Activated Carbon from Biomass Sustainable Sources
Abstract
1. Introduction
2. Processing Techniques
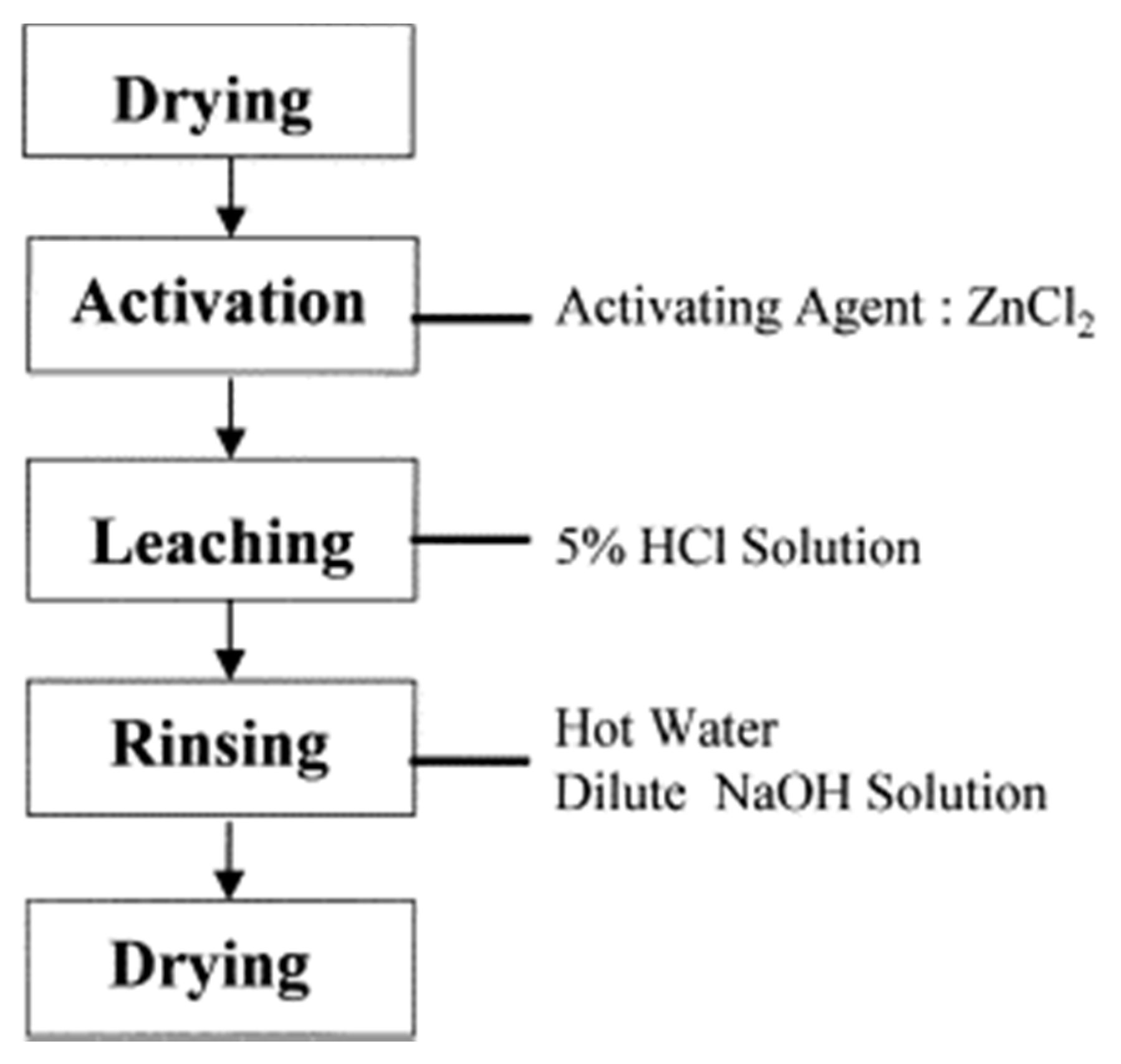
2.1. Pretreatment and Carbonization
