The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Processes
2.2. Electrochemical Evaluation Methods
3. Results and Discussion
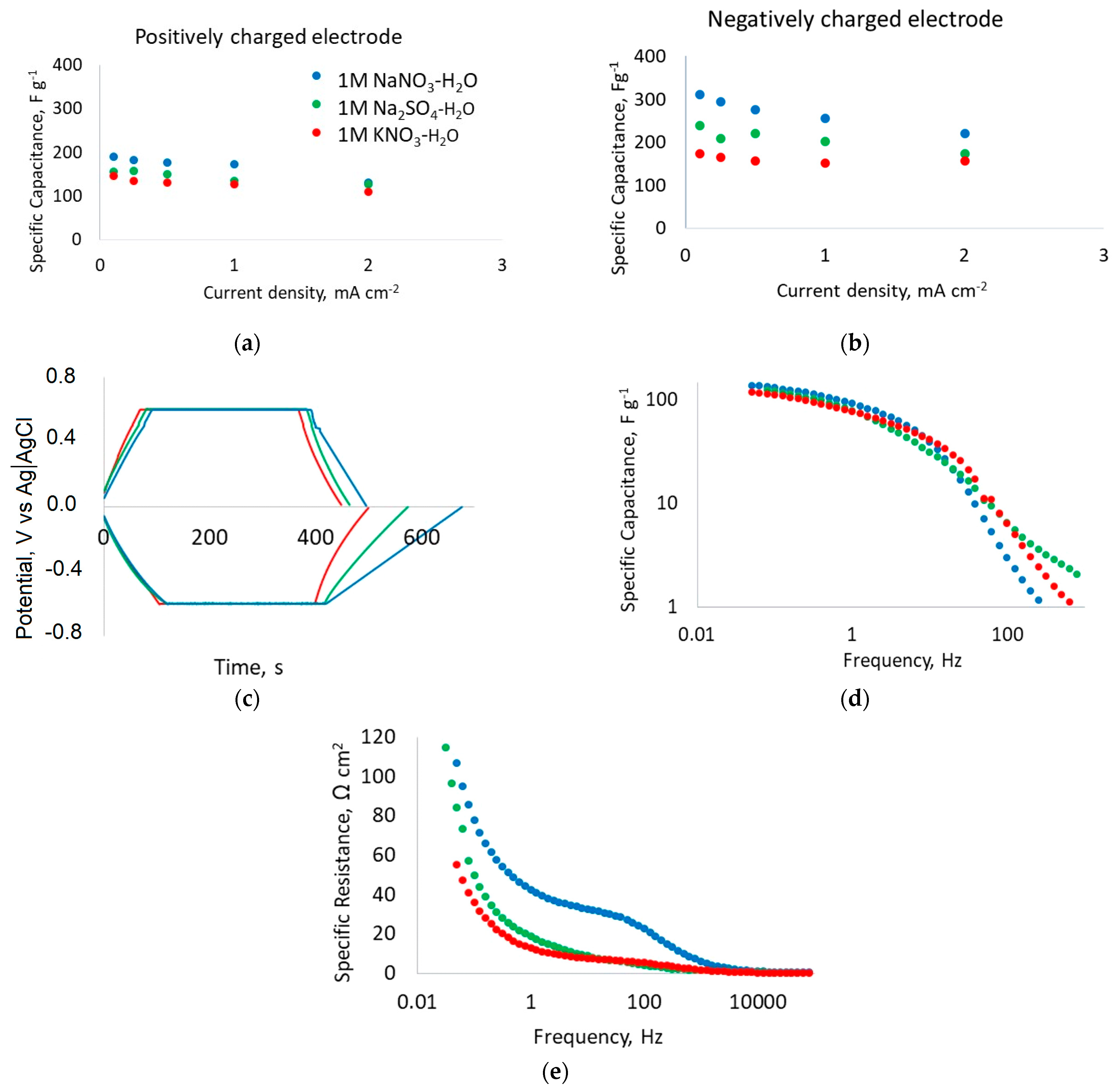
3.1. Electrochemical Evaluation of Fibrous Electrodes in Aqueous Electrolytes
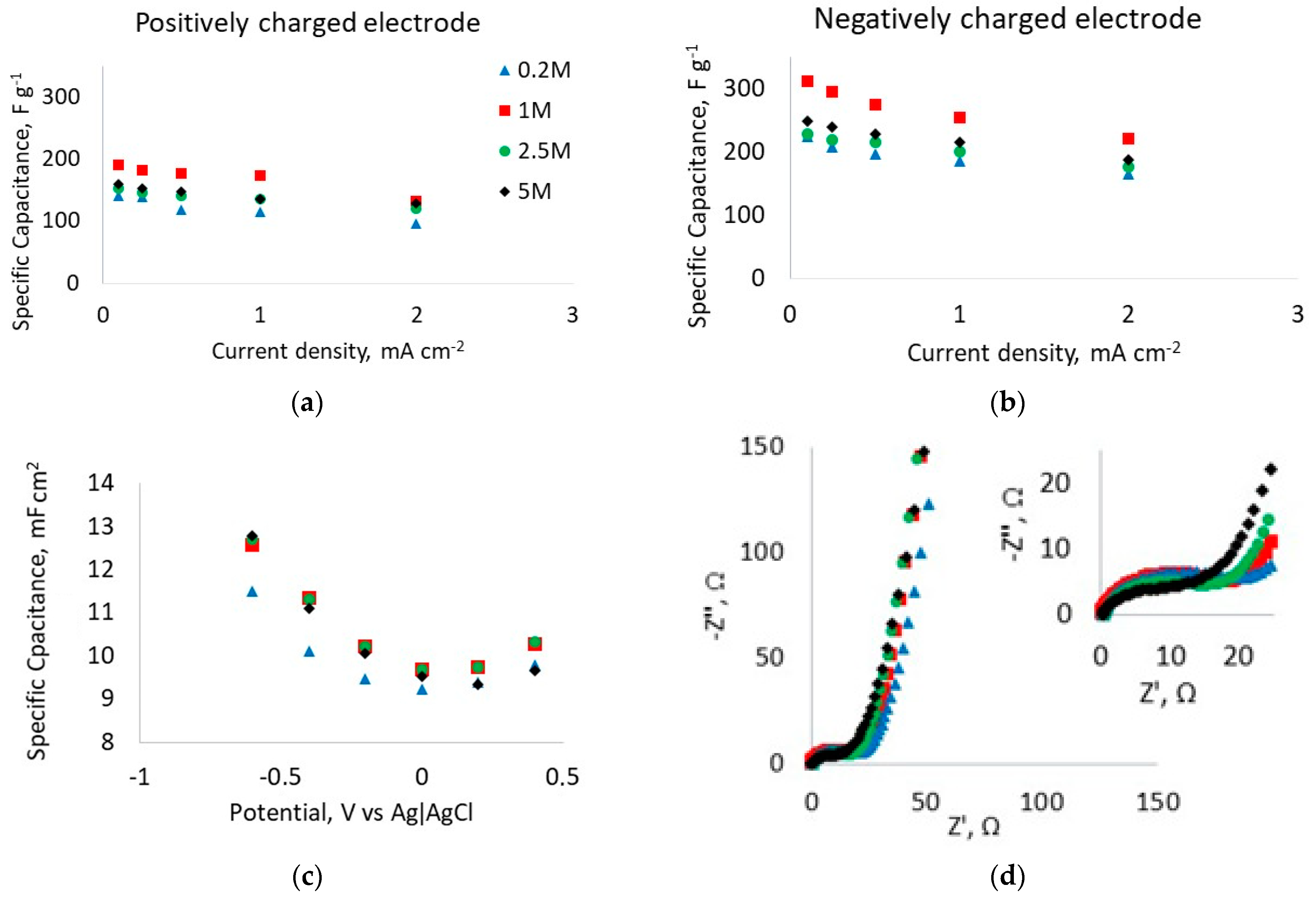
3.2. Effect of Electrolyte Concentration on Fibrous Electrode Performance
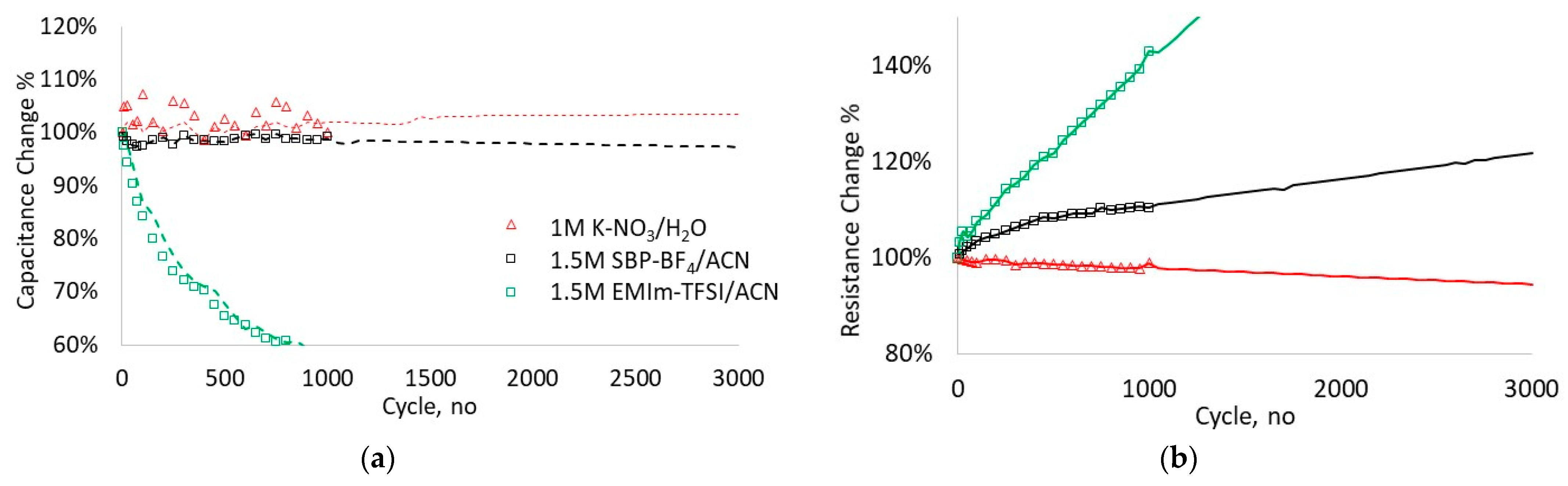
3.3. Cycle-Stability Analysis for Full Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Beguin, F.; Frackowiak, E. Supercapacitors: Materials, Systems, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Menzel, J.; Fic, K.; Frackowiak, E. Hybrid aqueous capacitors with improved energy/power performance. Prog. Nat. Sci. Mater. Int. 2015, 25, 642–649. [Google Scholar] [CrossRef]
- Guerrero-Martínez, M.Á.; Romero-Cadaval, E.; Minambres-Marcos, V.; Milanés-Montero, M.I. Supercapacitor Energy Storage System for Improving the Power flow in Photovoltaic Plants. Electron. Compon. Mater. 2014, 44, 3. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, W. A Review for Aqueous Electrochemical Supercapacitors. Front. Energy Res. 2015, 3, 1–5. [Google Scholar] [CrossRef]
- Horn, M.; MacLeod, J.; Liu, M.; Webb, J.; Motta, N. Supercapacitors: A new source of power for electric cars? Econ. Anal. Policy 2019, 61, 93–103. [Google Scholar] [CrossRef]
- Burke, A.; Zhao, H. Applications of Supercapacitors in Electric and Hybrid Vehicles; 5th European Symposium on Supercapacitor and Hybrid Solutions (ESSCAP): Brasov, Romania, 2015; p. 15. [Google Scholar]
