Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Andersson, J.; Gronkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energ. 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energ. 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Amer, W.A.; Yu, H.; Ji, J.; Huang, L.; Shan, J.; Tong, R. Hydrogen Storage in Metal-Organic Frameworks. J. Inorg. Organomet. Polym. Mater. 2012, 23, 270–285. [Google Scholar] [CrossRef]
- Schlichtenmayer, M.; Hirscher, M. Nanosponges for hydrogen storage. J. Mater. Chem. 2012, 22, 10134–10143. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.P.; Sun, Y. Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrogen Energ. 2004, 29, 319–322. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; de Faria, L.I.L.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energ. 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Aminorroaya-Yamini, S.; Nevirkovets, I.; Konstantinov, K.; Liu, H.K. Enhanced Hydrogen Storage in Graphene Oxide-MWCNTs Composite at Room Temperature. Adv. Energy Mater. 2012, 2, 1439–1446. [Google Scholar] [CrossRef]
- Shet, S.P.; Priya, S.S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energ. 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Fischer, R.A. Covalent organic frameworks and their metal nanoparticle composites: Prospects for hydrogen storage. Phys. Status Solidi B Basic Solid State Phys. 2013, 250, 1119–1127. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Yu, F.; Ma, J. Nanoconfinement engineering for enchanced adsorption of carbon materials, metal-organic frameworks, mesoporous silica, MXenes and porous organic polymers: A review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Poirier, E.; Dailly, A. On the nature of the adsorbed hydrogen phase in microporous metal-organic frameworks at supercritical temperatures. Langmuir 2009, 25, 12169–12176. [Google Scholar] [CrossRef][Green Version]
- Nalaparaju, A.; Babarao, R.; Zhao, X.S.; Jiang, J.W. Atomistic insight into adsorption, mobility, and vibration of water in ion-exchanged zeolite-like metal-organic frameworks. ACS Nano 2009, 3, 2563–2572. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Q.; Zhang, L.; Liu, Y.C.; Kang, Y. Adsorption sites of hydrogen in zeolitic imidazolate frameworks. J. Phys. Chem. B 2009, 113, 11049–11053. [Google Scholar] [CrossRef] [PubMed]
- Gygi, D.; Bloch, E.D.; Mason, J.A.; Hudson, M.R.; Gonzalez, M.I.; Siegelman, R.L.; Darwish, T.A.; Queen, W.L.; Brown, C.M.; Long, J.R. Hydrogen storage in the expanded pore metal-organic frameworks M2 (dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 28, 1128–1138. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Balderas-Xicohténcatl, R.; Schlichtenmayer, M.; Hirscher, M. Volumetric Hydrogen Storage Capacity in Metal-Organic Frameworks. Energy Technol. 2018, 6, 578–582. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef]
- Wang, T.C.; Wright, A.M.; Hoover, W.J.; Stoffel, K.J.; Richardson, R.K.; Rodriguez, S.; Flores, R.C.; Siegfried, J.P.; Vermeulen, N.A.; Fuller, P.E.; et al. Surviving Under Pressure: The Role of Solvent, Crystal Size, and Morphology During Pelletization of Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 52106–52112. [Google Scholar] [CrossRef] [PubMed]
- Severino, M.I.; Gkaniatsou, E.; Nouar, F.; Pinto, M.L.; Serre, C. MOFs industrialization: A complete assessment of production costs. Faraday Discuss. 2021, 231, 326–341. [Google Scholar] [CrossRef]
- Broom, D.P.; Hirscher, M. Improving Reproducibility in Hydrogen Storage Material Research. ChemPhysChem 2021, 22, 2141–2157. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S.G. Structural Stability of Metal Organic Framework MOF-177. J. Phys. Chem. Lett. 2010, 1, 73–78. [Google Scholar] [CrossRef]
- Ming, Y.; Purewal, J.; Yang, J.; Xu, C.C.; Veenstra, M.; Gaab, M.; Muller, U.; Siegel, D.J. Stability of MOF-5 in a hydrogen gas environment containing fueling station impurities. Int. J. Hydrogen Energ. 2016, 41, 9374–9382. [Google Scholar] [CrossRef]
- Bae, J.; Choi, J.S.; Hwang, S.; Yun, W.S.; Song, D.; Lee, J.; Jeong, N.C. Multiple Coordination Exchanges for Room-Temperature Activation of Open-Metal Sites in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 24743–24752. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Leachman, J.W.; Jacobsen, R.T.; Penoncello, S.G.; Lemmon, E.W. Fundamental Equations of State for Parahydrogen, Normal Hydrogen, and Orthohydrogen. J. Phys. Chem. Ref. Data 2009, 38, 721–748. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 6 March 2020).
