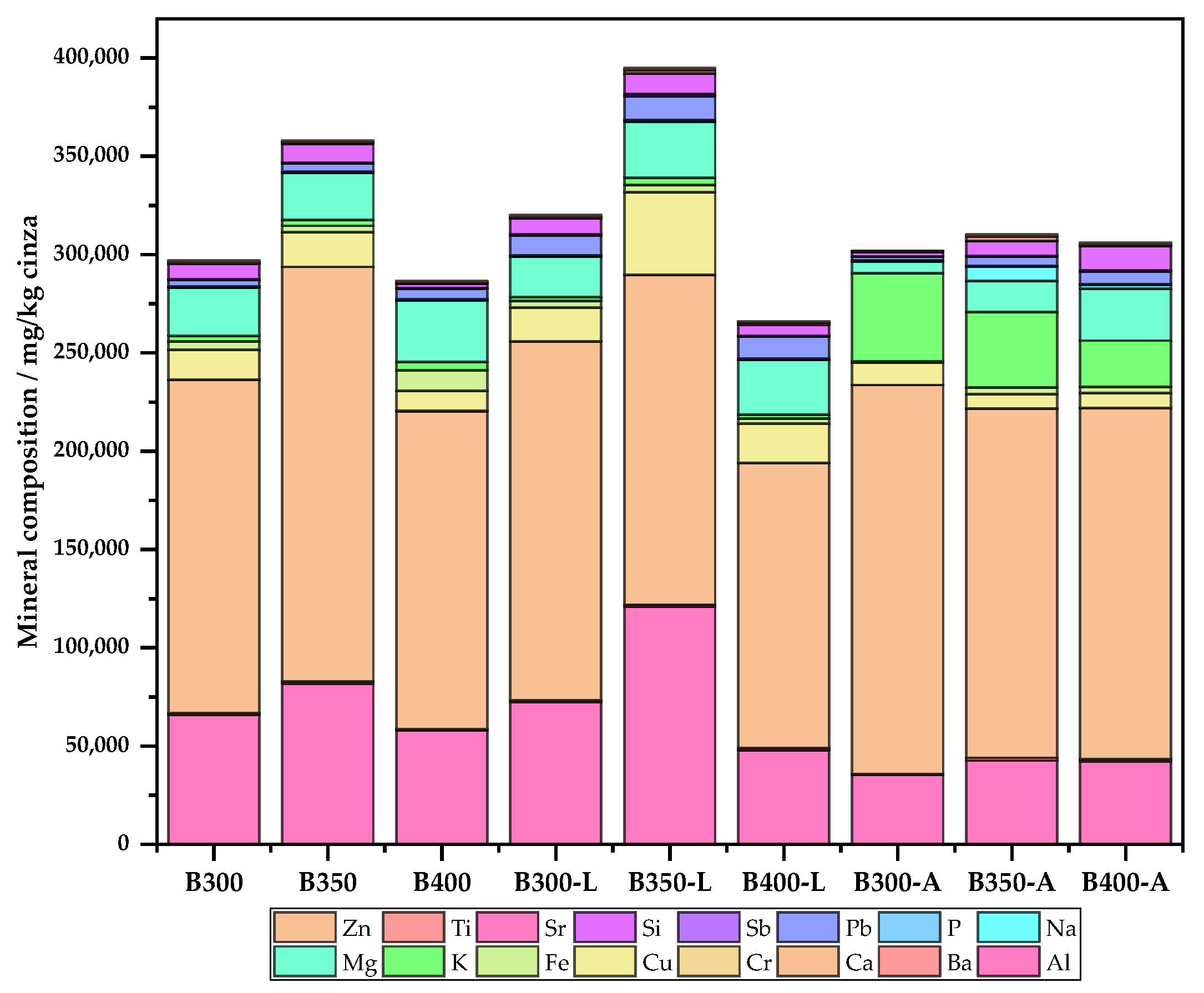
3.2.4. Mineral Composition
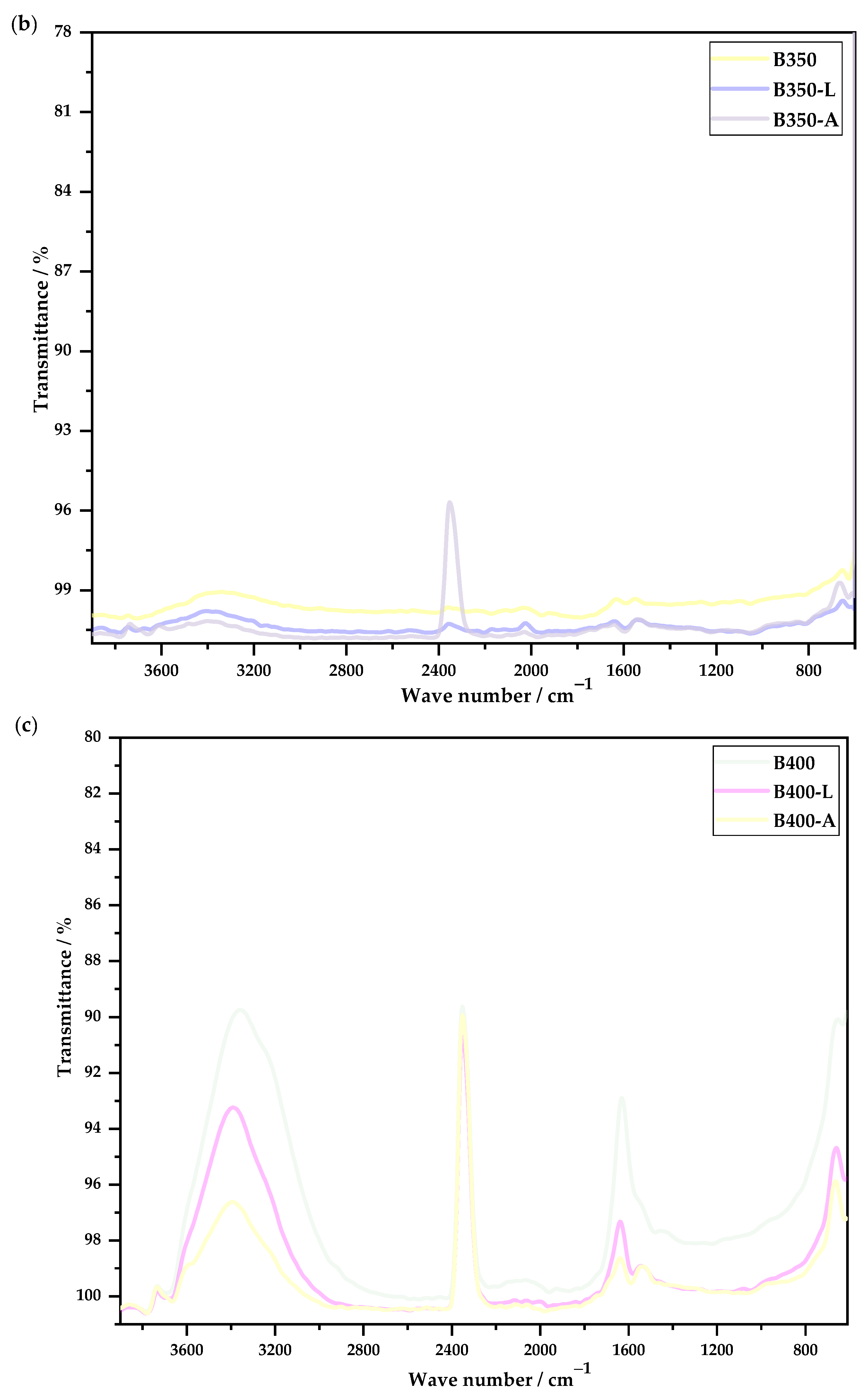
3.2.5. Fourier-Transform Infrared Spectroscopy