2.2. Simultaneously Carbonization and Activation
2.3. Activation
3. Biomass Resources for Activated Carbon
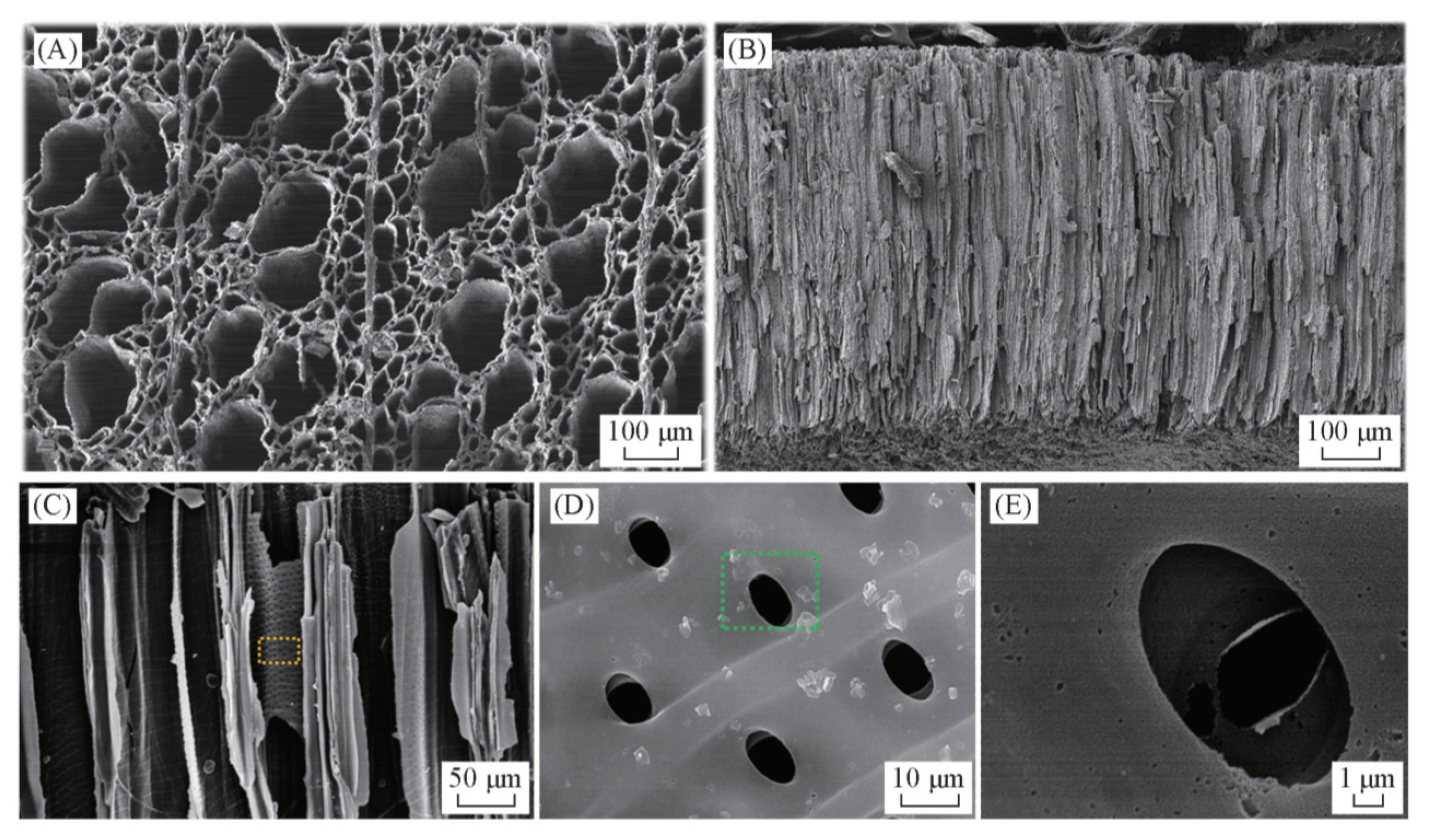
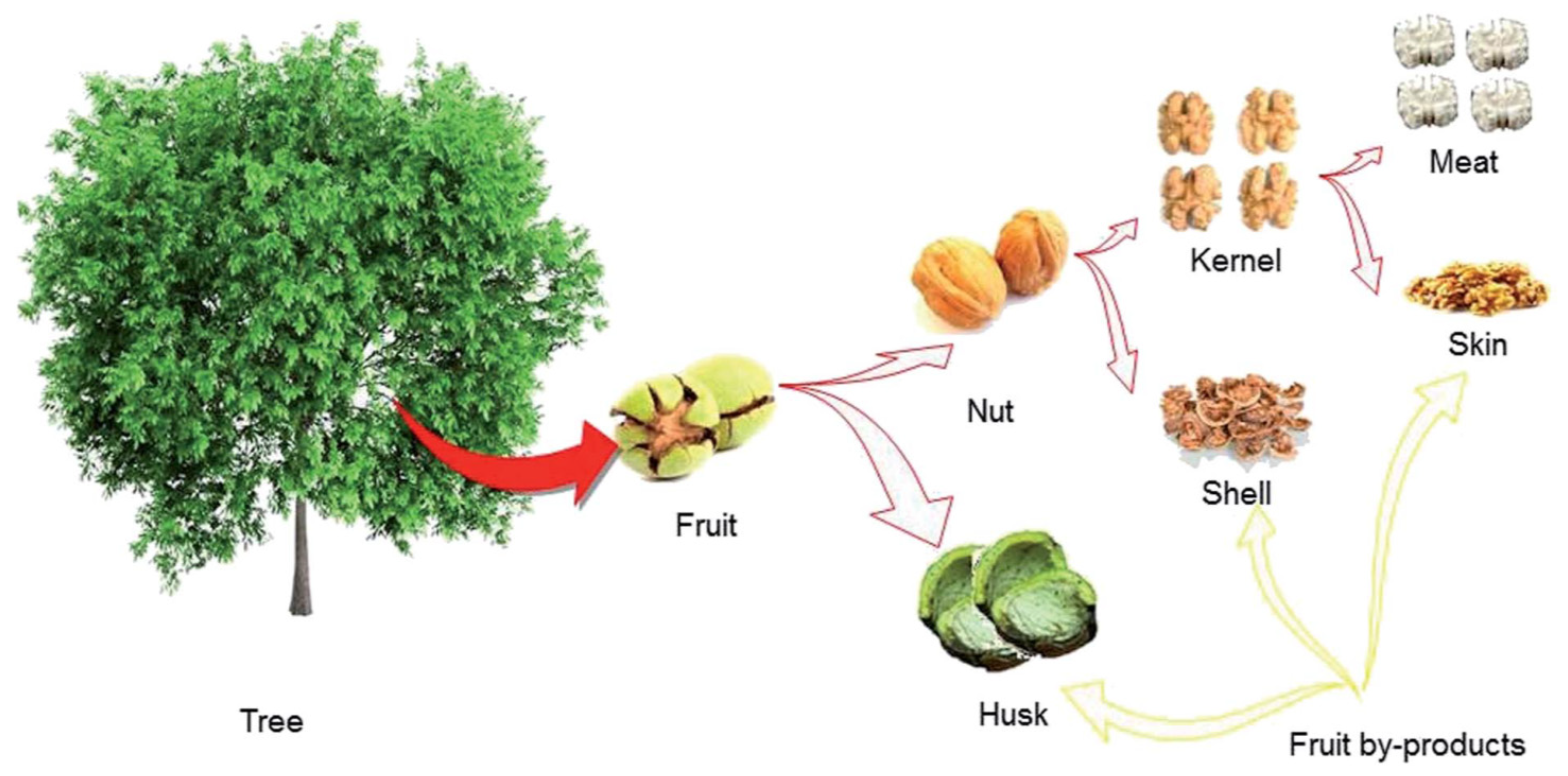
3.1. Walnut Shell