- Blach, T.P.; Gray, E.M. Sieverts apparatus and methodology for accurate determination of hydrogen uptake by light-atom hosts. J. Alloy. Compd. 2007, 446, 692–697. [Google Scholar] [CrossRef]
- Purewal, J.; Veenstra, M.; Tamburello, D.; Ahmed, A.; Matzger, A.J.; Wong-Foy, A.G.; Seth, S.; Liu, Y.Y.; Siegel, D.J. Estimation of system-level hydrogen storage for metal-organic frameworks with high volumetric storage density. Int. J. Hydrog. Energy 2019, 44, 15135–15145. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- de Lange, M.F.; van Velzen, B.L.; Ottevanger, C.P.; Verouden, K.J.; Lin, L.C.; Vlugt, T.J.; Gascon, J.; Kapteijn, F. Metal-Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef]
- Villajos, J.A.; Zimathies, A.; Prinz, C. A fast procedure for the estimation of the hydrogen storage capacity by cryoadsorption of metal-organic framework materials from their available porous properties. Int. J. Hydrogen Energ. 2020, 46, 29323–29331. [Google Scholar] [CrossRef]
- Horcajada, P.; Surble, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Greneche, J.M.; Margiolaki, I.; Ferey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Seo, Y.K.; Yoon, J.W.; Lee, J.S.; Lee, U.H.; Hwang, Y.K.; Jun, C.H.; Horcajada, P.; Serre, C.; Chang, J.S. Large scale fluorine-free synthesis of hierarchically porous iron(III) trimesate MIL-100(Fe) with a zeolite MTN topology. Microporous Mesoporous Mater. 