Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Determinations
2.3. Adsorption Experiments
2.3.1. Equilibrium Experiments
2.3.2. Kinetics Experiments
3. Results and Discussion
3.1. Batch Adsorption Experiments
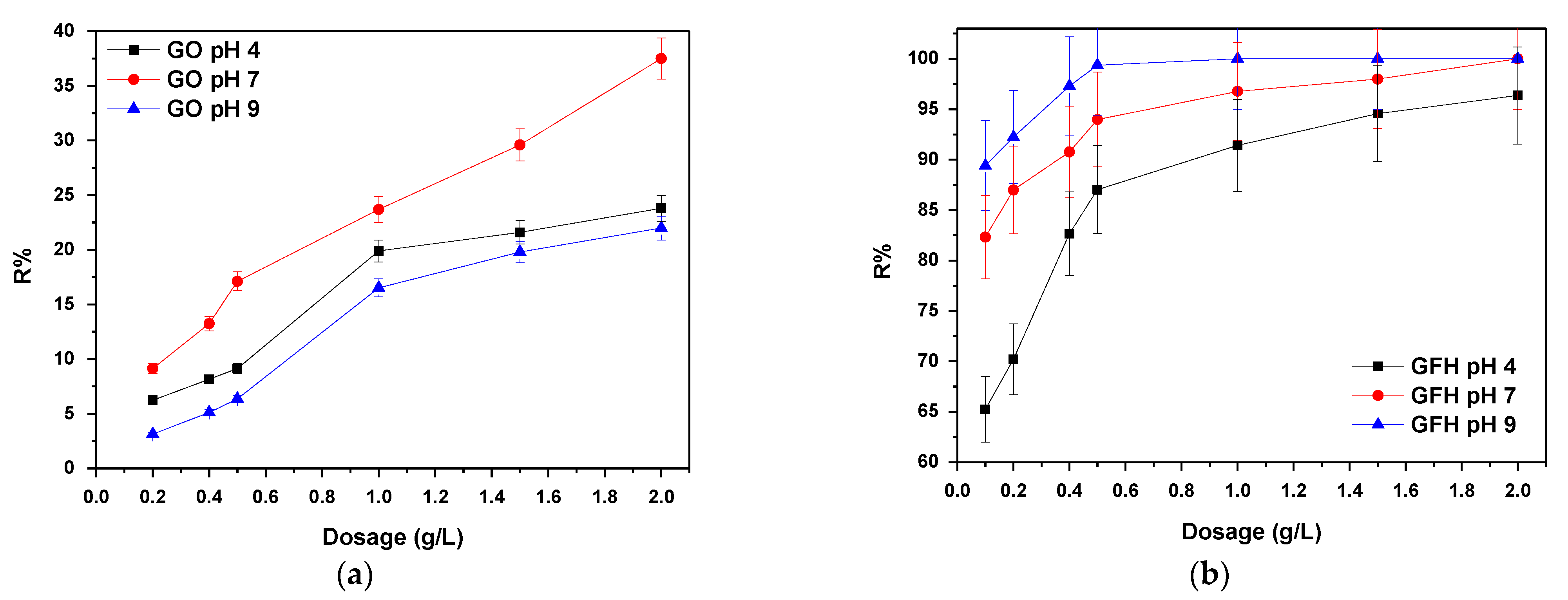
3.1.1. Effect of Adsorbent Dose
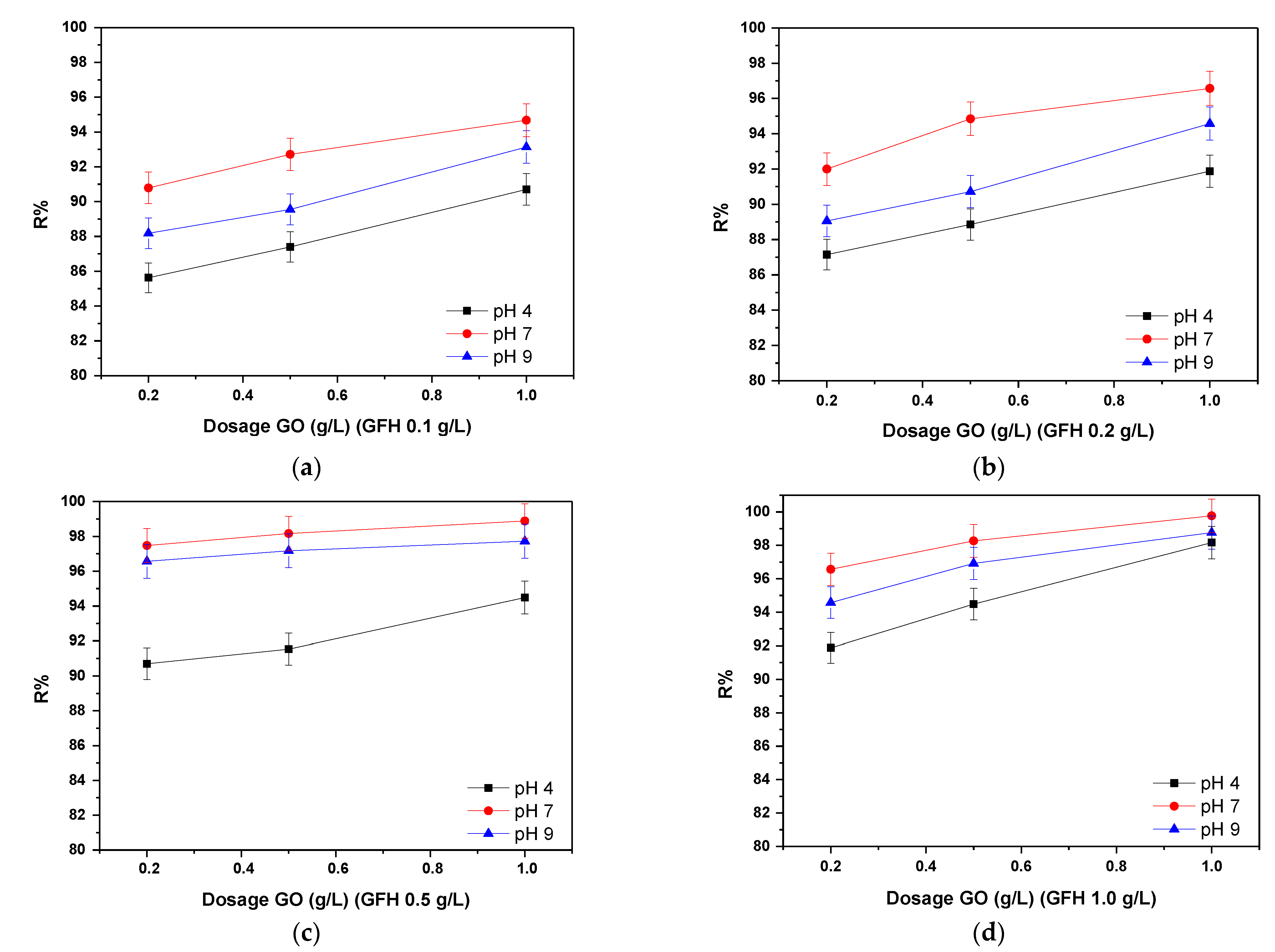
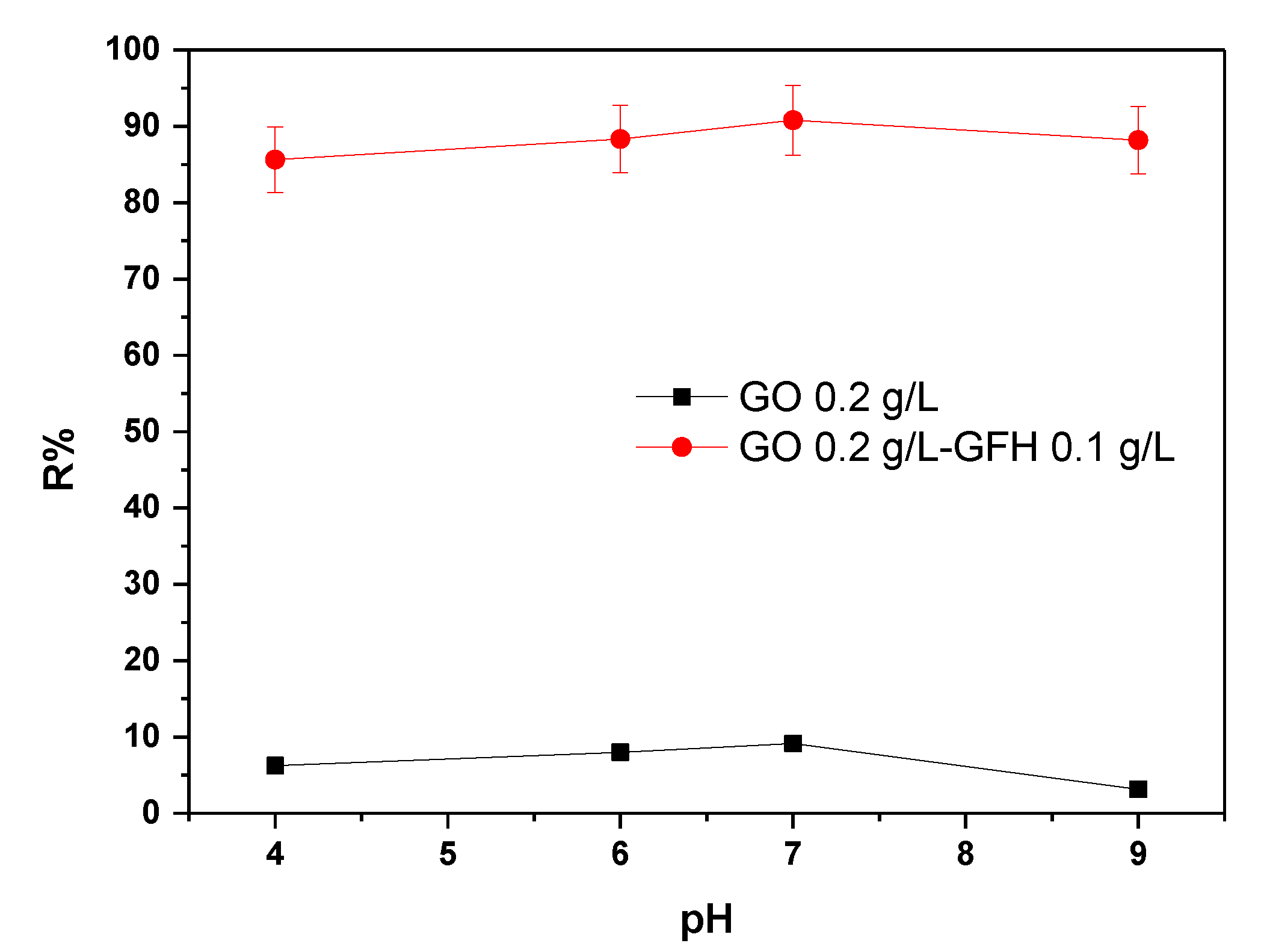
3.1.2. Effect of the Dose Ratio of GO/GFH to the Removal of As(III) from Waters
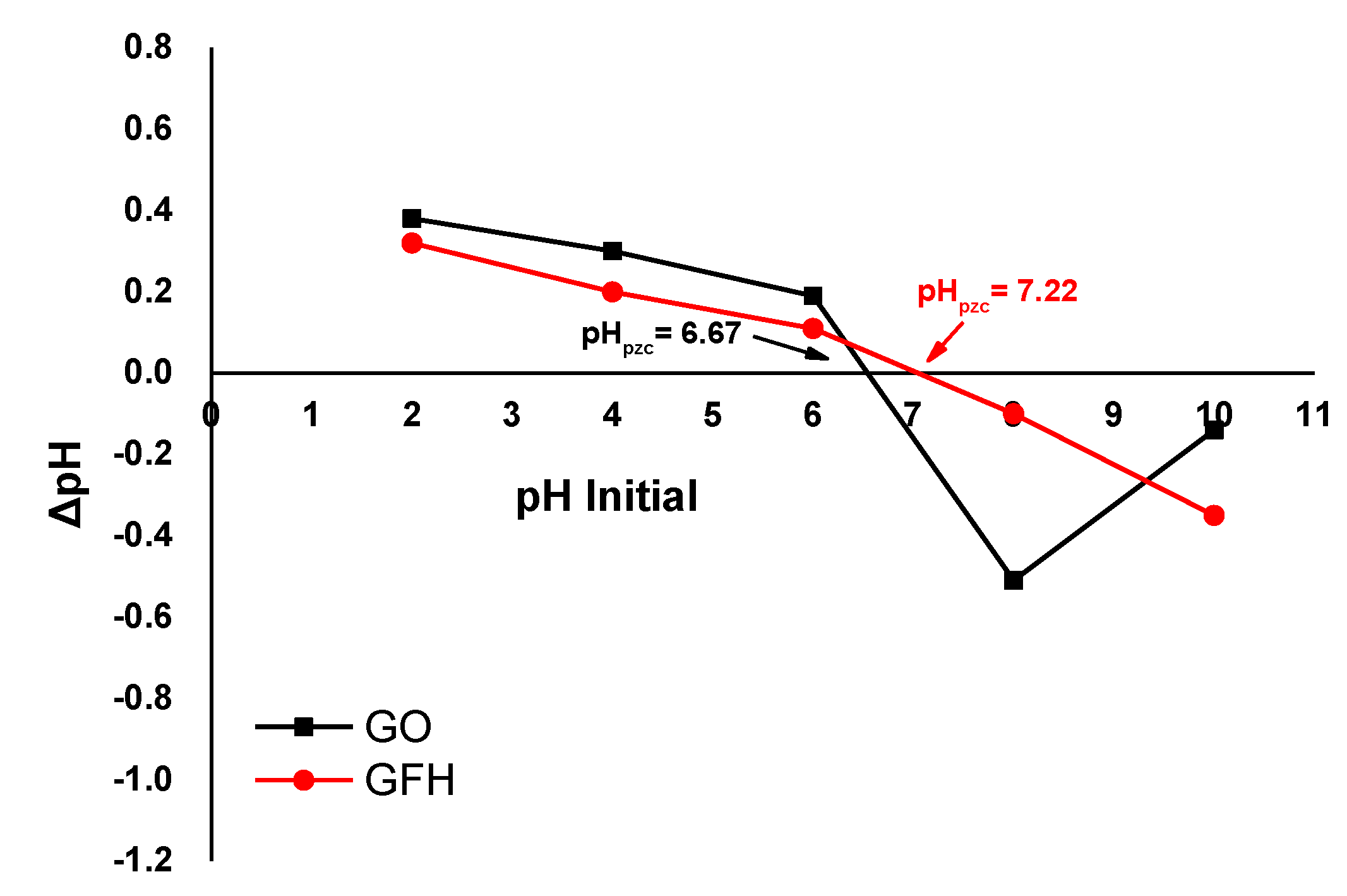
3.1.3. Effect of Initial pH Solution
3.2. Adsorption Isotherms
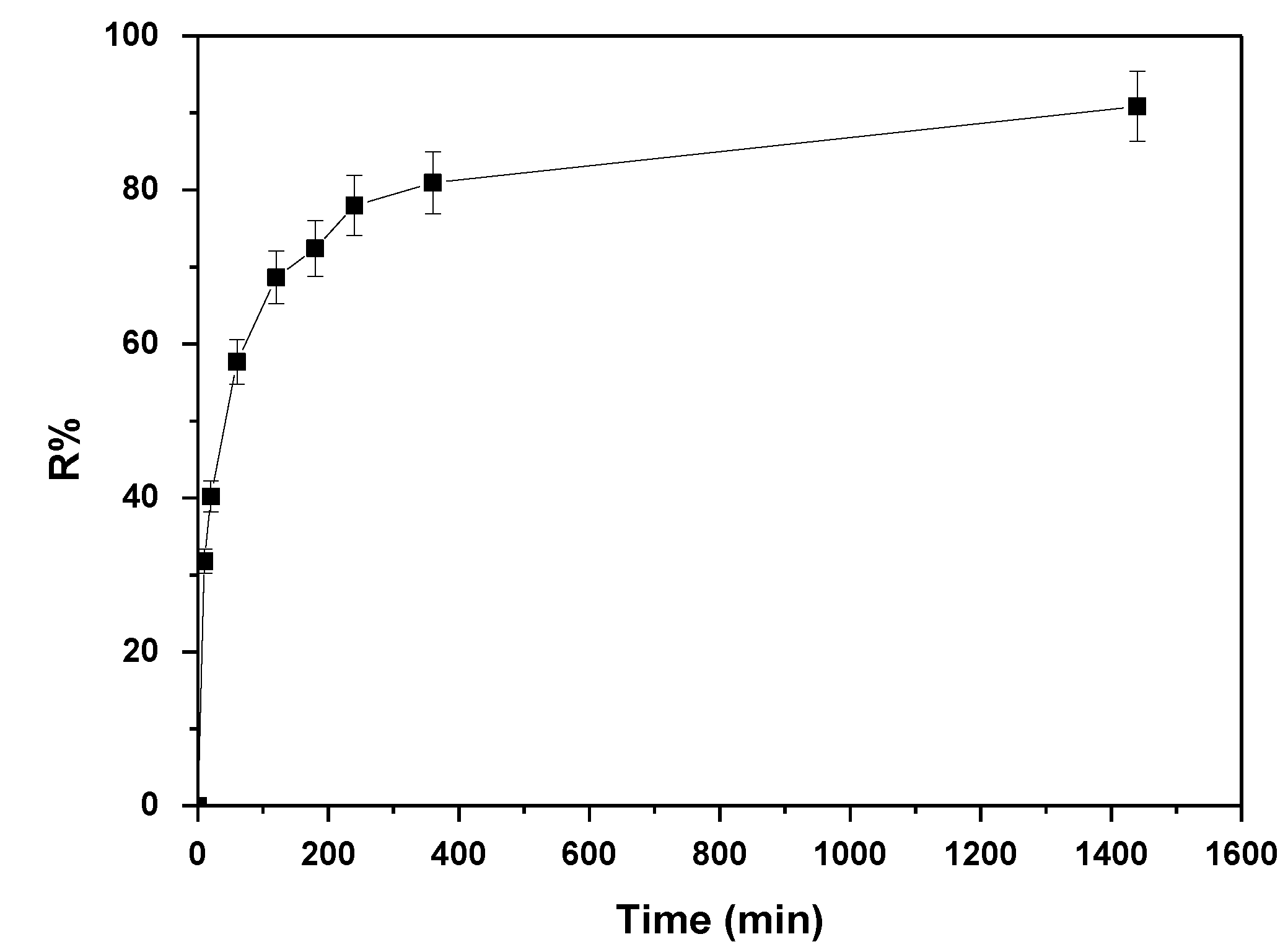
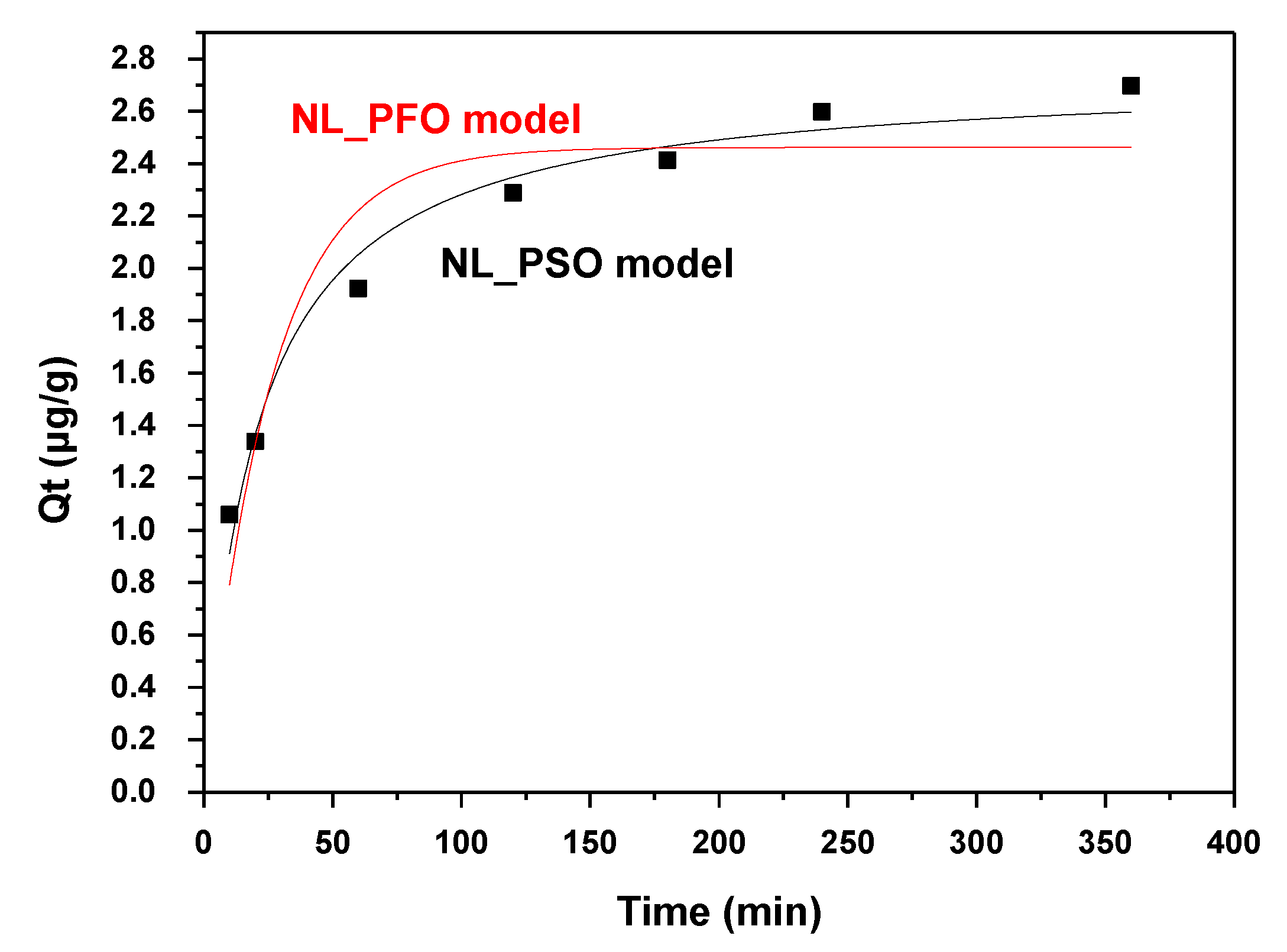
3.3. Effect of Contact Time and Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsoyiannis, I.A.; Zouboulis, A.I. Comparative evaluation of conventional