Atomic Layer Deposition of Nanolayered Carbon Films
Abstract
:1. Introduction
2. Experimental Details (Methods)
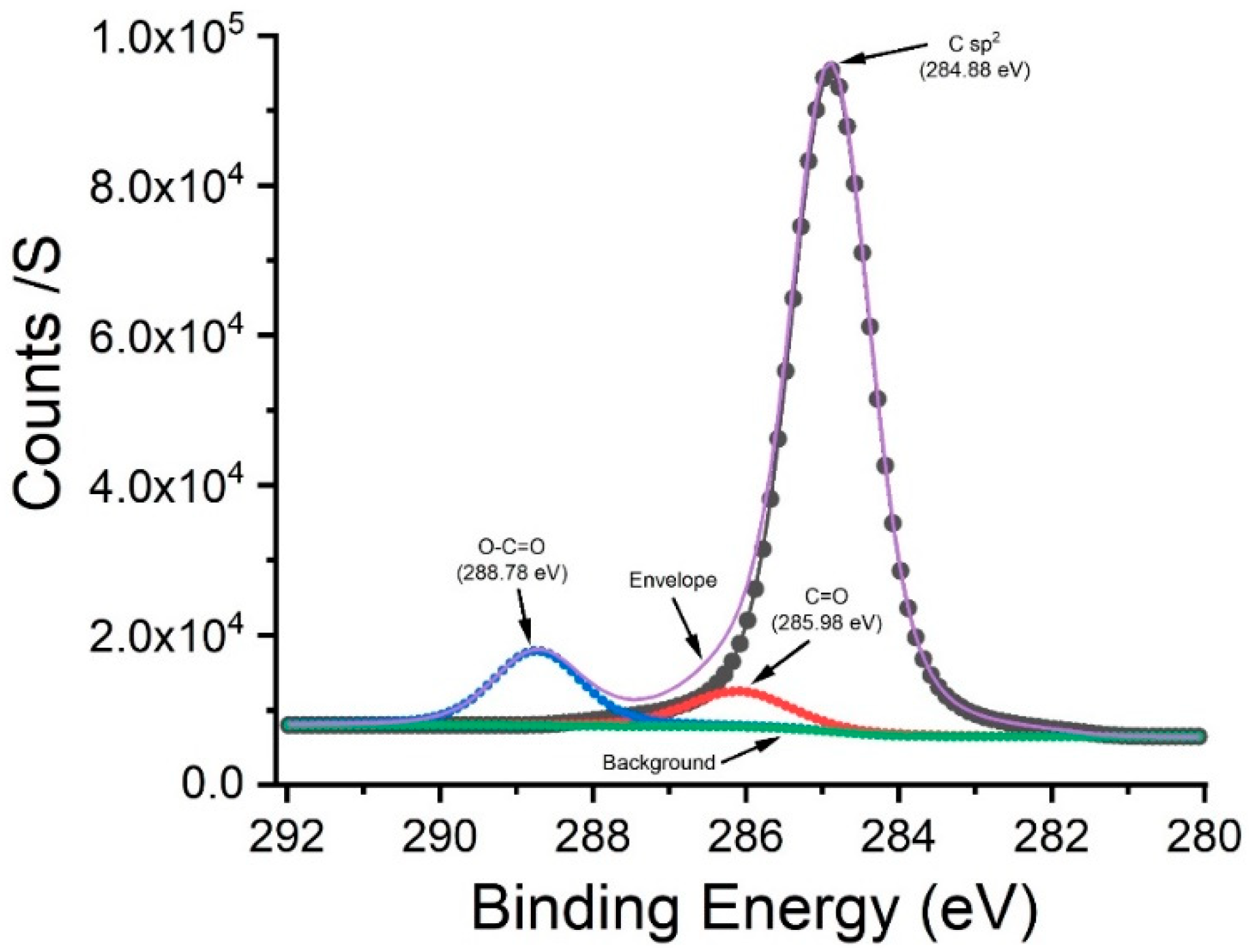
2.1. X-ray Photoelectron Spectroscopy (XPS) for Analysis of the Composition and Atomic Concentration of the Carbon Film
2.2. Scanning Electron Micrograph (SEM) for the Imaging and Analysis of the Surface Morphology of Carbon Films
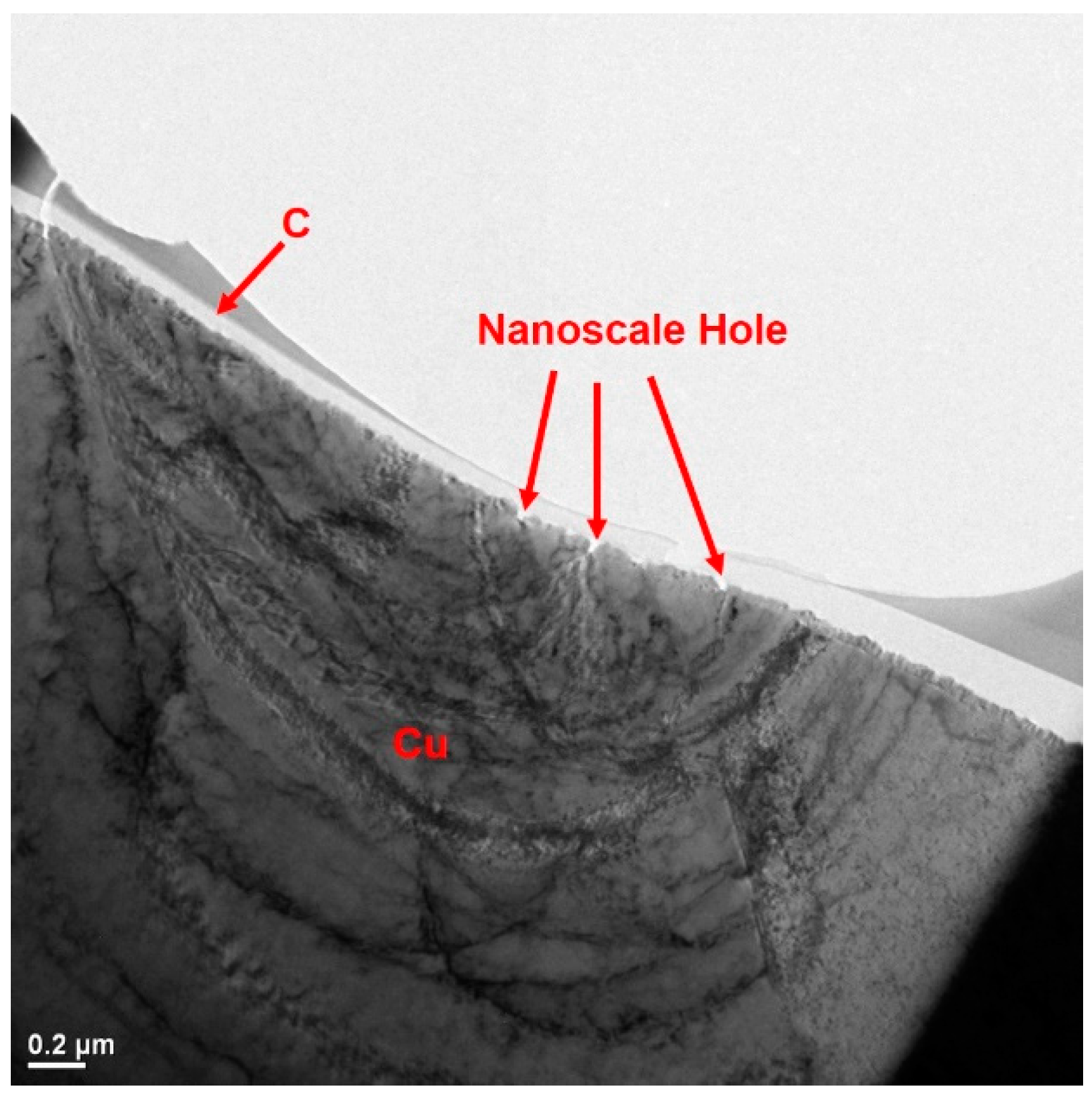
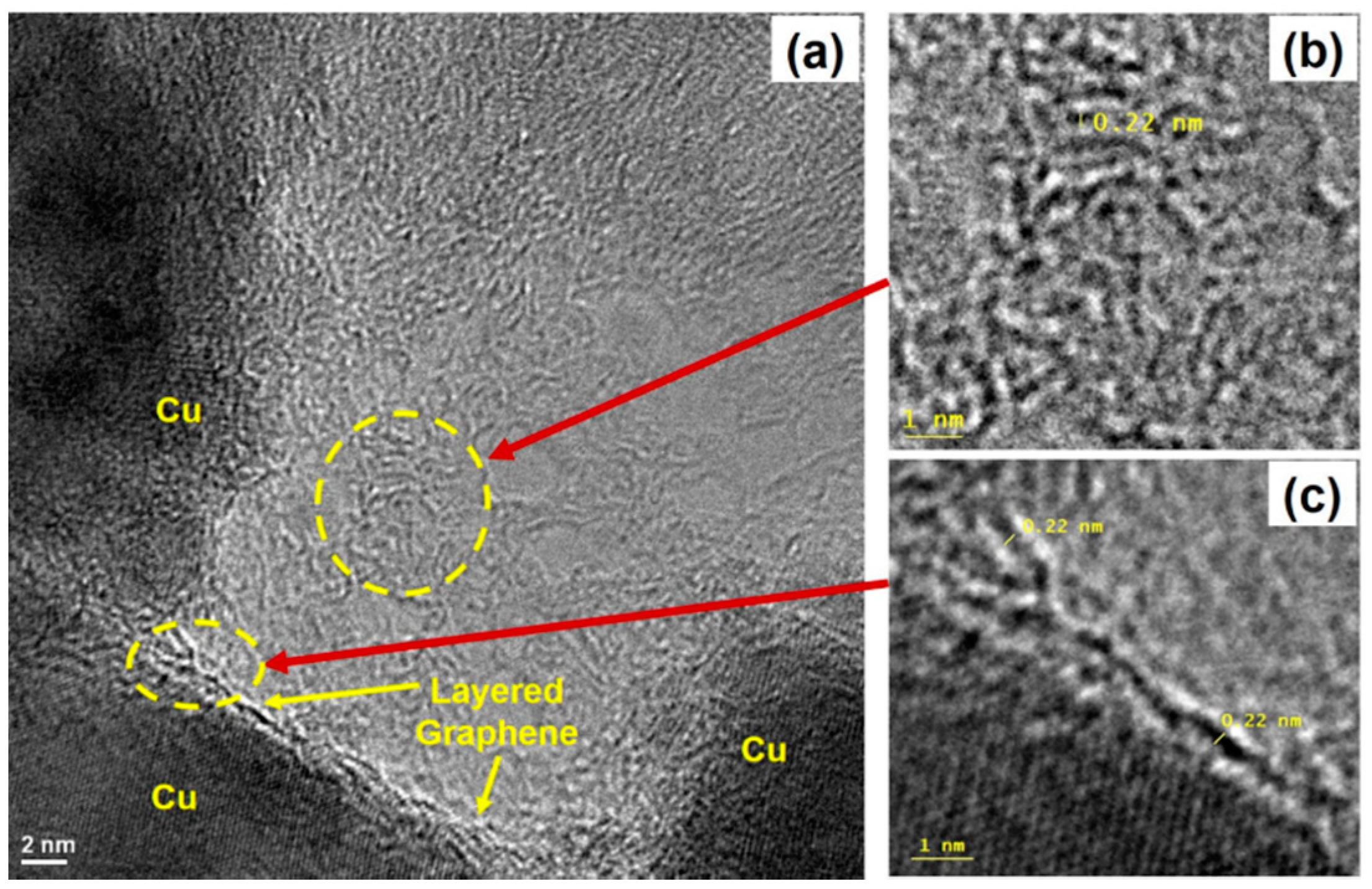
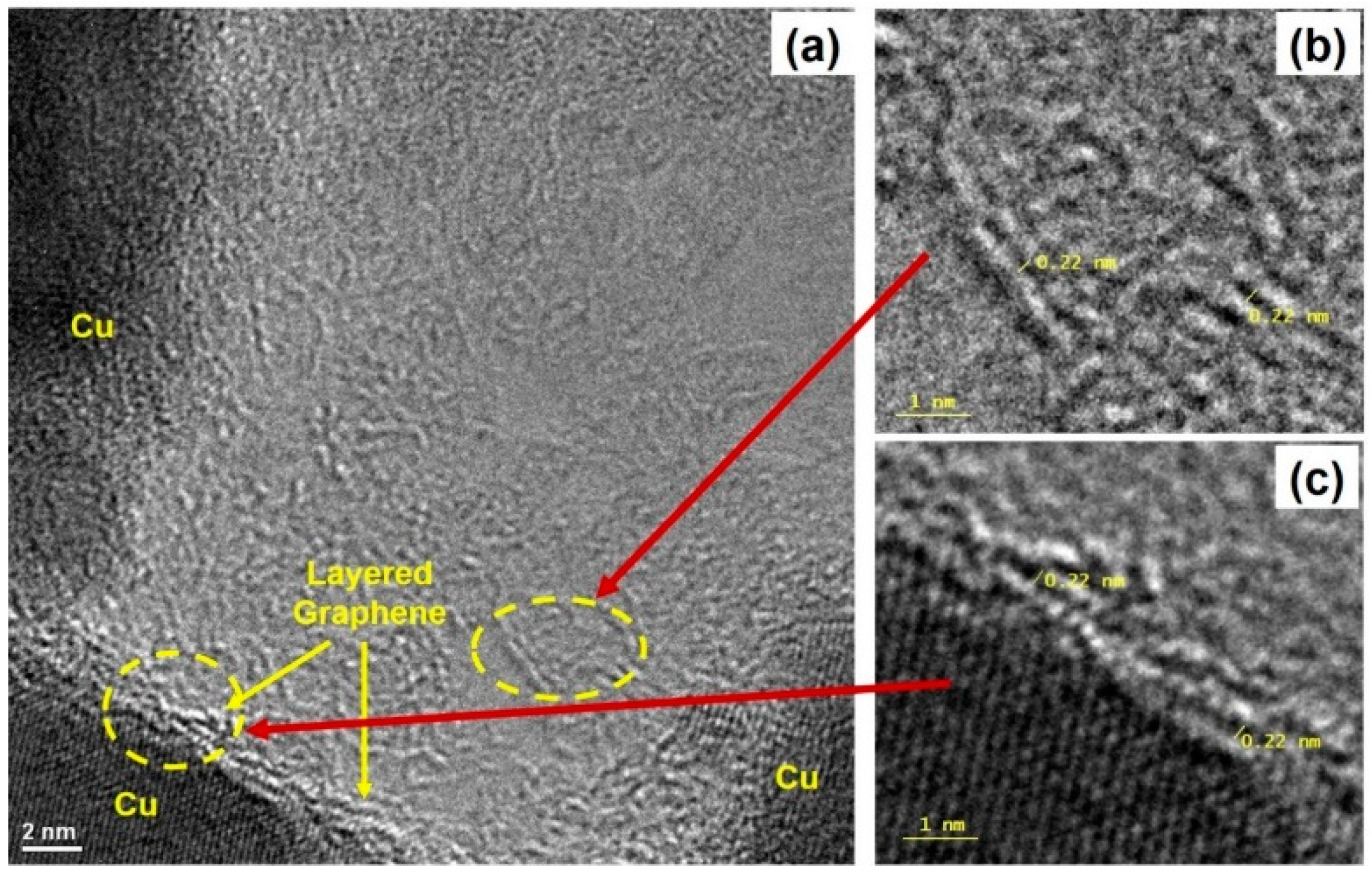
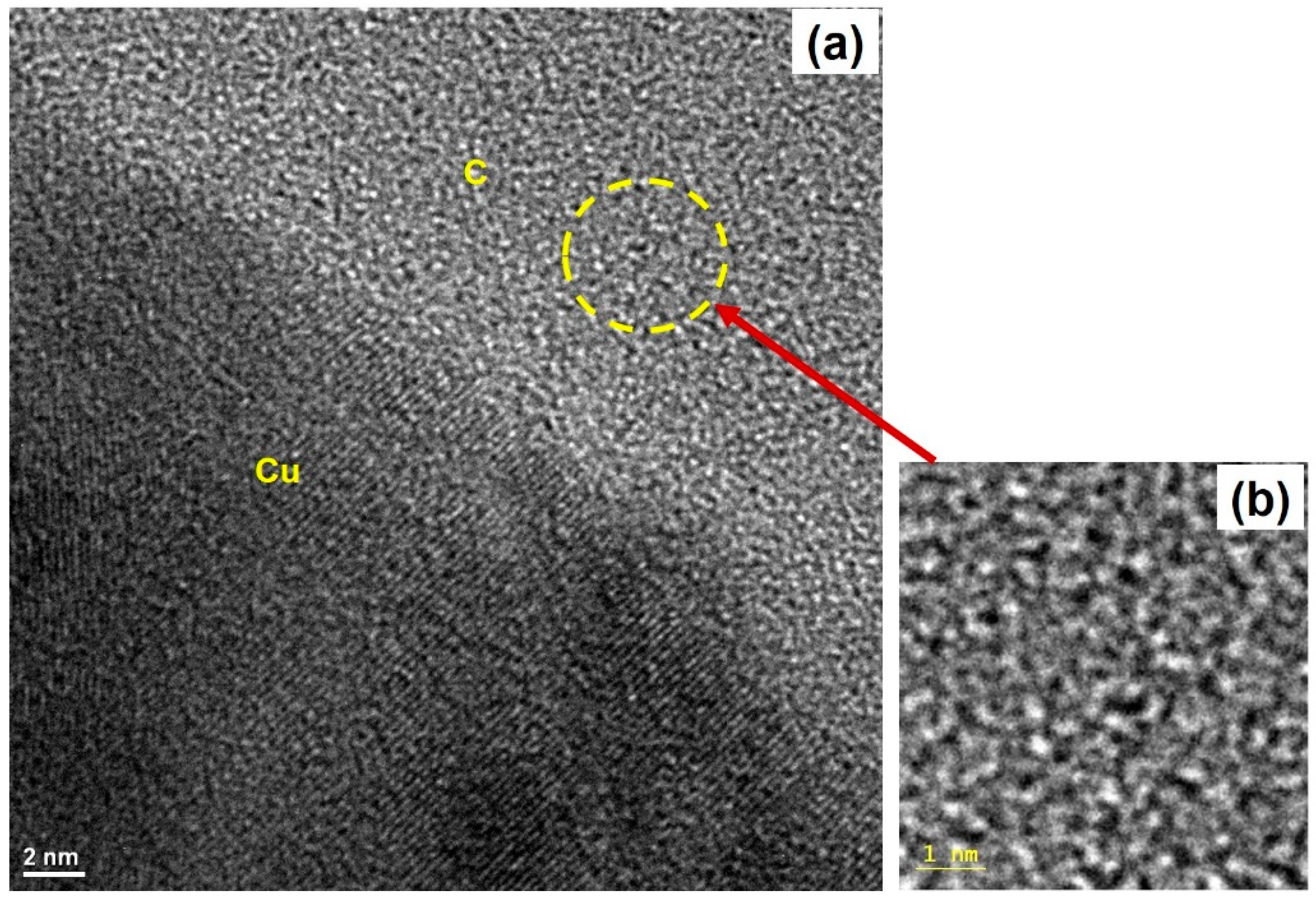
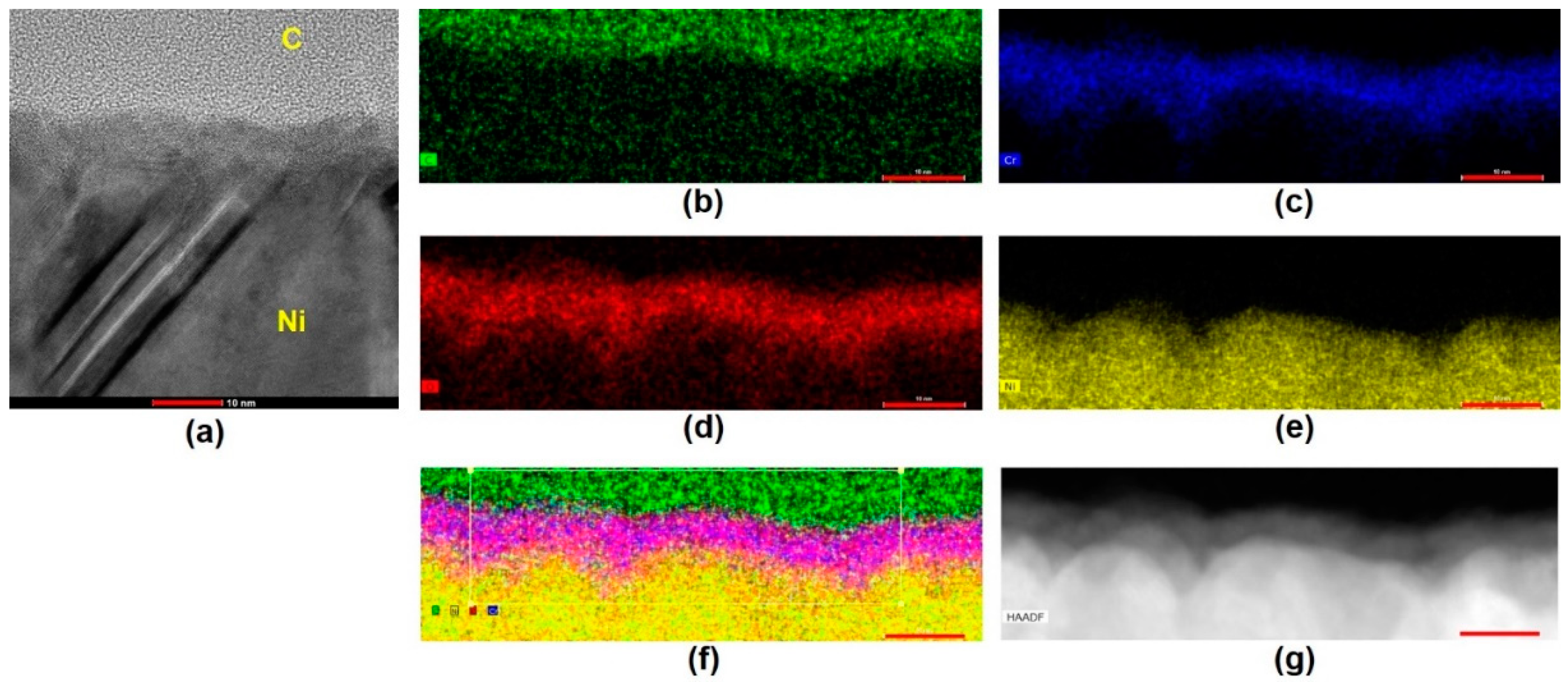
2.3. High-Resolution Tunneling Electron Micrograph (HRTEM) Imaging and Energy Dispersive Spectroscopy (EDS) Mapping of Carbon Films
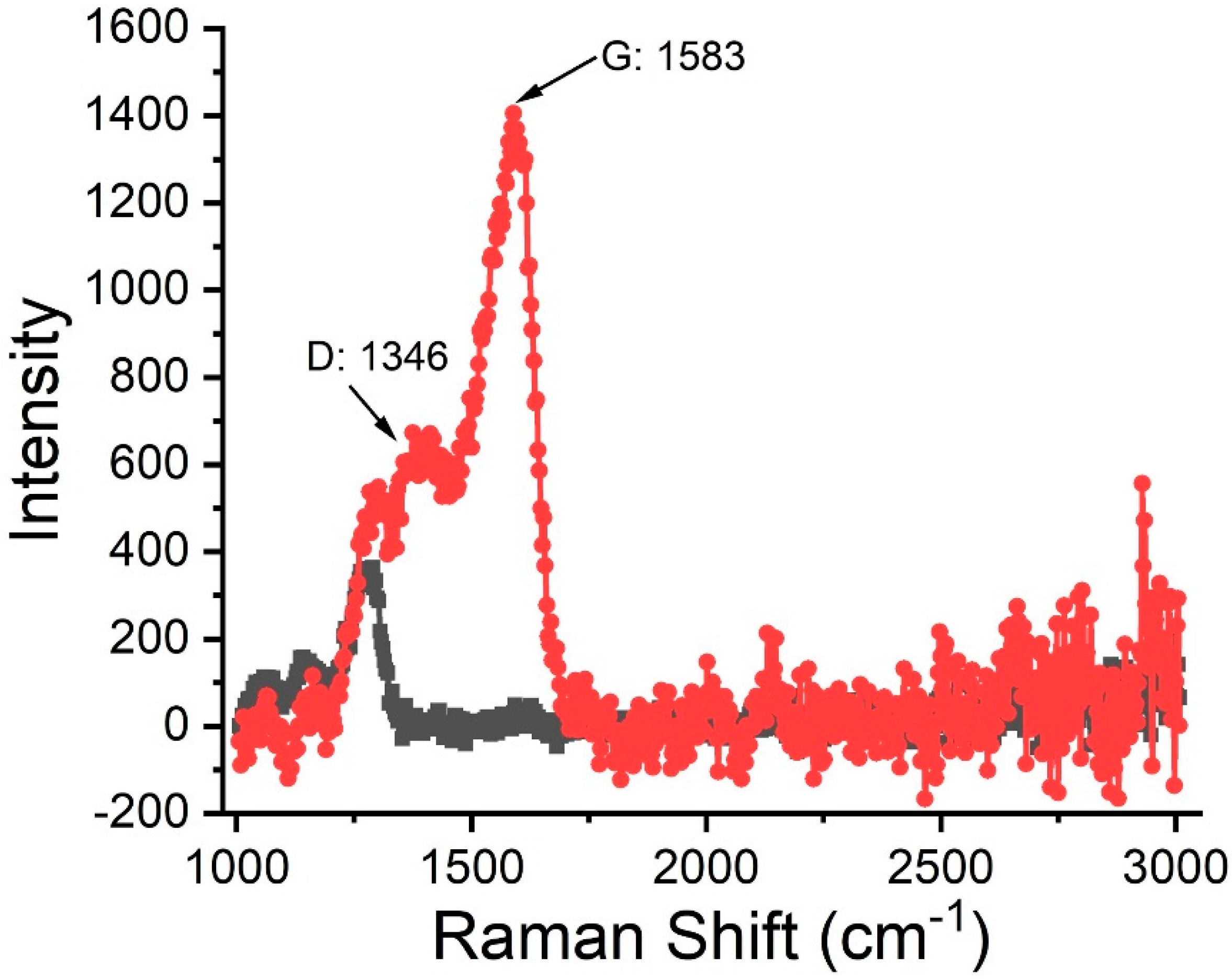
2.4. Raman Spectroscopy Analysis of Carbon Films
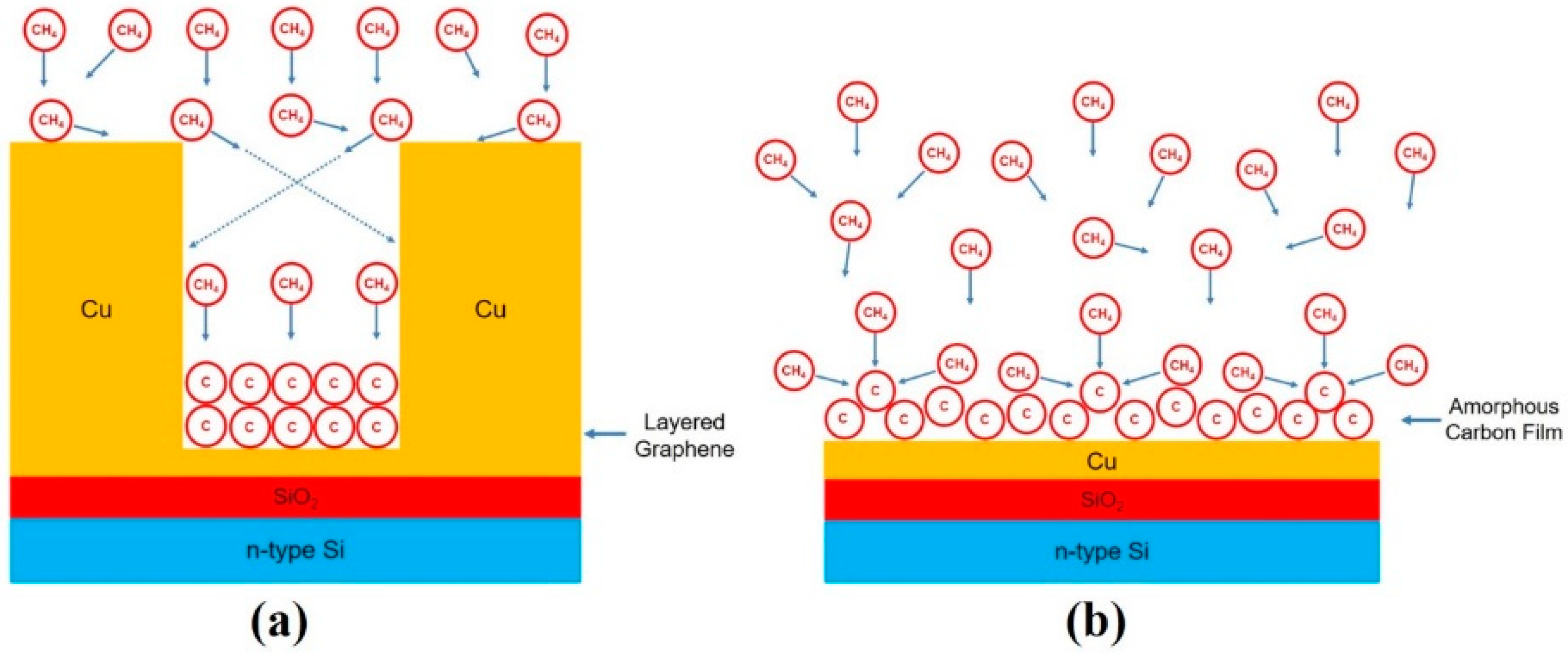
3. Results and Discussion
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Atomic Concentration (%) of Carbon Film | |||
|---|---|---|---|
| C | O | Cu | Others (F, Ca, Cl) |
| 72 | 20 | 3 | 5 |


