Conversion of Coal-Biomass into Diesel by Using Aspen Plus
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Specifications
- ○
- Proximate analysis results (PROXANAL in Aspen Plus);
- ○
- Ultimate analysis results (ULTANAL in Aspen Plus);
- ○
- Sulfur analysis results (SULFANAL in Aspen Plus).
2.2. Coal-Gasification Products
2.3. Syngas Ratio
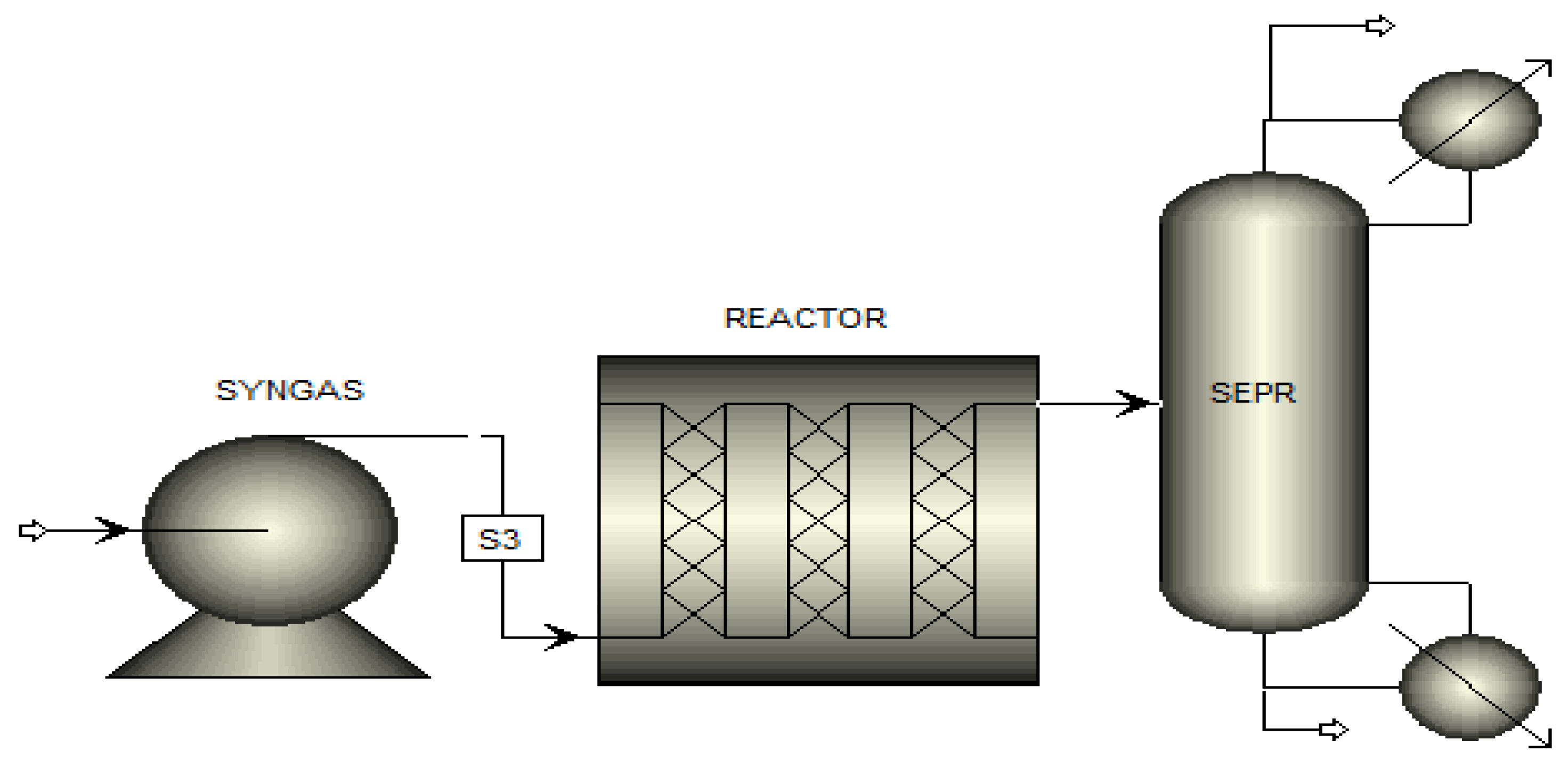
2.4. Modeling of FT Reactor
2.5. Heat Transfer Coefficient
2.6. Parametric Analysis
3. Results and Discussion
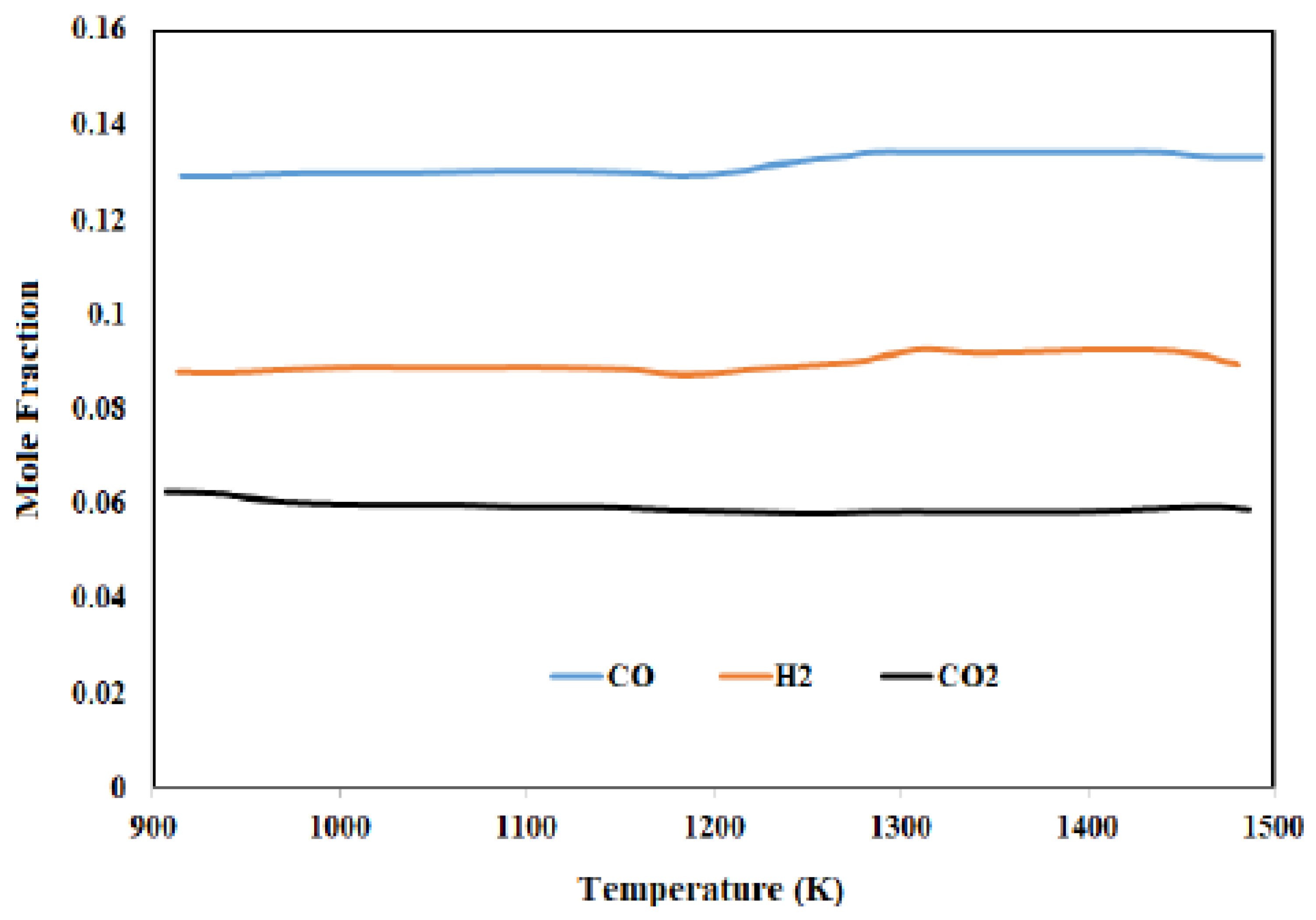
3.1. Syngas Component Profile “Lignite Coal” Thar Coal Pakistan
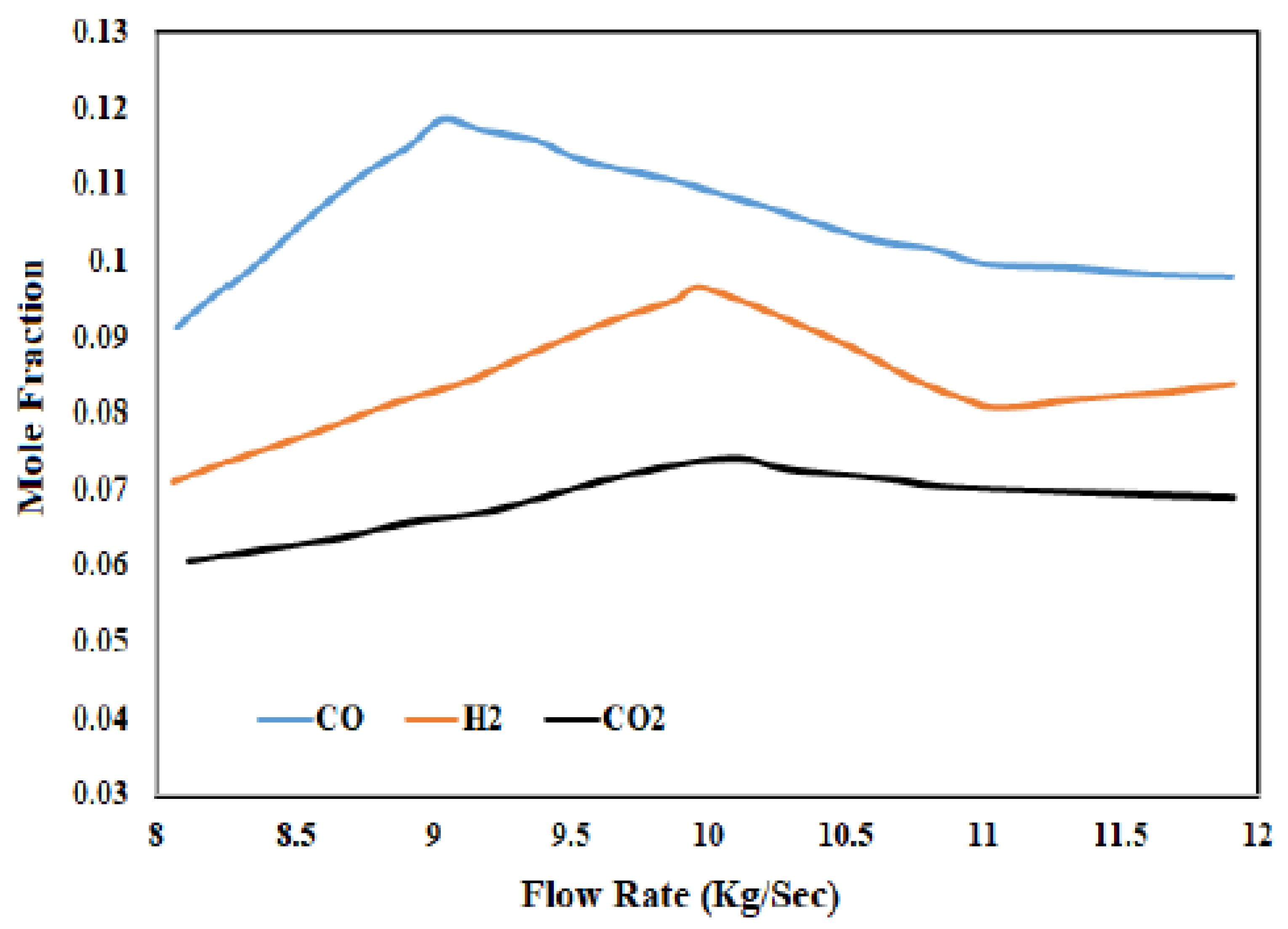
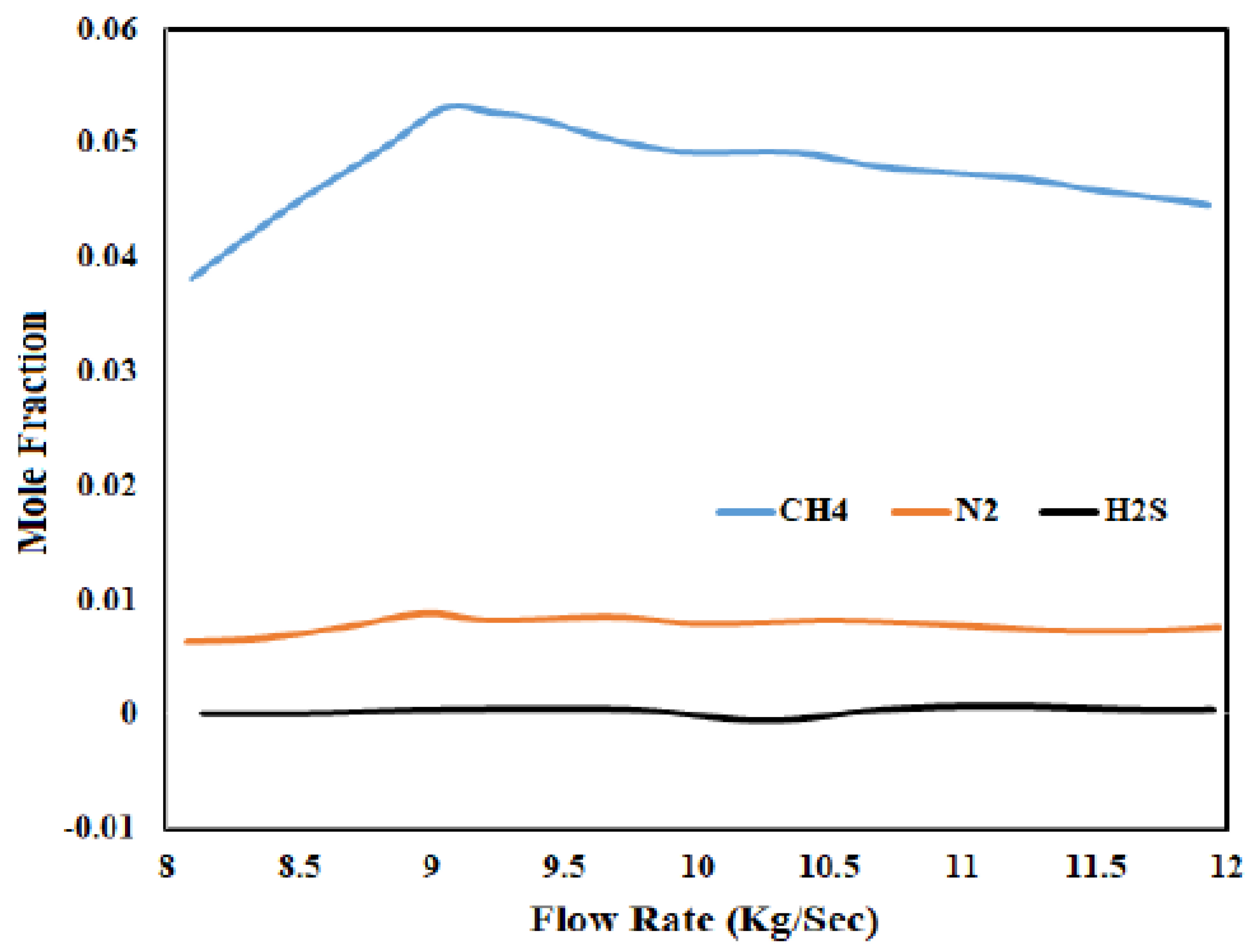
3.2. Importance of Steam Flow Rate
3.3. Optimization of Steam Flow with Thar Coal
3.4. Product Yield
3.5. Reduction of GHG Emissions in CTL FTS Process
3.6. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP. BP Statistical Review of World Energy globally consistent data on world energy markets and authoritative publications in the field of energy. In BP Energy Outlook; BP: London, UK, 2021; Volume 70, pp. 8–20. [Google Scholar]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Farooqi, Z.U.R.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2018, 19, 258–274. [Google Scholar] [CrossRef]