2012, 157, 137–145. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A decade of UiO-66 research: A historic review of dynamic structure, synthesis mechanisms, and characterization techniques of an archetypal metal-organic framework. Cryst. Growth Des. 2019, 20, 1347–1362. [Google Scholar] [CrossRef]
- Vo, T.K.; Le, V.N.; Yoo, K.S.; Song, M.; Kim, D.; Kim, J. Facile Synthesis of UiO-66(Zr) Using a Microwave-Assisted Continuous Tubular Reactor and Its Application for Toluene Adsorption. Cryst. Growth Des. 2019, 19, 4949–4956. [Google Scholar] [CrossRef]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Yildirim, T.; Farha, O.K.; Sholl, D.S.; Snurr, R.Q. Computation-Ready, Experimental Metal-Organic Frameworks: A Tool To Enable High-Throughput Screening of Nanoporous Crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Zlotea, C.; Moretto, P.; Steriotis, T. A Round Robin characterisation of the hydrogen sorption properties of a carbon based material. Int. J. Hydrogen Energ. 2009, 34, 3044–3057. [Google Scholar] [CrossRef]
- Broom, D.P.; Hirscher, M. Irreproducibility in hydrogen storage material research. Energy Environ. Sci. 2016, 9, 3368–3380. [Google Scholar] [CrossRef]