References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.S.; Liang, T.; Shi, M.M.; Chen, H.Z. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, Y. Atomically thin boron nitride: Unique properties and applications. Adv. Funct. Mater. 2016, 26, 2594–2608. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.; Eda, G.; Li, L.; Loh, K.; Zhang, H. The chemistry of two-dimensional transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Mallouk, T.T.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Stormer, H.L.; Kim, P. Experimental observation of the quantum hall effect and berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and breaking of ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Pimpolari, L.; Calabrese, G.; Worsley, R.; Majee, S.; Polyushkin, D.; Paur, M.; Pace, S.; Keum, D.; Fabbri, F.; et al. Low-voltage 2D materials-based printed field-effect transistors for integrated digital and analog electronics on paper. Nat. Commun. 2020, 11, 3566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Halim, U.; Ding, M.; Liu, Y.; Wang, Y.; Jia, C.; Chen, P.; Duan, X.; Wang, C.; Song, F.; et al. Solution-processable 2D semiconductors for high performance large-area electronics. Nature 2018, 562, 254–258. [Google Scholar]
- Liu, C.; Chen, H.; Wang, S.; Liu, Q.; Jiang, Y.; Zhang, D.; Liu, M.; Zhou, P. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 2020, 15, 545–557. [Google Scholar] [CrossRef]
