Abstract
Effective fluoride removal from water is a persistent global concern both for drinking water and wastewater treatment. According to World Health Organization (WHO), standards for the maximum contaminant level in drinking water cannot be higher than 1.5 mg F− L−1 since affects the skeletal and nervous systems of humans. Various technologies have been developed to decrease fluoride concentration from waters, such as adsorption, coagulation, precipitation and membrane separation. Membrane technology has been found to be a very effective technology, significantly reducing fluoride to desired standards levels; however, it has received less attention than other technologies because it is a costly process. This review aims to discuss the recent studies using modified membranes for fluoride removal. Emphasis is given on cellulose-, polymer- and graphene-based membranes and is further discussing the modification of membranes with several metals that have been developed in the last years. It was observed that the main focus of the total publications has been on the use of polymer-based membranes. Most of the membranes applied for defluoridation exhibit greater efficiency at pH values close to that of drinking water (i.e., 6–8), and maximum treatment capacity was obtained with the use of a cellulose modified membrane Fe-Al-Mn@chitosan with a permeate flux of 2000 L m−2 h−1, following the carbon-based amyloid fibril nano-ZrO2 composites (CAF-Zr) 1750 L m−2. A technical-economic comparison study of NF and RO is also referred, concluding that NF membrane is slightly less expensive.
1. Introduction
Fluorine is an active non-metal element that has negatively charged species i.e., fluoride ions, and affects human health, which includes teeth and bones. Up to a certain concentration level, it can have a positive effect; however, above 1.5 mg L−1, the impact is negative [1,2]. In particular, according to the International Standards Organization, the acceptable concentration values of fluoride in drinking water are usually 0.5–1.0 mg L−1. According to the World Health Organization (WHO) and the EU Directive 98/83/EC [3,4] the guideline value for fluoride in drinking water is 1.5 mg L−1, as at lower concentrations there is an increasing risk of prevention of teeth cavities and at progressively higher levels there is an increasing risk of dental and skeletal fluorosis [5,6]. The limit has remained constant also in the revised version of the drinking water directive of 16 December 2020 [7].
Figure 1a depicts the benefits of fluoridation of water on human teeth in three steps: First, fluoride ions replace hydroxide anions, resulting from food, inside the teeth; Second, small amounts of fluoride strengthens teeth, and third, enamel is repaired, because of the predominant presence of fluoride inions. In Figure 1b, the contrast with the disadvantages of excess concentration of fluoride ions, leading to dental fluorosis, is presented.
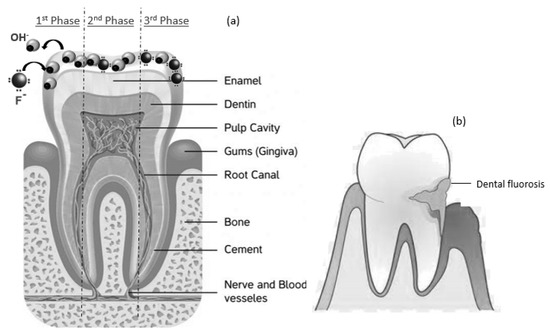
Figure 1.
(a) Benefits of fluoridation of water in three phases; (b) Dental fluorosis problem. This figure sows both aspects of fluoride presence in water.
Fluoride in the environment occurs both through its natural presence in the earth’s crust, i.e., natural rocks, mainly as sellaite (MgF2), fluorspar (CaF2), cryolite (Na3AlF6) and fluorapatite (Ca3(PO4)2Ca(FCl2) and through contamination of water bodies caused by anthropogenic actions, i.e., the excessive use of phosphate fertilizers that can reach the groundwater sources [2,8]. Among water sources, groundwater usually contains higher fluoride concentrations ranging from 1.0 mg L−1 to greater than 35.0 mg L−1 depending on the local geology, the physical and chemical behavior of the aquifer and its interface with the environment [9].
Drinking water contaminated with fluoride is considered an environmental issue affecting the worldwide population, as can be seen in Figure 2. Specifically, in regions such as India, Bangladesh, China, Pakistan and Africa [10], the displayed concentrations are too high, so many thousands of people suffer from fluoride-related diseases, with China and India being the most impacted countries. In Africa, particularly in Ghana and Tanzania, the local standard for fluoride is 4 mg L−1; thus, there is an indication of dental fluorosis and several times it exceeds the WHO permeable limit of 1.5 mg L−1, as illustrated in Figure 3 [11]. In developing countries, this problem is more obvious because the drinking water is derived mainly from untreated groundwater therefore, the issue of effective fluoride removal becomes problematic in these areas, mainly due to the lack of adequate and basic infrastructure but also due to the lack of relevant knowledge [12,13].
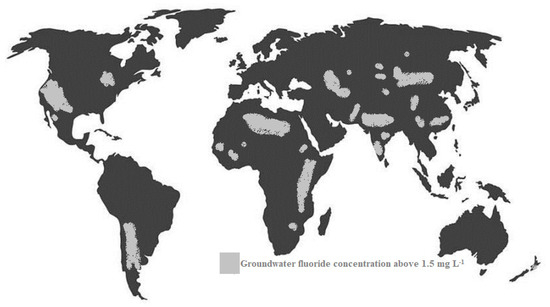
Figure 2.
Geographical areas (in light grey) associated with groundwater fluoride concentration above 1.5 mg L−1 (recommended WHO level) of naturally occurring fluoride. The figure shows that areas in north and South America, Africa and Asia exhibit groundwaters with elevated fluoride concentrations.
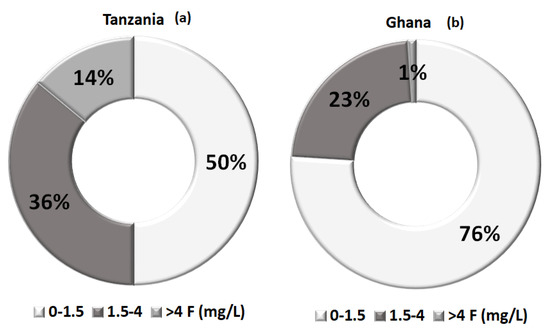
Figure 3.
Percentage of fluoride existence in relation to local standards (a) Tanzania case; (b) Ghana case, in Africa [11].