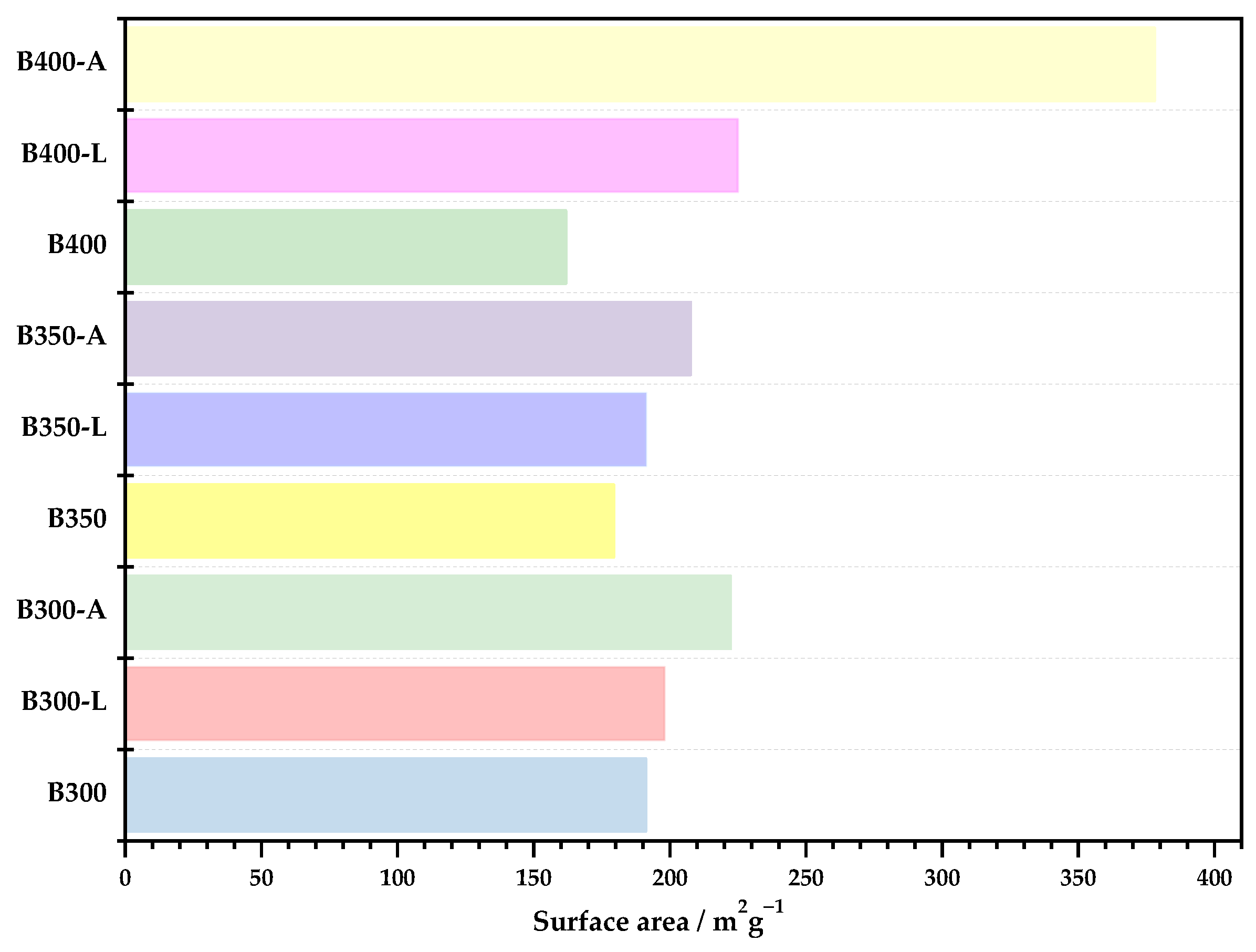
3.2.6. Nitrogen Adsorption at 77 K
4. Conclusions
- The percentage of carbon present in the original feedstock and in the produced biochars were similar, differing mainly in the percentage of oxygen, which was lower, and in the ash, which increased as the temperature increased.
