Solvent and Substituent Effect on Selectivity of Triphenylether-Based Ionophores: A Voltammetric Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
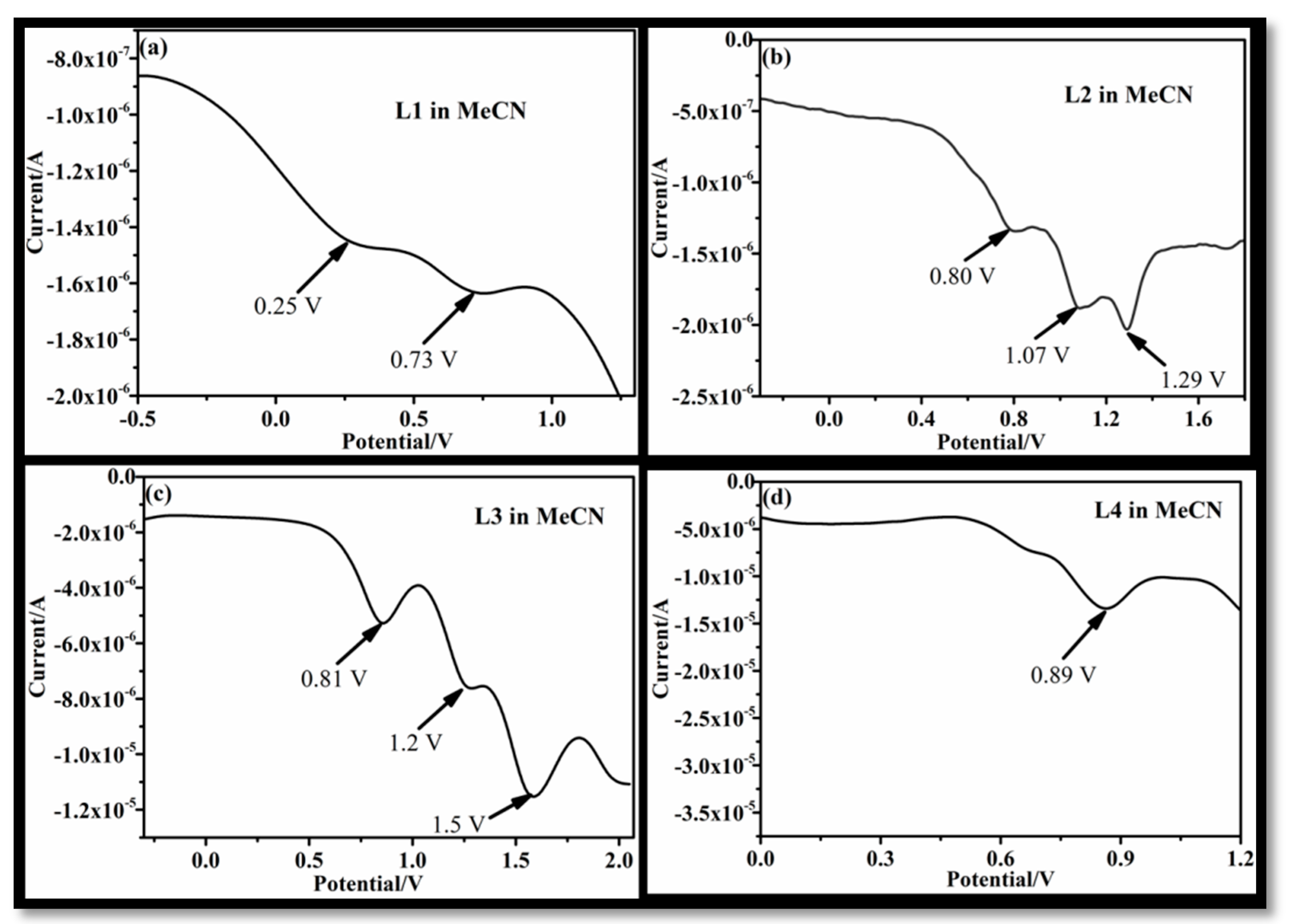
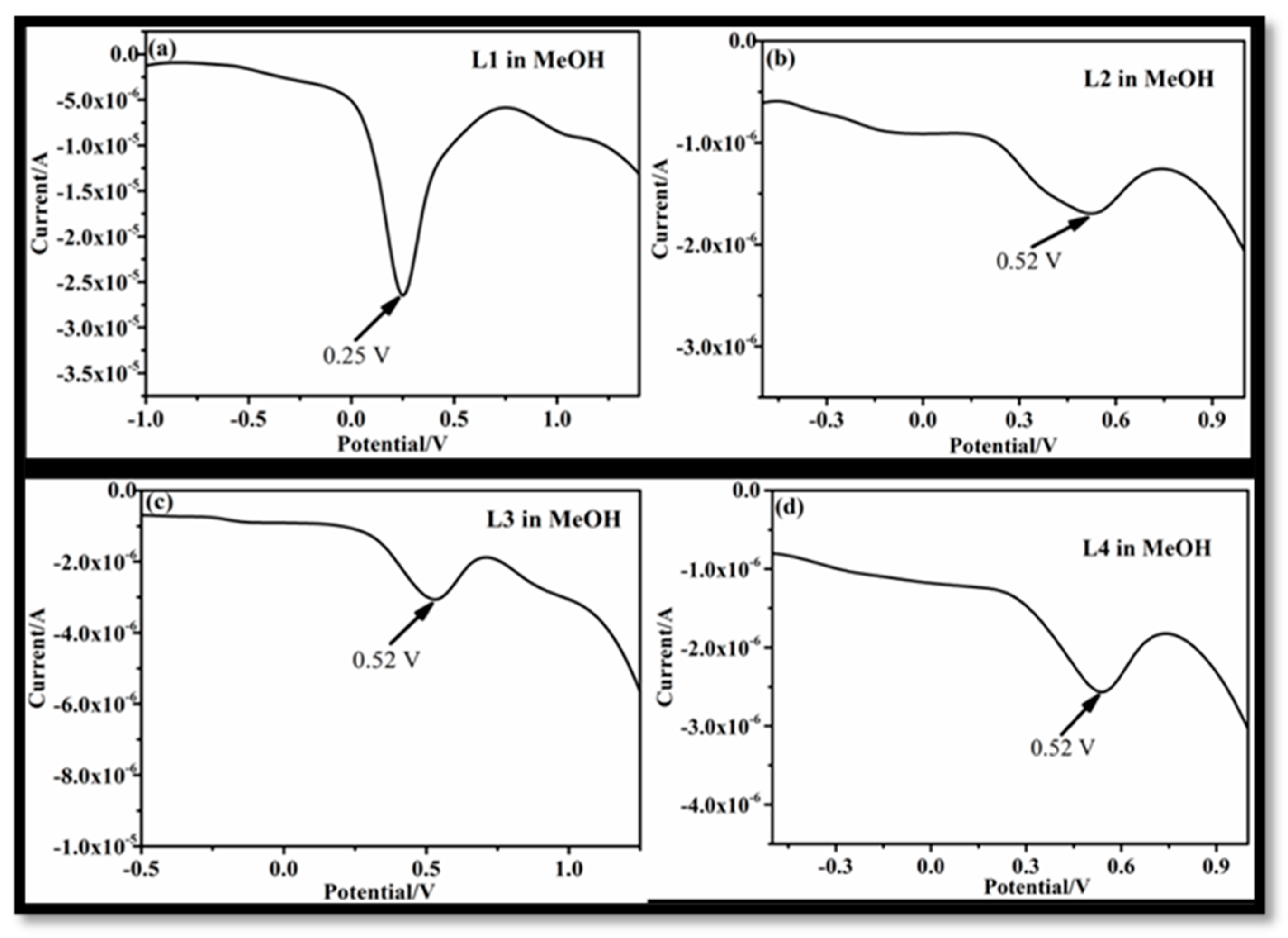
3.1. Effect of Solvent on Electrochemical Behavior of L1–L4
3.2. Behavior of L2–L4 towards Cyanide Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Eng. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.; Kim, H.; Kim, J.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef]
- Guth, U.; Vonau, W.; Zosel, J. Recent developments in electrochemical sensor application and technology—A review. Meas. Sci. Technol. 2009, 20, 042002. [Google Scholar] [CrossRef]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K.; Ghana Kumar, G.P. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Mittal, S.K.; Chhibber, M.; Gupta, S. Imine derivative as an analytical probe for Al+3, F− and CN− sensing with antibacterial activity against E. coli—An application of electrochemical and spectrofluorimetric techniques. Microchem. J. 2021, 168, 106500. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: The state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D.; Davis, J.J. Electrochemical anion sensing: Supramolecular approaches. Chem. Rev. 2020, 120, 1888–1935. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Bernhardt, P.V. A ferrocene functionalised macrocyclic receptor for cations and anions. J. Chem. Soc. Dalton Trans. 2001, 9, 1428–1431. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Creevey, N.L. Electrochemical anion recognition with ferrocene functionalised macrocycles. Dalton Trans. 2004, 6, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, L.-J.; Liao, J.-H.; Chen, C.-T.; Huang, C.-H.; Chen, C.-S.; Fang, J.-M. Two-arm ferrocene amide compounds: Synclinal conformations for selective sensing of dihydrogen phosphate ion. Org. Lett. 2003, 5, 1821–1824. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Deng, L.; Yu, H.; Huo, J.; Ma, L.; Wang, J. Electrochemical assessment of the interaction of dihydrogen phosphate with a novel ferrocenyl receptor. J. Phys. Chem. B 2009, 113, 15141–15144. [Google Scholar] [CrossRef] [PubMed]
- Nieto, D.; González-Vadillo, A.M.; Bruña, S.; Pastor, C.J.; Kaifer, A.E.; Cuadrado, I. Pt (II)-activated coupling of aminoethylferrocene with benzonitrile. A facile access route to a new redox-active bis (ferrocenyl-amidine) anion sensor. Chem. Commun. 2011, 47, 10398–10400. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, B.; Shaghaghi, Z. Anion-binding properties of two calix[4]arene derivatives containing two ferrocene imine or ferrocene amine units at the upper rim. Appl. Organomet. Chem. 2011, 25, 317–322. [Google Scholar] [CrossRef]