The aforementioned information demonstrates that the problem of the occurrence of fluoride ions in natural waters is of major importance. It is for this reason that various technologies have been developed to decrease fluoride concentration from waters, such as adsorption [13], coagulation [1], precipitation [14] and membrane separations [15,16], in order to render the treated water potable. Table 1 summarizes the commonly applied defluoridation techniques and their main advantages and disadvantages. Particularly, in the case of ion-exchange, the high cost of resin, the need of regeneration, the waste disposal requirements and lack of selectivity, exclude this method from being efficient and cost-effective [17]. On the other hand, coagulation is an inexpensive technology but requires high doses, resulting in high residual concentrations, i.e., of aluminum [18]. In the precipitation method calcium, aluminum and iron salts are reported in several studies; however, the problem associated with lime-based defluoridation is the low solubility of the formed salts, which prevents complete removal of fluoride [17,18]. Another method, adsorption, is a widely used technique [19] that has the advantage of being a manual method with a low-cost operation, with an increased selectivity and adsorbent readiness. Several materials such as alumina [20], activated carbon [21], ion exchange resins, silica gel, natural materials like clay, mud and low-cost alternative adsorbents like fly ash, bone charcoal, etc., are used [13,22,23].

Table 1.
Main advantages and disadvantages of commonly applied techniques for fluoride removal from drinking water.
Despite the numerous techniques that are available for fluoride removal, effective removal still remains a challenging issue, and therefore, adsorption is a technology that this review focuses on, because of certain advantages that makes this technology easier to apply, especially for remote areas in developing countries [24]. However, adsorption of fluoride has some points that need further investigation, such as the ability to adsorb in dilute solutions, pH and contact time, regeneration, adsorption stability and removal capacity in the presence of other ions, which can ultimately increase the overall cost of the fluoride removal method [25]. The use of bone char was studied [26] on real groundwater, providing a fluoride removal up to 90% and in recent years, residues obtained from palm trees [27] (e.g., palm kernel shell/midribs, and coconut husk/shell/fiber/root) have been considered as a possible alternative adsorbent material for the effective removal of fluorides from drinking water thanks to the low cost and its wide availability in some of the countries most affected by the fluoride problem. Despite the very interesting results on its optimal removal yields, this alternative adsorbent for fluoride removal will still have to be carefully studied also because of their disadvantages (high specific surface and low reduction in uptake capacity when regenerated) [27].
Membrane technology is an effective technology in reducing fluoride to acceptable concentrations. Nevertheless, it has received less attention than other technologies because it is supposed to be an expensive process [2], especially in comparison with adsorption techniques. However, the advantages of using the membrane process are numerous. Among these is the fact that it is effective in removing other contaminants from water simultaneously, providing perpetual water quality, and is also effective in a wider pH range, while being completed in one stage with the minimum use of chemical agents [2,30,31]. In Table 2 some recent applications of membrane engineering in water and wastewater treatment and some of the achievements that result from their use are presented.

Table 2.
Applications of membrane engineering in water and wastewater treatment.
As the cost is the most important disadvantage of this technology, in order to optimize the method applicability, many studies have been carried out to optimize the parameters that affect the efficiency and the applicability and also the cleaning of membranes, in order to extend the effective operation times [44]. The virus retention capabilities of membranes during operation is also a very important parameter affecting the ability of the membranes, and there are studies [45] focusing on this determination with standardized and molecular biology methods. Thus, the membrane technology, due to the large increase in the demand for drinking water but also to the difficulties that arise due to the complex composition of the water to be treated, was particularly developed, resulting in increased efficiency with reduced operating costs. This also found advantages in the application for the removal of fluoride from the water.
Widely used membranes for fluoride removal based on operational driving force [2,46] are classified as follows:
- pressure-driven membrane operations: reverse osmosis (RO); nano-filtration (NF); ultra-filtration (UF) and microfiltration (MF);
- electric potential gradient: electro dialysis (ED);
- temperature gradient: membrane distillation (MD); and
- concentration gradient: forward osmosis (FO); dialysis, and pervaporation.
Pressure-driven membrane processes are the most commonly applied for water and wastewater treatment [47,48]. Mostly, MF, UF, NF and RO (driven separation processes) are usually made from synthetic organic polymers, including, among others, polyethylene (PE), polytetrafluorethylene (PTFE), polypropylene (PP), and cellulose acetate (CA) [49]. Table 3 summarizes the advantages and disadvantages for the use of RO and NF membranes in water treatment [50].

Table 3.
Advantages and disadvantages of RO and NF methods in water treatment [50].
Consequently, the enhanced practical efficacy of the membrane-based separation technologies makes it more likely for further studies [15,51]. Mixed-matrix membranes (MMMs) consist of well-dispersed inorganic additives within the polymer matrices and can be used to adsorb many contaminants, including fluoride. These adsorptive membranes can provide increased filtration efficiency, long-term operation, ability to adsorb various contaminants from water, better antimicrobial properties, and are easy to reuse [30]. Besides, natural polysaccharide-based polymers and their derivatives [52], such as CA [30,53] and chitosan [54,55], have been widely used as a base for membrane modification. These natural polymers provide considerate properties such as high water flux, owing to their hydrophilic nature and compatible dissolution in suitable solvent [52].
Recently, graphene has attracted wide attention of researchers in various fields due to its excellent properties, such as chemical and thermal stability, as well as the mechanical flexibility and large specific surface area that make it a potential candidate as an effective adsorbent [56,57]. However, because graphene sheets consist of hexagonally arrayed sp2-bonded carbon atoms [58], they can only adsorb substances through van der Waals forces. Therefore, non-modified graphene is not an appropriate adsorbent for many contaminants. There are several studies on the potentials of using modified Graphene Oxide (GO)-based materials in water treatment for removal of toxic heavy metals [59,60], organic dyes, fluoride, and other organic pollutants [59,61,62] but only in some cases, GO-based membranes have been examined.
Considering the global need for safe drinking water and the problems that arise with the established legislation limits for drinking water, this review aims to discuss the recent studies using modified membranes for fluoride removal. Emphasis will be given to the ongoing effort and new MMM, focusing on cellulose, polymer and graphene-based membranes and discussing further the modification of membranes with several metals that have been developed and used in the aforementioned research fields in the last years.
2. Pressure Driven Modified Membranes
2.1. Polymer Membranes
A carbonized bone meal (CBM) impregnated polysulfone-based mixed matrix hollow fiber membrane has been examined for defluoridation and disinfection of groundwater [63]. The performance of CBM/UF/MMM provided a maximum fluoride uptake capacity of 5 mg g−1, at 34 kPa (0.34 bar) trans membrane pressure (TMP) drop and 10 L h−1 cross flow rate.
