Novel Ultrahard Extended Hexagonal C10, C14 and C18 Allotropes with Mixed sp2/sp3 Hybridizations: Crystal Chemistry and Ab Initio Investigations
Abstract
1. Introduction
2. Brief Presentation of the Computational Framework
3. Crystal Chemistry Results
4. Charge Density
5. Mechanical Properties from Elastic Constants
| Space Group | a=b (Å) | c (Å) | ρ (g/cm3) | HV (GPa) | B0 (GPa) | |
|---|---|---|---|---|---|---|
| Polytype 4H: C8 | P63/mmc | 2.5221 | 8.2371 | 3.5164 | 97 | 443 |
| Hybrid C10 | P63/mmc | 2.4960 | 11.1775 | 3.3073 | 92 | 417 |
| Polytype 6H: C12 | P63/mmc | 2.5221 | 12.3557 | 3.5164 | 97 | 443 |
| Hybrid C14 | P63/mmc | 2.5000 | 15.2330 | 3.3867 | 94 | 427 |
| Polytype 8H: C16 | P63/mmc | 2.5221 | 16.47429 | 3.5164 | 97 | 443 |
| Hybrid C18 | P63/mmc | 2.5019 | 19.3964 | 3.4146 | 94 | 430 |
| Lonsdaleite | P63/mmc | 2.5221 † | 4.1186 † | 3.5164 | 97 | 443 |
| Diamond | Fd-3m | 3.56661 ‡ | 3.5169 | 98 | 445 § | |
| HV | B | GV | E ** | ν ** | KIc‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T * | LO † | MO ‡ | CN § | B0 * | BV | |||||
| GPa | MPa·m½ | |||||||||