- Kanwal, S.; Mehran, M.T.; Hassan, M.; Anwar, M.; Naqvi, S.R.; Khoja, A.H. An integrated future approach for the energy security of Pakistan: Replacement of fossil fuels with syngas for better environment and socio-economic development. Renew. Sustain. Energy Rev. 2021, 156, 111978. [Google Scholar] [CrossRef]
- Mukherjee, S.; Rajabi, M.; Esterle, J. Relationship between coal composition, fracture abundance and initial reservoir permeability: A case study in the Walloon Coal Measures, Surat Basin, Australia. Int. J. Coal Geol. 2021, 240, 103726. [Google Scholar] [CrossRef]
- Jaffri, G.-R.; Zhang, J. Catalytic gasification of Pakistani Lakhra and Thar lignite chars in steam gasification. J. Fuel Chem. Technol. 2009, 37, 11–19. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy Globally Consistent Data on World Energy Markets; BP: London, UK, 2022. [Google Scholar]
- Shar, M.A.; Mahesar, A.A. Natural gas potential of Pakistan an important parameter in mitigating greenhouse gas emissions. Pak. J. Anal. Environ. Chem. 2020, 21, 209–218. [Google Scholar] [CrossRef]
- Nel, W.P.; Cooper, C.J. Implications of fossil fuel constraints on economic growth and global warming. Energy Policy 2009, 37, 166–180. [Google Scholar] [CrossRef]