The polyacrylonitrile hollow fiber membranes [64] AlFu metal organic frameworks (MOF), were produced combining a mixed matrix ultrafiltration hollow fiber membrane with aluminium fumarate. AlFu MOF MH10 (10 w/w % doped concentration) membrane indicated a likely performance in contaminated groundwater with a fluoride concentration of 4.1 mg L−1, at low TMP 0.35 bar, with a breakthrough time of 18 h and a permeate flux of 20 L m−2 h−1 with a small filtration surface of 0.026 m2. More than 10 w/w % doped concentration was not used due to the formation of agglomerate.
ABN/TPU-NFM is a modified membrane [65] that exhibited extensive adsorbance efficiency for fluoride removal from water, with maximum adsorption capacity to be 1.9 mg g−1. For the preparation of this membrane, initially, crystalline TiO2 nanoparticles were synthesized at room temperature using Bacillus licheniformis bacteria, and additionally, the modification continued by adding alumina to achieve hybrid biological and chemically synthesized Al2O3/TiO2 nanocomposite. Finally, this hybrid nanocomposite was impregnated onto electrospun thermoplastic polyurethane (TPU) nanofibers.
Mg–Al–Fe layered double hydroxides/polyether sulfone MMM (Mg–Al–Fe LDHs/PES) [66] is another type of modified membrane produced for fluoride removal. According to the results of this study, the Mg–Al–Fe LDHs/PES membranes exhibited a high adsorption capacity for fluoride, i.e., 1.61 mg g−1. The membranes could be reused four times.
A membrane has been synthesized by combining poly vinylidene fluoride with activated alumina and maifanite in order to finally obtain the (MFS-AA-PVDF) membrane that was tested as an adsorbent for the removal of fluoride from water [67]. The adsorption kinetics that were conducted showed that the adsorption into the membrane followed a pseudo-second-order model; moreover, the Langmuir model best fit the adsorption of fluoride into the membrane, which described the deposition of fluoride into the membrane as a monolayer. The optimum pH for this research was found to be 5.02 with an optimum initial fluoride concentration of 12.37 mg L−1 and a suitable temperature of 36.75 ℃. The percentage of fluoride removal by the synthesized membrane reached a maximum of 84%, under the various optimum conditions. In general, novel hybrid polyvinylidene fluoride membranes were successfully fabricated and have shown a good efficiency in the treatment of wastewaters from textile industry [68].
The commercial membrane NF90 was investigated in order to study its efficiency to remove fluoride from brackish waters [69]. The results showed that the rejection rate of the membrane was stabilized at a pressure of 11 bar. In addition, under optimum conditions of 42 mg L−1 of fluoride concentration and a pH of 7.2, 98% of fluoride was achieved while having a permeate flux of 64.8 L m−2 h−1. Taking into account the same conditions obtained from the results, the authors also found that NF90 was able to remove around 88% of fluoride concentration from a natural groundwater source at an almost neutral pH to retain a fluoride concentration of 0.35 mg L−1, which is lower than the concentration limit provided by the WHO.
A polyamide reverse osmosis membrane was investigated for the removal of fluoride from a binary water mixture, contaminated with fluoride and chromium(VI) [70]. For a solution containing a mixture of 5 mg L−1 of both fluoride and hexavalent chromium and under optimum conditions of a pH of 8 and a pressure of 16 bar, the reverse osmosis membrane was able to achieve a 99.97% rejection for fluoride. Both CFSK and CFSD models were used in order to evaluate in this work the performance of the membrane though different parameters such as the mass transfer coefficient and the permeate of the solute and results showed that the data obtained better fit the CFSK model.
A novel carboxylated polyacrylonitrile nanofibrous membrane (C-PAN NFM) [71] can be used as an excellent fluoride adsorption material. As a novel porous membrane material, C-PAN NFM has a wide application potential by using polyacrylonitrile as the raw material, because of the relative advantages in stability, cost and surface area.
Figure 4 shows the maximum fluoride uptake capacities (mg g−1) presented in this study. As depicted, the polyacrylonitrile hollow fiber membranes AlFu MOF exhibited the highest capacity (205 mg g−1), among them.
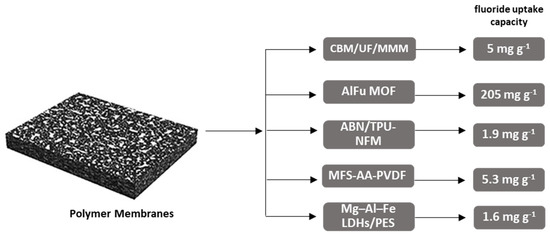
Figure 4.
Schematic graph depicting maximum fluoride uptake capacities (mg g−1) of presented. Polymer-based membranes, showing that MOF type materials show the highest capacity, far ahead by the second one.
2.2. Cellulose Membranes
Fe-Al-Mn@chitosan loaded CA-based mixed matrix membrane (MMM) [72] has been produced by the phase inversion method, in order to remove fluoride from groundwater. Respectable compatibility on chitosan and CA further enhances the homogeneous dispersion of nanoparticles (Fe-Al-Mn@chitosan) in the membrane matrix. The optimum molecular weight cut off (MWCO) for M8 (8% loading) membranes was found to be 8 kD, which can lead to a residual fluoride concentration within the permissible limit (<1.5 mg L−1) for 9 h at operating TMP of 2 bar, initially through adsorption and then due to electrostatic repulsion. M8 membrane has shown good antifouling properties and antibacterial prospective in water.
CDs-PyBA is an amino-modified cellulose membrane containing pyrene-boronic acid-based carbon dots that has been developed recently, by Li et al. [73], for the selective detection and removal of fluoride ion. This ratiometric nanosensor applied as the energy donor-acceptor pair for selectivity detecting F−, showed fast detection and excellent removal capacity for F− of 90.2%. Cellulose-based sensors could offer a dual-functionality for both sensing and removal of fluoride ions with the prospective benefits of processability, cost effectiveness and environmental friendliness.
Another cellulose membrane for defluoridation studies is the prepared PES/CA/Fe2O3, which is about an iron oxide (Fe2O3) modified polyethersulfone (PES)/CA blend flat sheet membrane [74]. According to the results obtained, this membrane exhibited the optimum pure water flux of 156 L m–2 h–1 and maximum fluoride removal efficiency of 70.3%. In addition, extended durability of the composite membrane was confirmed by re-usability studies for 8 cycles with constant rejection rate.