- Li, L.; Shao, L.; Liu, X.; Gao, A.; Wang, H.; Zheng, B.; Hou, G.; Shehzad, K.; Yu, L.; Miao, F. Room-temperature valleytronic transistor. Nat. Nanotechnol. 2020, 15, 743–749. [Google Scholar] [CrossRef]
- Qian, Q.; Lei, J.; Wei, J.; Zhang, Z.; Tang, G.; Zhong, K.; Zheng, Z.; Chen, K. 2D materials as semiconducting gate for field-effect transistors with inherent over-voltage protection and boosted ON-current. NPJ 2D Mater. Appl. 2019, 3, 24. [Google Scholar] [CrossRef]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 7, 768–779. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. 2D semiconductors for transistors. Nat. Rev. Mater. 2016, 1, 16052. [Google Scholar] [CrossRef]
- Moore, G.E. Cramming more components onto integrated circuits. Electronics 1965, 38, 114–117. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Zhang, W.; Chang, H. Synthesis of high quality two-dimensional materials via chemical vapor deposition. Chem. Sci. 2015, 6, 6705–6716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najmaei, S.; Liu, Z.; Zhou, W.; Zou, X.; Shi, G.; Lei, S.; Yakobson, B.I.; Idrobo, J.-C.; Ajayan, P.M.; Lou, J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. [Google Scholar] [CrossRef]