- Weingarth, D.; Noh, H.; Foelske-Schmitz, A.; Wokaun, A.; Kötz, R. A reliable determination method of stability limits for electrochemical double layer capacitors. Electrochim. Acta 2013, 103, 119–124. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Käärik, M.; Maran, U.; Leis, J. Characterization and prediction of double-layer capacitance of nanoporous carbon materials using the Quantitative nano-Structure-Property Relationship approach based on experimentally determined porosity descriptors. Carbon 2020, 158, 494–504. [Google Scholar] [CrossRef]
- Arulepp, M.; Leis, J.; Lätt, M.; Miller, F.; Rumma, K.; Lust, E.; Burke, A. The advanced carbide-derived carbon based supercapacitor. J. Power Sources 2006, 162, 1460–1466. [Google Scholar] [CrossRef]
- Ramachandran, R.; Wang, F. Electrochemical Capacitor Performance: Influence of Aqueous Electrolytes. Supercapacitors Theor. Pract. Solut. 2017. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Dyatkin, B.; Mamontov, E.; Cook, K.M.; Gogotsi, Y. Capacitance, charge dynamics, and electrolyte-surface interactions in functionalized carbide-derived carbon electrodes. Prog. Nat. Sci. 2015, 25, 631–641. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Kook, M.; Mäeorg, U.; Kozlova, J.; Sammelselg, V.; Perkson, A.; Leis, J. Characterisation of steam-treated nanoporous carbide-derived carbon of TiC origin: Structure and enhanced electrochemical performance. J. Porous Mater. 2017, 25, 1057–1070. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Kook, M.; Kozlova, J.; Ritslaid, P.; Aruväli, J.; Mäeorg, U.; Sammelselg, V.; Leis, J. High-performance microporous carbon from deciduous wood-origin metal carbide. Microporous Mesoporous Mater. 2019, 278, 14–22. [Google Scholar] [CrossRef]
- Lota, K.; Sierczynska, A.; Acznik, I. Effect of aqueous electrolytes on electrochemical capacitor capacitance. Chemik 2013, 67, 1138–1145. [Google Scholar]