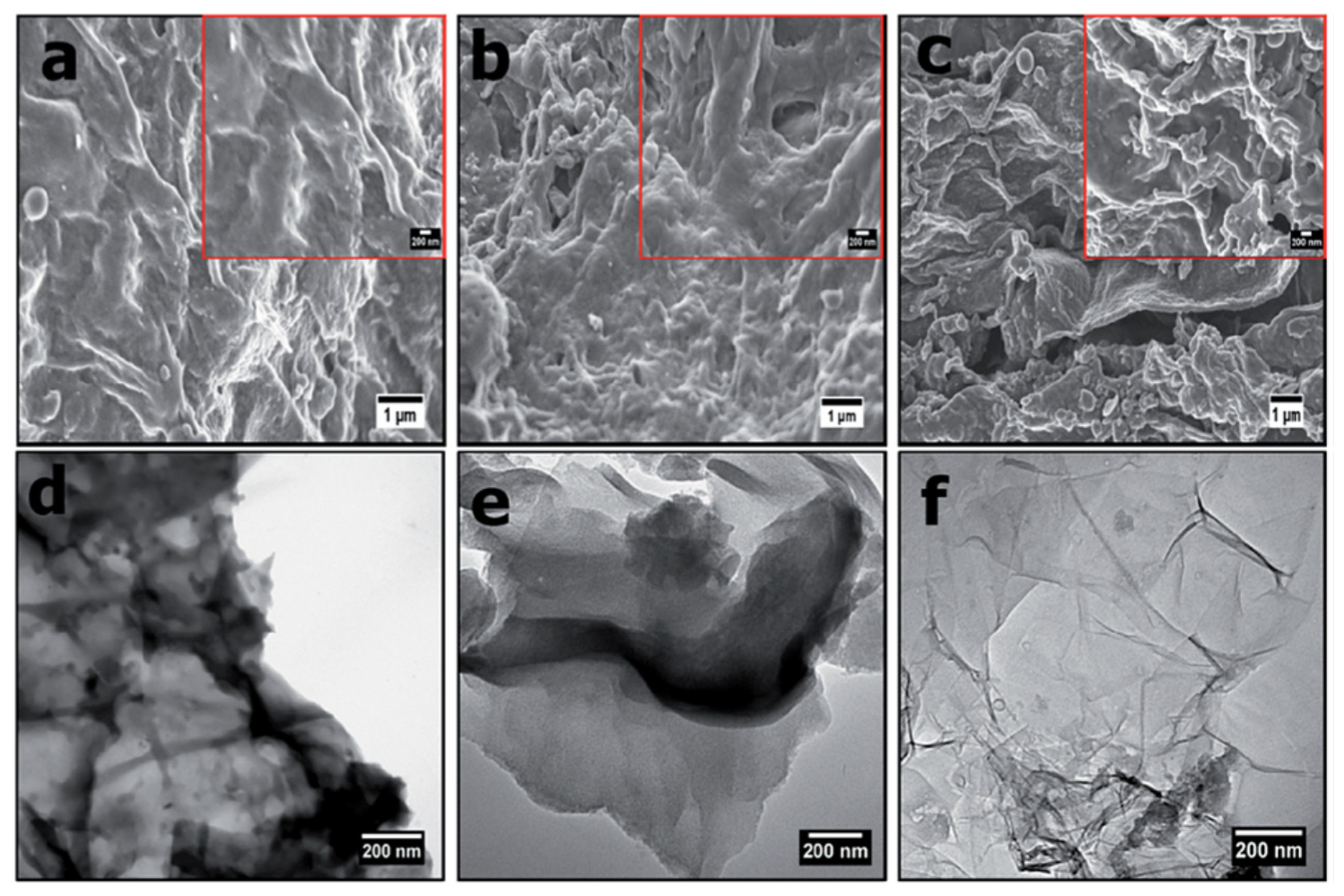
3.2. Citrus-Limon Tree Leaves
3.3. Guava Tree Wood
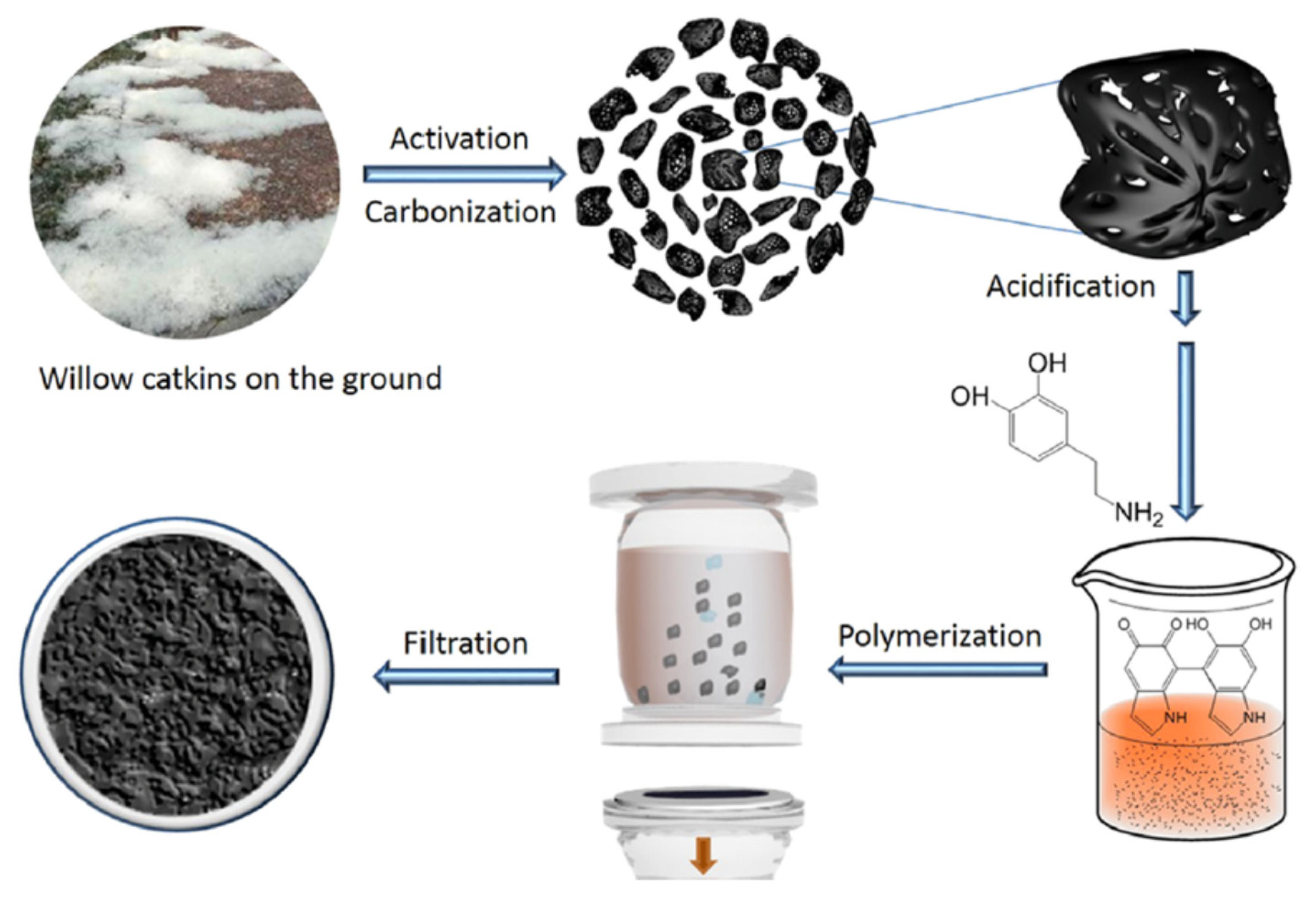
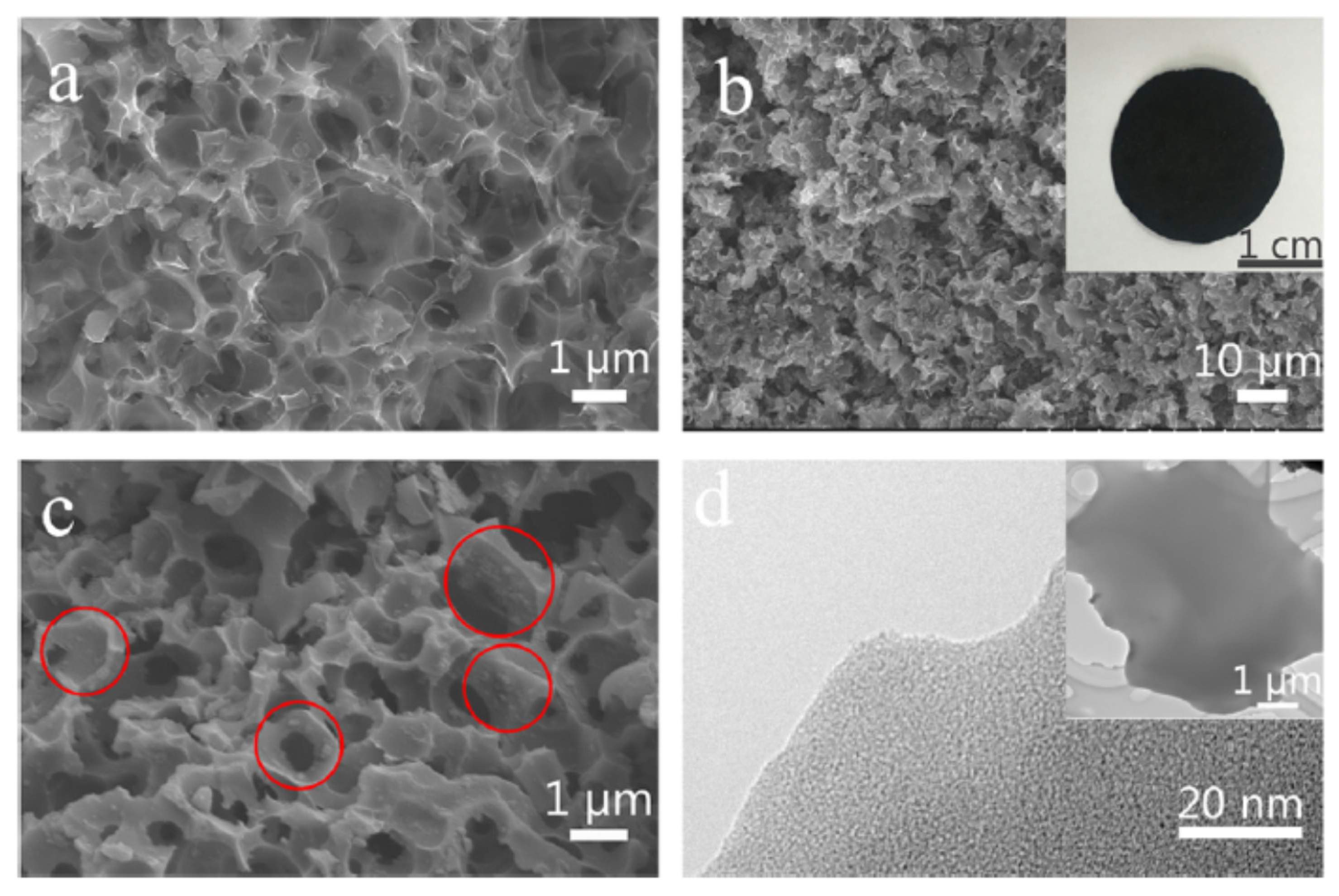