- Lin, S.; Sun, W.; Guo, L.; Cheng, P.; Sun, Y.; Zhang, H. Development of a reduced mechanism of a three components surrogate fuel for the coal-to-liquid and diesel combustion simulation. Fuel 2021, 294, 120370. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Guo, L.; Yan, Y.; Li, J.; Lin, S.; Wang, Q.; Sun, Y. An experimental study of using coal to liquid (CTL) and diesel as pilot fuels for gasoline dual-fuel combustion. Fuel 2020, 289, 119962. [Google Scholar] [CrossRef]
- Ma, G.; Wang, X.; Xu, Y.; Wang, Q.; Wang, J.; Lin, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced Conversion of Syngas to Gasoline-Range Hydrocarbons over Carbon Encapsulated Bimetallic FeMn Nanoparticles. ACS Appl. Energy Mater. 2018, 1, 4304–4312. [Google Scholar] [CrossRef]
- Dry, M. Catalytic aspects of industrial Fischer-Tropsch synthesis. J. Mol. Catal. 1982, 17, 133–144. [Google Scholar] [CrossRef]
- Martínez-Vargas, D.X.; Sandoval-Rangel, L.; Campuzano-Calderon, O.; Flores, M.R.; Lozano, F.J.; Nigam, K.D.P.; Mendoza, A.; Montesinos-Castellanos, A. Recent Advances in Bifunctional Catalysts for the Fischer–Tropsch Process: One-Stage Production of Liquid Hydrocarbons from Syngas. Ind. Eng. Chem. Res. 2019, 58, 15872–15901. [Google Scholar] [CrossRef]
- Sirikulbodee, P.; Ratana, T.; Sornchamni, T.; Phongaksorn, M.; Tungkamani, S. Catalytic performance of Iron-based catalyst in Fischer–Tropsch synthesis using CO2 containing syngas. Energy Procedia 2017, 138, 998–1003. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, M.; Yu, Y. Exergy and economic analyses of indirect coal-to-liquid technology coupling carbon capture and storage. J. Clean. Prod. 2017, 174, 87–95. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, Z.; Deng, C.; Liu, Z.; Zhou, H.; Ren, J.; Zhou, T. Techno-economic analysis of coal-to-liquid processes with different gasifier alternatives. J. Clean. Prod. 2020, 253, 120006. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ibok, U.J.; Garg, R.; Garg, R.; Iqbal, A.; Amin, M.; Mustafa, F.; Egilmez, M.; Galal, A.M. A Brief Review on Fruit and Vegetable Extracts as Corrosion Inhibitors in Acidic Environments. Molecules 2022, 27, 2991. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Shah, H.H.; Iqbal, A.; Farooqi, Z.U.R.; Krawczuk, M.; Zia, A. Conversion of Waste Biomass into Activated Carbon and Evaluation of Environmental Consequences Using Life Cycle Assessment. Appl. Sci. 2022, 12, 5741. [Google Scholar] [CrossRef]
- Amin, M.; Chung, E.; Shah, H.H. Effect of different activation agents for activated carbon preparation through characterization and life cycle assessment. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Ministry of Climate Change. Available online: https://mocc.gov.pk/ProjectDetail/M2QzOWJmMjUtZTU3MC00NmFkLWE4YmMtZDFhMmRlOGU2NGRh (accessed on 4 August 2022).