- Barzegar, F.; Momodu, D.Y.; Fashedemi, O.O.; Bello, A.; Dangbegnon, J.K.; Manyala, N. Investigation of different aqueous electrolytes on the electrochemical performance of activated carbon-based supercapacitors. RSC Adv. 2015, 5, 107482–107487. [Google Scholar] [CrossRef]
- Ibukun, O.; Jeong, H.K. Effects of Aqueous Electrolytes in Supercapacitors. New Phys. Sae Mulli 2019, 69, 154–158. [Google Scholar] [CrossRef]
- Lang, A.W.; Ponder, J.F.; Österholm, A.M.; Kennard, N.J.; Bulloch, R.H.; Reynolds, J.R. Flexible, aqueous-electrolyte supercapacitors based on water-processable dioxythiophene polymer/carbon nanotube textile electrodes. J. Mater. Chem. A 2017, 5, 23887–23897. [Google Scholar] [CrossRef]
- Mao, X.; Hatton, T.A.; Rutledge, G.C. A Review of Electrospun Carbon Fibers as Electrode Materials for Energy Storage. Curr. Org. Chem. 2013, 17, 1390–1401. [Google Scholar] [CrossRef]
- Frackowiak, E.; Abbas, Q.; Béguin, F. Carbon/carbon supercapacitors. J. Energy Chem. 2013, 22, 226–240. [Google Scholar] [CrossRef]
- Ruiz, V.; Santamaría, R.; Granda, M.; Blanco, C. Long-term cycling of carbon-based supercapacitors in aqueous media. Electrochim. Acta 2009, 54, 4481–4486. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Béguin, F. A new type of high energy asymmetric capacitor with nanoporous carbon electrodes in aqueous electrolyte. J. Power Sources 2010, 195, 4234–4241. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. A symmetric carbon/carbon supercapacitor operating at 1.6V by using a neutral aqueous solution. Electrochem. Commun. 2010, 12, 1275–1278. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-M.; Qin, Z.