- The amount of volatile matter in the biochars was lower as the temperature of biochar production increased from 300 to 400 °C.
- The mass yield of biochars was not influenced by the temperature increase, ranging between 70 and 75%.
- The chlorine removal potential for biochars that were washed and activated was above 80%, demonstrating the efficiency of carbonization as a pretreatment for thermochemical processes to remove chlorinated compounds.
- In the FTIR analysis, it was possible to observe that there was a great difference between the spectra of the untreated biochars and the washed biochars, indicating the removal of compounds that were on the surface, such as chlorine. Biochar produced at 400 °C showed the lowest peaks after washing.
- In the analysis of the surfaces of the biochar samples, the differences between temperatures were more noticeable when the biochars were washed. When the activation process was carried out, the biochar samples produced at 300 and 350 °C were very similar, with the biochar produced at 400 °C having a higher surface area.
- Some results for the 350 °C biochars were not similar to the behaviors of the biochars produced at 300 and 400 °C, indicating that, when making the feedstock mixture, the amount of plastic and small metals may have been higher, thus making the carbonization process more difficult.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Reen, S.; Chyuan, H.; Wayne, K.; Loke, P.; Phang, S.; Chuan, T.; Nagarajan, D.; Lee, D. Sustainable Approaches for Algae Utilisation in Bioenergy Production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the Recent Advances of Microwave Pretreatment Technologies for the Conversion of Lignocellulosic Biomass into Sustainable Biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Abro, R.; Harijan, K.; Zhao, Z.; Bazmi, A.A.; Abbas, T.; Yu, G. Progress in Production of Biomass-to-Liquid Biofuels to Decarbonize Transport Sector-Prospectus and Challenges. RSC Adv. 2016, 6, 32140–32170. [Google Scholar] [CrossRef]
- Tuan, A.; Pham, V.V. 2-Methylfuran (MF) as a Potential Biofuel: A Thorough Review on the Production Pathway from Biomass, Combustion Progress, and Application in Engines. Renew. Sustain. Energy Rev. 2021, 148, 111265. [Google Scholar] [CrossRef]
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research, International Telecommunication Union, and International Solid Waste Association: Geneva, Switzerland, 2020; Volume 3, pp. 1–120. [Google Scholar]
- Bhaskar, K.; Kumar, B. Electronic Waste Management and Sustainable Development Goals: Is There a Business Case for Linking the Two? J. Indian Bus. Res. 2019, 11, 120–137. [Google Scholar] [CrossRef]
- Khetriwal, D.S.; Kraeuchi, P.; Widmer, R. Producer Responsibility for E-Waste Management: Key Issues for Consideration—Learning from the Swiss Experience. J. Environ. Manag. 2009, 90, 153–165. [Google Scholar] [CrossRef]
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Böni, H. Global Perspectives on E-Waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Andrade, D.F.; Castro, J.P.; Garcia, J.A.; Machado, R.C.; Pereira-Filho, E.R.; Amarasiriwardena, D. Analytical and Reclamation Technologies for Identification and Recycling of Precious Materials from Waste Computer and Mobile Phones. Chemosphere 2022, 286, 131739. [Google Scholar] [CrossRef]
- Perkins, D.N.; Brune Drisse, M.N.; Nxele, T.; Sly, P.D. E-Waste: A Global Hazard. Ann. Glob. Health 2014, 80, 286–295. [Google Scholar] [CrossRef]