| Polytype 4H: C8 | 97 | 90 | 106 | 102 | 443 | 445 | 557 | 1179 | 0.058 | 6.5 |
| Hybrid C10 | 92 | 79 | 82 | 74 | 417 | 408 | 440 | 971 | 0.103 | 5.4 |
| Polytype 6H: C12 | 97 | 90 | 94 | 85 | 443 | 445 | 502 | 1094 | 0.090 | 6.2 |
| Hybrid C14 | 94 | 72 | 88 | 80 | 427 | 417 | 467 | 1020 | 0.092 | 5.7 |
| Polytype 8H: C16 | 97 | 90 | 103 | 100 | 443 | 432 | 539 | 1142 | 0.059 | 6.3 |
| Hybrid C18 | 94 | 66 | 92 | 88 | 430 | 412 | 488 | 1050 | 0.075 | 5.7 |
| Lonsdaleite | 97 | 90 | 99 | 94 | 443 | 432 | 521 | 1115 | 0.070 | 6.2 |
| Diamond | 98 | 90 | 100 | 93 | 445 †† | 530 †† | 1138 | 0.074 | 6.4 | |
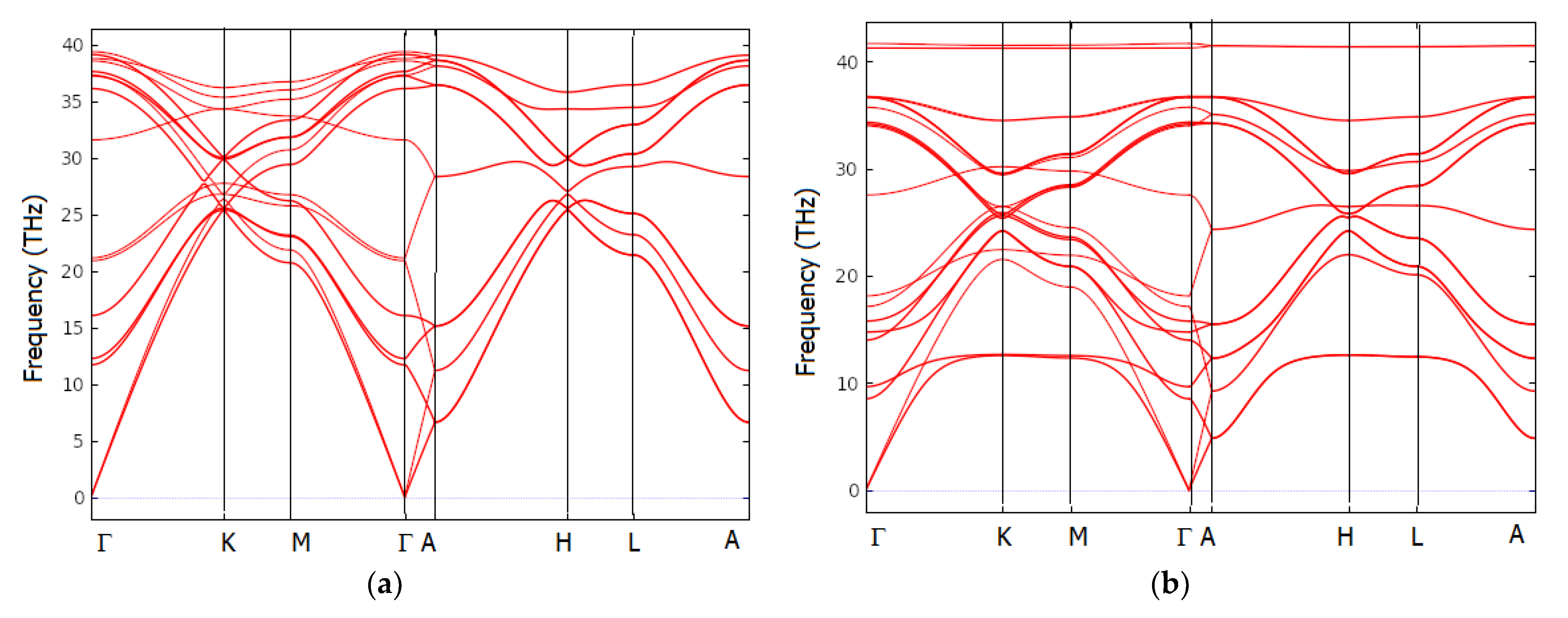
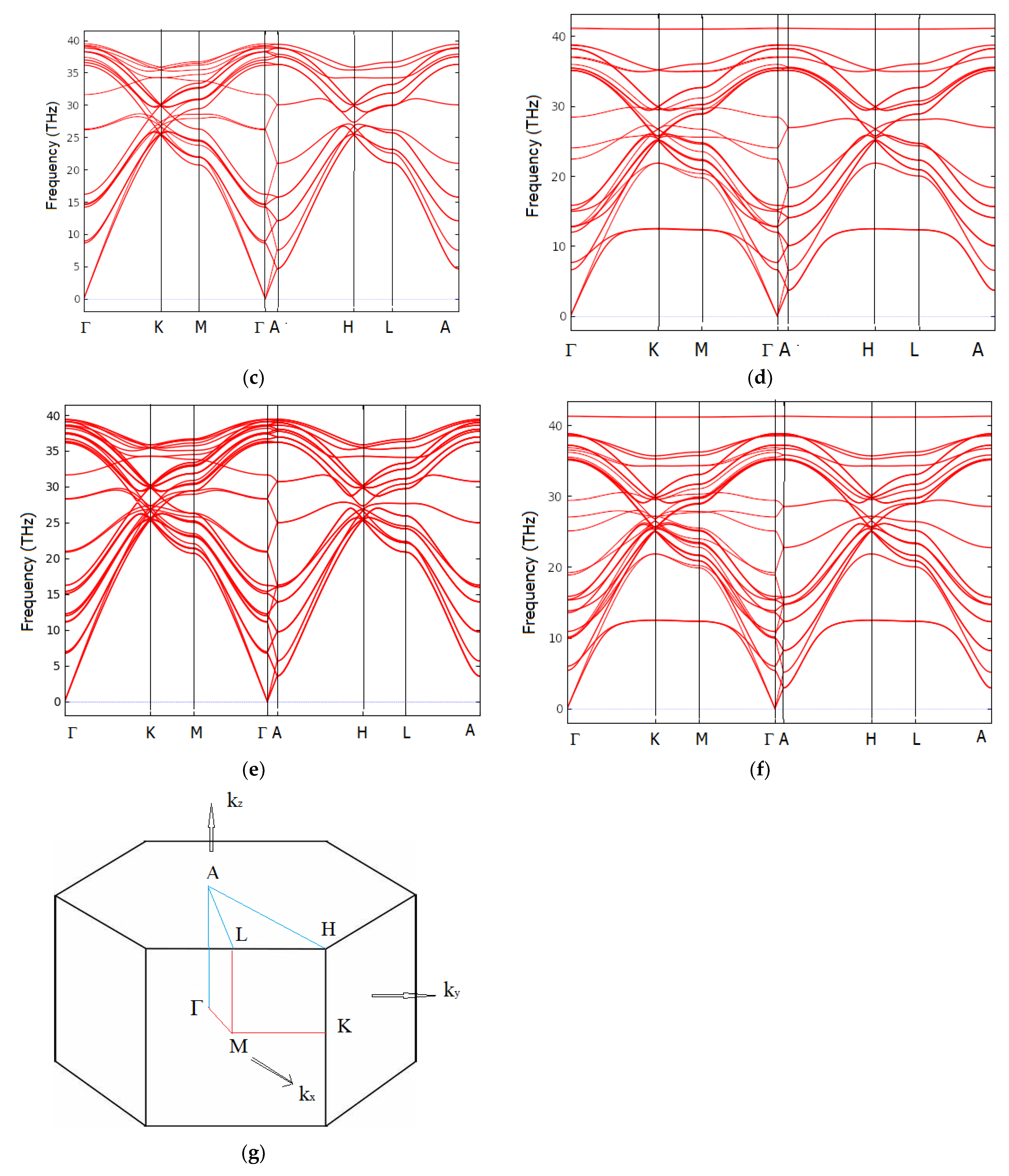
6. Dynamic Properties from the Phonons
7. Electronic Band Structures and Density of States
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, P.; Fornari, R.; Kamimura, H. (Eds.) Comprehensive Semiconductor Science and Technology; Reference work; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ownby, P.D.; Yang, X.; Liu, J. Calculated X-ray diffraction data for diamond polytypes. J. Am. Ceram. Soc. 1992, 75, 1876–1883. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Duan, X.F.; Shang, N.G.; Li, Q.; Wan, L.; Bello, I.; Lee, S.T. Epitaxial diamond polytypes on silicon. Nature 2001, 412, 404. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, J.; Bucknum, M.J.; Yao, P.; Li, T. First-principles studies of diamond polytypes. Diam. Relat. Mater. 2008, 17, 356–364. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Su, D.S.; Centi, G.; Perathoner, S. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials. Chem. Soc. Rev. 2018, 47, 8438–8473. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, N.; Jiang, X. Progress in electrochemistry of hybrid diamond/sp2-C nanostructures. Curr. Opin. Electrochem. 2022, 32, 100884. [Google Scholar] [CrossRef]
- Hu, M.; Huang, Q.; Zhao, Z.; Xu, B.; Yu, D.; He, J. Superhard and high-strength yne-diamond semimetals. Diam. Relat. Mater. 2014, 46, 15–20. [Google Scholar] [CrossRef]
- Matar, S.F. C5 as simplest ultrahard allotrope with mixed sp2/sp3 carbon hybridizations from first principles. arXiv 2022, arXiv:2210.04210. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. A 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Perdew, J.; Burke, K.; Ernzerhof, M. The Generalized Gradient Approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes, 2nd ed.; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Blöchl, P.E.; Jepsen, O.; Anderson, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special k-points for Brillouin zone integration. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Voigt, W. Über die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper. Annal. Phys. 1889, 274, 573–587. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Eyert, V. The Augmented Spherical Wave Method, Lect. Notes Phys. 849; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Brazhkin, V.V.; Solozhenko, V.L. Myths about new ultrahard phases: Why materials that are significantly superior to diamond in elastic moduli and hardness are impossible. J. Appl. Phys. 2019, 125, 130901. [Google Scholar] [CrossRef]
- Mukhanov, V.A.; Kurakevych, O.O.; Solozhenko, V.L. The interrelation between hardness and compressibility of substances and their structure and thermodynamic properties. J. Superhard Mater. 2008, 30, 368–378. [Google Scholar] [CrossRef]
