Nanocomposite of Ellagic Acid with Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Detection of Six Biomolecules
Abstract
1. Introduction
Reagents and Solutions
2. Instrumentation
Preparation of MGPE/MWCNTs-EA
3. Results and Discussion
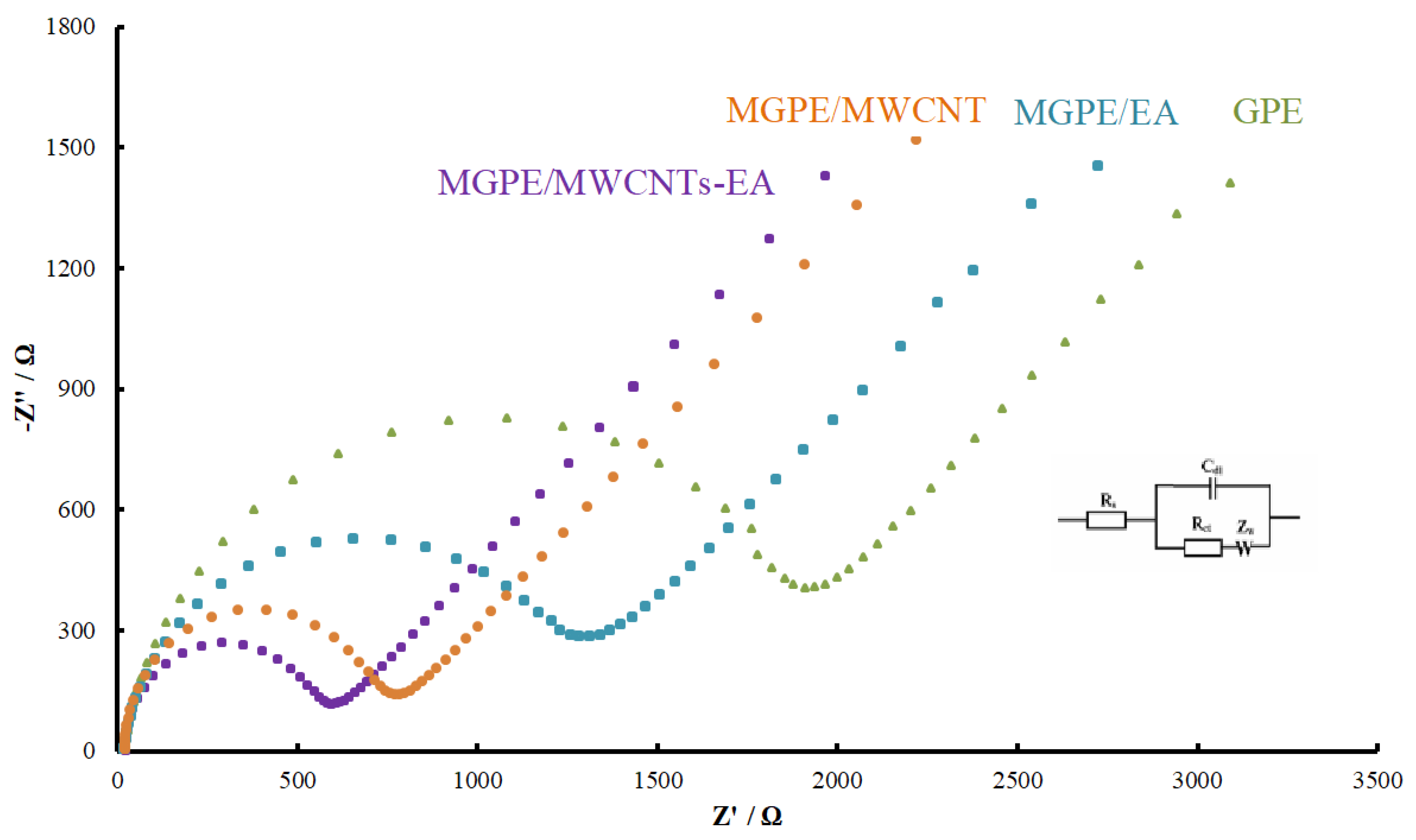
3.1. Characterization
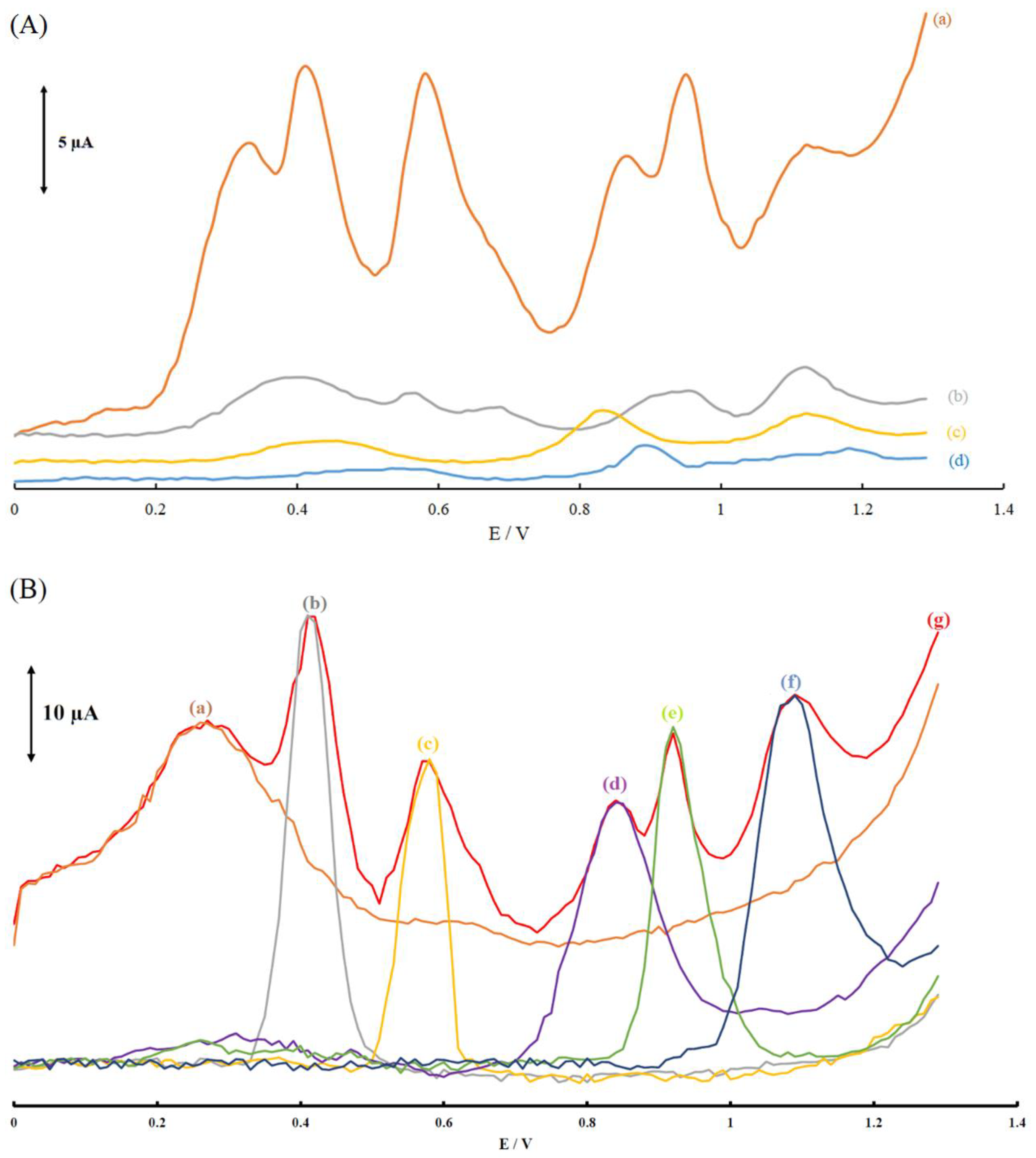
3.2. Electrochemical Behavior of the Modified Electrodes
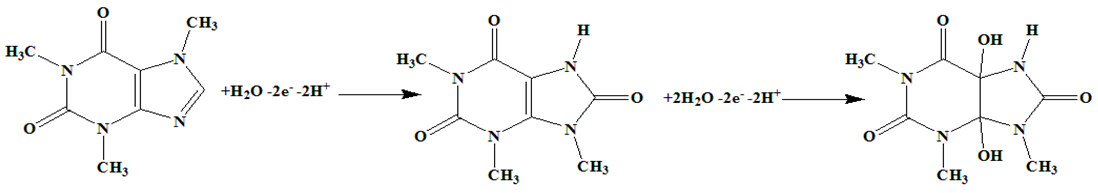
3.3. Electrochemical Behavior of AA, DA, UA, Trp, XN, and CA
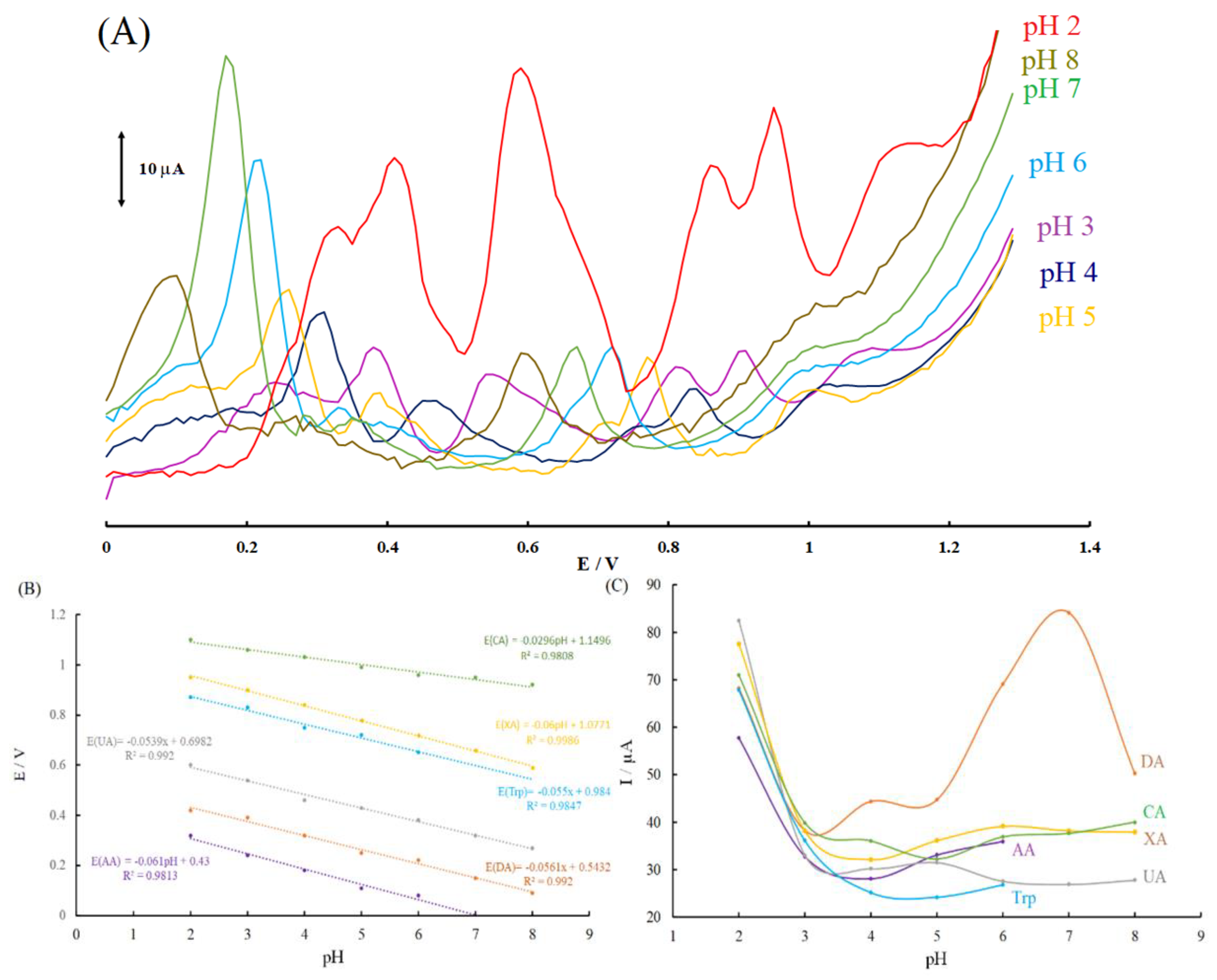
3.4. Influence of pH on the Simultaneous Electrooxidation of AA, DA, UA, Trp, XN, and CA
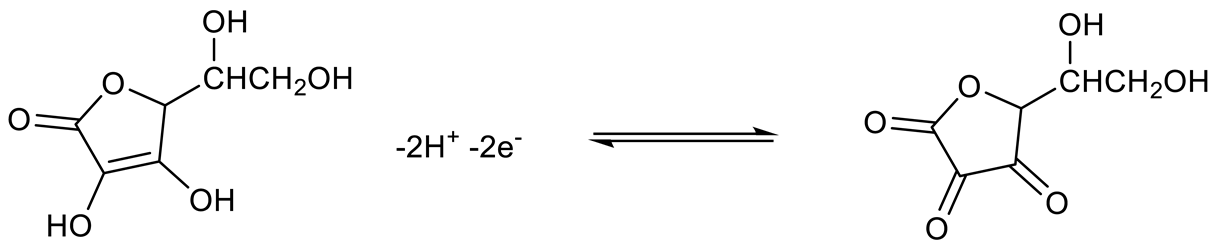
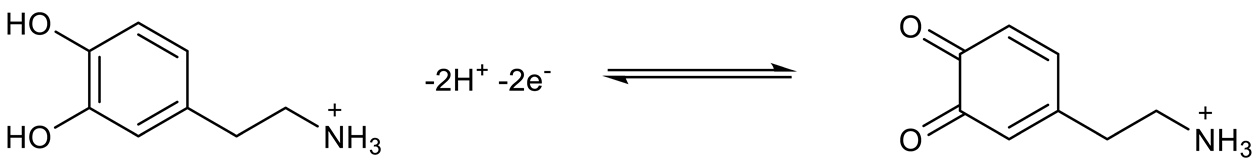
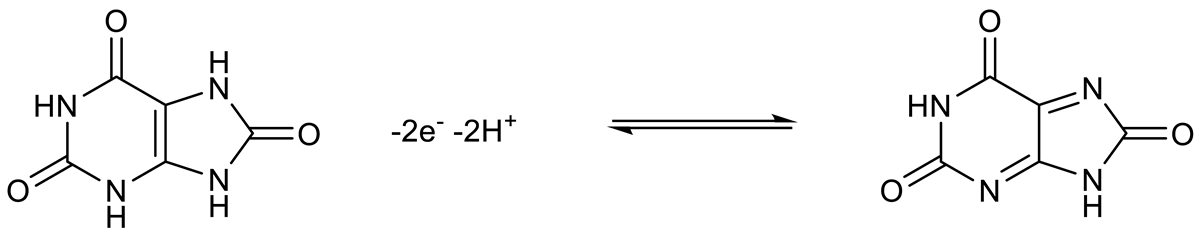
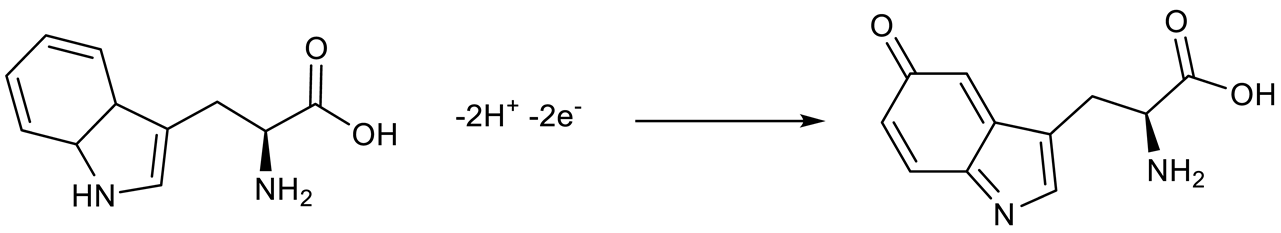
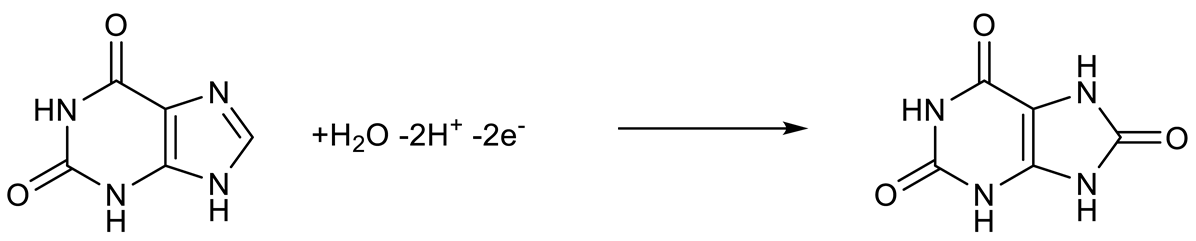
3.5. Interference Studies
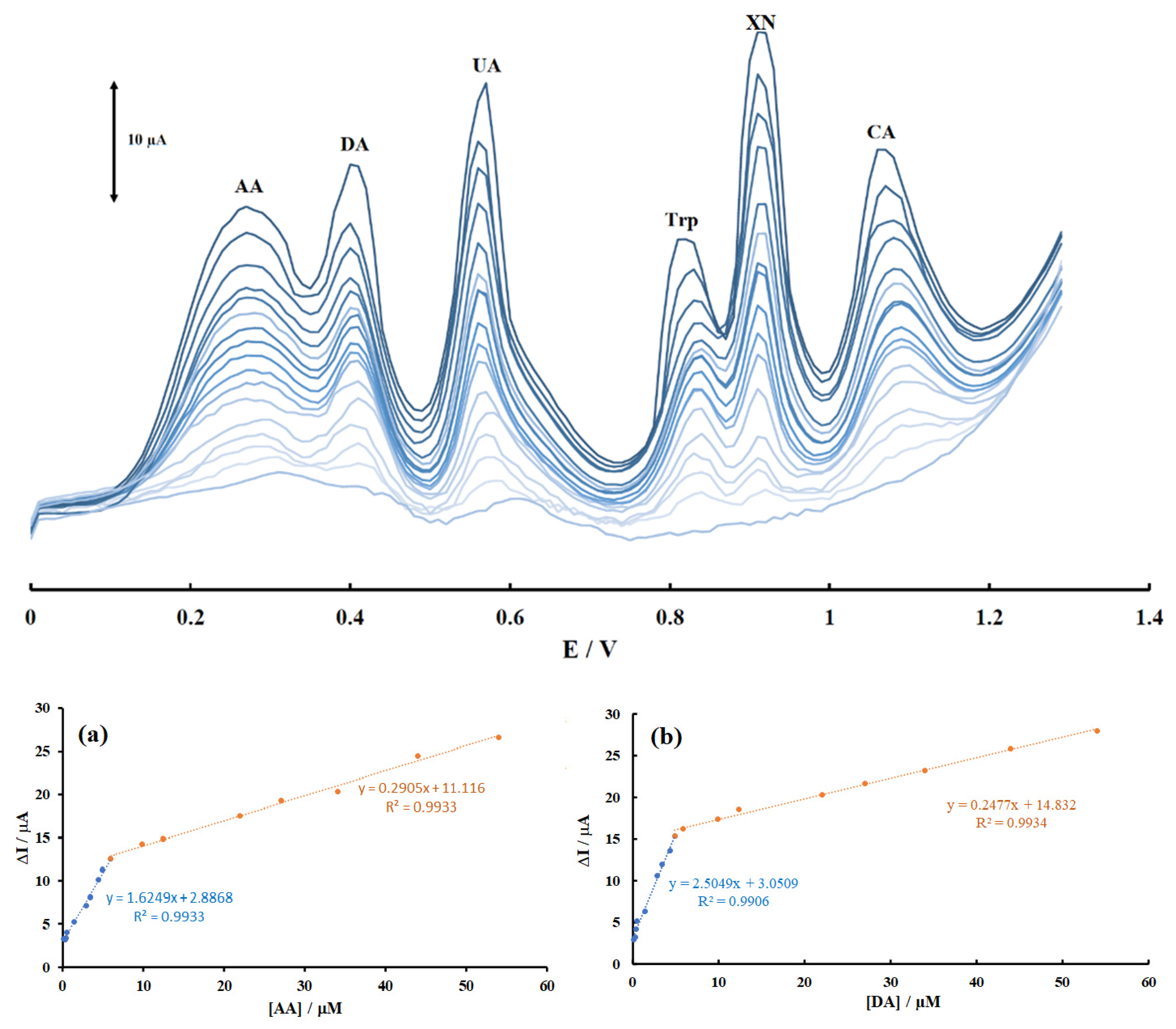
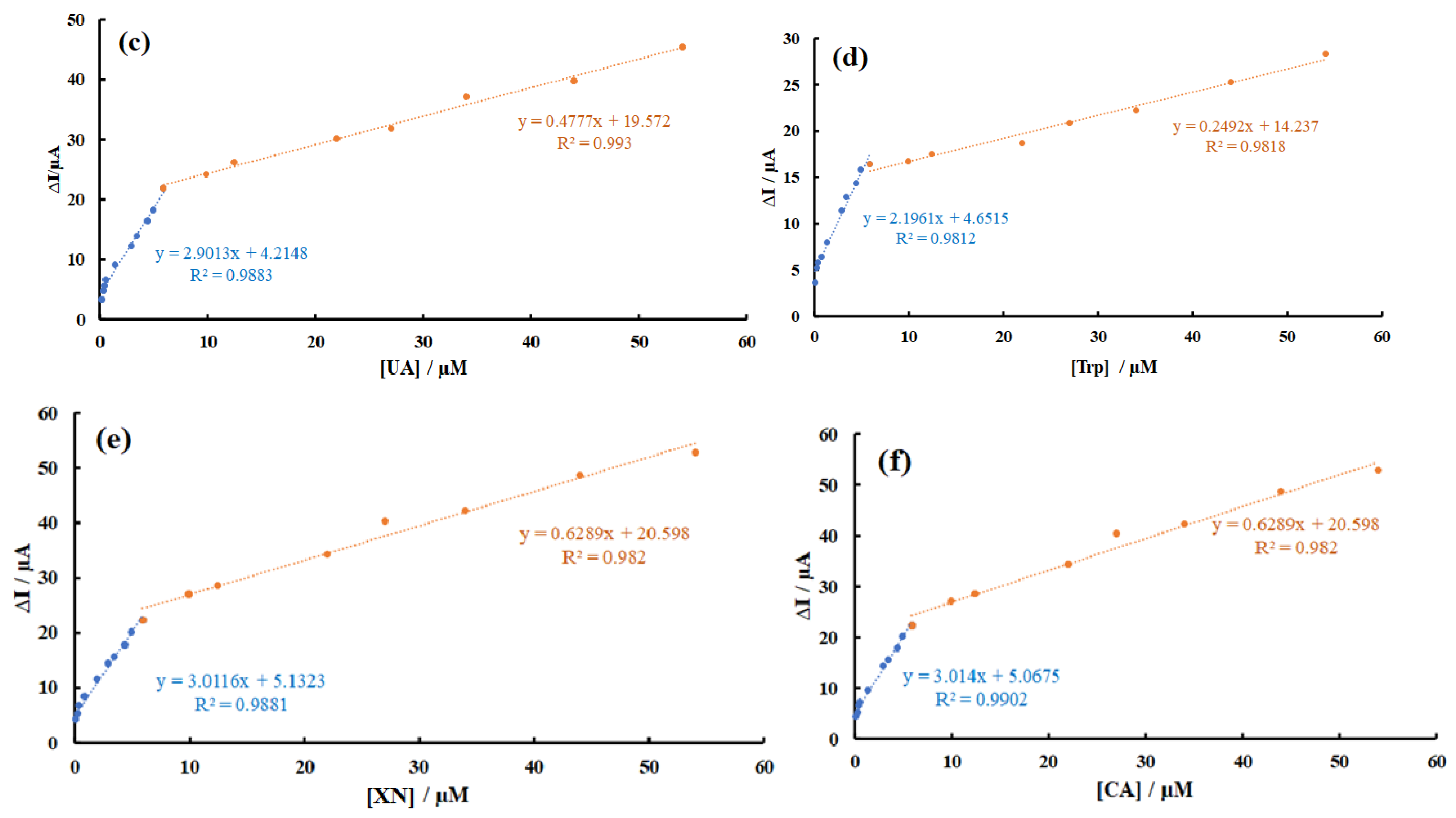
3.6. Calibration Curves
3.7. Stability and Reproducibility
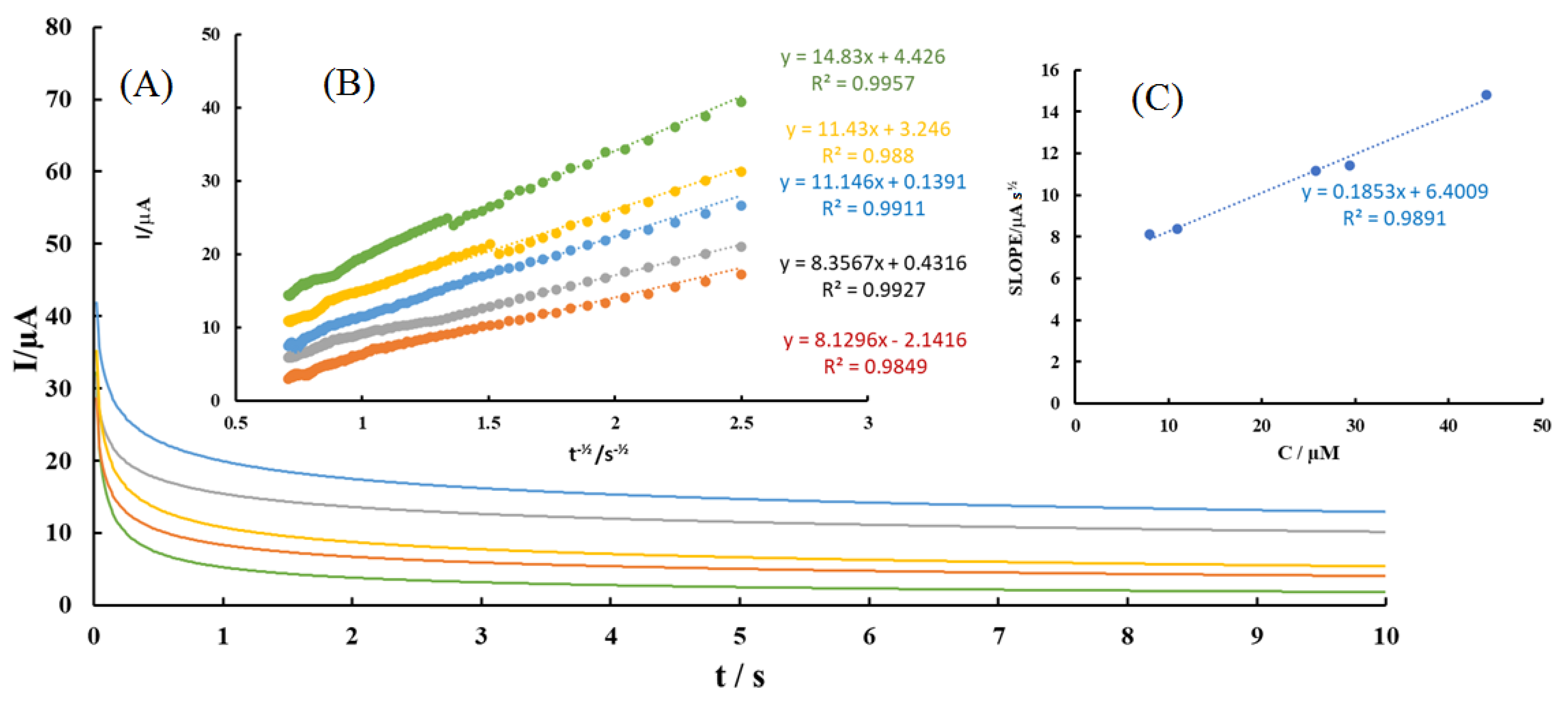
3.8. Chronoamperometric Studies
3.9. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Zhou, J.; Noroozifar, M.; Kerman, K. Gold-platinum core-shell nanoparticles with thiolated polyaniline and multi-walled carbon nanotubes for the simultaneous voltammetric determination of six drug molecules. Chemosensors 2021, 9, 24. [Google Scholar] [CrossRef]
- Spataru, N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Anodic voltammetry of xanthine, theophylline, theobromine and caffeine at conductive diamond electrodes and its analytical application. Electroanalysis 2002, 14, 721–728. [Google Scholar] [CrossRef]
- Kan, X.; Liu, T.; Li, C.; Zhou, H.; Xing, Z.; Zhu, A. A novel electrochemical sensor based on molecularly imprinted polymers for caffeine recognition and detection. J. Solid State Electrochem. 2002, 16, 3207–3213. [Google Scholar] [CrossRef]
- Okonny, U.L.P.; Wang, S.X.; Stubbs, R.J.; Guzman, N.A. Determination of caffeine and its metabolites in urine by capillary electrophoresis–mass spectrometry. Electrophoresis 2005, 26, 2652–2663. [Google Scholar] [CrossRef] [PubMed]
- Mandel, H.G. Update on caffeine consumption, disposition and action. Food Chem. Toxicol. 2002, 40, 1231–1234. [Google Scholar] [CrossRef]