-Y.; Chen, L. Effect of electrolytes on electrochemical properties of graphene sheet covered with polypyrrole thin layer. Prog. Nat. Sci. 2011, 21, 460–466. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Wei, C.; Li, B.; Kang, F.; Guan, Z. Charge storage mechanism of manganese dioxide for capacitor application: Effect of the mild electrolytes containing alkaline and alkaline-earth metal cations. J. Power Sources 2011, 196, 7854–7859. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Thakur, K.P. Reappraisal of thermochemical radii for complex ions. J. Chem. Educ. 1979, 56, 576. [Google Scholar] [CrossRef]
- Park, J.-S. Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 043002. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Tan, K.L.; Lim, S.H.; Ramakrishna, S. Electrospun Nanofibers for Air Filtration Applications. Procedia Eng. 2014, 75, 159–163. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tahkar, A.I. Use of Nano Fibers in Filtration—A review. IJSRD-Int. J. Sci. Res. Dev. 2016, 4, 7. [Google Scholar]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of Nanofibers for Energy Applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Bai, Y.; Zhao, X.; Wang, R.; Huang, Y.; Liang, Q.; Huang, Z. A Non-Woven Network of Porous Nitrogen-doping Carbon Nanofibers as a Binder-free Electrode for Supercapacitors. Electrochim. Acta 2017, 230, 445–453. [Google Scholar] [CrossRef]
- He, T.; Fu, Y.; Meng, X.; Yu, X.; Wang, X. A novel strategy for the high performance supercapacitor based on polyacrylonitrile-derived porous nanofibers as electrode and separator in ionic liquid electrolyte. Electrochim. Acta 2018, 282, 97–104. [Google Scholar] [CrossRef]