and alternative methods for the removal of arsenic from contaminated groundwaters. Rev. Environ. Health 2006, 21, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Smith, A.H.; Steinmaus, C.M. Health effects of arsenic and chromium in drinking water: Recent human findings. Annu. Rev. Public Health 2009, 30, 107–122. [Google Scholar] [CrossRef]
- Chunhui, L.; Jin, T.; Puli, Z.; Bin, Z.; Duo, B.; Xuebin, L. Simultaneous removal of fluoride and arsenic in geothermal water in Tibet using modified yak dung biochar as an adsorbent. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Arsenic occurrence in Europe: Emphasis in Greece and description of the applied full-scale treatment plants. Desalin. Water Treat. 2015, 54, 2100–2107. [Google Scholar] [CrossRef]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated Carbons for Arsenic Removal from Natural Waters and Wastewaters: A Review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Tzollas, N.M.; Tolkou, A.K.; Mitrakas, M.; Ernst, M.; Zouboulis, A.I. Use of novel composite coagulants for arsenic removal from waters-experimental insight for the application of polyferric sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef]
- Cañas Kurz, E.E.; Hellriegel, U.; Figoli, A.; Gabriele, B.; Bundschuh, J.; Hoinkis, J. Small-scale membrane-based arsenic removal for decentralized applications–Developing a conceptual approach for future utilization. Water Res. 2021, 196, 116978. [Google Scholar] [CrossRef]