- Mehdi, S.N.; Khan, Z.M.; Farid, H.U.; Hussain, S. Investigating Compatible Drying Technique for Safe Utilization of Thar Coal, Pakistan. Int. J. Coal Prep. Util. 2021, 42, 3303–3324. [Google Scholar] [CrossRef]
- Fletcher, T.H.; Kerstein, A.R.; Pugmire, R.J.; Solum, M.S.; Grant, D.M. Chemical percolation model for devolatilization. 3. Direct use of carbon-13 NMR data to predict effects of coal type. Energy Fuels 1992, 6, 414–431. [Google Scholar] [CrossRef]
- Suuberg, E.M.; Peters, W.A.; Howard, J.B. Product Composition and Kinetics of Lignite Pyrolysis. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 37–46. [Google Scholar] [CrossRef]
- Kulkarni, M.; Ganguli, R. Moving Bed Gasification of Low Rank Alaska Coal. J. Combust. 2012, 2012, 918754. [Google Scholar] [CrossRef]
- Claeys, M.; van Steen, E. Basic Studies. Stud. Surf. Sci. Catal. 2004, 152, 601–680. [Google Scholar] [CrossRef]
- Amin, M.; Munir, S.; Iqbal, N.; Wabaidur, S.M.; Iqbal, A. The Conversion of Waste Biomass into Carbon-Supported Iron Catalyst for Syngas to Clean Liquid Fuel Production. Catalysts 2022, 12, 1234. [Google Scholar] [CrossRef]
- Pöhlmann, F.; Jess, A. Influence of Syngas Composition on the Kinetics of Fischer-Tropsch Synthesis of using Cobalt as Catalyst. Energy Technol. 2015, 4, 55–64. [Google Scholar] [CrossRef]