- Mazhnik, E.; Oganov, A.R. A model of hardness and fracture toughness of solids. J. Appl. Phys. 2019, 126, 125109. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R. Evolutionary search for superhard materials: Methodology and applications to forms of carbon and TiO2. Phys. Rev. B 2011, 84, 092103. [Google Scholar] [CrossRef]
- Matar, S.F.; Solozhenko, V.L. Crystal chemistry and ab initio prediction of ultrahard rhombohedral B2N2 and BC2N. Solid State Sci. 2021, 118, 106667. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Le Godec, Y. A hunt for ultrahard materials. J. Appl. Phys. 2019, 126, 230401. [Google Scholar] [CrossRef]
- Bindzus, N.; Straasø, T.; Wahlberg, N.; Becker, J.; Bjerg, L.; Lock, N.; Dippel, A.-C.; Iversen, B.B. Experimental determination of core electron deformation in diamond. Acta Cryst. A 2014, 70, 39–48. [Google Scholar] [CrossRef]
- Krishnan, R.S. Raman spectrum of diamond. Nature 1945, 155, 171. [Google Scholar] [CrossRef]
- Matar, S.F. Crystal chemistry rationale and DFT investigations of novel hard tetragonal C6 built from tetrahedral C(sp3) lattice embedding allene-like sp2 linear tricarbon. ChemArxiv 2022. [Google Scholar] [CrossRef]