- Mazer, M.; Perrone, J. Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J. Med. Toxicol. 2008, 4, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, M.L.; Niittynen, L.; Korpela, R.; Vapaatalo, H. Coffee, caffeine and blood pressure: A critical review. Eur. J. Clin. Nutr. 1999, 53, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Lindsey, T. Fatal caffeine overdose: Two case reports. Forensic Sci. Int. 2005, 153, 67–69. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Palanisamy, S.; Mani, V.; Chen, S.M. Simultaneous electrochemical determination of dopamine and paracetamol on multiwalled carbon nanotubes/graphene oxide nanocomposite-modified glassy carbon electrode. Talanta 2003, 117, 297–304. [Google Scholar] [CrossRef]
- Turak, F.; Guzel, R.; Dinc, E. Simultaneous determination of ascorbic acid and caffeine in commercial soft drinks using reversed-phase ultraperformance liquid chromatography. J. Food Drug Anal. 2017, 25, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, M.; Kowalczyk, J. Quantification of allantoin, uric acid, xanthine and hypoxanthine in ovine urine by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 129–138. [Google Scholar] [CrossRef]
- Alves, A.C.; Meinhart, A.D.; Teixeira Filho, J.; Godoy, H.T. Development of a method for simultaneous analysis of caffeine and taurine in energy drinks by micellar electrokinetic chromatography with diode-array detector. Food Sci. Technol. 2019, 39, 673–682. [Google Scholar] [CrossRef]
- Khoshayand, M.R.; Abdollahi, H.; Shariatpanahi, M. Simultaneous spectrophotometric determination of paracetamol, ibuprofen and caffeine in pharmaceuticals by chemometric methods. Spectrochim. Acta Part A 2008, 70, 491–499. [Google Scholar] [CrossRef]
- Wang, H.Y.; Sun, Y.; Tang, B. Study on fluorescence property of dopamine and determination of dopamine by fluorimetry. Talanta 2002, 57, 899–907. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, H.; Ding, M.; Shang, Z. Large phase ratio spontaneous extraction followed by GC-MS for the determination of caffeine in beverages. Chromatographia 2010, 71, 323–326. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, C.; Kokot, S. Simultaneous kinetic spectrophotometric determination of acetaminophen and phenobarbital by artificial neural networks and partial least squares. Anal. Chim. Acta 2000, 419, 185–196. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Noroozifar, M. Copper polydopamine complex/multiwalled carbon nanotubes as novel modifier for simultaneous electrochemical determination of ascorbic acid, dopamine, acetaminophen, nitrite and xanthine. J. Solid State Electrochem. 2018, 22, 3049–3057. [Google Scholar] [CrossRef]