- Van der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.; Heinz, T.F.; Reichman, D.R.; Muller, D.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.; Wang, S.; Chu, L.; Toh, M.; Kumar, R.; Zhao, W.; Neto, A.H.C.; Martin, J.; Adam, S.; Özyilmaz, B.; et al. Transport properties of monolayer MoS2 grown by chemical vapor deposition. Nano Lett. 2014, 14, 1909–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Zhang, W.; Zhu, Y.; Han, Y.; Pu, J.; Chang, J.; Hsu, W.; Huang, J.; Hsu, C.; Chiu, M.; et al. Monolayer MoSe2 grown by chemical vapor deposition for fast photodetection. ACS Nano 2014, 8, 8582–8590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Huang, X.; Liu, L.; Wang, M.; Wang, L.; Huang, B.; Zhu, D.; Li, J.; Gu, C.; Meng, X. CVD synthesis of large-area, highly crystalline MoSe2 atomic layers on diverse substrates and application to photodetectors. Nanoscale 2014, 6, 8949–8955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Tweet, D.J.; Conley, J.F., Jr. Atomic layer deposition of two dimensional MoS2 on 150 mm substrates. J. Vac. Sci. Technol. 2016, 34, 021515. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Shin, S.; Kwon, D.; Hana, S.; Min, Y. Novel chemical route for atomic layer deposition of MoS2 thin film on SiO2/Si substrate. Nanoscale 2014, 6, 14453–14458. [Google Scholar] [CrossRef]
- Dai, T.; Liu, Y.; Fan, X.; Liu, X.; Xie, D.; Li, Y. Synthesis of few-layer 2H-MoSe2 thin films with wafer-level homogeneity for high-performance photodetector. Nanophotonics 2018, 7, 1959–1969. [Google Scholar] [CrossRef]
- Dai, T.; Liu, Y.; Liu, X.; Xie, D.; Li, Y. High performance photodetectors constructed on atomically thin few-layer MoSe2 synthesized using atomic layer deposition and a chemical vapor deposition chamber. J. Alloys Compd. 2019, 785, 951–957. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, W.; Jiang, Z.; Yang, S.; Jing, W.; Shi, P.; Wu, X.; Ye, Z. Low-temperature remote plasma-enhanced atomic layer deposition of graphene and characterization of its atomic-level structure. J. Mater. Chem. C 2014, 2, 7570–7574. [Google Scholar] [CrossRef]
- Xiao, Z.; Kisslinger, K.; Chance, S.; Banks, S. Comparison of hafnium dioxide and zirconium dioxide grown by plasma-enhanced atomic layer deposition for the application of electronic materials. Crystals 