- Arita, R.; Ikeda, H. Is Fermi-surface nesting the origin of superconductivity in iron pnictides?: A fluctuation–exchange-approximation study. J. Phys. Soc. Japan 2009, 78, 113707. [Google Scholar] [CrossRef]
- Dugdale, S.B. Life on the edge: A beginner’s guide to the Fermi surface. Phys. Scr. 2016, 91, 053009. [Google Scholar] [CrossRef]
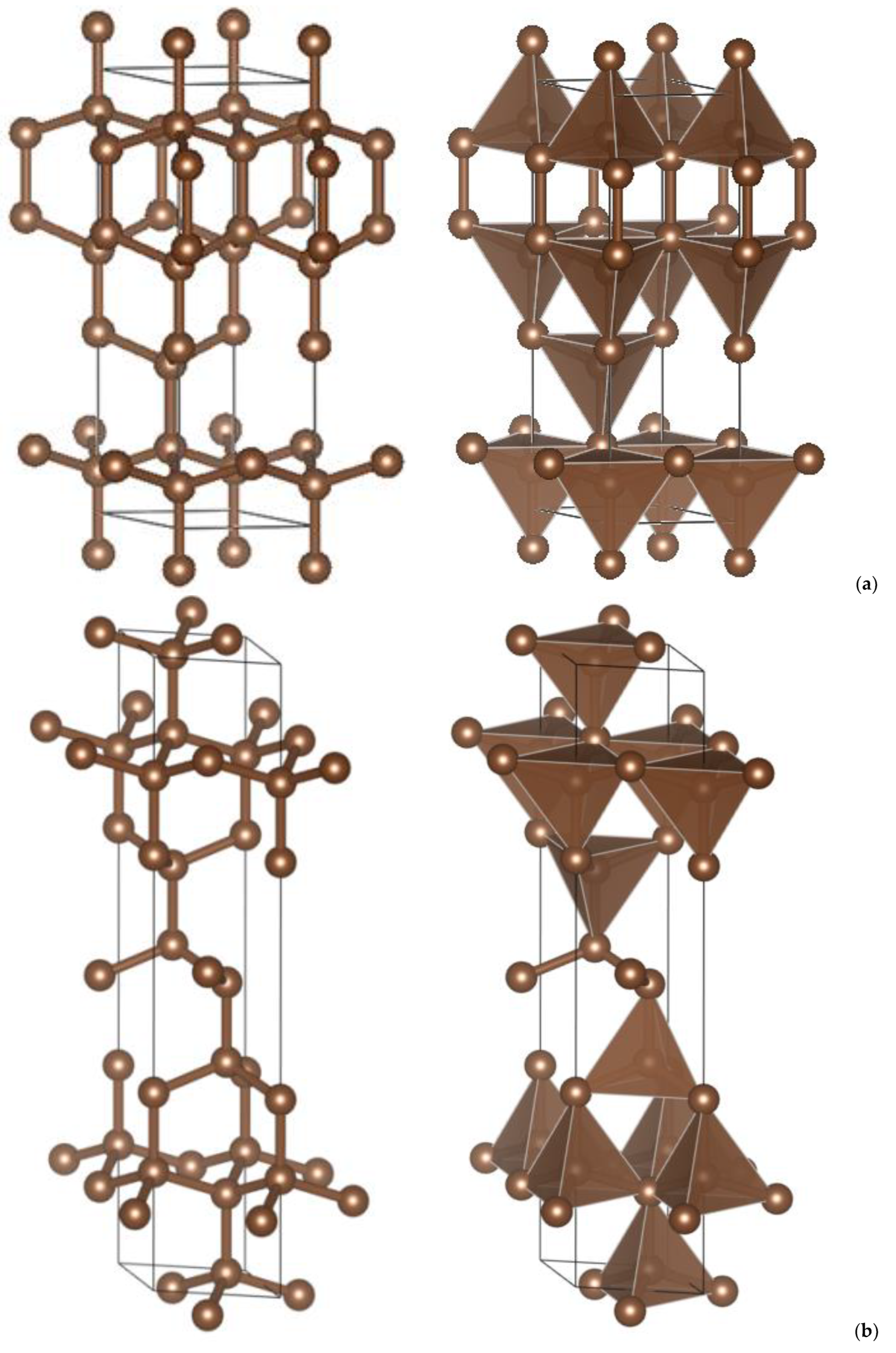
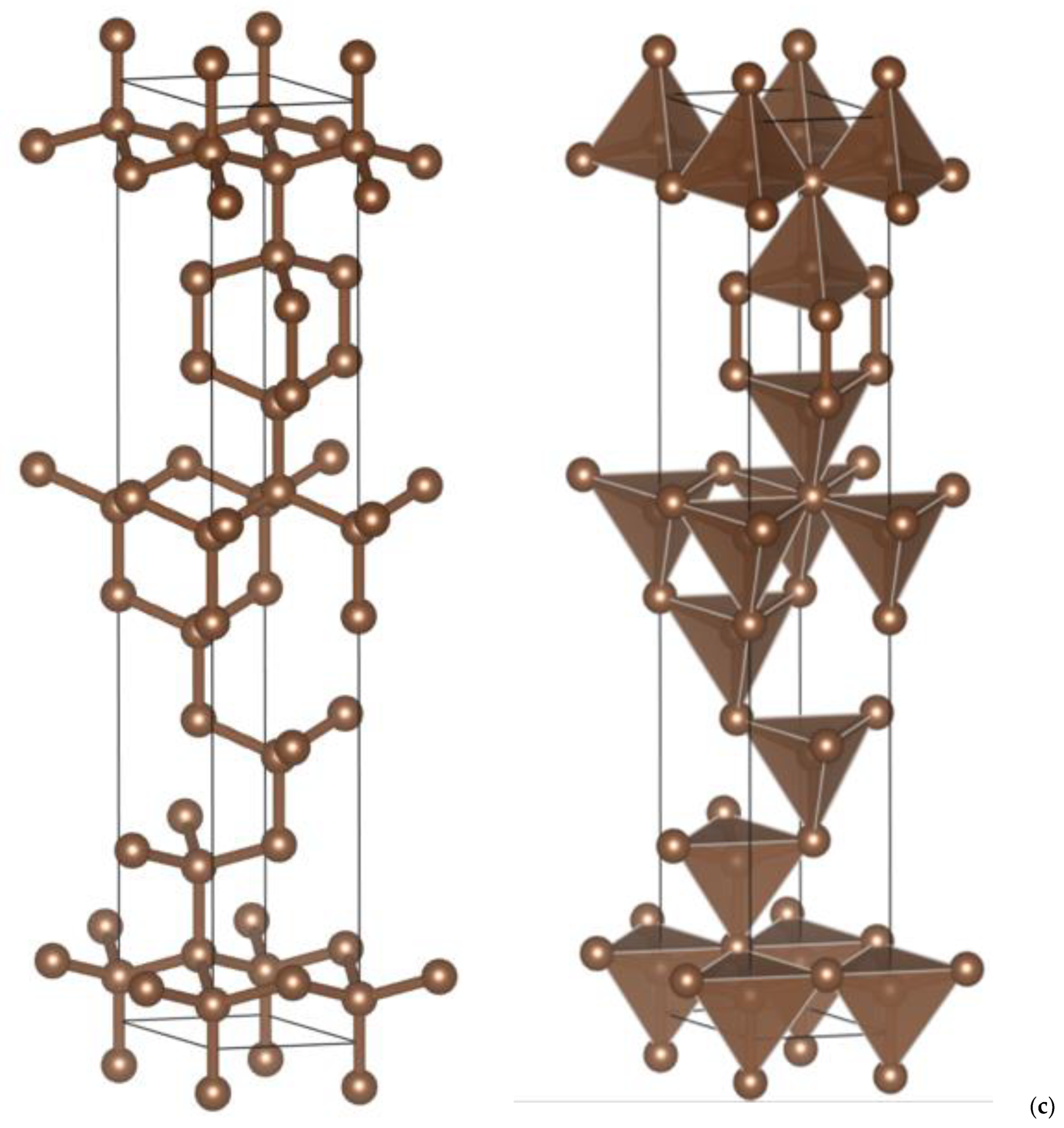
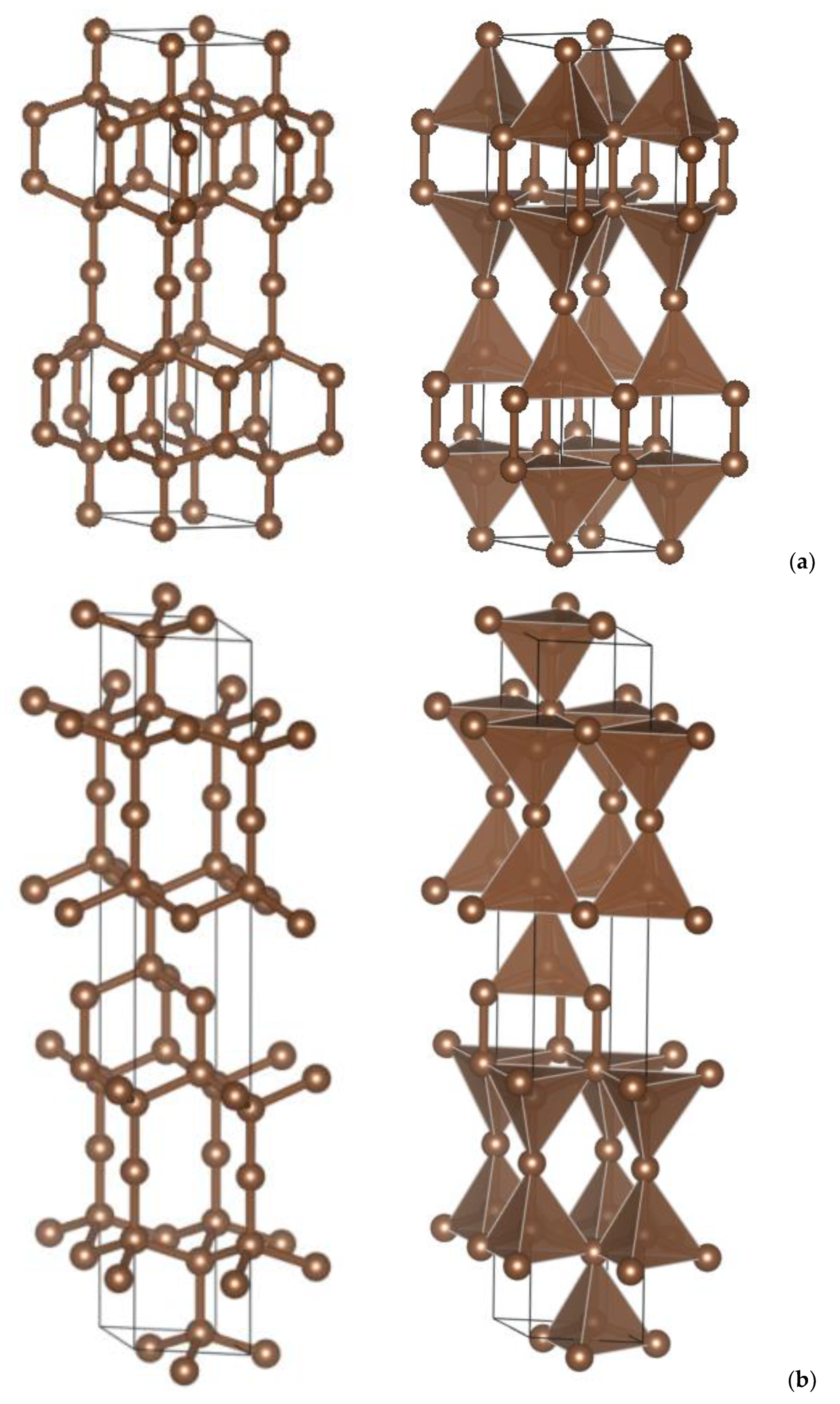
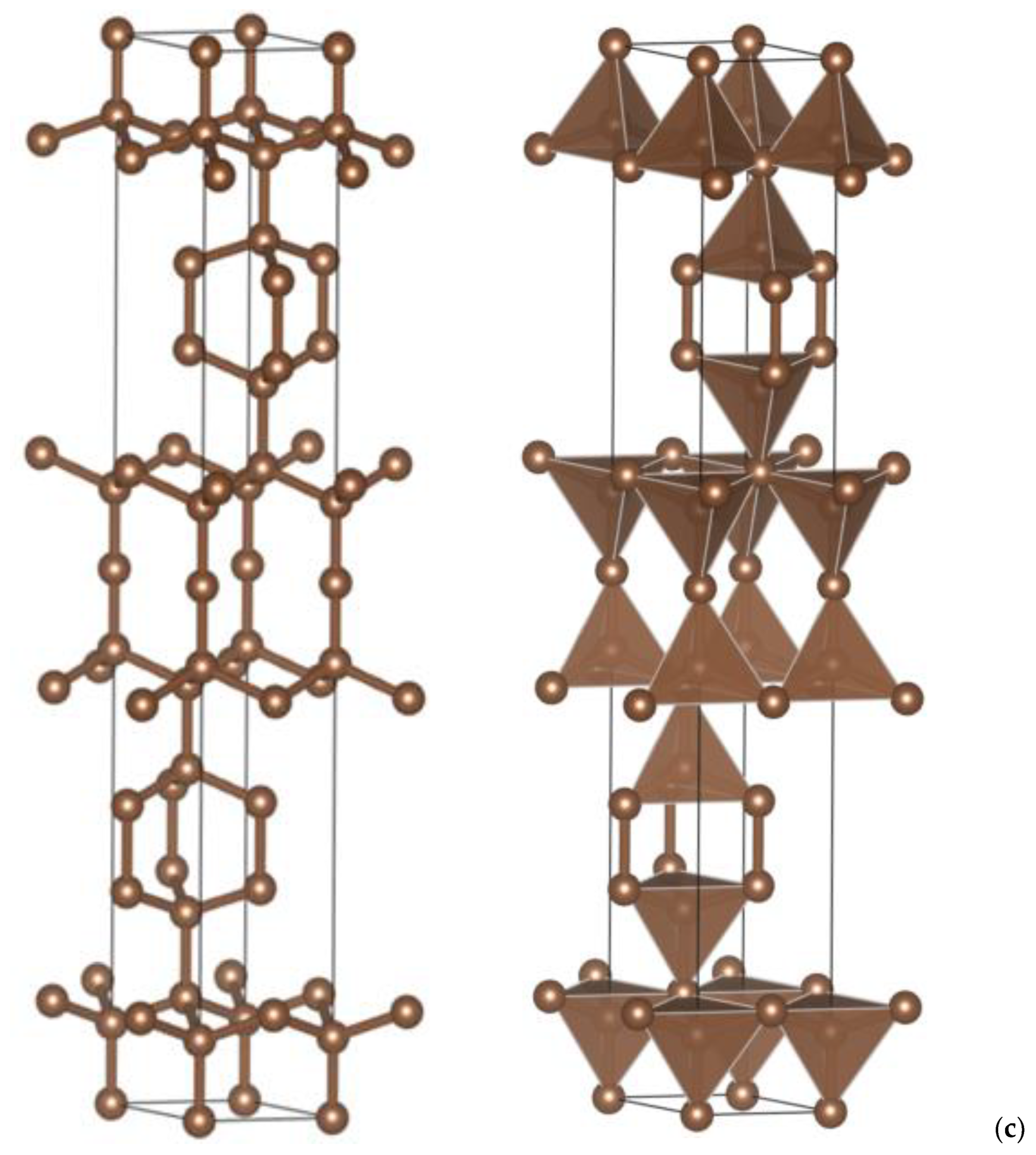
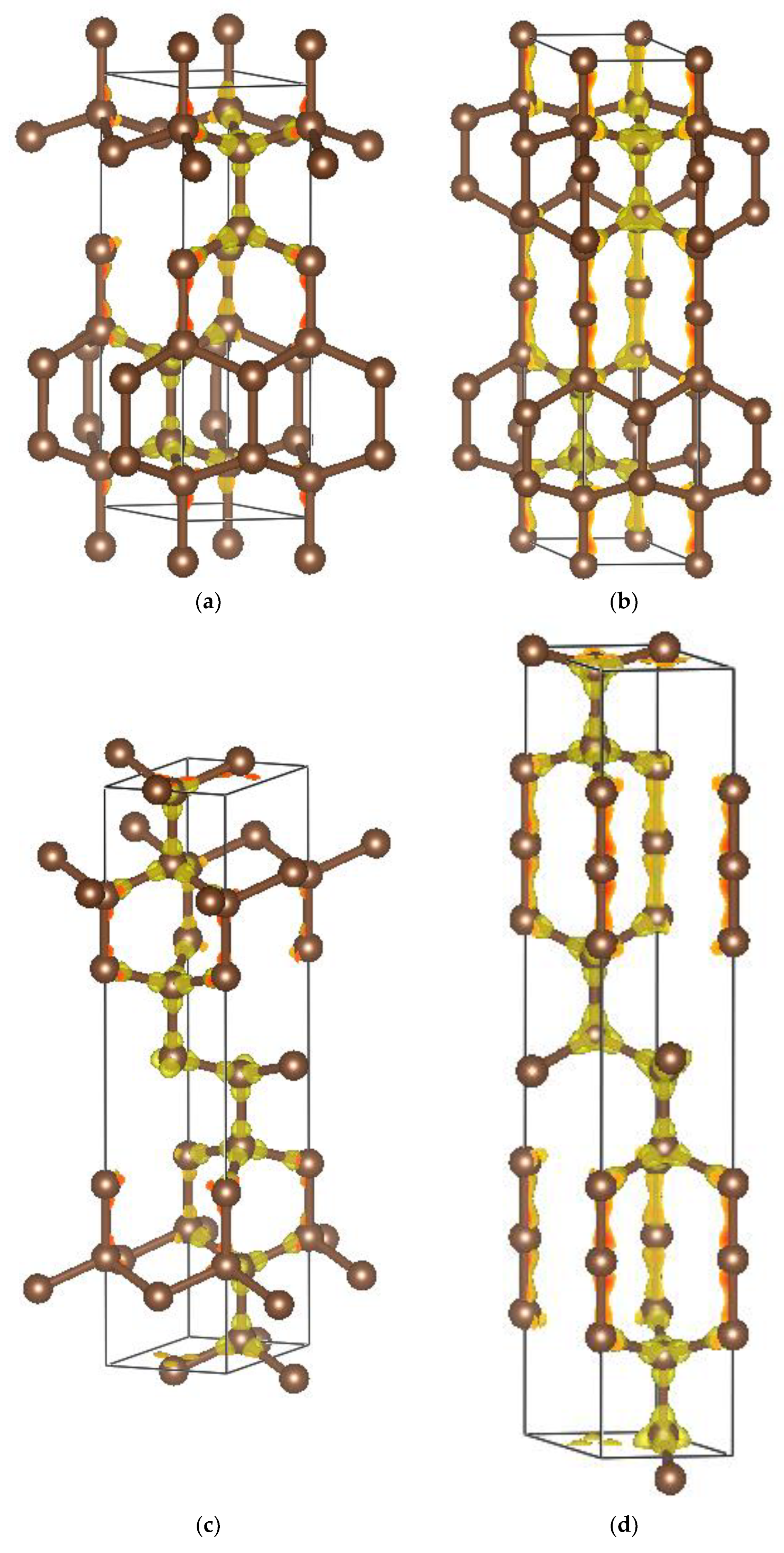
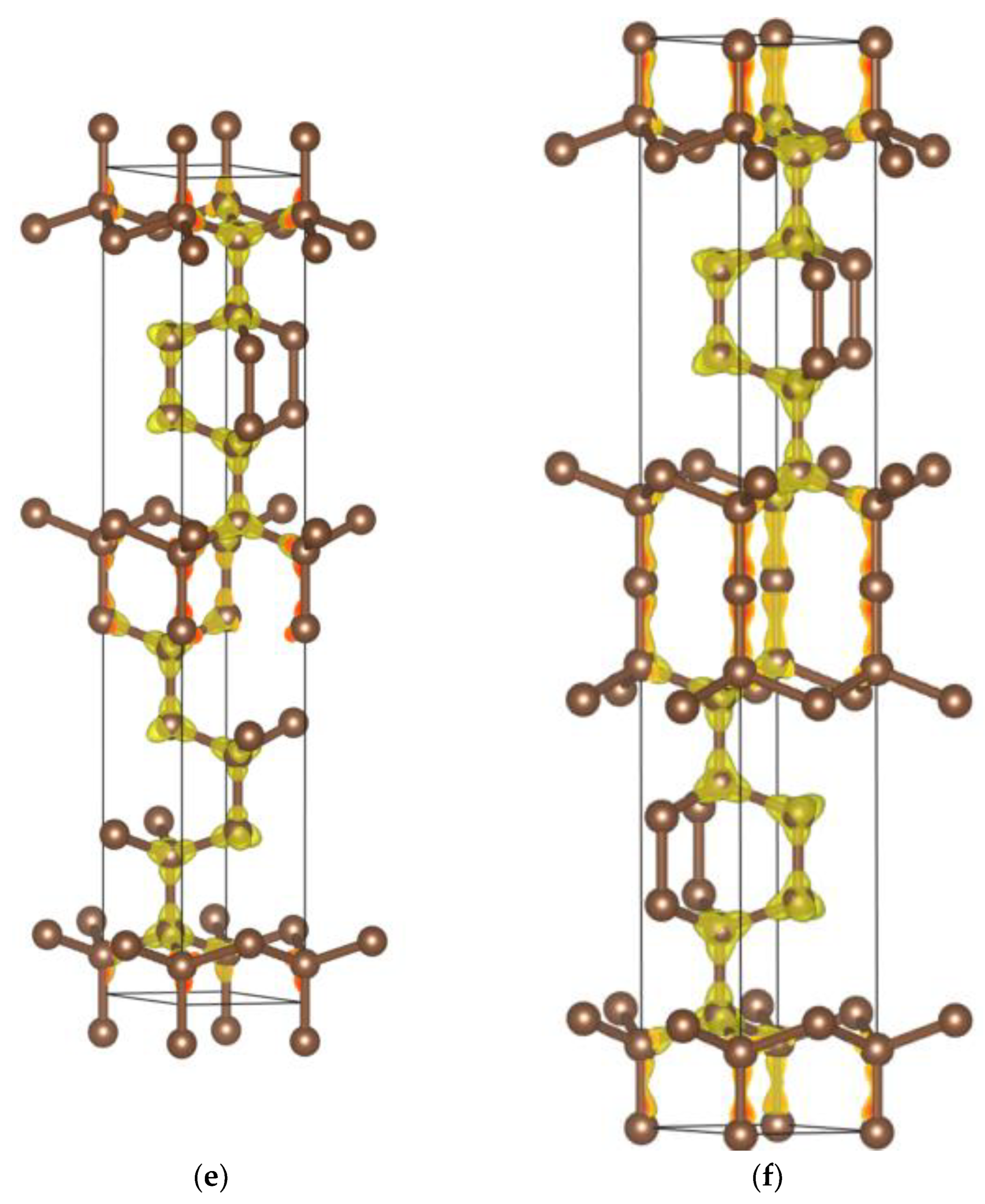


| (a) 4H, 6H and 8H Polytypes [2] (Presently Calculated Values Are in Parentheses) | |||
| P63/mmc N°194 | Polytype 4H: C8 | Polytype 6H: C12 | Polytype 8H: C16 |
| a, Å | 2.522 (2.511) | 2.522 (2.514) | 2.522 (2.516) |
| c, Å | 8.237 (8.279) | 12.356 (12.394) | 16.474 (16.505) |
| C1(4e) 0 0 z | 0.0938 (0.093) | 0.1875 (0.187) | 0.0469 (0.047) |
| C2(4f) 1/3 2/3 z | 0.1563 (0.156) | 0.5208 (0.521) | 0.0781 (0.0780) |
| C3(4f) 1/3 2/3 z | – | 0.6458 (0.645) | 0.1719 (0.1710) |
| C4(4f) 1/3 2/3 z | – | – | 0.797 (0.797) |