- Hurst, K.E.; Gennett, T.; Adams, J.; Allendorf, M.D.; Balderas-Xicohtencatl, R.; Bielewski, M.; Edwards, B.; Espinal, L.; Fultz, B.; Hirscher, M.; et al. An International Laboratory Comparison Study of Volumetric and Gravimetric Hydrogen Adsorption Measurements. ChemPhysChem 2019, 20, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 adsorption in a microporous metal-organic framework with open metal sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Liu, D.-J. Final Project Report for DOE/EERE High-Capacity and Low-Cost Hydrogen-Storage Sorbents for Automotive Applications; Texas A & M Univ.: College Station, TX, USA, 2017. [Google Scholar]
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled reducibility of a metal-organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. Engl. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Liu, Y.; Kabbour, H.; Brown, C.M.; Neumann, D.A.; Ahn, C.C. Increasing the density of adsorbed hydrogen with coordinatively unsaturated metal centers in metal-organic frameworks. Langmuir 2008, 24, 4772–4777. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Duren, T. Opening the gate: Framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wharmby, M.T.; Parra, J.B.; Ania, C.O.; Fairen-Jimenez, D. Role of crystal size on swing-effect and adsorption induced structure transition of ZIF-8. Dalton Trans. 2016, 45, 6893–6900. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Fanourgakis, G.S.; Froudakis, G.E.; Trikalitis, P.N.; Hirscher, M. Concepts for improving hydrogen storage in nanoporous materials. Int. J. Hydrogen Energ. 2019, 44, 7768–7779. [Google Scholar] [CrossRef]
- Gomez-Gualdron, D.A.; Wang, T.C.; Garcia-Holley, P.; Sawelewa, R.M.; Argueta, E.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; Farha, O.K. Understanding Volumetric and Gravimetric Hydrogen Adsorption Trade-off in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 33419–33428. [Google Scholar] [CrossRef]
- Yan, Y.; da Silva, I.; Blake, A.J.; Dailly, A.; Manuel, P.; Yang, S.; Schroder, M. High Volumetric Hydrogen Adsorption in a Porous Anthracene-Decorated Metal-Organic Framework. Inorg. Chem. 2018, 57, 12050–12055. [Google Scholar] [CrossRef]
- Parilla, P.A.; Gross, K.; Hurst, K.; Gennett, T. Recommended volumetric capacity definitions and protocols for accurate, standardized and unambiguous metrics for hydrogen storage materials. Appl. Phys. A-Mater. 2016, 122, 201. [Google Scholar] [CrossRef]
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 30 November 2021).
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef]