- Amiri-Aref, M.; Raoof, J.B.; Ojani, R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens. Actuat. B 2014, 192, 634–641. [Google Scholar] [CrossRef]
- Primo, E.N.; Gutierrez, F.A.; Luque, G.L. Comparative study of the electrochemical behavior and analytical applications of (bio)sensing platforms based on the use of multi-walled carbon nanotubes dispersed in different polymers. Anal. Chim. Acta 2013, 805, 19–35. [Google Scholar] [CrossRef]
- Hossieny, I.; Temerk, Y. Sensitive electrochemical sensor for simultaneous determination of uric acid and xanthine in human biological fluids based on the nano-boron doped ceria modified glassy carbon paste electrode. J. Electroanal. Chem. 2016, 780, 176–186. [Google Scholar]
- Levine, M. New concepts in the biology and biochemistry of ascorbic acid. N. Engl. J. Med. 1986, 314, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-H.; Wu, Y.-F.; Liu, X.-W.; Rong, M.-C.; Chen, X.-M.; Chen, X. An electrochemical ascorbic acid sensor based on palladium nanoparticles supported on graphene oxide. Anal. Chim. Acta 2012, 745, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, A.K.; Shoora, S.K. Multiwall carbon nanotube modified glassy carbon electrode as voltammetric sensor for the simultaneous determination of ascorbic acid and caffeine. Electrochim. Acta 2013, 93, 248–253. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Chen, L. Mesoporous silica-coated gold nanorods: Towards sensitive colorimetric sensing of ascorbic acid via target-induced silver overcoating. Nanoscale 2011, 3, 1756–1759. [Google Scholar] [CrossRef]
- Hallberg, L. Bioavailability of dietary iron in man. Annu. Rev. Nutr. 1981, 1, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr. Rev. 1999, 57, 71–77. [Google Scholar] [CrossRef]
- Matei, N.; Birghila, S.; Popescu, V.; Dobrinas, S.; Soceanu, A.; Oprea, C.; Magearu, V. Kinetic study of vitamin C degradation from pharmaceutical products. Rom. J. Phys. 2008, 53, 343–351. [Google Scholar]
- Zhang, M.; Liao, C.; Yao, Y.; Liu, Z.; Gong, F.; Yan, F. High-Performance Dopamine Sensors Based on Whole-Graphene Solution-Gated Transistor. Adv. Funct. Mater. 2014, 24, 978–985. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, S.; Ding, Y. Simultaneous detection of roxithromycin and dopamine using a sensor platform based on poly (sulfosalicylic acid) and its application in human serum studies. Anal. Methods 2014, 6, 3316–3321. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, H.K.; You, J.M.; Jeong, H.; Jeon, S. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO-porphyrin. Sens. Actuat. B 2014, 190, 886–895. [Google Scholar] [CrossRef]