- Tõnurist, K.; Vaas, I.; Thomberg, T.; Jänes, A.; Kurig, H.; Romann, T.; Lust, E. Application of multistep electrospinning method for preparation of electrical double-layer capacitor half-cells. Electrochim. Acta 2014, 119, 72–77. [Google Scholar] [CrossRef]
- Malmberg, S.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Arulepp, M.; Krumme, A. Fully Elecrospun Durable Electrode and Electrochemical Double-Layer Capacitor for High Frequency Applications; ESA SPCD: Lanskroun, Czech Republic, 2018; p. 10. [Google Scholar]
- Malmberg, S.; Arulepp, M.; Savest, N.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Käärik, M.; Mikli, V.; Krumme, A.; Malmberg, S. Directly electrospun electrodes for electrical double-layer capacitors from carbide-derived carbon. J. Electrost. 2020, 103, 103396. [Google Scholar] [CrossRef]
- Malmberg, S.; Arulepp, M.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Krumme, A. Electrochemical Evaluation of Directly Electrospun Carbide-Derived Carbon-Based Electrodes in Different Nonaqueous Electrolytes for Energy Storage Applications. C J. Carbon Res. 2020, 6, 59. [Google Scholar] [CrossRef]
- Balbuena, P.B. Electrolyte Materials-Issues and Challenges. In Proceedings of the AIP Conference Proceedings, Freiberg, Germany, 17 February 2014; pp. 82–97. [Google Scholar] [CrossRef]
- Conway, B.E. Behavior of the Double Layer in Nonaqueous Electrolytes and Nonaqueous Electrolyte Capacitors. In Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999; pp. 169–181. [Google Scholar]