- Schwob, T.; Kempe, R. A reusable Co catalyst for the selective hydrogenation of functionalized nitroarenes and the direct synthesis of imines and benzimidazoles from nitroarenes and aldehydes. Angew. Chem. Int. Ed. 2016, 55, 15175–15179. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Senthamarai, T.; Sohail, M.; Alshammari, A.S.; Pohl, M.-M.; Beller, M.; Jagadeesh, R.V. Cobalt-based nanoparticles prepared from MOF–carbon templates as efficient hydrogenation catalysts. Chem. Sci. 2018, 9, 8553–8560. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chhibber, M.; Mittal, S.K. Amine derivative of triphenyl ether as an optical sensor for the detection of cyanide ions and traces of water in acetonitrile supported with voltammetric studies. J. Appl. Electrochem. 2020, 50, 185–195. [Google Scholar] [CrossRef]
- Sharma, R.; Chhibber, M.; Mittal, S.K. A new ionophore for chemical sensing of F−, CN− and Co2+ using voltammetric, colorimetric and spectrofluorimetric techniques. RSC Adv. 2016, 6, 51153–51160. [Google Scholar] [CrossRef]
- Gupta, S.; Mittal, S.K.; Chhibber, M. Triphenyl Ether Amide as a Probe for Electrochemical and Optical Sensing of Copper, Cyanide and Arginine. J. Electrochem. Soc. 2020, 167, 167506. [Google Scholar] [CrossRef]
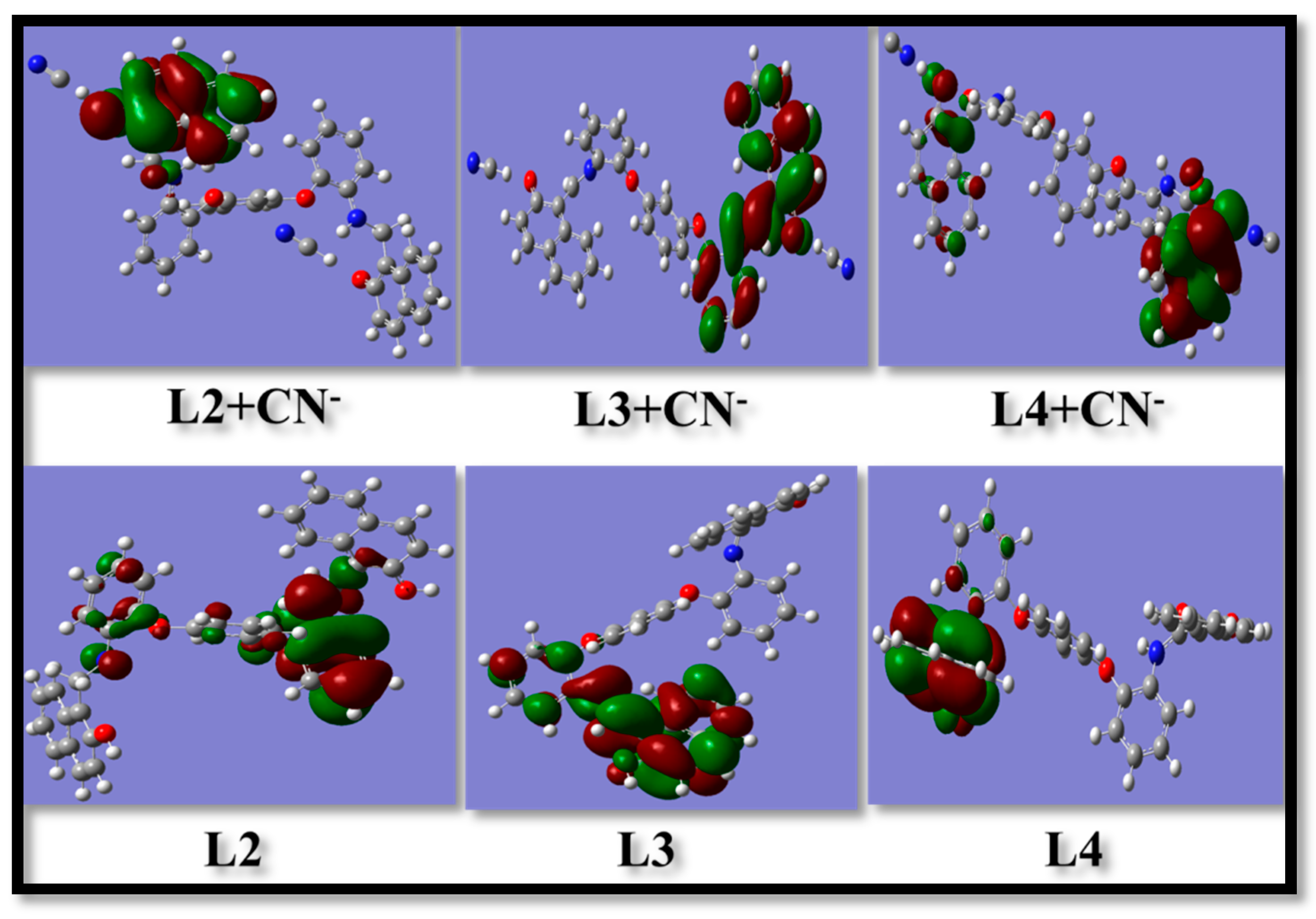
| Receptor/Complex System | Energy in a.u | Receptor/Complex System | Energy in a.u | Receptor/Complex System | Energy in a.u |
|---|---|---|---|---|---|
| L2 | 0.355 | L3 | 0.126 | L4 | 0.152 |
| L2-CN− | 0.141 | L3-CN− | 0.118 | L4-CN− | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, S.K.; Gupta, S.; Chhibber, M. Solvent and Substituent Effect on Selectivity of Triphenylether-Based Ionophores: A Voltammetric Study. C 2021, 7, 85. https://doi.org/10.3390/c7040085
Mittal SK, Gupta S, Chhibber M. Solvent and Substituent Effect on Selectivity of Triphenylether-Based Ionophores: A Voltammetric Study. C. 2021; 7(4):85. https://doi.org/10.3390/c7040085
Chicago/Turabian StyleMittal, Susheel K., Shivali Gupta, and Manmohan Chhibber. 2021. "Solvent and Substituent Effect on Selectivity of Triphenylether-Based Ionophores: A Voltammetric Study" C 7, no. 4: 85. https://doi.org/10.3390/c7040085
APA StyleMittal, S. K., Gupta, S., & Chhibber, M. (2021). Solvent and Substituent Effect on Selectivity of Triphenylether-Based Ionophores: A Voltammetric Study. C, 7(4), 85. https://doi.org/10.3390/c7040085