- Lario, B.Á.; Vicente, J.M. Uric acid and evolution. Rheumatology 2010, 49, 2010–2015. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Taei, M.; Khayamian, T. A differential pulse voltammetric method for simultaneous determination of ascorbic acid, dopamine, and uric acid using poly (3-(5-chloro-2-hydroxyphenylazo)-4,5-dihydroxynaphthalene-2,7-disulfonic acid) film modified glassy carbon electrode. J. Electroanal. Chem. 2009, 633, 212–220. [Google Scholar] [CrossRef]
- Lian, W.; Ma, D.J.; Xu, X.; Chen, Y.; Wu, Y.L. Rapid high-performance liquid chromatography method for determination of tryptophan in gastric juice. J. Dig. Dis. 2012, 13, 100–106. [Google Scholar] [CrossRef]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin. Chim. Acta 2005, 356, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, X.; Ji, X.; Lin, R.; Lin, W. Simultaneous determination of ascorbic acid, dopamine and uric acid using poly(4-aminobutyric acid) modified glassy carbon electrode. Sens. Actuat. B 2013, 178, 359–365. [Google Scholar] [CrossRef]
- Ganesh, H.V.S.; Noroozifar, M.; Kerman, K. Epigallocatechin Gallate-Modified Graphite Paste Electrode for Simultaneous Detection of Redox-Active Biomolecules. Sensors 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Bahmanzadeh, S.; Noroozifar, M. Fabrication of modified carbon paste electrodes with Ni-doped Lewatit FO36nano ion exchange resin for simultaneous determination of epinephrine, paracetamol and tryptophan. J. Electroanal. Chem. 2018, 809, 153–162. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Narouie, S.; Noroozifar, M. Modified glassy carbon electrode with Polydopamine-multiwalled carbon nanotubes for simultaneous electrochemical determination of biomolecules in biological fluids. J. Pharm. Biomed. Anal. 2018, 161, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Omara Shastan, Z.; Ganesh, H.; Noroozifar, M.; Kerman, K. Carbon ceramic microelectrodes modified with buckyballs for simultaneous determination of redox-active biomolecules. RSC Adv. 2018, 8, 5960–5966. [Google Scholar] [CrossRef]
- Tohidinia, M.; Farsadrooh, M.; Bahmanzadeh, S.; Sabbaghi, N.; Noroozifar, M. Poly(quercetin)-bismuth nanowires as a new modifier for simultaneous voltammetric determination of dihydroxybenzene isomers and nitrite. RSC Adv. 2018, 8, 1237–1245. [Google Scholar] [CrossRef]