- Balducci, A. Electrolytes for high voltage electrochemical double layer capacitors: A perspective article. J. Power Sources 2016, 326, 534–554. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Shikata, H.; Takao, K.; Asanuma, N.; Taruta, S.; Park, Y.-Y. An aqueous electrolyte of the widest potential window and its superior capability for capacitors. Sci. Rep. 2017, 7, srep45048. [Google Scholar] [CrossRef]
- Bu, X.; Su, L.; Dou, Q.; Lei, S.; Yan, X. A low-cost “water-in-salt” electrolyte for a 2.3 V high-rate carbon-based supercapacitor. J. Mater. Chem. A 2019, 7, 7541–7547. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Chang, D.; Kim, J.; Moon, S.; Oh, K.; Park, K.-Y.; Seong, W.M.; Park, H.; Kwon, G.; et al. Toward a low-cost high-voltage sodium aqueous rechargeable battery. Mater. Today 2019, 29, 26–36. [Google Scholar] [CrossRef]
- Stojanovska, E.; Pampal, E.S.; Kilic, A.; Quddus, M.; Candan, Z. Developing and characterization of lignin-based fibrous nanocarbon electrodes for energy storage devices. Compos. Part B Eng. 2019, 158, 239–248. [Google Scholar] [CrossRef]
- Khosrozadeh, A.; Singh, G.; Wang, Q.; Luo, G.; Xing, M. Supercapacitor with extraordinary cycling stability and high rate from nano-architectured polyaniline/graphene on Janus nanofibrous film with shape memory. J. Mater. Chem. A 2018, 6, 21064–21077. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, D.Q.; Yang, S.Y. Electrochemical Insight into Cycle Stability of Organic Electrolyte Supercapacitors. Adv. Mater. Res. 2011, 347–353, 467–471. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Ku, B.-C.; Kim, J.; Joh, H.-I. Structural Evolution of Polyacrylonitrile Fibers in Stabilization and Carbonization. Adv. Chem. Eng. Sci. 2012, 2, 275–282. [Google Scholar] [CrossRef]