- Worou, C.N.; Chen, Z.L.; Bacharou, T. Arsenic removal from water by nanofiltration membrane: Potentials and limitations. Water Pract. Technol. 2021, 16, 291–319. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Arsenic(III) and Arsenic(V) Removal from Water Sources by Molecularly Imprinted Polymers (MIPs): A Mini Review of Recent Developments. Sustainability 2022, 14, 5222. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Ramanan, R.N. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367–390. [Google Scholar] [CrossRef]
- Damtie, M.M.; Choi, J.; Technology, B. Fluoride Removal and Nitrogen Recovery from Wastewater by Membrane Distillation Process. Ph.D. Thesis, University of Science and Technology, Daejeon, Republic of Korea, 2020. [Google Scholar]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2020, 28, 59063–59075. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic(V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef]
- Langmuir, D.A.O. Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn 2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Hug, S.J.; Leupin, O.X. Iron-catalyzed oxidation of arsenic (III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction RN. Environ. Sci. Technol. 2003, 37, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, H.; Chen, G.; Jefferson, W.A.; Qu, J. As(III) oxidation by active chlorine and subsequent removal of As(V) by Al 13 polymer coagulation using a novel dual function reagent. Environ. Sci. Technol. 2012, 46, 6776–6782. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent manganese feroxyhyte: A novel nanoadsorbent equally selective for As(III) and As(V) removal from drinking water. Environ. Sci. Technol. 2013, 47, 9699–9705. [Google Scholar] [CrossRef]
- Raptopoulou, C.; Kalaitzidou, K.; Tolkou, A.; Palasantza, P.A.; Mitrakas, M.; Zouboulis, A. Phosphate Removal from Effluent of Secondary Wastewater Treatment: Characterization of Recovered Precipitates and Potential Re-use as Fertilizer. Waste Biomass Valorization 2016, 7, 851–860. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance evaluation of small sized powdered ferric hydroxide as arsenic adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef]
- Szlachta, M.; Wójtowicz, P. Treatment of arsenic-rich waters using granular iron hydroxides. Desalin. Water Treat. 2016, 57, 26376–26381. [Google Scholar] [CrossRef]
- World Health Organization European Standards for Drinking-Water. Am. J. Med. Sci. 1970, 242, 56.