A CA-alumina flat sheet mixed matrix membrane has been delivered and examined for fluoride removal, achieving a removal of 88–92% by using as feed fluoride concentration 12 mg L−1 [30]. It is perceived that the molecular weight cut off (MWCO) of the produced membranes decreases with alumina concentration, with an initial value of 24 kDa. Cellulose acetate phthalate (CAP)-alumina membrane could be regenerated for five cycles of operation. In addition, simultaneously with the removal of fluoride, the removal of microorganisms was achieved by applying this type of membrane. CAP as the base polymer and activated alumina as an additive, were also used in order to produce a mixed matrix membrane (MMM), for cross flow ultrafiltration of fluoride, by Mondal et al. [75].
Aluminum fumarate metal oxide framework was added to CAP in order to synthesize a novel mixed matrix membrane suitable for the defluoridation process of groundwater [76]. Depending on the membrane MOF concentration within the membrane, the adsorption of fluoride varied, as it reached values of 107 mg g−1 to 179 mg g−1 for MOF weight percentage ranging between 2% and 10%. For a 10% concentration of aluminium fumarate, a 99% rejection of fluoride was achieved by the membrane; in addition, it was found that the life of the membrane was about 17 h for a fluoride concentration 10 mg L−1. The most desired transmembrane pressure was found to be 138 kPa, which lead to a breakthrough time of 15.5 h and resulted in a permeate flux of 17 L m−2 h−1.
Figure 5 summarizes the cellulose-based membrane categories, analyzed in this review. Maximum fluoride uptake capacities (mg g−1) are compared. The results indicate that the aluminum fumarate metal oxide membrane AlFu MOF exhibited the highest capacity (179 mg g−1).
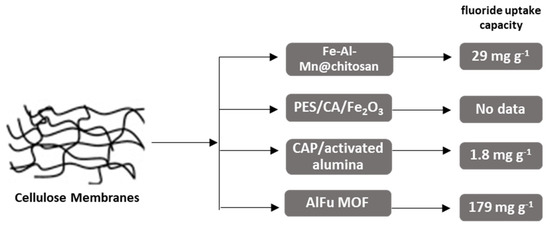
Figure 5.
Schematic graph, depicting maximum fluoride uptake capacities (mg g−1) of presented. Cellulose-based membranes. MOF type materials show the highest efficiencies for fluoride removal.
2.3. Graphene Membranes
The Zirconium-Chitosan/Graphene Oxide Membrane (Zr-CTS/GO) [31] appears to be an innovative and recent emerging material having an effective application in removing fluoride ions from aqueous media, in a wide pH range of 3–11 and quite short contact time of 45 min. According to this study, the Zr-CTS/GO membrane could effectively remove fluoride with an adsorption capacity 29.06 mg g−1, in a multilayer form as exhibited by the Freundlich model.
A further investigation from the same authors was conducted for the purpose of material characterization and its implementation for anion removal from groundwater [77]. Results indicated that mixing GO and zirconium chitosan to form the previously mentioned membrane led to an increase in the thermal stability of the components alongside mechanical strength. Moreover, the removal of fluoride from groundwater was efficient throughout a temperature range between 15 ℃ and 40 ℃, and equilibrium adsorption was reached within 1 h.
An innovative graphene-based nanocomposite flat-sheet cross-flow module membrane was manufactured by interfacial polymerization (IP) over chemical bonding of GO layer to polyethersulfone (PES) surface [78]. The application of this type membrane prospered in removal of more than 80% of fluoride from water. A significant advantage of the application of this graphene-based nanocomposite membrane is the preservation of beneficial calcium and magnesium minerals in drinking water. The practically high flux of about 150 L m−2 h−1 at a TMP of 15 bar shows that the aforementioned membrane provides a sustainable operation without any substantial drop in flux.
Figure 6 presents the maximum adsorption capacity (mg g−1) only for Zirconium-Chitosan/ GO Membrane (Zr-CTS/GO), while there are no relative data for graphene oxide (GO) layer/polyethersulfone (PES) membrane.
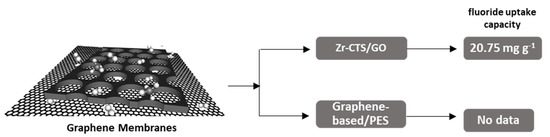
Figure 6.
Schematic graph of depicting maximum fluoride uptake capacities (mg g−1) of presented. Graphene-based membranes.
2.4. Other Modified Membranes
Carbon-based amyloid fibril nano-ZrO2 (<10 nm) composite (CAF-Zr) is a recently developed hybrid membrane for fluoride removal both from wastewater and drinking water [79]. This type of membrane exhibited excellent fluoride removal capacity above 99% for feeding concentrations up to 200 mg L−1, high selectivity and easy and fast purification via filtration. Hybrid CAF-Zr membranes are effective in fluoride removal either in low or high initial concentrations, typical of the tap and waste waters infection status, performing a treatment capacity of 1750 L water m−2, with a prospect effective regeneration of the membrane by alkaline treatment.
The use of zirconium for the modification of membranes was studied very recently by Mohamed et al. [16], according to whose research, composite nanofiber membranes (CNMs) were modified by using zirconium based on UiO-66 and its amino version (UiO-66-NH2) powders, resulting in UiO-66-NH2 CNM membranes. According to the results of this study, high fluoride removal occurred owing to both electrostatic interactions between the fluoride ions and the metal sites in MOF and the hydrogen bonds formed with MOF amino groups, with an adsorption capacity of 95 mg g−1 at pH 8.
Another modified membrane was produced by He et al. [80], who studied its application in the removal of fluoride from drinking water. Therefore, the Al-HAP membrane produced by modification of hydroxyapatite with Al(OH)3 nanoparticles is a biocompatible and novel membrane that exhibited a testament capacity of 1568 L m−2 for feed fluoride concentration of 5 mg L−1. An important factor in increasing the efficiency of the membrane was its thickness, the study of which showed that the fluoride removal efficiency increased with the thickness of Al-HAP membrane, providing as optimal the 0.3 mm. It is worth mentioning that this membrane, after six adsorption-desorption cycles, appeared to be suitable for regeneration.
One study synthesized a membrane based on zirconium metal organic framework (Zr-MOF) for the purpose of fluoride remediation from water [81]. After obtaining good results of the adsorption capabilities of the zirconium based MOF, the membrane experiments revealed that the Zr-MOF membrane was able to remove fluoride in an efficient manner through a dynamic input where the removal efficiency depended on the flow rate of the fluoride feed. When fluoride concentrations of 5, 8 and 10 mg L−1 were fed to the membrane of 20 μm thickness, the resulting removal capabilities were 5510, 5173 and 4664 L m−2 respectively. It was also found that the membrane can deal with a maximum water volume of 2700 mL when the fluoride concentration was 5 mg L−1 at a flow rate of 15 mL min−1. Regeneration studies showed that this amount dropped to 2235 mL after six cycles, which promotes this membrane as suitable for regeneration.