- Li, J.; Su, S.; Zhou, L.; Kundrát, V.; Abbot, A.M.; Mushtaq, F.; Ouyang, D.; James, D.; Roberts, D.; Ye, H. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition during the carbonization of polyacrylonitrile fibers. J. Appl. Phys. 2013, 113, 024313. [Google Scholar] [CrossRef]
- Surianarayanan, M.; Vijayaraghavan, R.; Raghavan, K.V. Spectroscopic investigations of polyacrylonitrile thermal degradation. J. Polym. Sci. Part Polym. Chem. 1998, 36, 2503–2512. [Google Scholar] [CrossRef]
- Conley, R.T.; Bieron, J.F. Examination of the oxidative degradation of polyacrylonitrile using infrared spectroscopy. J. Appl. Polym. Sci. 1963, 7, 1757–1773. [Google Scholar] [CrossRef]
- Henrici-Olivé, G.; Olivé, S. Molecular interactions and macroscopic properties of polyacrylonitrile and model substances. In Chemistry; Springer: Berlin/Heidelberg, Gemany, 1979; Volume 32, pp. 123–152. [Google Scholar] [CrossRef]
- Torop, J.; Palmre, V.; Arulepp, M.; Sugino, T.; Asaka, K.; Aabloo, A. Flexible supercapacitor-like actuator with carbide-derived carbon electrodes. Carbon 2011, 49, 3113–3119. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. (Eds.) Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Feng, X. Nanocarbons for Advanced Energy Storage, Volume 1; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- He, M.; Fic, K.; Frąckowiak, E.; Novák, P.; Berg, E.J. Influence of aqueous electrolyte concentration on parasitic reactions in high-voltage electrochemical capacitors. Energy Storage Mater. 2016, 5, 111–115. [Google Scholar] [CrossRef]

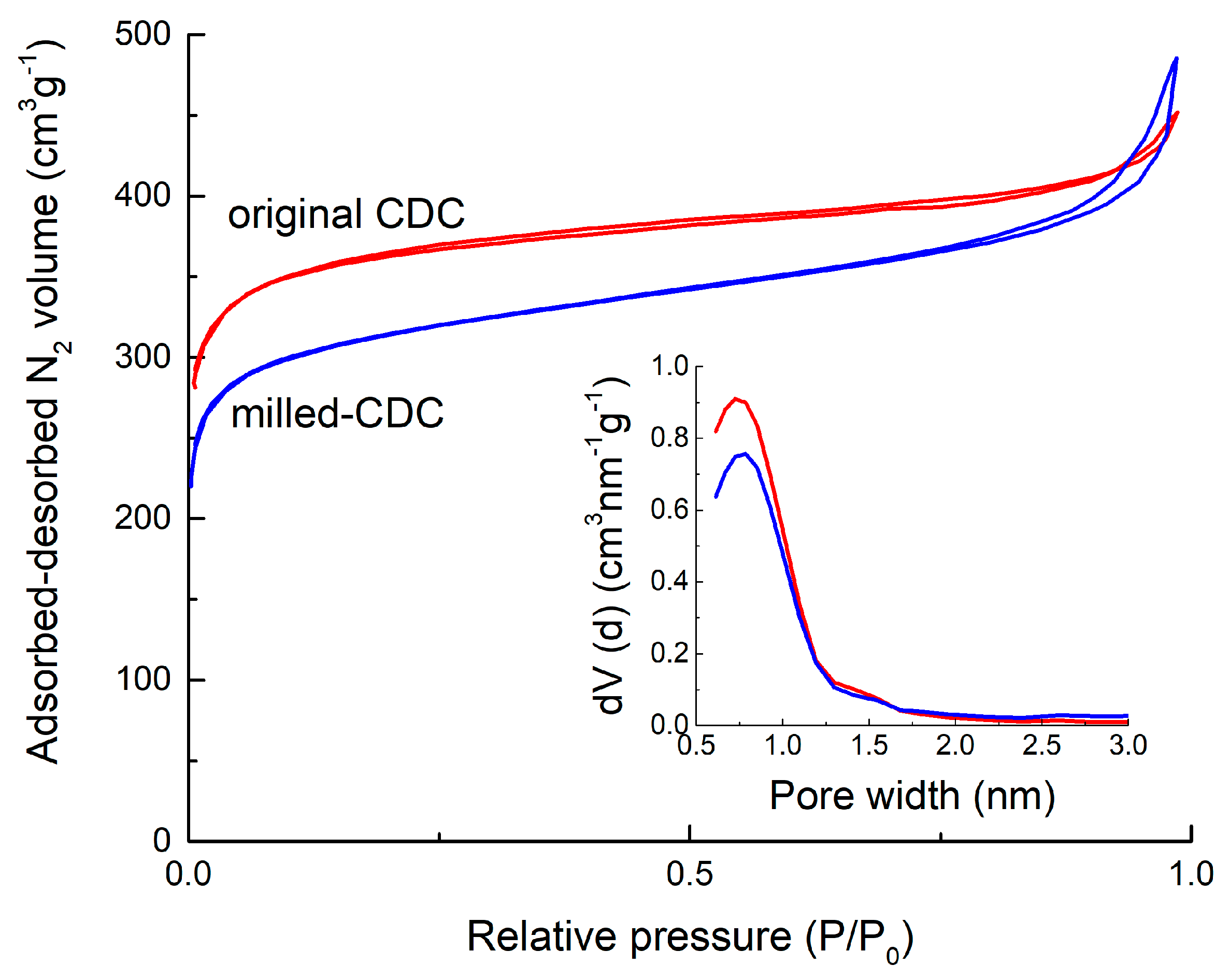
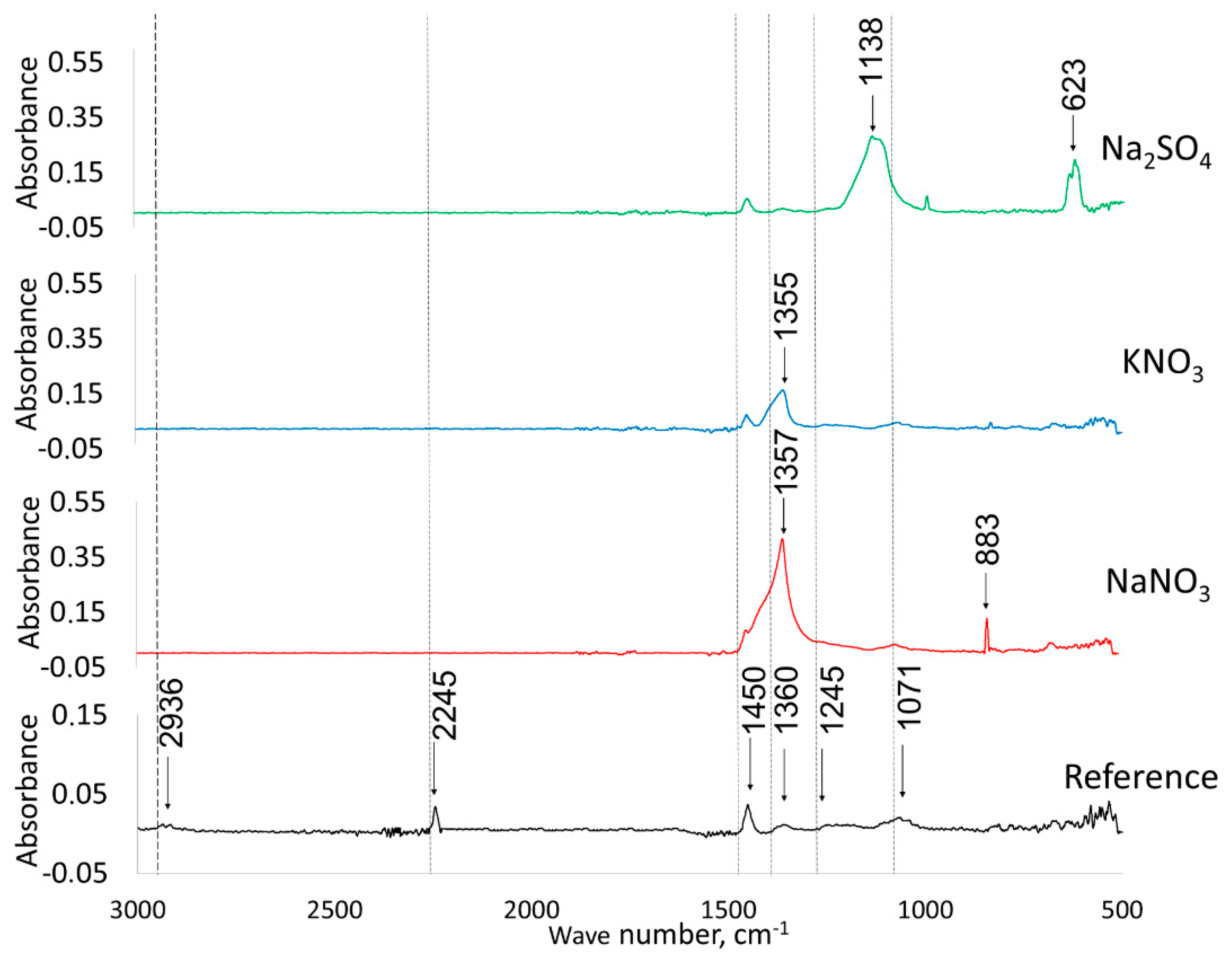
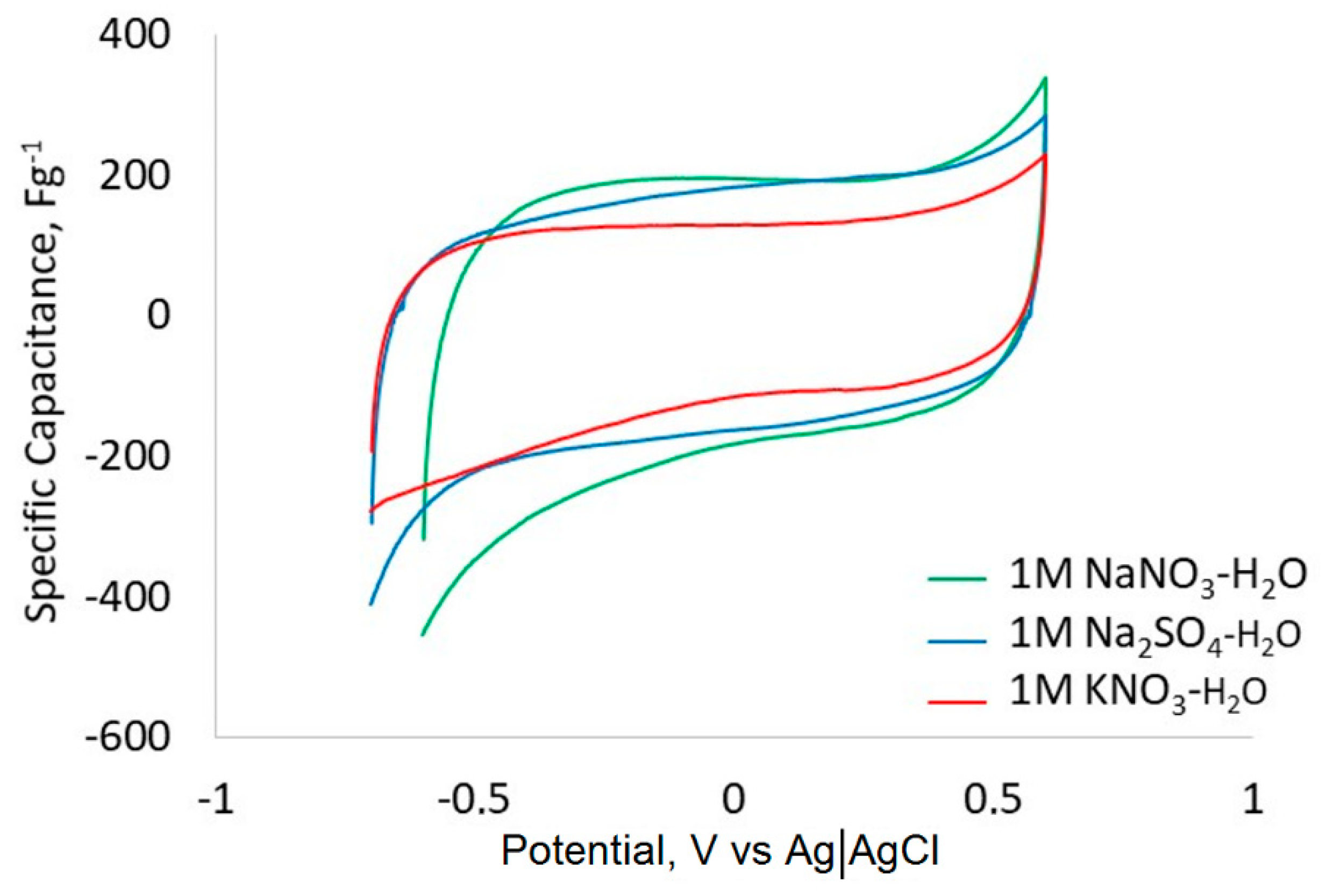
| Material | SaBET | Sadft | Vtot | Vµ dft |
|---|---|---|---|---|
| m2 g−2 | m2 g−2 | cm3 g−2 | cm3 g−2 | |
| Non-milled | 1282 | 1390 | 0.67 | 0.53 |