- DOE Technical Targets for Hydrogen Storage Systems for Portable Power Equipment. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-storage-systems-portable-power-equipment (accessed on 30 November 2021).
- Collings, I.E.; Goodwin, A.L. Metal-organic frameworks under pressure. J. Appl. Phys. 2019, 126, 181101. [Google Scholar] [CrossRef]
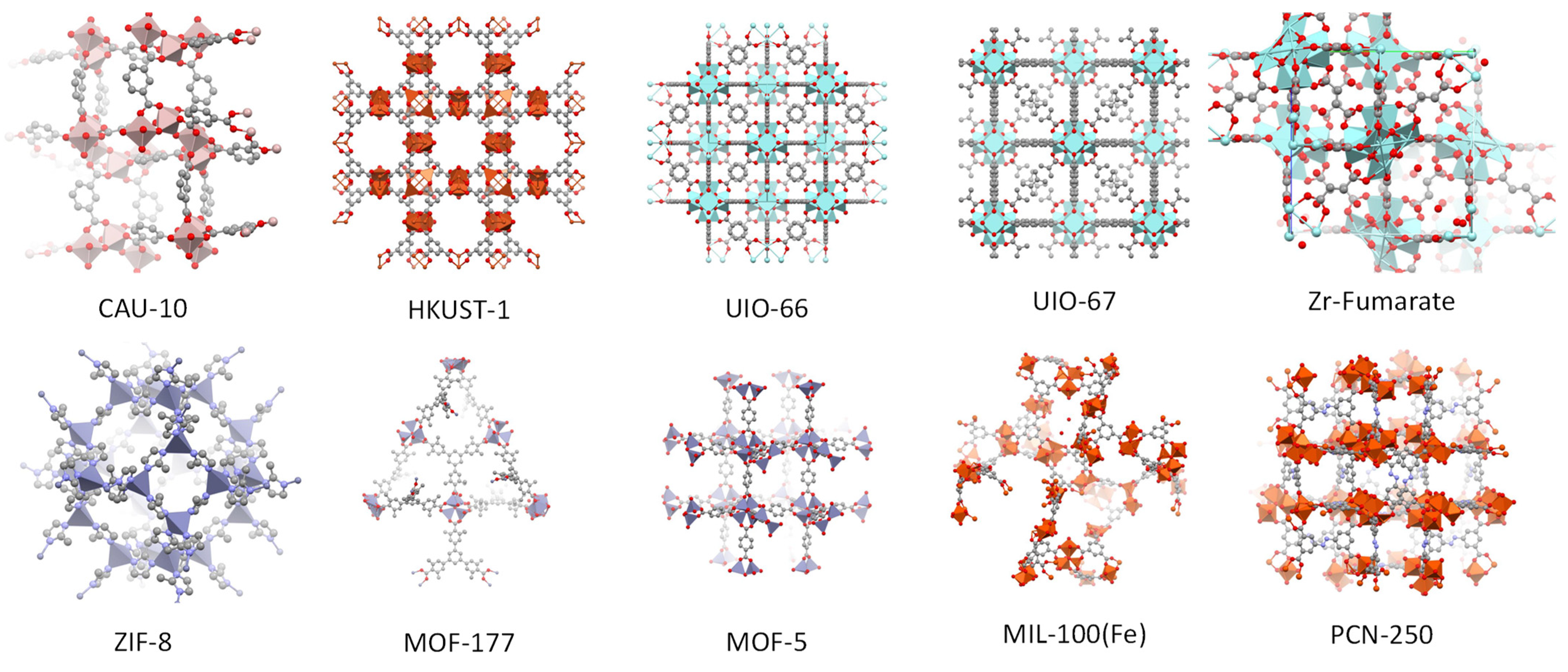


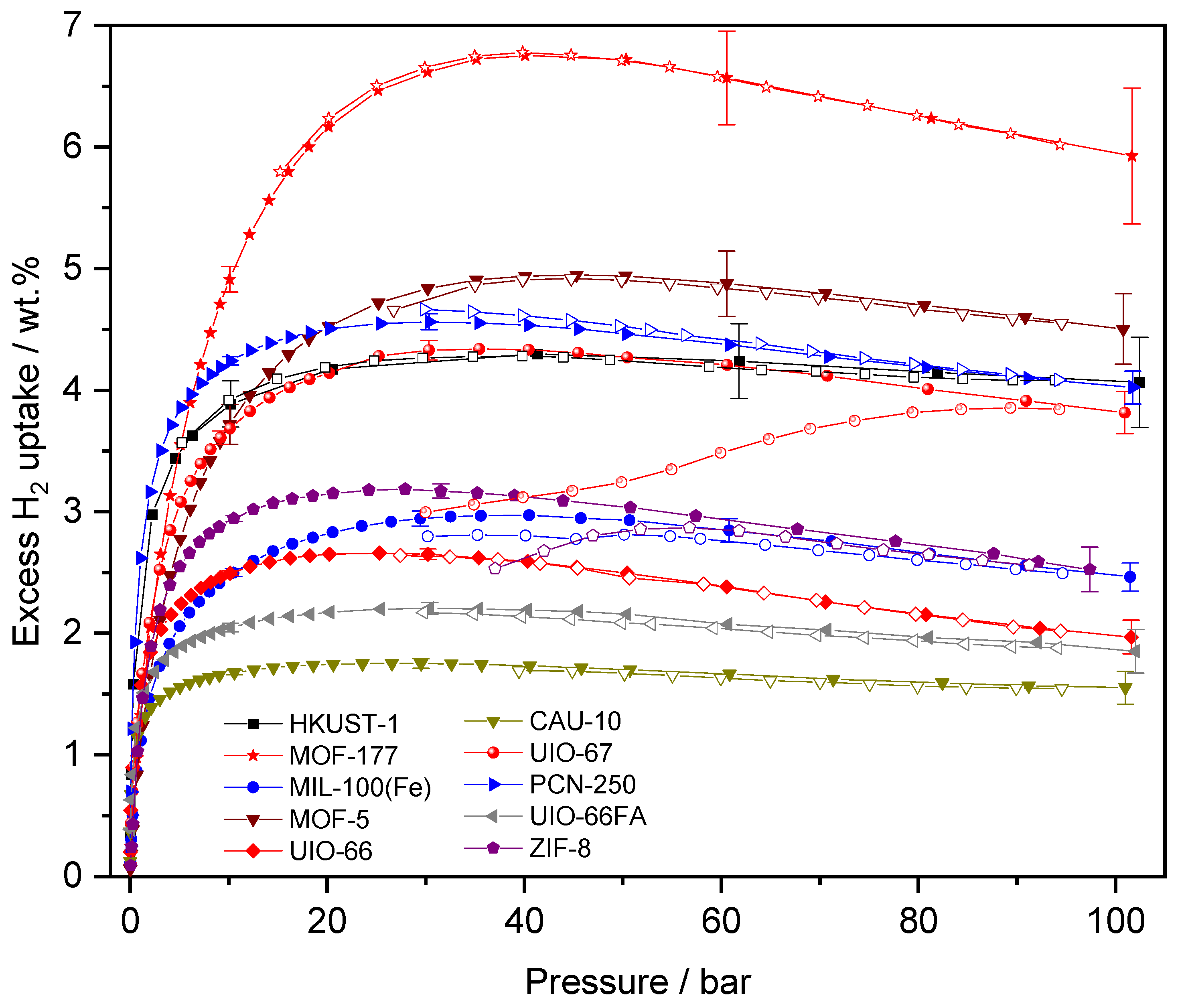
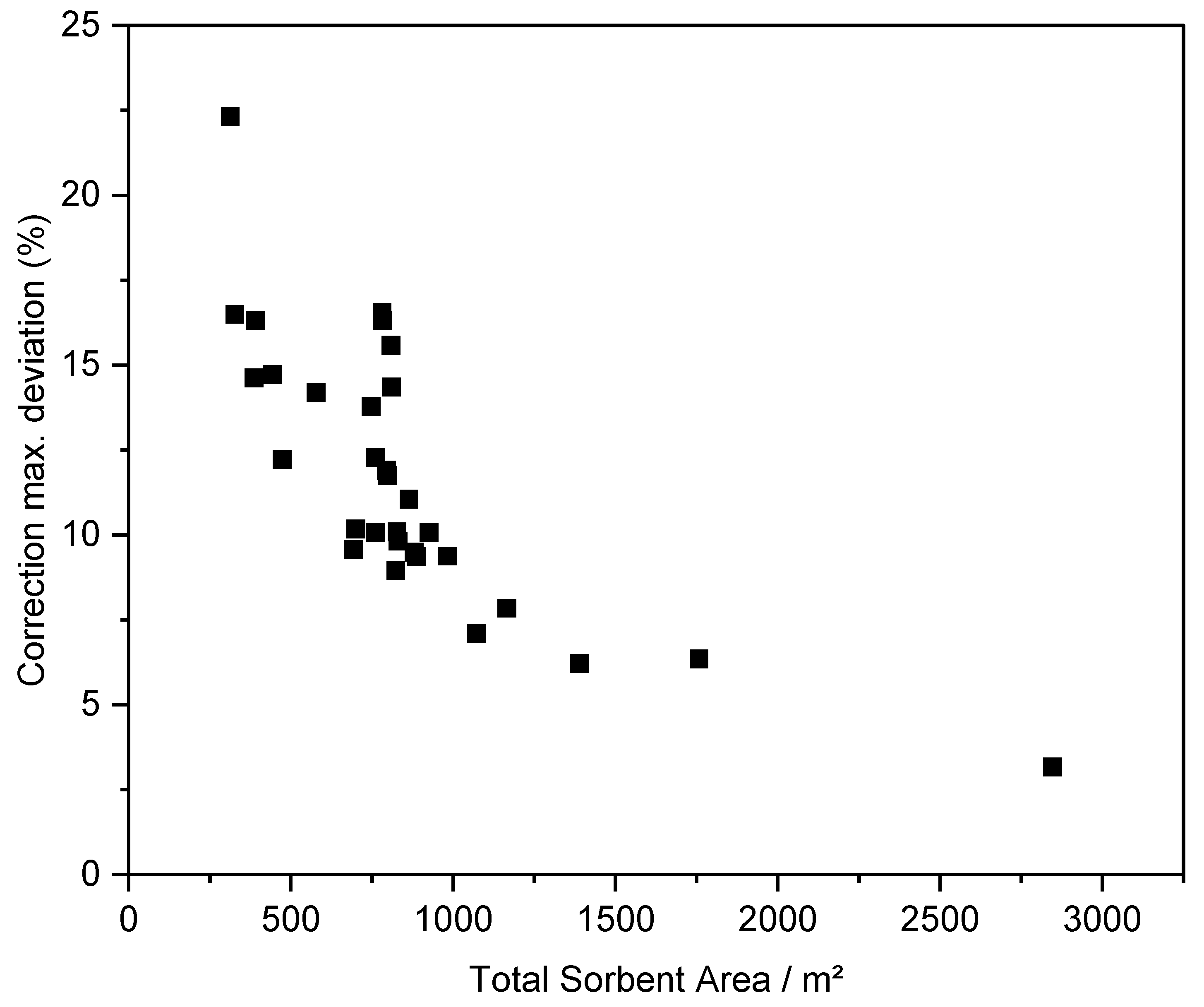
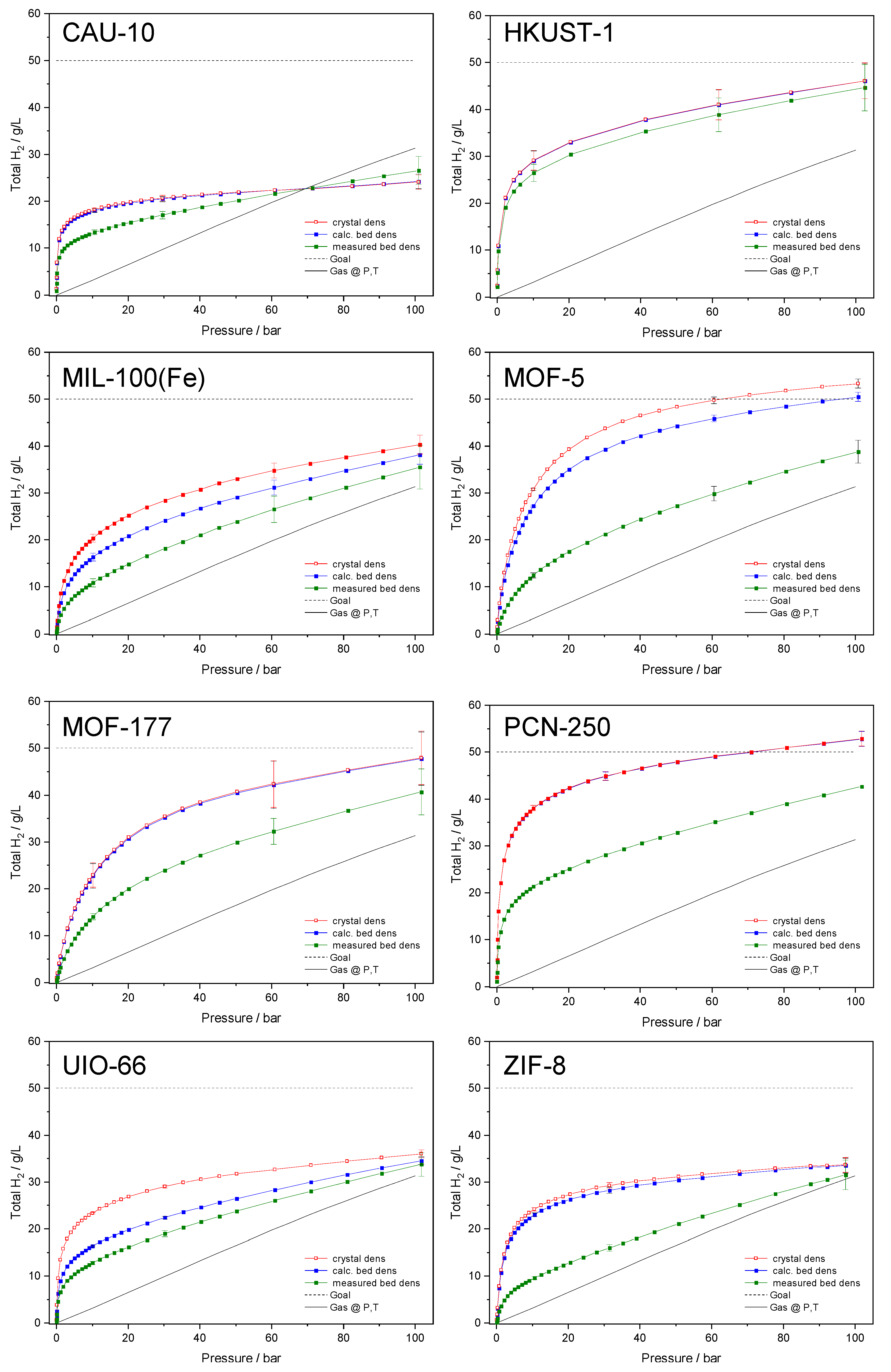
| Name | ABET (m²·g−1) | Vp 1 (cm³·g−1) | Vµp 2 (cm³·g−1) | Tactivation 3 (°C) |
|---|---|---|---|---|
| MOF-177 | 4449.29 | 1.82 ± 0.01 | 1.80 ± 0.01 | 120 |
| MOF-5 | 1976.50 | 1.00 ± 0.01 | 0.80 ± 0.01 | 120 |
| UIO-67 | 2135.00 | 0.85 ± 0.01 | 0.84 ± 0.01 | 120 |
| MIL-100(Fe) | 2015.00 | 1.28 ± 0.01 | 0.88 ± 0.01 | 150 |
| PCN-250(Fe) | 1811.30 | 0.72 ± 0.01 | 0.72 ± 0.01 | 190 |
| HKUST-1 | 1800.16 | 0.73 ± 0.01 | 0.73 ± 0.01 | 200 |
| ZIF-8 | 1734.03 | 0.71 ± 0.01 | 0.63 ± 0.01 | 200 |
| UIO-66 | 1201.54 | 1.22 ± 0.01 | 0.62 ± 0.01 | 150 |
| Zr-FA | 717.91 | 0.32 ± 0.01 | 0.31 ± 0.01 | 120 |
| CAU-10 | 629.65 | 0.26 ± 0.01 | 0.25 ± 0.01 | 100 |
| Name | ρsk 1 (g·cm−3) | ρcr 2 (g·cm−3) | ρbed,calc 3 (g·cm−3) | ρcr 4 (g·cm−3) | ρbed,meas 5 (g·cm−3) |
|---|---|---|---|---|---|