- Forti, V.; Baldé, K.; Kuehr, R. E-Waste Statistics: Guidelines on Classifications, Reporting and Indicators; United Nations University: Bonn, Germany, 2018; ISBN 9789280890662. [Google Scholar]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Nan, M.; Herath, G.; Moyo, T. E-Waste in the International Context—A Review of Trade Flows, Regulations, Hazards, Waste Management Strategies and Technologies for Value Recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ali, S.H.; Tian, J.; Li, J. Mapping Anthropogenic Mineral Generation in China and Its Implications for a Circular Economy. Nat. Commun. 2020, 11, 1544. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Yao, L.; Xu, Z. Auto-Sorting Commonly Recovered Plastics from Waste Household Appliances and Electronics Using near-Infrared Spectroscopy. J. Clean. Prod. 2020, 246, 118732. [Google Scholar] [CrossRef]
- Martinho, G.; Pires, A.; Saraiva, L.; Ribeiro, R. Composition of Plastics from Waste Electrical and Electronic Equipment (WEEE) by Direct Sampling. Waste Manag. 2012, 32, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, X.; Feng, Y.; Zhang, L.; Yuan, Z. Recycled WEEE Plastics in China: Generation Trend and Environmental Impacts. Resour. Conserv. Recycl. 2022, 177, 105978. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S. A Comprehensive Review on Thermochemical, Biological, Biochemical and Hybrid Conversion Methods of Bio-Derived Lignocellulosic Molecules into Renewable Fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical Conversion Pathways for Carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.; Tang, T. Recent Progress in Controlled Carbonization of (Waste) Polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Taherymoosavi, S.; Verheyen, V.; Munroe, P.; Joseph, S.; Reynolds, A. Characterization of Organic Compounds in Biochars Derived from Municipal Solid Waste. Waste Manag. 2017, 67, 131–142. [Google Scholar] [CrossRef]
- Huang, J.; Qiao, Y.; Wei, X.; Zhou, J.; Yu, Y.; Xu, M. Effect of Torrefaction on Steam Gasification of Starchy Food Waste. Fuel 2019, 253, 1556–1564. [Google Scholar] [CrossRef]
- Zheng, A.; Fan, Y.; Wei, G.; Zhao, K.; Huang, Z.; Zhao, Z.; Li, H. Chemical Looping Gasification of Torrefied Biomass Using NiFe2O4 as an Oxygen Carrier for Syngas Production and Tar Removal. Energy Fuels 2020, 34, 6008–6019. [Google Scholar] [CrossRef]
- Laird, D.A. The Charcoal Vision: A Win-Win-Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, While Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Brown, R.C.; Amonette, J.E.; National, N. Review of the Pyrolysis Platform for Coproducing Bio-Oil and Biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar]
- Brewer, C.E.; Unger, R.; Schmidt-rohr, K.; Brown, R.C. Criteria to Select Biochars for Field Studies Based on Biochar Chemical Properties. BioEnergy Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Bioresource Technology Hydochar and Biochar: Production, Physicochemical Properties and Techno- Economic Analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of Reaction Conditions and Feedstock on Hydrochar Properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An Overview of the Effect of Pyrolysis Process Parameters on Biochar Stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L. A Review on Selective Production of Value-Added Chemicals via Catalytic Pyrolysis of Lignocellulosic Biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Atienza-martínez, M.; Gea, G.; Plaza, D.; Lu, F. Pyrolysis of Cashew Nutshells: Characterization of Products and Energy Balance. Energy 2018, 158, 72–80. [Google Scholar] [CrossRef]
- Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Calado, H.; Goncalves, M.M.; Brito, P. Co-Carbonization of a Mixture of Waste Insulation Electric Cables (WIEC) and Lignocellulosic Waste, for the Removal of Chlorine: Biochar Properties and Their Behaviors. Fuel 2022, 320, 123932. [Google Scholar] [CrossRef]