3.4. Willow Catkin
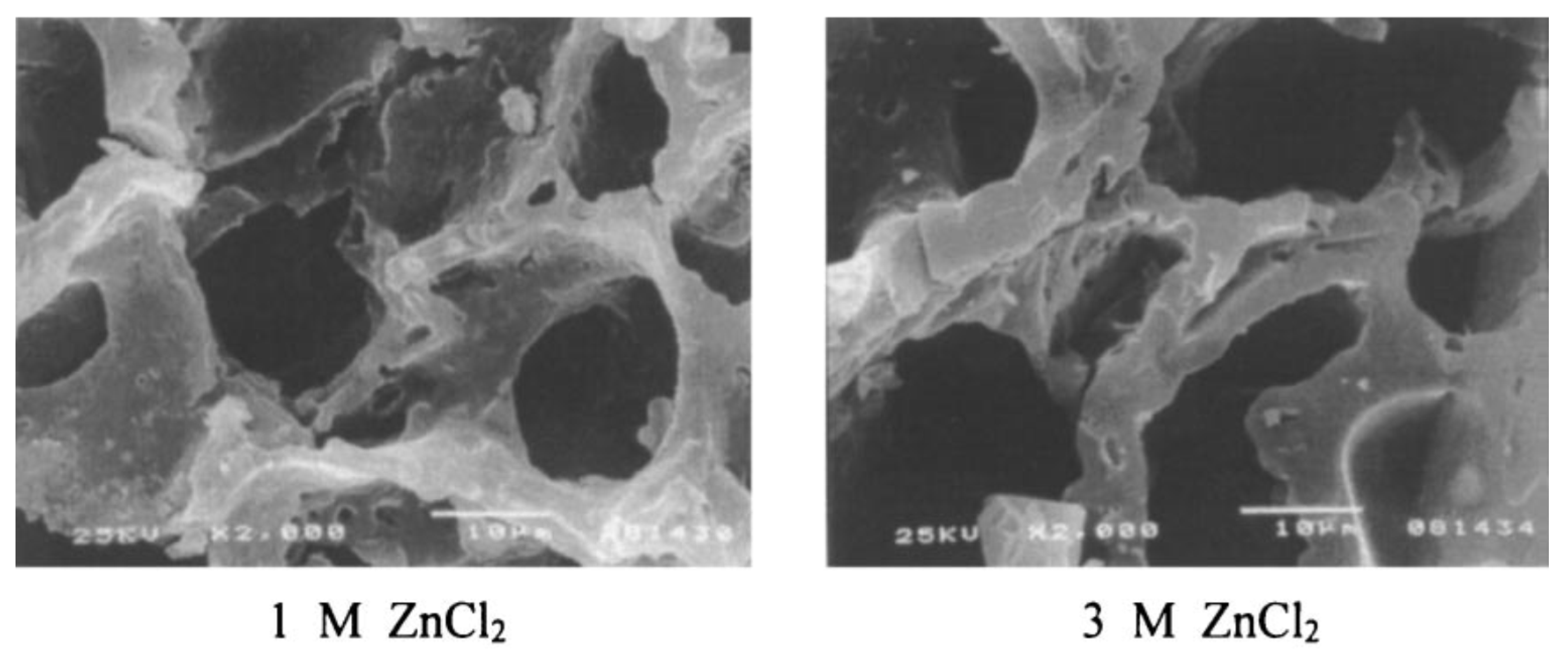
3.5. Onion Peel
4. Functionalization of Activated Carbon
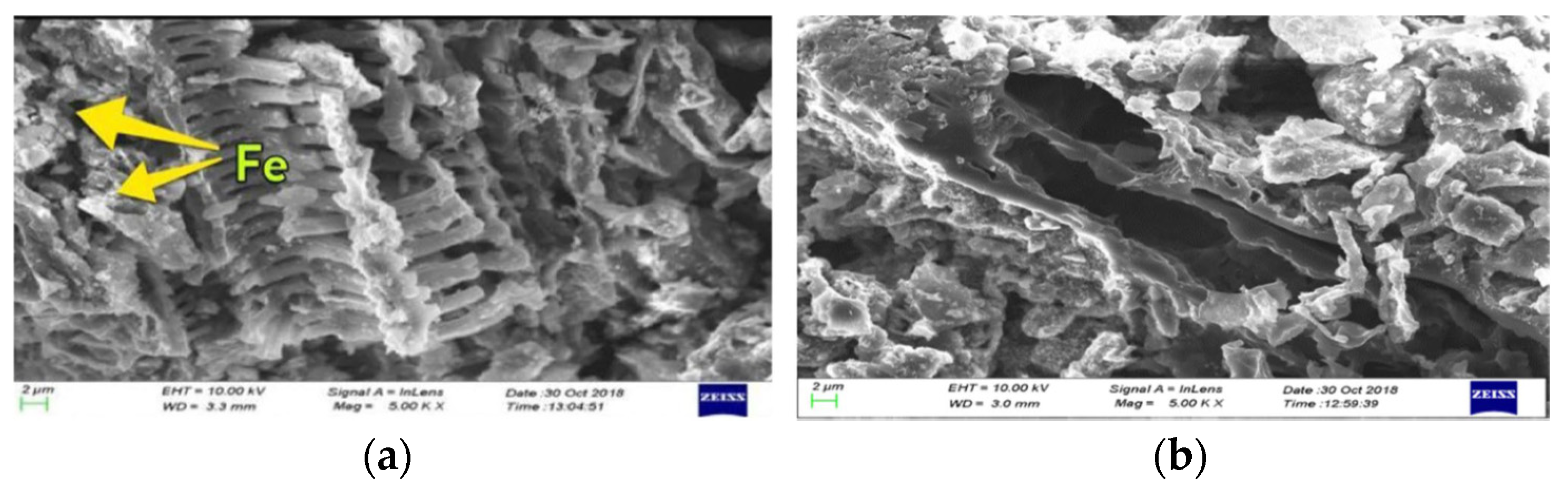
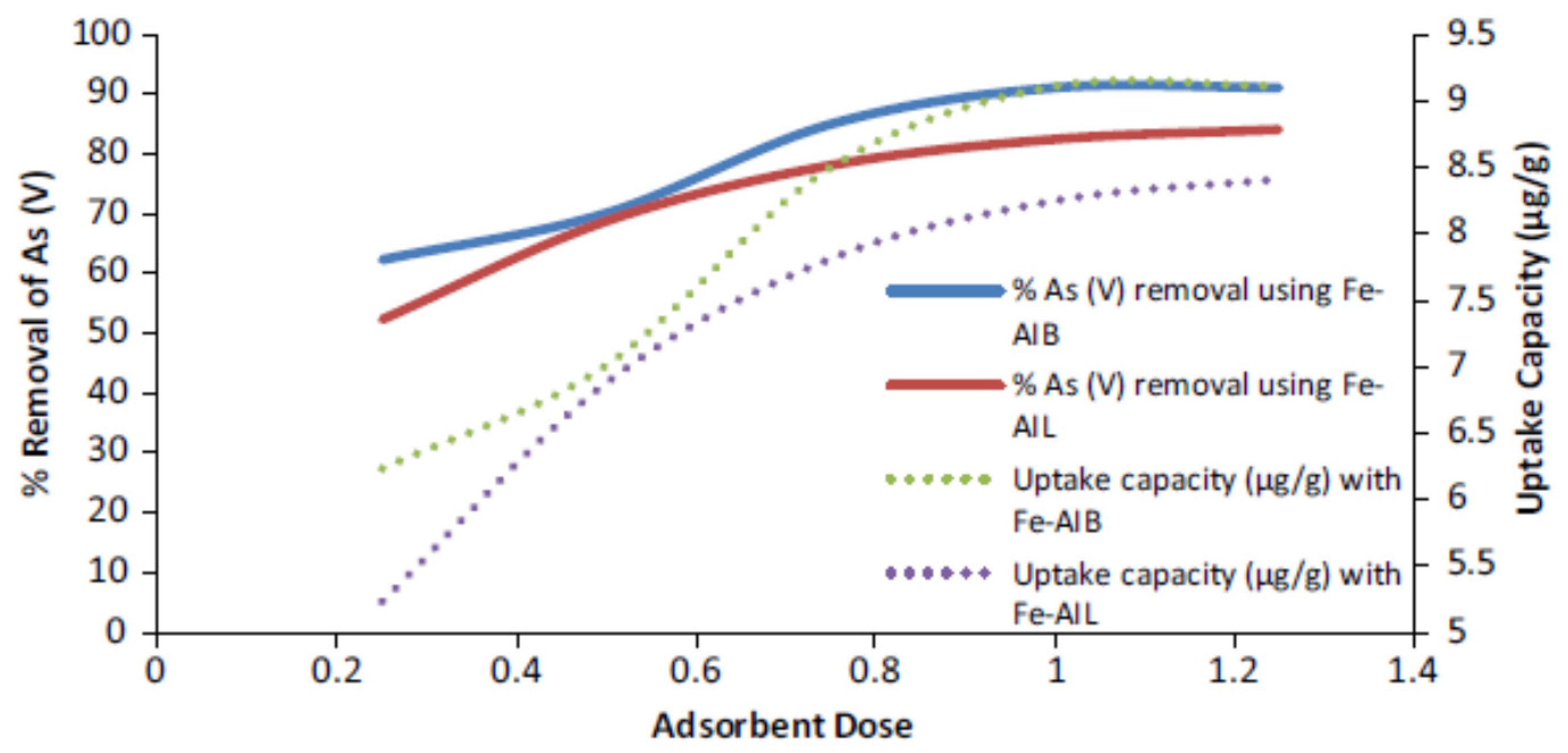