| Volume, Å3 | 45.376 (45.25) | 68.064 (67.87) | 90.75 (90.45) |
| dC1(tet)-C2(tet), Å | 1.544 | 1.544 | 1.544 |
| Etotal, eV | −72.68 | −109.06 | −145.43 |
| Ecoh/atom, eV | −2.49 | −2.49 | −2.49 |
| (b) Hybrid C8, C14 and C16 | |||
| P63/mmc No. 194 | C10 | C14 | C18 |
| a, Å | 2.496 | 2.500 | 2.502 |
| c, Å | 11.178 | 15.233 | 19.396 |
| C(2a) (trig) | 0 0 0 | – | 0 0 0 |
| C(2b) (trig) | – | 0 0 ¼ | – |
| C1(4e) 0 0 z | 0.3692 | 0.1541 | 0.0753 |
| C2(4f) 1/3 2/3 z | 0.1807 | 0.5171 | 0.1035 |
| C3(4f) 1/3 2/3 z | – | 0.6180 | 0.1828 |
| C4(4f) 1/3 2/3 z | – | – | 0.7903 |
| Volume, Å3 | 60.307 | 82.451 | 105.14 |
| dC1(tet)-C2(tet), Å | 1.545 | 1.544 | 1.536 |
| dC(tet)-C(trig), Å | 1.462 | 1.462 | 1.461 |
| Etotal, eV | −84.94 | −121.45 | −157.73 |
| Ecoh/atom, eV | −1.89 | −2.08 | −2.16 |
| C11 | C12 | C13 | C33 | C44 | BV | GV | |
|---|---|---|---|---|---|---|---|
| Polytype 4H: C8 | 1190 | 105 | 37 | 1271 | 542 | 445 | 557 |
| Hybrid C10 | 922 | 101 | 39 | 1469 | 410 | 408 | 440 |
| Polytype 6H: C12 | 1129 | 160 | 43 | 1256 | 485 | 445 | 502 |
| Hybrid C14 | 972 | 93 | 69 | 1343 | 439 | 417 | 467 |
| Polytype 8H: C16 | 1152 | 99 | 48 | 1211 | 526 | 432 | 539 |