- Liu, Z. The Fate of Fluorine and Chlorine during Thermal Treatment of Coals. Environ. Sci. Technol. 2006, 40, 7886–7889. [Google Scholar]
- Longo, A.; Nobre, C.; Sen, A.; Panizio, R.; Brito, P.; Gonçalves, M. Torrefaction Upgrading of Heterogenous Wastes Containing Cork and Chlorinated Polymers. Environments 2022, 9, 99. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel Properties and Combustion Kinetics of Hydrochar Prepared by Hydrothermal Carbonization of Bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Hua, Y.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Lokman, M.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and Its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F.; Berlin, T.U. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Hall, W.; Box, P.O. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Manyà, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimental Study on the Effect of Pyrolysis Pressure, Peak Temperature, and Particle Size on the Potential Stability of Vine Shoots-Derived Biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O: C Molar Ratios Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon Manag. 2014, 3004, 289–303. [Google Scholar] [CrossRef]
- Budai, A.; Calucci, L.; Rasse, D.P.; Tau, L.; Pengerud, A.; Wiedemeier, D.; Abiven, S.; Forte, C. Effects of Pyrolysis Conditions on Miscanthus and Corncob Chars: Characterization by IR, Solid State NMR and BPCA Analysis. J. Anal. Appl. Pyrolysis 2017, 128, 335–345. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J. Method for Determining Black Carbon in Residues of Vegetation Fires. Environ. Sci. Technol. 1995, 29, 2695–2702. [Google Scholar] [CrossRef]
- Yasuhara, A.; Amano, Y.; Shibamoto, T. Investigation of the Self-Heating and Spontaneous Ignition of Refuse-Derived Fuel (RDF) during Storage. Waste Manag. 2010, 30, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Aamer, M.; Taha, S.; Taqvi, H. Pyrolysis, Kinetics Analysis, Thermodynamics Parameters and Reaction Mechanism of Typha Latifolia to Evaluate Its Bioenergy Potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, Nutrient Availability and Hydrophobicity of Biochars Derived from Manure, Crop Residues, and Municipal Solid Waste for Their Use as Soil Amendments. Chemosph. J. 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T. Effect of the Heating Rate on the Thermochemical Behavior and Biofuel Properties of Sewage Sludge Pyrolysis. Eenrgy Fuels 2016, 30, 1564–1570. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Díaz, R.M. Influence of Sewage Sludge Treatment on Pyrolysis and Combustion of Dry Sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- López, A.; De Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of Time and Temperature on Pyrolysis of Plastic Wastes in a Semi-Batch Reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Zaheer-uddin, M.; Guo, J. Co-Pyrolysis of Sewage Sludge and Low-Density Polyethylene—A Thermogravimetric Study of Thermo-Kinetics and Thermodynamic Parameters. J. Environ. Chem. Eng. 2021, 9, 104554. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of Refuse Derived Fuel through Torrefaction and Carbonization: Evaluation of RDF Char Fuel Properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Preston, C.M.; Schmidt, M.W.I. Black (Pyrogenic) Carbon: A Synthesis of Current Knowledge and Uncertainties with Special Consideration of Boreal Regions. Biogeosciences 2006, 3, 397–420. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html#ir-spectrum-table-by-range (accessed on 5 May 2023).