An economically efficient hybrid filtration cell (HFC), which utilizes limestone and activated carbons for fluoride’s removal from water, was examined [82], demonstrating that fluoride could be completely removed from artificial water at pH of 5.0, within 1.5 h, with an initial fluoride concentration of 30 mg L−1, adsorbent dosage of 30 mg L−1 and water temperature of 313 K.
NF/RO membranes under variable solar irradiance conditions were used to treat natural Tanzanian waters of high F- concentrations (17–60 mg L−1). These batteryless directly powered photovoltaic membrane filtration (PV-membrane) systems [83] demonstrated resilience that was more dependent on membrane type, and as it turned out, tighter NF/RO membranes showed high resilience to variations in permeate quality. The MWCO of the used NF90 and NF270 membranes was 90–180 and 150–340 kDa, respectively, demonstrating a permeability of 6.0–11.0 and 14.0–19.0 L h−1 m−2 bar−1.
Table 4 shows the reviewed membranes when employed for the removal of fluoride from water and offers evidence about their applications in water treatment. As shown in this Table, most of the membrane applied for defluoridation exhibit greater efficiency at pH values close to that of drinking water (i.e., 6–8). Maximum treatment capacity was obtained with the use of a cellulose modified membrane (Fe-Al-Mn@chitosan [72]) with a permeate flux of 2000 L m−2 h−1 similar to those obtained from the membrane produced by modification of hydroxyapatite with Al(OH)3 nanoparticles [80] (1568 L m−2 h−1) and zirconium-based MOF [79] (1750 L m−2 h−1).

Table 4.
Comparison of membranes in literature.
A technical-economic comparison study of NF and RO in the reduction of fluoride from groundwater occurred [85]. One NF and two RO polyamide membranes were used, and the results showed that NF membrane is slightly less expensive, with the following order: NF90 (NF) < BW30LE (RO) < TM710 (RO). The total annualized cost of water treatment by the NF membrane was calculated to be 0.43 $ m−3 [86].
Analysing the reviewed data in this paper, relative to the percentage % of publications on each membrane category (Figure 7), it was observed that the main focus of the reviewed publications, has been on the use of polymer-based membranes, with 46%, followed by cellulose-based and other type modified membranes at the same percentage % of occurrence (39% of the total publications) and only some cases (~15%) of GO-based membranes have been examined. The studies are mainly focusing on the membranes technique effect on the performance of fluoride removal addressing their possible impact on human health and the environment.
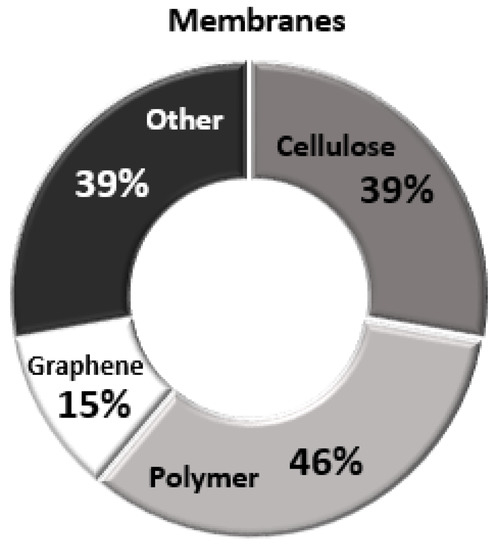
Figure 7.
Percentage % of publications on each membrane category with application on the fluoride removal. The main focus of the reviewed publications has been on the use of polymer-based membranes.
3. Conclusions
This review article presents the recently developed modified membranes for fluoride removal. Cellulose-, polymer- and graphene-based membranes were compared, as well as several modified membranes with metals, developed in the last years. The major findings are listed in the following points:
- Most of the membranes applied for fluoride removal reveal higher efficiency at the pH range relevant to drinking water treatment (i.e., 6–8).
- The polyacrylonitrile hollow fiber membrane AlFu MOF exhibited the highest fluoride capacity (205 mg g−1).
- Maximum treatment capacity was obtained with the use of a cellulose-modified membrane Fe-Al-Mn@chitosan with a permeate flux of 2000 L m−2 h−1. The carbon-based amyloid fibril nano-ZrO2 composites (CAF-Zr) and Al-HAP membrane produced by modification of hydroxyapatite with Al(OH)3 follows, with values for treatment capacity of 1750 and 1568 L m−2, respectively.
- Main focus of the reviewed studies has been on the use of polymer-based membranes.
- A technical-economic comparison study of NF and RO in the reduction of fluoride from groundwater showed that NF membrane is slightly less expensive.
Author Contributions
Conceptualization, A.K.T., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; methodology, A.K.T., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; validation, I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; formal analysis, A.K.T., E.M., F.M.C., I.A.K., G.Z.K. and E.A.D.; investigation, A.K.T., E.M., F.M.C. and I.A.K.; resources, A.K.T., E.M., F.M.C., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; data curation, A.K.T., E.M. and F.M.C.; writing—original draft preparation, A.K.T., E.M. and F.M.C.; writing—review and editing, A.K.T., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; visualization, A.K.T., E.M., F.M.C., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C.; supervision, A.K.T., I.A.K., G.Z.K., E.A.D., V.T. and M.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
| ABN/TPU-NFM | Al2O3/bio-TiO2 nanocomposite impregnated electrospunTPU nanofiber membrane |
| Al Fu MOF | Aluminium fumarate metal organic frameworks |
| CAF-Zr | Carbon based amyloid fibril nano-ZrO2 composites |
| CBM | Carbonized bone meal |
| CA | Cellulose acetate |
| CAP | Cellulose acetate phthalate |
| GO | Graphene Oxide |
| MOF | Metal organic frameworks |
| MF | Microfiltration |
| MFS-AA-PVDF | Polyvinylidene fluoride-activated alumina-maifanite membranes |
| MMM | Mixed matrix membrane |
| MWCO | Molecular weight cut off |
| NF | Nanofiltration |
| PES | Polyethersulfone |
| PV-membrane | Photovoltaic membrane |
| RO | Reverse osmosis |
| TPU | Thermoplastic polyurethane |
| TMP | Trans membrane pressure |
| UF | Ultrafiltration |
| Zr-CTS/GO | Zirconium-chitosan/graphene oxide membrane |
| Zr-MOF | Zirconium metal organic framework |
References
- Tolkou, A.K.; Mitrakas, M.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Fluoride removal from water by composite Al/Fe/Si/Mg pre-polymerized coagulants: Characterization and application. Chemosphere 2019, 231, 528–537. [Google Scholar] [CrossRef]
- Damtie, M.M.; Woo, Y.C.; Kim, B.; Hailemariam, R.H.; Park, K.D.; Shon, H.K.; Park, C.; Choi, J.S. Removal of fluoride in membrane-based water and wastewater treatment technologies: Performance review. J. Environ. Manag. 2019, 251, 109524. [Google Scholar] [CrossRef]
- World Health Organization European Standards for Drinking-Water. Am. J. Med. Sci. 1970, 242, 56.