| MOF-177 | 1.46 ± 0.03 | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.43 | 0.22 ± 0.01 |
| MOF-5 | 1.87 ± 0.04 | 0.75 ± 0.02 | 0.65 ± 0.02 | 0.60 | 0.25 ± 0.02 |
| UIO-67 | 1.70 ± 0.03 | 0.70 ± 0.02 | 0.69 ± 0.02 | 0.73 | n.a. |
| MIL-100(Fe) | 2.28 ± 0.05 | 0.77 ± 0.02 | 0.59 ± 0.02 | 0.68 | 0.34 ± 0.02 |
| PCN-250(Fe) | 1.98 ± 0.04 | 0.82 ± 0.03 | 0.81 ± 0.03 | 0.89 | 0.42 ± 0.03 |
| HKUST-1 | 1.69 ± 0.03 | 0.74 ± 0.02 | 0.74 ± 0.02 | 0.88 | 0.67 ± 0.04 |
| ZIF-8 | 1.41 ± 0.03 | 0.75 ± 0.03 | 0.71 ± 0.02 | 0.92 | 0.22 ± 0.01 |
| UIO-66 | 2.09 ± 0.04 | 0.92 ± 0.03 | 0.59 ± 0.02 | 1.20 | 0.43 ± 0.03 |
| Zr-FA | 2.12 ± 0.04 | 1.28 ± 0.07 | 1.26 ± 0.06 | 1.57 | n.a. |
| CAU-10 | 1.37 ± 0.03 | 1.02 ± 0.06 | 1.00 ± 0.06 | 3.20 | 0.68 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villajos, J.A. Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials. C 2022, 8, 5. https://doi.org/10.3390/c8010005
Villajos JA. Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials. C. 2022; 8(1):5. https://doi.org/10.3390/c8010005
Chicago/Turabian StyleVillajos, Jose A. 2022. "Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials" C 8, no. 1: 5. https://doi.org/10.3390/c8010005
APA StyleVillajos, J. A. (2022). Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials. C, 8(1), 5. https://doi.org/10.3390/c8010005