- Li, S.; Noroozifar, M.; Kerman, K. Nanocomposite of ferricyanide-doped chitosan with multi-walled carbon nanotubes for simultaneous senary detection of redox-active biomolecules. J. Electroanal. Chem. 2019, 849, 113376. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanatsu, K.; Iino, T.; Kato, S.; Jeong, Y.I.; Shibata, N.; Takada, K.; Takeuchi, K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002, 71, 827. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agri. Food Chem. 2002, 50, 2200. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomas-Barberan, F.A.; Espın, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.C.; Huang, M.T.; Chang, R.L.; Sayer, J.M.; Jerina, D.M.; Conney, A.H. Disposition of the naturally occurring antimutagenic plant phenol, ellagic acid, and its synthetic derivatives, 3-O-decylellagic acid and 3,3′-di-O-methylellagic acid in mice. Carcinogenesis 1986, 7, 1663. [Google Scholar] [CrossRef]
- Paivarinta, E.; Pajari, A.M.; Torronen, R.; Mutanen, M. Ellagic Acid and Natural Sources of Ellagitannins as Possible Chemopreventive Agents Against Intestinal Tumorigenesis in the Min Mouse. Nutr. Cancer 2006, 54, 79. [Google Scholar] [CrossRef]
- Bisen, P.S.; Bundela, S.S.; Sharma, A. Ellagic Acid—Chemopreventive Role in Oral Cancer. J. Cancer Sci. Ther. 2012, 4, 023. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Hassani-Nadiki, H.; Hadavi, M.S.; Foroughi, M.M. Modified fluorine-doped tin oxide electrode with inorganic ruthenium red dye-multiwalled carbon nanotubes for simultaneous determination of a dopamine, uric acid, and tryptophan. Sens. Actuat. B 2014, 204, 333–341. [Google Scholar] [CrossRef]
- Minh, T.T.; Phong, N.H.; Van Duc, H.; Khieu, D.Q. Microwave synthesis and voltammetric simultaneous determination of paracetamol and caffeine using an MOF-199-based electrode. J. Mater. Sci. 2018, 53, 2453–2471. [Google Scholar] [CrossRef]
- Mekassa, B.; Tessema, M.; Chandravanshi, B.S. Simultaneous determination of caffeine and theophylline using square wave voltammetry at poly (l-aspartic acid)/functionalized multi-walled carbon nanotubes composite modified electrode. Sens. Biosens. Res. 2017, 16, 46–54. [Google Scholar] [CrossRef]