- Herschy, R.W. Water quality for drinking: WHO guidelines. Encycl. Earth Sci. Ser. 2012, 876–883. [Google Scholar] [CrossRef]
- Arya, S.; Subramani, T.; Vennila, G.; Karunanidhi, D. Health risks associated with fluoride intake from rural drinking water supply and inverse mass balance modeling to decipher hydrogeochemical processes in Vattamalaikarai River basin, South India. Environ. Geochem. Health 2021, 43, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Premathilaka, R.W.; Liyanagedera, N.D. Fluoride in drinking water and nanotechnological approaches for eliminating excess fluoride. J. Nanotechnol. 2019, 2019, 2192383. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and the Council of the European Union Directive (EU) 2020/2184, EU (revised) Drinking Water Directive. Off. J. Eur. Communities 2020, 2019, 1–62.
- Kabay, N.; Arar, Ö.; Samatya, S.; Yüksel, Ü.; Yüksel, M. Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species. J. Hazard. Mater. 2008, 153, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Hosamani, K.M.; Reddy, H.R.S.; Nataraj, S.K.; Aminabhavi, T.M. Application of the electrodialytic pilot plant for fluoride removal. J. Water Chem. Technol. 2011, 33, 293–300. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, C.B.; Hoque, M.A.; Islam, M.A.; Dewan, A. Groundwater Constituents and Trace Elements in the Basement Aquifers of Africa and Sedimentary Aquifers of Asia: Medical Hydrogeology of Drinking Water Minerals and Toxicants. Earth Syst. Environ. 2020, 4, 369–384. [Google Scholar] [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Ali, S.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Health 2017, 40, 1265–1281. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, X.; Zhang, L.; Wu, B.; Zhang, G.; Zhang, S. Coagulation removal of fluoride by zirconium tetrachloride: Performance evaluation and mechanism analysis. Chemosphere 2019, 218, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Roy, A.; Sarkar, S.; Ghosh, S.; Majumdar, S.; Chakraborty, S.; Mandal, S.; Mukhopadhyay, A.; Bandyopadhyay, S. Combination technology of ceramic microfiltration and reverse osmosis for tannery wastewater recovery. Water Resour. Ind. 2013, 3, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Valadez Sanchez, E.P.; Bogdanova, E.; Bergfeldt, B.; Mahmood, A.; Ostvald, R.V.; Hashem, T. Efficient Fluoride Removal from Aqueous Solution Using Zirconium-Based Composite Nanofiber Membranes. Membranes 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Suganya, S.; Srinivas, S.; Priyadharshini, S.; Karthika, M.; Karishma Sri, R.; Swetha, V.; Naushad, M.; Lichtfouse, E. Treatment of fluoride-contaminated water. A review. Environ. Chem. Lett. 2019, 17, 1707–1726. [Google Scholar] [CrossRef] [Green Version]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in drinking water and defluoridation of water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Manousi, N.; Zachariadis, G.A.; Katsoyiannis, I.A. Recently Developed Adsorbing Materials for Fluoride Removal from Water and Fluoride Analytical Determination Techniques: A Review. Sustainability 2021, 13, 7061. [Google Scholar] [CrossRef]
- Alhassan, S.I.; Huang, L.; He, Y.; Yan, L.; Wu, B.; Wang, H. Fluoride removal from water using alumina and aluminum-based composites: A comprehensive review of progress. Crit. Rev. Environ. Sci. Technol. 2020, 1–35. [Google Scholar] [CrossRef]
- Gallios, G.P.; Tolkou, A.K.; Katsoyiannis, I.A.; Stefusova, K.; Vaclavikova, M.; Deliyanni, E.A. Adsorption of arsenate by nano scaled activated carbon modified by iron and manganese oxides. Sustainability 2017, 9, 1684. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.X.; Qu, J.H.; Liu, R.P.; Lan, H.C. Effect of aluminum fluoride complexation on fluoride removal by coagulation. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 395, 88–93. [Google Scholar] [CrossRef]
- He, Z.; Liu, R.; Xu, J.; Liu, H.; Qu, J. Defluoridation by Al-based coagulation and adsorption: Species transformation of aluminum and fluoride. Sep. Purif. Technol. 2015, 148, 68–75. [Google Scholar] [CrossRef]
- Hug, S.J.; Winkel, L.H.E.; Voegelin, A.; Berg, M.; Johnson, A.C. Arsenic and Other Geogenic Contaminants in Groundwater-A Global Challenge. Chimia 2020, 74, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Sorlini, S.; Palazzini, D.; Collivignarelli, C. Fluoride removal from drinking water in Senegal: Laboratory and pilot experimentation on bone char-based treatment. J. Water Sanit. Hyg. Dev. 2011, 1, 213–223. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Torretta, V.; Rada, E.C.; Caccamo, F.M.; Sorlini, S. Adsorption of fluorides in drinking water by palm residues. Sustainability 2020, 12, 3786. [Google Scholar] [CrossRef]
- Du, Y.; Wang, D.; Wang, W.; Fu, J.; Chen, X.; Wang, L.; Yang, W.; Zhang, X. Electrospun Nanofibrous Polyphenylene Oxide Membranes for High-Salinity Water Desalination by Direct Contact Membrane Distillation. ACS Sustain. Chem. Eng. 2019, 7, 20060–20069. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Chatterjee, S.; De, S. Adsorptive removal of fluoride by activated alumina doped cellulose acetate phthalate (CAP) mixed matrix membrane. Sep. Purif. Technol. 2014, 125, 223–238. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, N.; Su, P.; Li, M.; Feng, C. Fluoride removal from aqueous solution by Zirconium-Chitosan/Graphene Oxide Membrane. React. Funct. Polym. 2017, 114, 127–135. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Memb. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Ochando Pulido, J.M.; Stoller, M.; Victor-Ortega, M.D.; Martinez-Ferez, A. Analysis of the Fouling Build-up of a Spiral Wound Reverse Osmosis Membrane in the Treatment of Two-phase Olive Mill Wastewater. Chem. Eng. Trans. 2016, 47, 403–408, SE-Research Articles. [Google Scholar] [CrossRef]
- Zuo, K.; Chen, M.; Liu, F.; Xiao, K.; Zuo, J.; Cao, X.; Zhang, X.; Liang, P.; Huang, X. Coupling microfiltration membrane with biocathode microbial desalination cell enhances advanced purification and long-term stability for treatment of domestic wastewater. J. Memb. Sci. 2018, 547, 34–42. [Google Scholar] [CrossRef]