| Hybrid C18 | 1012 | 90 | 45 | 1325 | 461 | 412 | 488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matar, S.F.; Eyert, V.; Solozhenko, V.L. Novel Ultrahard Extended Hexagonal C10, C14 and C18 Allotropes with Mixed sp2/sp3 Hybridizations: Crystal Chemistry and Ab Initio Investigations. C 2023, 9, 11. https://doi.org/10.3390/c9010011
Matar SF, Eyert V, Solozhenko VL. Novel Ultrahard Extended Hexagonal C10, C14 and C18 Allotropes with Mixed sp2/sp3 Hybridizations: Crystal Chemistry and Ab Initio Investigations. C. 2023; 9(1):11. https://doi.org/10.3390/c9010011
Chicago/Turabian StyleMatar, Samir F., Volker Eyert, and Vladimir L. Solozhenko. 2023. "Novel Ultrahard Extended Hexagonal C10, C14 and C18 Allotropes with Mixed sp2/sp3 Hybridizations: Crystal Chemistry and Ab Initio Investigations" C 9, no. 1: 11. https://doi.org/10.3390/c9010011
APA StyleMatar, S. F., Eyert, V., & Solozhenko, V. L. (2023). Novel Ultrahard Extended Hexagonal C10, C14 and C18 Allotropes with Mixed sp2/sp3 Hybridizations: Crystal Chemistry and Ab Initio Investigations. C, 9(1), 11. https://doi.org/10.3390/c9010011