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P.; Knappe, D.R.U. Intraparticle diffusion and adsorption of arsenate onto granular ferric hydroxide (GFH). Water Res. 2004, 38, 4002–4012. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nanosci. Technol. A Collect. Rev. Nat. J. 2009, 11–19. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Acoustic cavitation induced synthesis of zirconium impregnated activated carbon for effective fluoride scavenging from water by adsorption. Ultrason. Sonochem. 2018, 45, 65–77. [Google Scholar] [CrossRef]
- Rudzinski, W.; Everett, D.H. Multilayer Adsorption on Heterogeneous Surfaces. In Adsorption of Gases on Heterogeneous Surfaces; Academic Press: Cambridge, MA, USA, 1992; pp. 351–419. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Graphene Oxide/Fe-Based Composite Pre-Polymerized Coagulants: Synthesis, Characterization, and Potential Application in Water Treatment. C 2020, 6, 44. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene oxide synthesis, properties and characterization techniques: A comprehensive review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Dos Santos, O.A.L.; Hazeem, L.; Backx, B.P.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and Graphene Oxide-Based Composites for Removal of Organic Pollutants: A Review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Driehaus, W.; Dupont, F. Arsenic removal—Solutions for a world wide health problem using iron based adsorbents. J. Eur. Hydrol. 2005, 36, 119–132. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1957, 208, 1937. [Google Scholar] [CrossRef]
- Debnath, S.; Maity, A.; Pillay, K. Impact of process parameters on removal of Congo red by graphene oxide from aqueous solution. J. Environ. Chem. Eng. 2014, 2, 260–272. [Google Scholar] [CrossRef]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Tai, M.H.; Saha, B.; Streat, M. Determination of Point Zero Charge (PZC) of Homemade Charcoals of Shorea Robusta (Sakhuwa) and Pinus Roxburghii (Salla). Int. J. Eng. Res. Technol. 2020, 9, 153–155. [Google Scholar]