4.1. Iron Decorated Activated Carbon
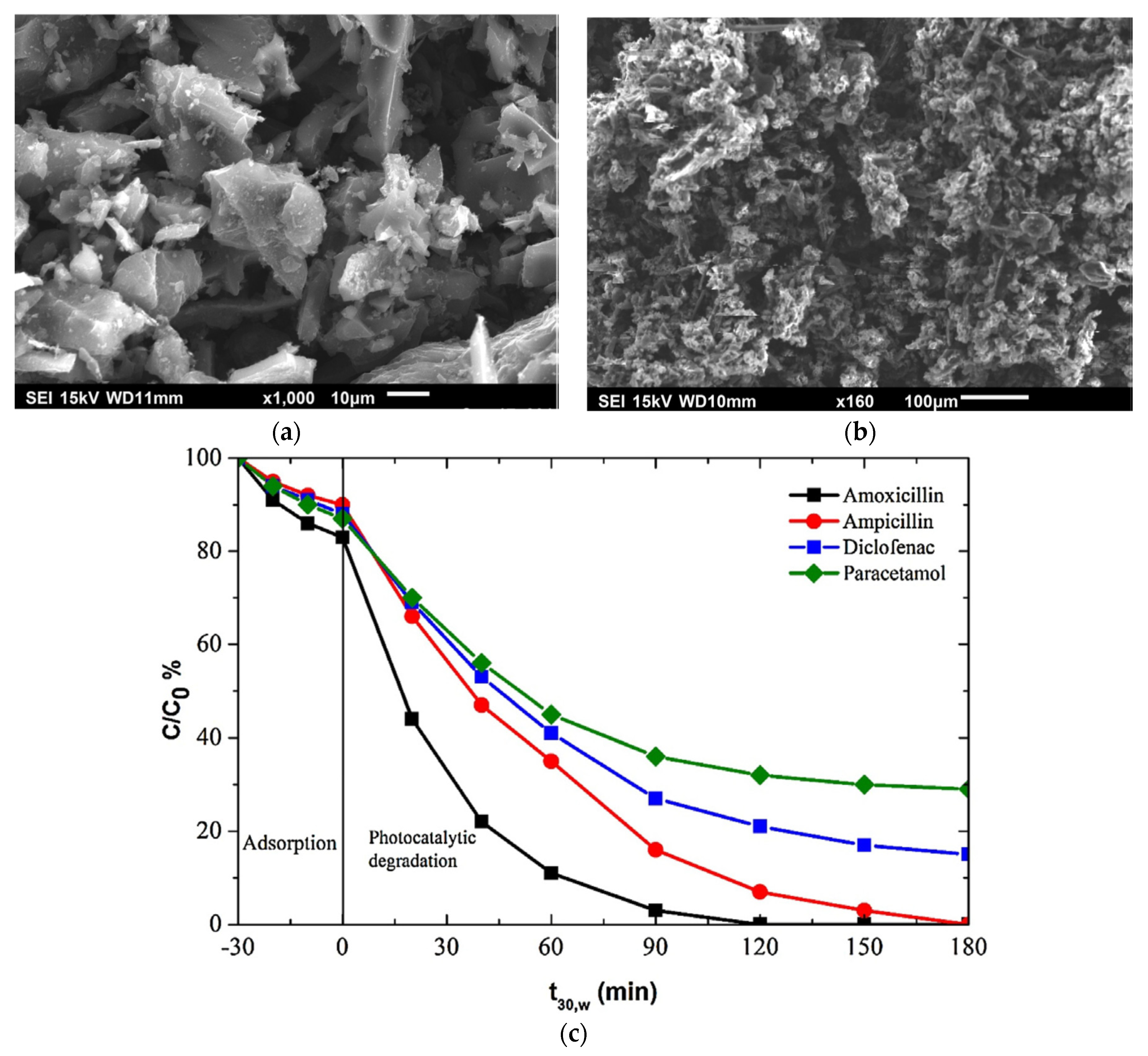
4.2. TiO2 Decorated Activated Carbon
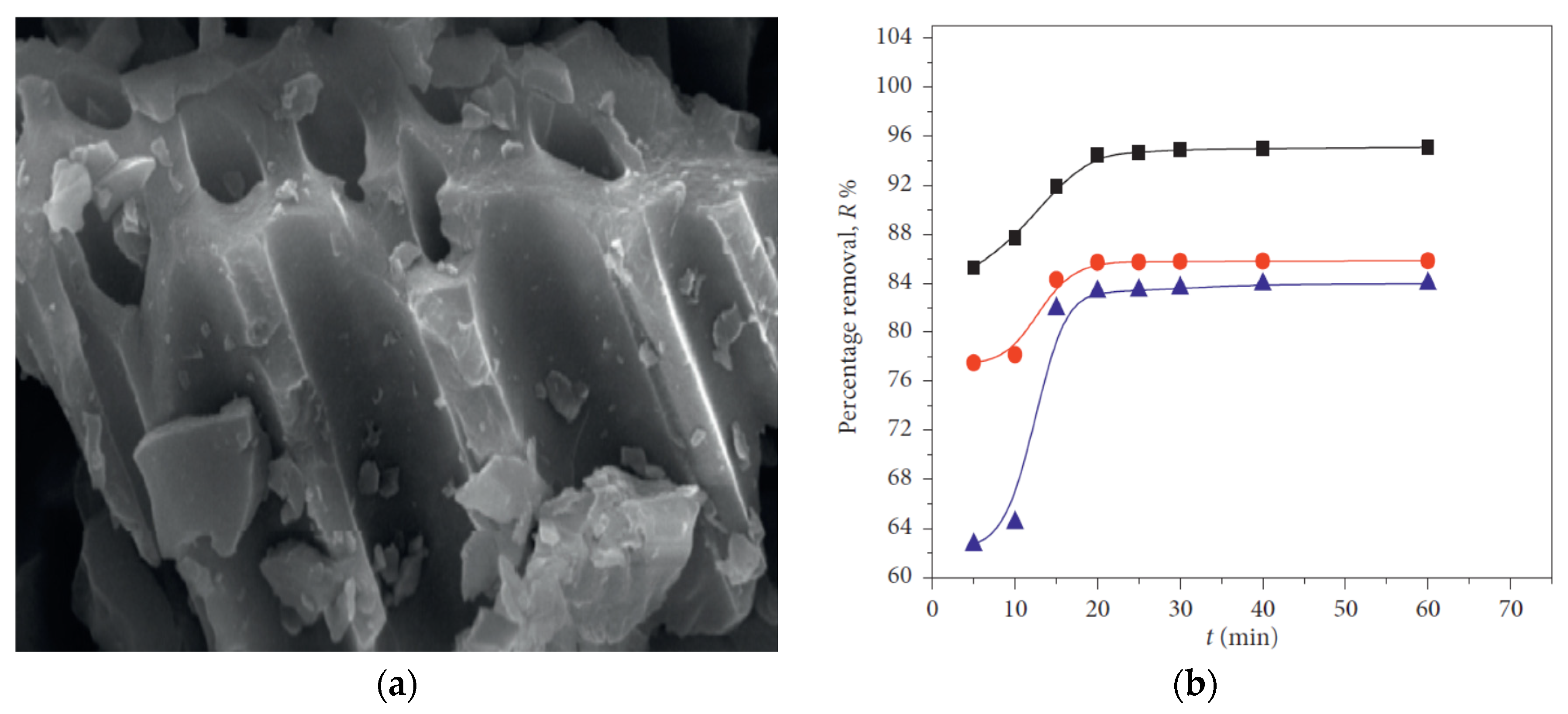
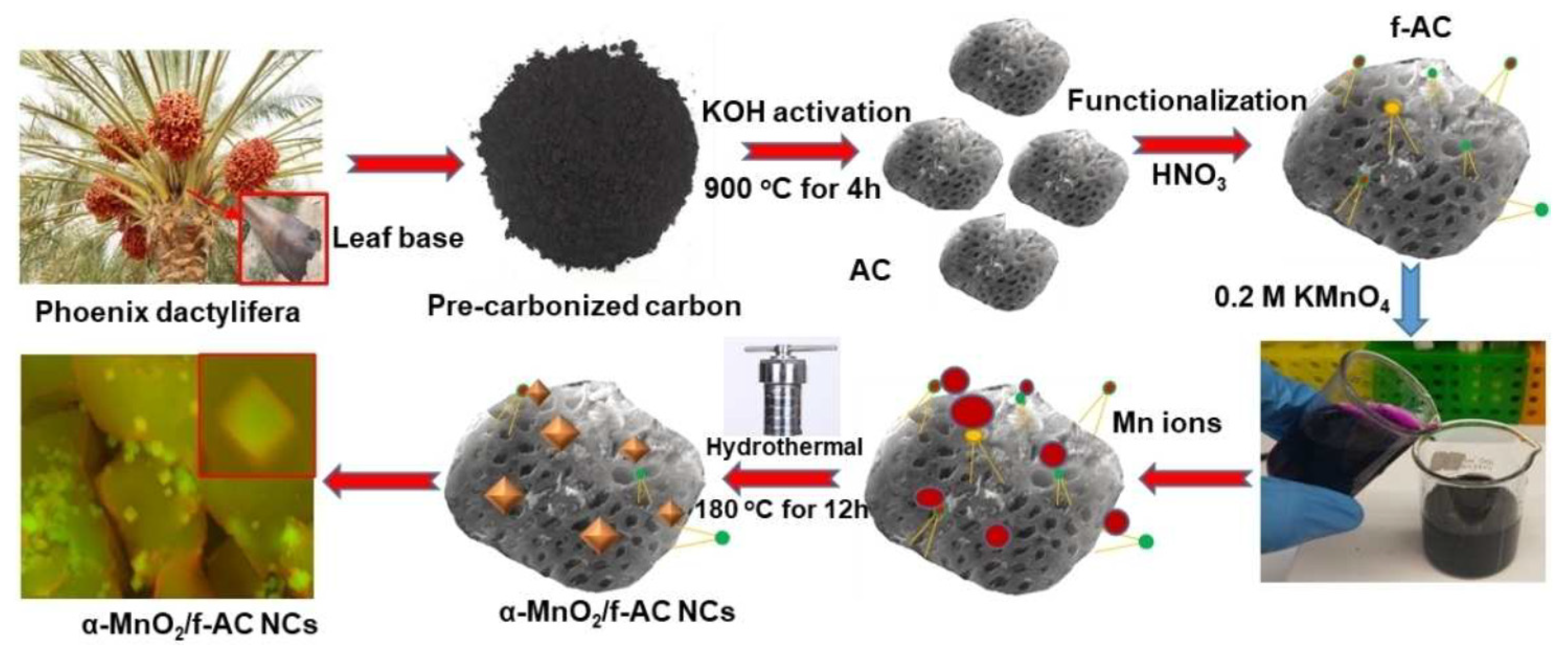