- Švorc, L.U.; Tomčík, P.; Svítková, J.; Rievaj, M.; Bustin, D. Voltammetric determination of caffeine in beverage samples on bare boron-doped diamond electrode. Food Chem. 2012, 135, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, T.; Bi, C.-Y. Simultaneous determination of acetaminophen, theophylline and caffeine using a glassy carbon disk electrode modified with a composite consisting of poly (Alizarin Violet 3B), multiwalled carbon nanotubes and graphene. Microchim. Acta 2016, 183, 731–739. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Chen, S.; Hu, F.; Zhang, M. Simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan on gold nanoparticles/overoxidized-polyimidazole composite modified glassy carbon electrode. Anal. Chim. Acta 2012, 741, 15–20. [Google Scholar] [CrossRef]
- Narouei, F.H.; Tammandani, H.K.; Ghalandarzehi, Y.; Sabbaghi, Y.; Noroozifar, M. An Electrochemical Sensor based on conductive polymers/Graphite Paste Electrode for Simultaneous Determination of Dopamine, Uric acid and Tryptophan in Biological Samples. Int. J. Electrochem. Sci. 2017, 12, 7739–7753. [Google Scholar] [CrossRef]
- Habibi, B.; Abazari, M.; Pournaghi-Azar, M.H. Simultaneous determination of codeine and caffeine using single-walled carbon nanotubes modified carbon-ceramic electrode. Coll. Surf. B Biointerfaces 2014, 114, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Nitrogen-doped carbon nanotubes decorated poly (L-Cysteine) as a novel, ultrasensitive electrochemical sensor for simultaneous determination of theophylline and caffeine. Talanta 2018, 178, 449–457. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Taheri, A. Preparation of silver hexacyanoferrate nanoparticles and its application for the simultaneous determination of ascorbic acid, dopamine and uric acid. Talanta 2010, 80, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Srdjenovic, B.; Djordjevic-Milic, V.; Grujic, N.; Injac, R.; Lepojevic, Z. Simultaneous HPLC Determination of Caffeine, Theobromine, and Theophylline in Food, Drinks, and Herbal Products. J. Chromatogr. Sci. 2008, 46, 144–149. [Google Scholar] [CrossRef]
- Cooper, N.; Khosravan, R.; Erdmanna, C.; Fiene, J.; Lee, J.W. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J. Chromatogr. B 2006, 837, 1–10. [Google Scholar] [CrossRef]
- Benedetto, G.E.D.; Fico, D.; Pennetta, A.; Malitesta, C.; Nicolardi, G.; Lofrumento, D.D.; Nuccio, F.D.; Pesa, V.L. A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J. Pharm. Biomed. Anal. 2014, 98, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Emadi-Konjin, P.; Verjee, Z.; Levin, A.V.; Adeli, K. Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC). Clin. Biochem. 2005, 38, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.F.; Freire, T.B.; Pinto, C.A.S.D.O.; Prado, M.S.A.; Baby, A.R.; Velasco, M.V.R. Tryptophan and kynurenine determination in human hair by liquid chromatography. J. Chromatogr. B 2017, 1065–1066, 59–62. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Linear Range/µM | Detection Limit/µM | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPE-MWCNTs | _ | 2.00–170.0 | 0.40–100.0 | 0.60–100.0 | _ | _ | _ | 0.36 | 0.27 | 0.065 | _ | _ | [47] |