- Praveen, P.; Heng, J.Y.P.; Loh, K.C. Tertiary wastewater treatment in membrane photobioreactor using microalgae: Comparison of forward osmosis & microfiltration. Bioresour. Technol. 2016, 222, 448–457. [Google Scholar] [CrossRef]
- Al-Obaidi, M.A.; Li, J.P.; Kara-Zaïtri, C.; Mujtaba, I.M. Optimisation of reverse osmosis based wastewater treatment system for the removal of chlorophenol using genetic algorithms. Chem. Eng. J. 2017, 316, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Indika, S.; Wei, Y.; Hu, D.; Ketharani, J.; Ritigala, T.; Cooray, T.; Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Weragoda, S.K.; et al. Evaluation of performance of existing ro drinking water stations in the north central province, Sri Lanka. Membranes 2021, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Kasongo, G. The removal of selected inorganics from municipal membrane bioreactor wastewater using UF/NF/RO membranes for water reuse application: A pilot-scale study. Membranes 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for arsenic removal: Challenges, recent developments, and perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Roy, S.; Majumdar, S.; Sahoo, G.C.; Bhowmick, S.; Kundu, A.K.; Mondal, P. Removal of As(V), Cr(VI) and Cu(II) using novel amine functionalized composite nanofiltration membranes fabricated on ceramic tubular substrate. J. Hazard. Mater. 2020, 399, 122841. [Google Scholar] [CrossRef]
- Szczuka, A.; Chuang, Y.-H.; Chen, F.C.; Zhang, Z.; Desormeaux, E.; Flynn, M.; Parodi, J.; Mitch, W.A. Removal of Pathogens and Chemicals of Emerging Concern by Pilot-Scale FO-RO Hybrid Units Treating RO Concentrate, Graywater, and Sewage for Centralized and Decentralized Potable Reuse. ACS ES&T Water 2021, 1, 89–100. [Google Scholar] [CrossRef]
- Bolto, B.; Zhang, J.; Wu, X.; Xie, Z. A review on current development of membranes for oil removal from wastewaters. Membranes 2020, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Diogo Januário, E.F.; de Camargo Lima Beluci, N.; Vidovix, T.B.; Vieira, M.F.; Bergamasco, R.; Salcedo Vieira, A.M. Functionalization of membrane surface by layer-by-layer self-assembly method for dyes removal. Process Saf. Environ. Prot. 2020, 134, 140–148. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpour, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochem. 2019, 58, 104633. [Google Scholar] [CrossRef] [PubMed]
- Humbert, H.; Machinal, C.; Labaye, I.; Schrotter, J.C. Virus removal retention challenge tests performed at lab scale and pilot scale during operation of membrane units. Water Sci. Technol. 2011, 63, 255–261. [Google Scholar] [CrossRef]
- Yuchi, A.; Matsunaga, K.; Niwa, T.; Terao, H.; Wada, H. Separation and preconcentration of fluoride at the ng ml-1 level with a polymer complex of zirconium(IV) followed by potentiometric determination in a flow system. Anal. Chim. Acta 1999, 388, 201–208. [Google Scholar] [CrossRef]
- Muro, C.; Riera, F.; del Carmen Diaz, M. Membrane Separation Process in Wastewater Treatment of Food Industry. In Food Industrial Processes–Methods and Equipment; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Singh, P.; Singh, A. Fluoride ions vs removal technologies: A study. Arab. J. Chem. 2016, 9, 815–824. [Google Scholar] [CrossRef]
- Aliyu, U.M.; Rathilal, S.; Isa, Y.M. Membrane desalination technologies in water treatment: A review. Water Pract. Technol. 2018, 13, 738–752. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Pandian, S.; Panchal, H. Overview of fluoride removal from water using separation techniques. Environ. Technol. Innov. 2021, 21, 101246. [Google Scholar] [CrossRef]
- Verma, S.P.; Sarkar, B. Simultaneous removal of Cd (II) and p-cresol from wastewater by micellar-enhanced ultrafiltration using rhamnolipid: Flux decline, adsorption kinetics and isotherm studies. J. Environ. Manag. 2018, 213, 217–235. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ingole, P.G.; Yun, S.; Choi, W.; Kim, J.; Lee, H. Water vapor removal using CA/PEG blending materials coated hollow fiber membrane. J. Chem. Technol. Biotechnol. 2015, 90, 1117–1123. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Hegab, H.M.; Wimalasiri, Y.; Ginic-Markovic, M.; Zou, L. Improving the fouling resistance of brackish water membranes via surface modification with graphene oxide functionalized chitosan. Desalination 2015, 365, 99–107. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 1–4. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Graphene Oxide/Fe-Based Composite Pre-Polymerized Coagulants: Synthesis, Characterization, and Potential Application in Water Treatment. C—J. Carbon Res. 2020, 6, 44. [Google Scholar] [CrossRef]
- Harker, A.H. Electrical conduction in graphene and nanotubes, by Shigeji Fujita and Akira Suzuki. Contemp. Phys. 2016, 57, 276–277. [Google Scholar] [CrossRef]
- Rashidi Nodeh, H.; Wan Ibrahim, W.A.; Ali, I.; Sanagi, M.M. Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As(III) and As(V) species from environmental water samples. Environ. Sci. Pollut. Res. 2016, 23, 9759–9773. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Katsoyiannis, I.A.; Zouboulis, A.I. Removal of arsenic, chromium and uranium from water sources by novel nanostructured materials including graphene-based modified adsorbents: A mini review of recent developments. Appl. Sci. 2020, 10, 3241. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Bi, D. Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ. Sci. Pollut. Res. 2015, 22, 4715–4724. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mukherjee, M.; De, S. Groundwater defluoridation and disinfection using carbonized bone meal impregnated polysulfone mixed matrix hollow-fiber membranes. J. Water Process Eng. 2020, 33, 101002. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhattacharjee, S.; De, S. Aluminium fumarate metal organic framework incorporated polyacrylonitrile hollow fiber membranes: Spinning, characterization and application in fluoride removal from groundwater. Chem. Eng. J. 2018, 334, 41–53. [Google Scholar] [CrossRef]