- Destainville, A.; Champion, E.; Laborde, E. Synthesis, Characterization and Thermal Behavior of Apatitic Tricalcium Phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Tehreem, S.; Yousra, M.; Alamer, K.H.; Alsudays, I.M.; Sarwar, S.; Kamal, A.; Naeem, S. Analysis of the Role of Various Biochar in the Remediation of Heavy Metals in Contaminated Water and Its Kinetics Study. J. Saudi Chem. Soc. 2022, 26, 101518. [Google Scholar] [CrossRef]
- Kakuta, Y. Study on Chlorine Removal from Mixture of Waste Plastics. Waste Manag. 2008, 28, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The Economic Value of Biochar in Crop Production and Carbon Sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A. Desorption of Arsenic from Exhaust Activated Carbons Used for Water Purification. J. Hazard. Mater. 2013, 260, 451–458. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Alicia, R.F.; Abdullah, E.C.; Sahu, J.N.; Haslija, A.B.A.; Tan, J. Statistical Optimization and Kinetic Studies on Removal of Zn 2 + Using Functionalized Carbon Nanotubes and Magnetic Biochar. J. Environ. Chem. Eng. 2013, 1, 486–495. [Google Scholar] [CrossRef]
- Bernard, B.; He, X.; Wang, S.; Abomohra, A.E.; Hu, Y.; Wang, Q. Co-Pyrolysis of Biomass and Waste Plastics as a Thermochemical Conversion Technology for High-Grade Biofuel Production: Recent Progress and Future Directions Elsewhere Worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]




| Sample | Temperature | Features |
|---|---|---|
| B300 | 300 | Biochars were produced at different temperatures. |
| B350 | 350 | |
| B400 | 400 | |
| B300-L | 300 | Biochars were washed in hot water, filtered and dried. |
| B350-L | 350 | |
| B400-L | 400 | |
| B300-A | 300 | Biochars were washed in hot water, filtered and dried and were submitted to an activation process with KOH 2 N. |
| B350-A | 350 | |
| B400-A | 400 |
| Parameters | WIEC/WLB | B300 | B350 | B400 | B300-L | B350-L | B400-L | B300-A | B350-A | B400-A |
|---|---|---|---|---|---|---|---|---|---|---|
| C (wt.%, db) | 52.3 | 40.77 | 42.64 | 43.95 | 42.38 | 43.06 | 43.14 | 41.59 | 35.22 | 47.44 |
| H (wt.%, db) | 2.5 | 4.02 | 3.74 | 2.83 | 2.81 | 3.33 | 3.76 | 4.34 | 3.56 | 5.12 |
| N (wt.%, db) | 0.2 | 5.08 | 4.24 | 4.01 | 12.9 | 11.38 | 10.41 | 0.9 | 0.85 | 0.51 |
| S (wt.%, db) | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| O (wt.%, db) | 45.0 | 50.13 | 49.38 | 49.21 | 41.91 | 42.23 | 42.69 | 53.17 | 60.37 | 46.93 |
| HHV (MJ/kg, db) | 21.23 | 18.15 | 18.27 | 18.45 | 19.6 | 19.67 | 19.71 | - | - | - |
| LHV (MJ/kg, db) | 19.88 | 15.98 | 16.25 | 16.92 | 18.08 | 17.87 | 17.68 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Panizio, R.; Assis, A.; Carmo-Calado, L.; Nobre, C.; Longo, A.; Silveira, J.; Goncalves, M.M.; Brito, P. Production, Characterization, and Activation of Biochars from a Mixture of Waste Insulation Electric Cables (WIEC) and Waste Lignocellulosic Biomass (WLB). C 2023, 9, 49. https://doi.org/10.3390/c9020049
Mota-Panizio R, Assis A, Carmo-Calado L, Nobre C, Longo A, Silveira J, Goncalves MM, Brito P. Production, Characterization, and Activation of Biochars from a Mixture of Waste Insulation Electric Cables (WIEC) and Waste Lignocellulosic Biomass (WLB). C. 2023; 9(2):49. https://doi.org/10.3390/c9020049
Chicago/Turabian StyleMota-Panizio, Roberta, Ana Assis, Luís Carmo-Calado, Catarina Nobre, Andrei Longo, José Silveira, Maria Margarida Goncalves, and Paulo Brito. 2023. "Production, Characterization, and Activation of Biochars from a Mixture of Waste Insulation Electric Cables (WIEC) and Waste Lignocellulosic Biomass (WLB)" C 9, no. 2: 49. https://doi.org/10.3390/c9020049
APA StyleMota-Panizio, R., Assis, A., Carmo-Calado, L., Nobre, C., Longo, A., Silveira, J., Goncalves, M. M., & Brito, P. (2023). Production, Characterization, and Activation of Biochars from a Mixture of Waste Insulation Electric Cables (WIEC) and Waste Lignocellulosic Biomass (WLB). C, 9(2), 49. https://doi.org/10.3390/c9020049