| CBNB/ CNT/GCE | 20–400 | 0.05–2.75 | 1.0–45 | 0.025–4.8 | _ | _ | 5.71 | 1.7 × 10−2 | 0.42 | 0.11 | _ | _ | [52] |
| nano-B-CeO2/ GCPE | _ | _ | 0.42–11.9 | _ | 0.07–2.02 | _ | _ | _ | 5.39 × 10−3 | _ | 2.36 × 10−3 | _ | [33] |
| PTh/GPE | _ | 10–180 | 6-180 | 6–180 | _ | _ | _ | 1 | 0.57 | 0.61 | _ | _ | [53] |
| SWCNT/ CCE | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | 0.25 | [54] |
| PLCY/N-CNT/GCE | _ | _ | _ | _ | _ | 0.4-300 | _ | _ | _ | _ | _ | 0.20 | [55] |
| GC/CNT-AgHCFNP | 4.0–78 | 2.4–130 | 2.0–15 | _ | _ | _ | 4.2 × 10−2 | 1.4 × 10−2 | 0.6 | _ | _ | _ | [56] |
| FC/Chi-MWCNT-GPE | 10–2057 | 0.99–94.1 | 0.99–193.7 | 0.99–198.9 | 1–191.3 | 10–2439.0 | 5.26 | 0.0011 | 0.0027 | 0.0037 | 0.0073 | 2.05 | [41] |
| MGPE/ MWCNTs-EA | 0.099–54 | 0.099–54 | 0.099–54 | 0.099–54 | 0.099–54 | 0.099–54 | 0.091 | 0.0099 | 0.013 | 0.011 | 0.014 | 0.078 | This Work |
| Sample | Target | Detected (µM) ± SD * (n = 3) | Spike (µM) | Found (µM) ± SD * (n = 3) | Recovery (%) | Standard (HPLC) Method [59,60,61,62,63] | ||
|---|---|---|---|---|---|---|---|---|
| Detected (µM) ± SD * (n = 3) | Found (µM) ± SD * (n = 3) | Recovery (%) | ||||||
| Serum | AA | _ | 4.0 | 3.92 ± 0.26 | 98.0 | _ | 3.90 ± 0.26 | 97.5 |
| _ | 15.0 | 14.75 ± 1.0 | 98.3 | _ | 14.84 ± 1.03 | 98.9 | ||
| _ | 35.0 | 34.3 ± 1.45 | 98.0 | _ | 34.70 ± 1.96 | 99.1 | ||
| DA | _ | 4.0 | 4.03 ± 0.09 | 100.7 | _ | 3.99 ± 0.09 | 99.8 | |
| _ | 15.0 | 14.90 ± 0.99 | 99.3 | _ | 15.50 ± 0.46 | 103.3 | ||
| _ | 35.0 | 35.10 ± 2.0 | 100.2 | _ | 35.10 ± 1.47 | 100.3 | ||
| UA | 7.7 ± 0.4 | 4.0 | 11.8 ± 0.5 | 102.5 | 7.9 ± 0.2 | 12.1 ± 0.3 | 105.0 | |
| 7.7 ± 0.4 | 15.0 | 22.8 ± 0.3 | 100.6 | 7.9 ± 0.2 | 22.8 ± 0.6 | 99.3 | ||
| 7.7 ± 0.4 | 35.0 | 43.1 ± 0.6 | 101.1 | 7.9 ± 0.2 | 43.2 ± 0.4 | 100.8 | ||
| Trp | 1.2 ± 0.1 | 4.0 | 5.1 ± 0.2 | 97.5 | 1.0 ± 0.5 | 5.2 ± 0.1 | 105.0 | |
| 1.2 ± 0.1 | 15.0 | 16.7 ± 0.8 | 103.3 | 1.0 ± 0.5 | 16.3 ± 0.5 | 102.0 | ||
| 1.2 ± 0.1 | 35.0 | 36.1 ± 0.1 | 99.7 | 1.0 ± 0.5 | 35.9 ± 0.2 | 99.7 | ||
| XN | _ | 4.0 | 3.94 ± 0.17 | 98.5 | _ | 4.05 ± 0.21 | 101.2 | |
| _ | 15.0 | 15.30 ± 1.11 | 102.0 | _ | 15.25 ± 0.55 | 101.7 | ||