| Milled | 1098 | 1173 | 0.66 | 0.44 |
| Salt | Molarity, M | Conductivity, mS cm−1 | pH | Ion Radius, nm | |
|---|---|---|---|---|---|
| Cation | Anion | ||||
| Na2SO4 | 1.0 | 78.2 | 7.13 | 0.12 | 0.24 |
| KNO3 | 1.0 | 73.2 | 7.34 | 0.15 | 0.17 |
| NaNO3 | 0.2 | 73.0 | 6.85 | 0.12 | 0.17 |
| NaNO3 | 1.0 | 89.2 | 7.10 | ||
| NaNO3 | 2.5 | 103.0 | 7.08 | ||
| NaNO3 | 5.0 | 117.2 | 7.02 | ||
| C+ Electrode, | C+ CDC, | C− Electrode, | C− CDC, | |
|---|---|---|---|---|
| Electrolyte | F g−1 | F g−1 | F g−1 | F g−1 |
| NaNO3-H2O | 40.8 | 177.5 | 63.5 | 276.1 |
| KNO3-H2O | 47.7 | 132.0 | 57.0 | 157.6 |
| Na2SO4-H2O | 34.6 | 150.7 | 51.0 | 221.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malmberg, S.; Arulepp, M.; Laanemets, K.; Käärik, M.; Laheäär, A.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Krumme, A. The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes. C 2021, 7, 46. https://doi.org/10.3390/c7020046
Malmberg S, Arulepp M, Laanemets K, Käärik M, Laheäär A, Tarasova E, Vassiljeva V, Krasnou I, Krumme A. The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes. C. 2021; 7(2):46. https://doi.org/10.3390/c7020046
Chicago/Turabian StyleMalmberg, Siret, Mati Arulepp, Krista Laanemets, Maike Käärik, Ann Laheäär, Elvira Tarasova, Viktoria Vassiljeva, Illia Krasnou, and Andres Krumme. 2021. "The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes" C 7, no. 2: 46. https://doi.org/10.3390/c7020046
APA StyleMalmberg, S., Arulepp, M., Laanemets, K., Käärik, M., Laheäär, A., Tarasova, E., Vassiljeva, V., Krasnou, I., & Krumme, A. (2021). The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes. C, 7(2), 46. https://doi.org/10.3390/c7020046