- Mahmood, S.; Iqbal, A.; Din, R.U.; Wadood, A.; Mateen, A.; Amin, M.; Yahia, I.S.; Zahran, H.Y. Influence of Homogenizing Methodology on Mechanical and Tribological Performance of Powder Metallurgy Processed Titanium Composites Reinforced by Graphene Nanoplatelets. Molecules 2022, 27, 2666. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Pareek, V.K.; O Tade, M. Modern Trends in CFD Simulations: Application to GTL Technology. Chem. Prod. Process Model. 2006, 1. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Visconti, C.G.; Groppi, G.; Lietti, L.; Tronconi, E. Intensifying heat transfer in Fischer-Tropsch tubular reactors through the adoption of conductive packed foams. Chem. Eng. J. 2018, 349, 829–837. [Google Scholar] [CrossRef]
- Sarwar, A.; Khan, M.N.; Azhar, K.F. Coal Chemistry and Morphology of Thar Reserves, Pakistan. J. Miner. Mater. Charact. Eng. 2012, 11, 817–824. [Google Scholar] [CrossRef]
- Makhura, E.; Rakereng, J.; Rapoo, O.; Danha, G. Effect of the operation parameters on the Fischer Tropsch synthesis process using different reactors. Procedia Manuf. 2019, 35, 349–355. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H. Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment. C 2022, 8, 57. [Google Scholar] [CrossRef]
- Jiang, Y.; Bhattacharyya, D. Plant-wide modeling of an indirect coal–biomass to liquids (CBTL) plant with CO2 capture and storage (CCS). Int. J. Greenh. Gas Control 2014, 31, 1–15. [Google Scholar] [CrossRef]
- Huffman, G.P. Incorporation of catalytic dehydrogenation into Fischer–Tropsch synthesis of liquid fuels from coal to minimize carbon dioxide emissions. Fuel 2011, 90, 2671–2676. [Google Scholar] [CrossRef]