| _ | 35.0 | 35.94 ± 2.60 | 102.7 | _ | 35.40 ± 1.34 | 101.1 | ||
| CA | 4.5 ± 0.3 | 4.0 | 8.5 ± 0.3 | 100.0 | 4.7 ± 0.1 | 8.8 ± 0.6 | 107.5 | |
| 4.5 ± 0.3 | 15.0 | 20.2 ± 0.7 | 104.6 | 4.7 ± 0.1 | 19.9 ± 0.4 | 102.6 | ||
| 4.5 ± 0.3 | 35.0 | 39.8 ± 0.4 | 100.8 | 4.7 ± 0.1 | 40.2 ± 0.2 | 102.0 | ||
| Urine | AA | _ | 4.0 | 4.06 ± 0.22 | 101.5 | _ | 4.11 ± 0.33 | 102.8 |
| _ | 15.0 | 14.89 ± 1.45 | 99.3 | _ | 15.09 ± 0.61 | 100.6 | ||
| _ | 35.0 | 34.85 ± 2.44 | 99.6 | _ | 34.95 ± 0.26 | 99.9 | ||
| DA | _ | 4.0 | 3.89 ± 0.20 | 97.3 | _ | 3.96 ± 0.25 | 99.0 | |
| _ | 15.0 | 15.5 ± 0.96 | 103.3 | _ | 15.02 ± 0.75 | 100.1 | ||
| _ | 35.0 | 35.90 ± 1.70 | 102.6 | _ | 35.50 ± 1.36 | 101.4 | ||
| UA | 1.4 ± 0.9 | 4.0 | 5.40 ± 0.7 | 100.0 | 1.8 ± 0.5 | 5.7 ± 0.1 | 97.5 | |
| 1.4 ± 0.9 | 15.0 | 16.9 ± 0.5 | 103.3 | 1.8 ± 0.5 | 17.0 ± 0.2 | 101.3 | ||
| 1.4 ± 0.9 | 35.0 | 35.7 ± 0.3 | 98.0 | 1.8 ± 0.5 | 37.4 ± 0.6 | 101.7 | ||
| Trp | 1.0 ± 0.2 | 4.0 | 4.9 ± 0.3 | 97.5 | 0.7 ± 0.2 | 4.5 ± 0.4 | 95.0 | |
| 1.0 ± 0.2 | 15.0 | 16.9 ± 0.7 | 106.0 | 0.7 ± 0.2 | 16.7 ± 0.2 | 106.0 | ||
| 1.0 ± 0.2 | 35.0 | 37.1 ± 0.8 | 103.1 | 0.7 ± 0.2 | 35.9 ± 0.1 | 100.5 | ||
| XN | _ | 4.0 | 3.93 ± 0.19 | 98.2 | _ | 4.04 ± 0.11 | 101.0 | |
| _ | 15.0 | 14.85 ± 0.88 | 99.0 | _ | 14.93 ± 0.77 | 99.5 | ||
| _ | 35.0 | 34.50 ± 1.10 | 98.6 | _ | 35.50 ± 1.05 | 101.4 | ||
| CA | 3.8 ± 0.2 | 4.0 | 8.0 ± 0.3 | 105.0 | 3.3 ± 0.5 | 7.1 ± 0.4 | 95.0 | |
| 3.8 ± 0.2 | 15.0 | 17.9 ± 0.6 | 94.0 | 3.3 ± 0.5 | 19.1 ± 0.7 | 105.3 | ||
| 3.8 ± 0.2 | 35.0 | 39.2 ± 0.2 | 101.1 | 3.3 ± 0.5 | 38.9 ± 0.3 | 101.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbaghi, N.; Noroozifar, M.; Kerman, K. Nanocomposite of Ellagic Acid with Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Detection of Six Biomolecules. C 2021, 7, 43. https://doi.org/10.3390/c7020043
Sabbaghi N, Noroozifar M, Kerman K. Nanocomposite of Ellagic Acid with Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Detection of Six Biomolecules. C. 2021; 7(2):43. https://doi.org/10.3390/c7020043
Chicago/Turabian StyleSabbaghi, Najmeh, Meissam Noroozifar, and Kagan Kerman. 2021. "Nanocomposite of Ellagic Acid with Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Detection of Six Biomolecules" C 7, no. 2: 43. https://doi.org/10.3390/c7020043
APA StyleSabbaghi, N., Noroozifar, M., & Kerman, K. (2021). Nanocomposite of Ellagic Acid with Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Detection of Six Biomolecules. C, 7(2), 43. https://doi.org/10.3390/c7020043