- Suriyaraj, S.P.; Bhattacharyya, A.; Selvakumar, R. Hybrid Al2O3/bio-TiO2 nanocomposite impregnated thermoplastic polyurethane (TPU) nanofibrous membrane for fluoride removal from aqueous solutions. RSC Adv. 2015, 5, 26905–26912. [Google Scholar] [CrossRef]
- Jia, Z.; Hao, S.; Lu, X. Exfoliated Mg–Al–Fe layered double hydroxides/polyether sulfone mixed matrix membranes for adsorption of phosphate and fluoride from aqueous solutions. J. Environ. Sci. (China) 2018, 70, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zheng, X.; Hou, J.; Wang, C. Preparation and Defluoridation Effectiveness of Composite Membrane Sorbent MFS-AA-PVDF. Water. Air. Soil Pollut. 2020, 231, 1–10. [Google Scholar] [CrossRef]
- Ben Dassi, R.; Chamam, B.; Méricq, J.P.; Faur, C.; El Mir, L.; Trabelsi, I.; Heran, M. Novel polyvinylidene fluoride/lead-doped zinc oxide adsorptive membranes for enhancement of the removal of reactive textile dye. Int. J. Environ. Sci. Technol. 2021, 18, 2793–2804. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, X.; Gao, S.; Wang, X.; Zhang, R. Progress in research and application of nanofiltration (Nf) technology for brackish water treatment. Membranes 2021, 11, 662. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Balomajumder, C. Simultaneous rejection of fluoride and Cr(VI) from synthetic fluoride-Cr(VI) binary water system by polyamide flat sheet reverse osmosis membrane and prediction of membrane performance by CFSK and CFSD models. J. Mol. Liq. 2017, 234, 194–200. [Google Scholar] [CrossRef]
- Chen, X.; Wan, C.; Yu, R.; Meng, L.; Wang, D.; Chen, W.; Duan, T.; Li, L. A novel carboxylated polyacrylonitrile nanofibrous membrane with high adsorption capacity for fluoride removal from water. J. Hazard. Mater. 2021, 411, 125113. [Google Scholar] [CrossRef]
- Chaudhary, M.; Maiti, A. Fe–Al–Mn@chitosan based metal oxides blended cellulose acetate mixed matrix membrane for fluoride decontamination from water: Removal mechanisms and antibacterial behavior. J. Memb. Sci. 2020, 611, 118372. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Jiang, M.; Liu, X.; Chen, Z.; Wang, S.; James, T.D.; Wang, L.; Xiao, H. Engineering a ratiometric fluorescent sensor membrane containing carbon dots for efficient fluoride detection and removal. Chem. Eng. J. 2020, 399, 125741. [Google Scholar] [CrossRef]
- Evangeline, C.; Pragasam, V.; Rambabu, K.; Velu, S.; Monash, P.; Arthanareeswaran, G.; Banat, F. Iron oxide modified polyethersulfone/cellulose acetate blend membrane for enhanced defluoridation application. Desalin. Water Treat. 2019, 156, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Chatterjee, S.; De, S. Theoretical investigation of cross flow ultrafiltration by mixed matrix membrane: A case study on fluoride removal. Desalination 2015, 365, 347–354. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhattacharjee, S.; De, S. Experimental and modeling of fluoride removal using aluminum fumarate (AlFu) metal organic framework incorporated cellulose acetate phthalate mixed matrix membrane. J. Environ. Chem. Eng. 2017, 5, 6087–6097. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, N.; Li, M.; Feng, C. Synthesis and environmental application of zirconium–chitosan/graphene oxide membrane. J. Taiwan Inst. Chem. Eng. 2017, 77, 106–112. [Google Scholar] [CrossRef]
- Pal, M.; Mondal, M.K.; Paine, T.K.; Pal, P. Purifying arsenic and fluoride-contaminated water by a novel graphene-based nanocomposite membrane of enhanced selectivity and sustained flux. Environ. Sci. Pollut. Res. 2018, 25, 16579–16589. [Google Scholar] [CrossRef]
- Zhang, Q.; Bolisetty, S.; Cao, Y.; Handschin, S.; Adamcik, J.; Peng, Q.; Mezzenga, R. Selective and Efficient Removal of Fluoride from Water: In Situ Engineered Amyloid Fibril/ZrO2 Hybrid Membranes. Angew. Chemie—Int. Ed. 2019, 58, 6012–6016. [Google Scholar] [CrossRef]
- He, J.; Chen, K.; Cai, X.; Li, Y.; Wang, C.; Zhang, K.; Jin, Z.; Meng, F.; Wang, X.; Kong, L.; et al. A biocompatible and novelly-defined Al-HAP adsorption membrane for highly effective removal of fluoride from drinking water. J. Colloid Interface Sci. 2017, 490, 97–107. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cai, X.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Meng, F.; Liu, N.; Wang, X.; Kong, L.; et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal. J. Colloid Interface Sci. 2016, 484, 162–172. [Google Scholar] [CrossRef]
- Alhendal, M.; Nasir, M.J.; Hashim, K.S.; Amoako-Attah, J.; Al-Faluji, D.; Muradov, M.; Kot, P.; Abdulhadi, B. Cost-effective hybrid filter for remediation of water from fluoride. IOP Conf. Ser. Mater. Sci. Eng. 2020, 888, 012038. [Google Scholar] [CrossRef]
- Boussouga, Y.A.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: System resilience under solar irradiance fluctuations during the treatment of fluoride-rich natural waters by different nanofiltration/reverse osmosis membranes. J. Memb. Sci. 2021, 617, 118452. [Google Scholar] [CrossRef]
- Ramdani, A.; Gassara, S.; Deratani, A.; Taleb, S. Proceedings of the Third International Symposium on Materials and Sustainable Development; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319897073. [Google Scholar]
- Tahaikt, M.; El-Ghzizel, S.; Essafi, N.; Hafsi, M.; Taky, M.; Elmidaoui, A. Technical-economic comparison of nanofiltration and reverse osmosis in the reduction of fluoride ions from groundwater: Experimental, modeling, and cost estimate. Desalin. Water Treat. 2021, 216, 83–95. [Google Scholar] [CrossRef]
- Fatehizadeh, A.; Amin, M.M.; Sillanpää, M.; Hatami, N.; Taheri, E.; Baghaei, N.; Mahajan, S. Modeling of fluoride rejection from aqueous solution by nanofiltration process: Single and binary solution. Desalin. Water Treat. 2020, 193, 224–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).