- Katsoyiannis, I.A.; Voegelin, A.; Zouboulis, A.I.; Hug, S.J. Enhanced As(III) oxidation and removal by combined use of zero valent iron and hydrogen peroxide in aerated waters at neutral pH values. J. Hazard. Mater. 2015, 297, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Nour, S.; Selbie, M.; Prevost, M.; Blumenschein, C.D.; Chen, H.; Amy, G.L. Optimization of Process Parameters for Arsenic Treatment with Granular Ferric Hydroxide. In Proceedings of the AWWA Annual Conference, Anaheim, CA, USA, 15–19 June 2003. [Google Scholar]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
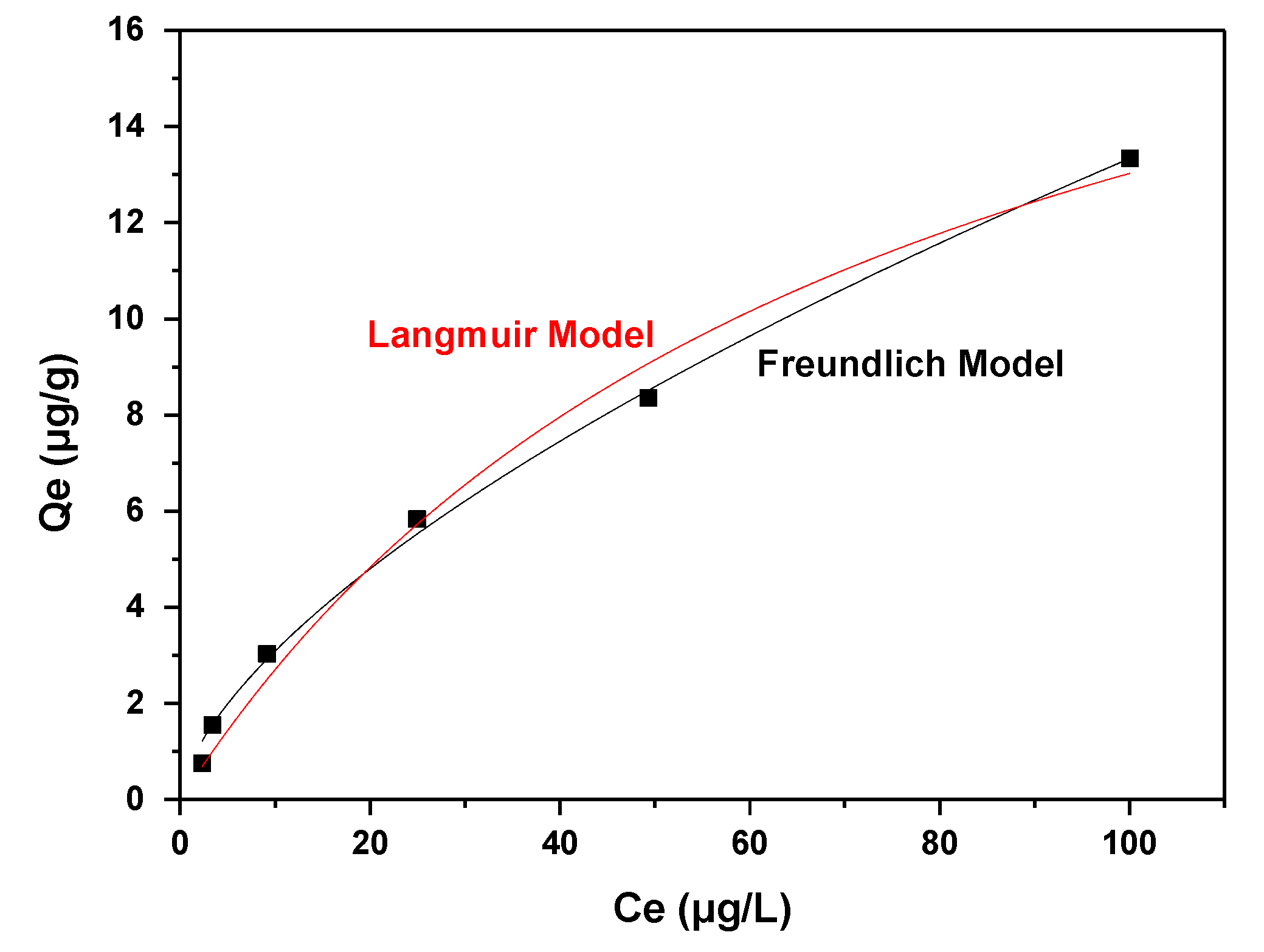
| Langmuir Isotherm Model | |||
| Qm (μg/g) | KL (L/g) | R2 | |
| 22.62 | 0.0136 | 0.9868 | |
| Freundlich Isotherm Model | |||
| 1/n | n | KF (μg/g) (L/μg)1/n | R2 |
| 0.6349 | 1.5752 | 0.7167 | 0.9962 |
| Pseudo-First-Order Model (PFO) | |||
| Qe.exp (μg/g) | K1 (L/μg∙min) | Qe.cal (μg/g) | R2 |
| 3.0283 | 0.0386 | 2.4621 | 0.8708 |
| Pseudo-Second-Order Model (PSO) | |||
| Qe.exp (μg/g) | K2 (L/μg∙min) | Qe.cal (μg/g) | R2 |
| 3.0283 | 0.0181 | 2.7421 | 0.9696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Rada, E.C.; Torretta, V.; Xanthopoulou, M.; Kyzas, G.Z.; Katsoyiannis, I.A. Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio. C 2023, 9, 10. https://doi.org/10.3390/c9010010
Tolkou AK, Rada EC, Torretta V, Xanthopoulou M, Kyzas GZ, Katsoyiannis IA. Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio. C. 2023; 9(1):10. https://doi.org/10.3390/c9010010
Chicago/Turabian StyleTolkou, Athanasia K., Elena Cristina Rada, Vincenzo Torretta, Maria Xanthopoulou, George Z. Kyzas, and Ioannis A. Katsoyiannis. 2023. "Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio" C 9, no. 1: 10. https://doi.org/10.3390/c9010010
APA StyleTolkou, A. K., Rada, E. C., Torretta, V., Xanthopoulou, M., Kyzas, G. Z., & Katsoyiannis, I. A. (2023). Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio. C, 9(1), 10. https://doi.org/10.3390/c9010010