- Nawaz, Z.; Ramzan, N.; Nawaz, S.; Naveed, S.; Khan, M.B. Green Processing of Coal to Liquid Fuels: Pakistan’s Perspective. Pak. Acad. Sci. 2012, 49, 165. [Google Scholar]
- Feng, Y.; Yang, B.; Hou, Y.; Duan, T.-H.; Yang, L.; Wang, Y. Comparative environmental benefits of power generation from underground and surface coal gasification with carbon capture and storage. J. Clean. Prod. 2021, 310, 127383. [Google Scholar] [CrossRef]
- Bińczak, G.; Pohorecki, R.; Moniuk, W.; Możeński, C. Amine activators of CO2 absorption in industrial conditions. Chem. Process Eng. 2019, 40, 157–165. [Google Scholar]
- Konopacka-Łyskawa, D.; Czaplicka, N.; Szefer, A. CaO-based high temperature CO2 sorbents–Literature review. Chem. Process Eng.-Inżynieria Chem. I Proces 2021, 42, 411–438. [Google Scholar]
- Raza, M.A.; Khatri, K.L.; Memon, M.A.; Rafique, K.; Haque, M.I.U.; Mirjat, N.H. Exploitation of Thar coal field for power generation in Pakistan: A way forward to sustainable energy future. Energy Explor. Exploit. 2022, 40, 1173–1196. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Ahmad, I.; Ditta, A.; Ilic, P.; Amin, M.; Naveed, A.B.; Gulzar, A. Types, sources, socioeconomic impacts, and control strategies of environmental noise: A review. Environ. Sci. Pollut. Res. 2022, 29, 81087–81111. [Google Scholar] [CrossRef]
- Lemly, A.D. Environmental hazard assessment of coal ash disposal at the proposed Rampal power plant. Hum. Ecol. Risk Assess. Int. J. 2017, 24, 627–641. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Elgowainy, A.A.; Bafana, A.; Wang, M. Performance and cost analysis of liquid fuel production from H2 and CO2 based on the Fischer-Tropsch process. J. CO2 Util. 2021, 46, 101459. [Google Scholar] [CrossRef]