2020, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Stevie, F.A.; Gianuzzi, L.A.; Prenitzer, B.I. Introduction to Focused Ion Beams: Instrumentation, Theory, Techniques and Practice; Springer International Publishing: New York, NY, USA, 2005. [Google Scholar]
- Bulusheva, L.G.; Kanygin, M.A.; Arkhipov, V.E.; Popov, K.M.; Fedoseeva, Y.V.; Smirnov, D.A.; Okotrub, A.V. In situ X-ray photoelectron spectroscopy study of lithium interaction with graphene and nitrogen-doped graphene films produced by chemical vapor deposition. J. Phys. Chem. C 2017, 121, 5108. [Google Scholar] [CrossRef]
- Patil, U.V.; Pawbake, A.S.; Machuno, L.G.B.; Gelamo, R.V.; Jadkark, S.R.; Rout, C.S.; Late, D.J. Effect of plasma treatment on multilayer graphene: X-ray photoelectron spectroscopy, surface morphology investigations and work function measurements. RSC Adv. 2016, 6, 48843. [Google Scholar] [CrossRef]
- Matei, D.G.; Weber, N.; Kurasch, S.; Wundrack, S.; Woszczyna, M.; Grothe, M.; Weimann, T.; Ahlers, F.; Stosch, R.; Kaiser, U.; et al. Functional single-layer graphene sheets from aromatic monolayers. Adv. Mater. 2013, 25, 4146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy. Carbon 2014, 70, 59–74. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Yu, K.; Chang, J.; Steeber, D.A.; Ocola, L.E.; Chen, J. Direct growth of vertically-oriented graphene for field-effect transistor biosensor. Sci. Rep. 2013, 3, 1696. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Bhowmick, S.; Sen, F.G.; Banerji, A.; Alpas, A.T. Roles of sliding-induced defects and dissociated water molecules on low friction of graphene. Sci. Rep. 2018, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, Y.; Xie, D.; Cui, Y.; Mi, W.; Zhang, Y.; Ren, T. Wafer-scale integration of graphene-based electronic, optoelectronic and electroacoustic devices. Sci. Rep. 2014, 4, 3598. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, D.; Wei, D.; Song, X.; We, D.; Wee, A. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef]
- Shakerzadeh, M.; Teo, E.H.T.; Sorkin, A.; Bosman, M.; Tay, B.K.; Su, H. Plasma density induced formation of nanocrystals in physical vapor deposited carbon films. Carbon 2011, 49, 1733. [Google Scholar] [CrossRef]
- Shakerzadeh, M.; Loh, G.C.; Xu, N.; Chow, W.L.; Tan, C.W.; Lu, C.; Yap, R.C.C.; Tan, D.; Tsang, S.H.; Teo, E.H.T.; et al. Re-ordering chaotic carbon: Origins and application of textured carbon. Adv. Mater. 