- UNFCCC. Paksitan Updated NDC 2021; UNFCCC: Bonn, Germany, 2021. [Google Scholar]

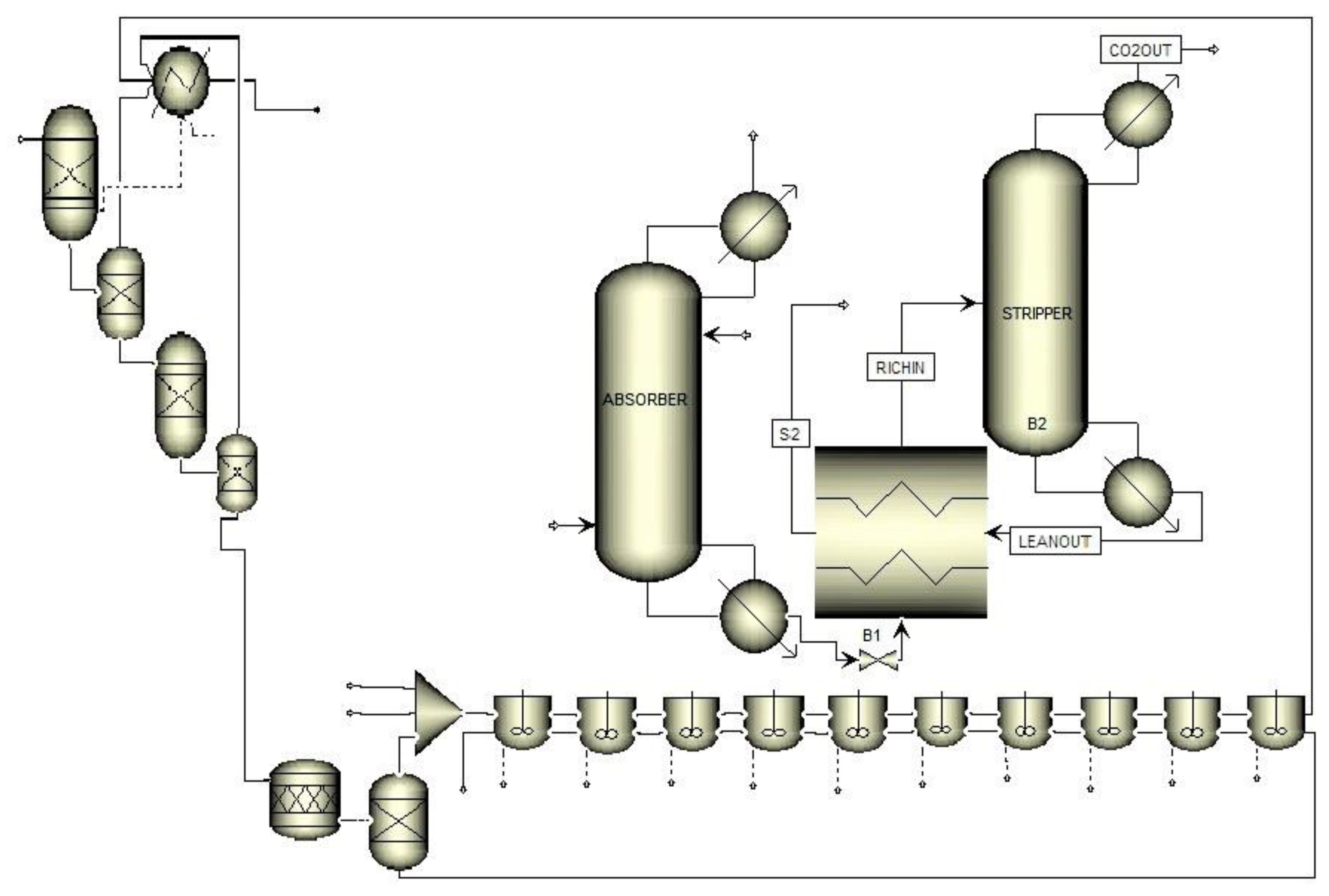
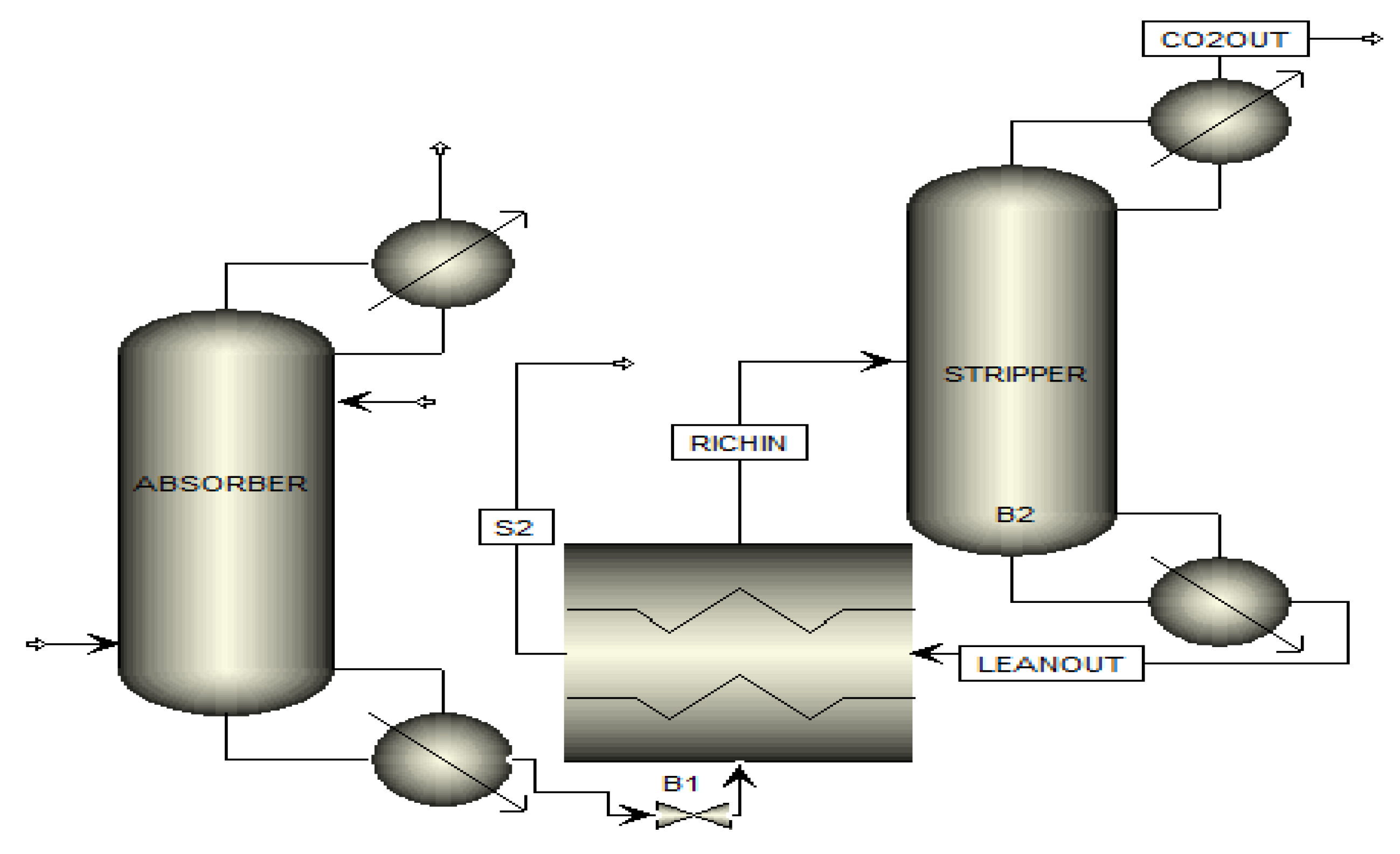
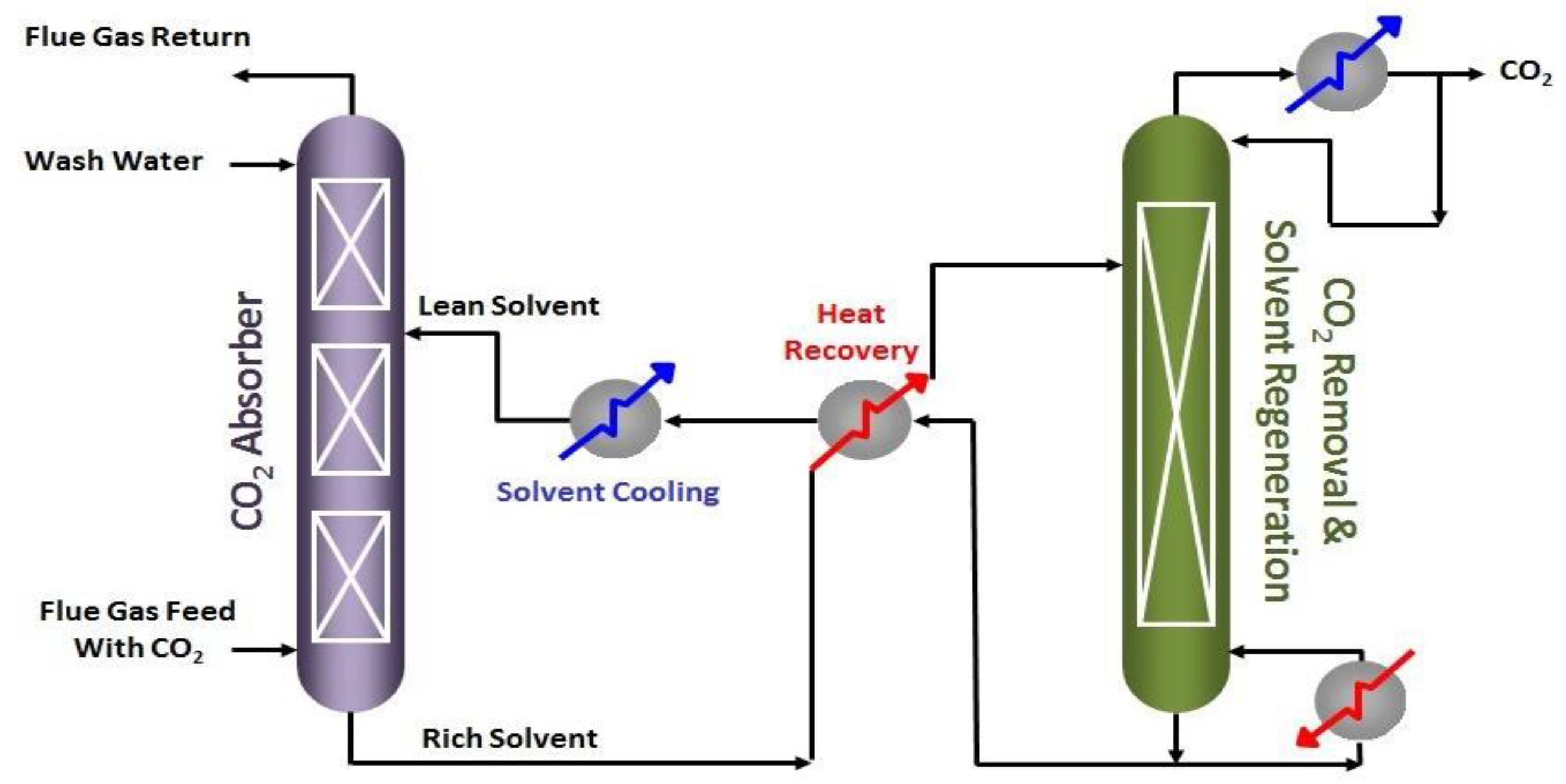
| Reactor | Conversion per Path (%) | Capacity per Reactor (bbl/day) | Characteristics |
|---|---|---|---|
| Tubular fixed-bed | 30–35 | ≤6000 | ≤30,000 tubes with catalyst pellet or extradites |
| Content | Carbon | Oxygen | Hydrogen | Sulphur | Nitrogen | Moisture | Ash |
|---|---|---|---|---|---|---|---|
| (%) | 27.1 | 11.0 | 2.1 | 1.4 | 0.4 | 38 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, B.; Amin, M.; Fareed, A.G.; Farooqi, Z.U.R. Conversion of Coal-Biomass into Diesel by Using Aspen Plus. C 2022, 8, 63. https://doi.org/10.3390/c8040063
Bashir B, Amin M, Fareed AG, Farooqi ZUR. Conversion of Coal-Biomass into Diesel by Using Aspen Plus. C. 2022; 8(4):63. https://doi.org/10.3390/c8040063
Chicago/Turabian StyleBashir, Bilal, Muhammad Amin, Anaiz Gul Fareed, and Zia Ur Rahman Farooqi. 2022. "Conversion of Coal-Biomass into Diesel by Using Aspen Plus" C 8, no. 4: 63. https://doi.org/10.3390/c8040063
APA StyleBashir, B., Amin, M., Fareed, A. G., & Farooqi, Z. U. R. (2022). Conversion of Coal-Biomass into Diesel by Using Aspen Plus. C, 8(4), 63. https://doi.org/10.3390/c8040063