2012, 24, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, S.; Deng, D.; Deng, S.; Chen, J.; Xu, N. Growth direction manipulation of few-layer graphene in the vertical plane with parallel arrangement. Carbon 2013, 56, 103–108. [Google Scholar] [CrossRef]
- Manfrinato, V.R.; Camino, F.E.; Stein, A.; Zhang, L.; Lu, M.; Stach, E.A.; Black, C.T. Patterning Si at the 1 nm length scale with aberration corrected electron-beam lithography: Tuning of plasmonic properties by design. Adv. Funct. Mater. 2019, 29, 1903429. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Kisslinger, K.; Monikandan, R. Atomic Layer Deposition of Nanolayered Carbon Films. C 2021, 7, 67. https://doi.org/10.3390/c7040067
Xiao Z, Kisslinger K, Monikandan R. Atomic Layer Deposition of Nanolayered Carbon Films. C. 2021; 7(4):67. https://doi.org/10.3390/c7040067
Chicago/Turabian StyleXiao, Zhigang, Kim Kisslinger, and Rebhadevi Monikandan. 2021. "Atomic Layer Deposition of Nanolayered Carbon Films" C 7, no. 4: 67. https://doi.org/10.3390/c7040067
APA StyleXiao, Z., Kisslinger, K., & Monikandan, R. (2021). Atomic Layer Deposition of Nanolayered Carbon Films. C, 7(4), 67. https://doi.org/10.3390/c7040067