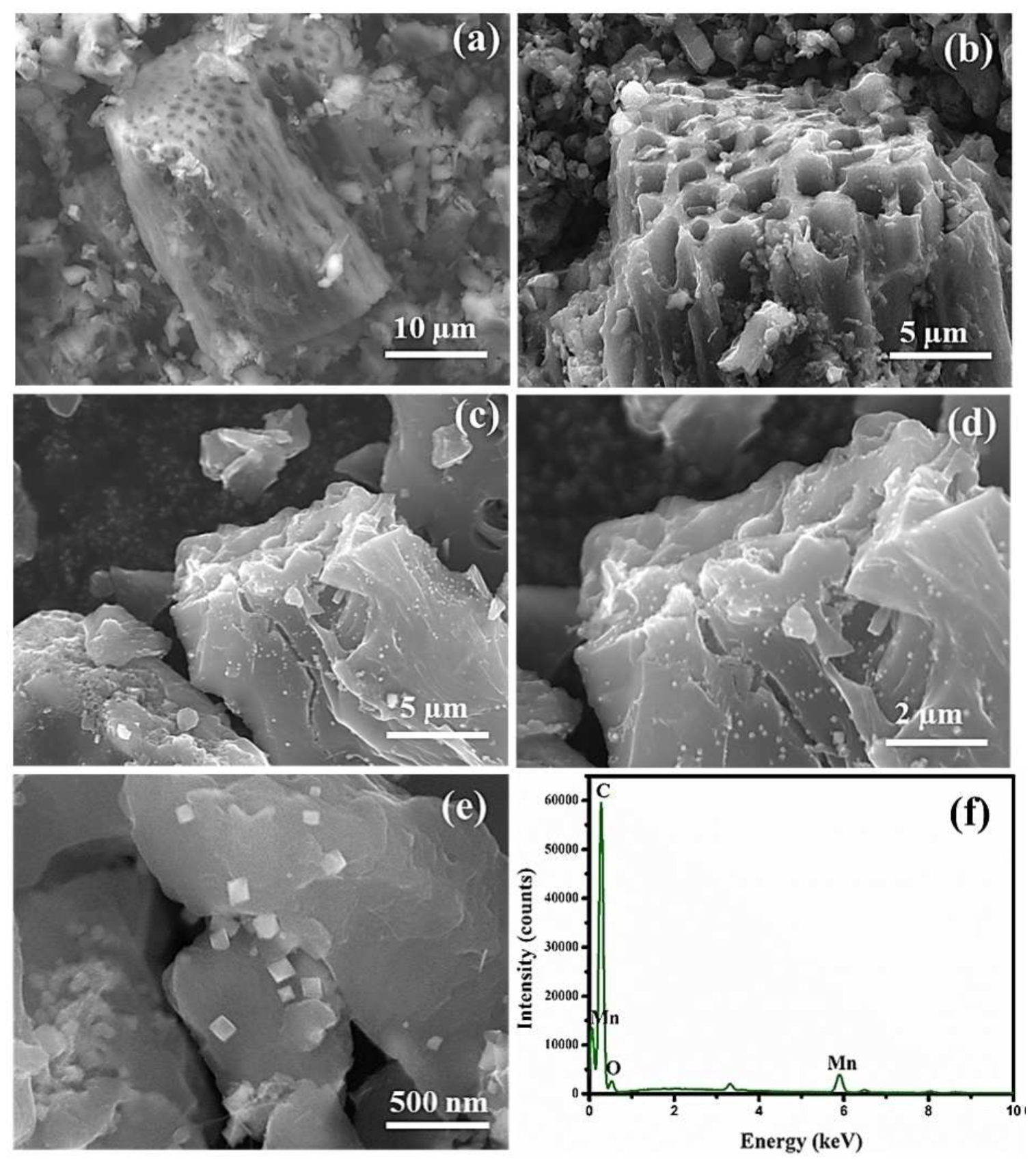
4.3. MnO2 Decorated Activated Carbon
4.4. Noble Metal Particles-Decorated Activated Carbon
5. Applications
6. Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated porous carbon derived from tea and plane tree leaves biomass for the removal of pharmaceutical compounds from wastewaters. Antibiotics 2021, 10, 65. [Google Scholar] [CrossRef]
- Egirani, D.; Latif, M.T.; Wessey, N.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for mercury removal. Appl. Water Sci. 2021, 11. [Google Scholar] [CrossRef]
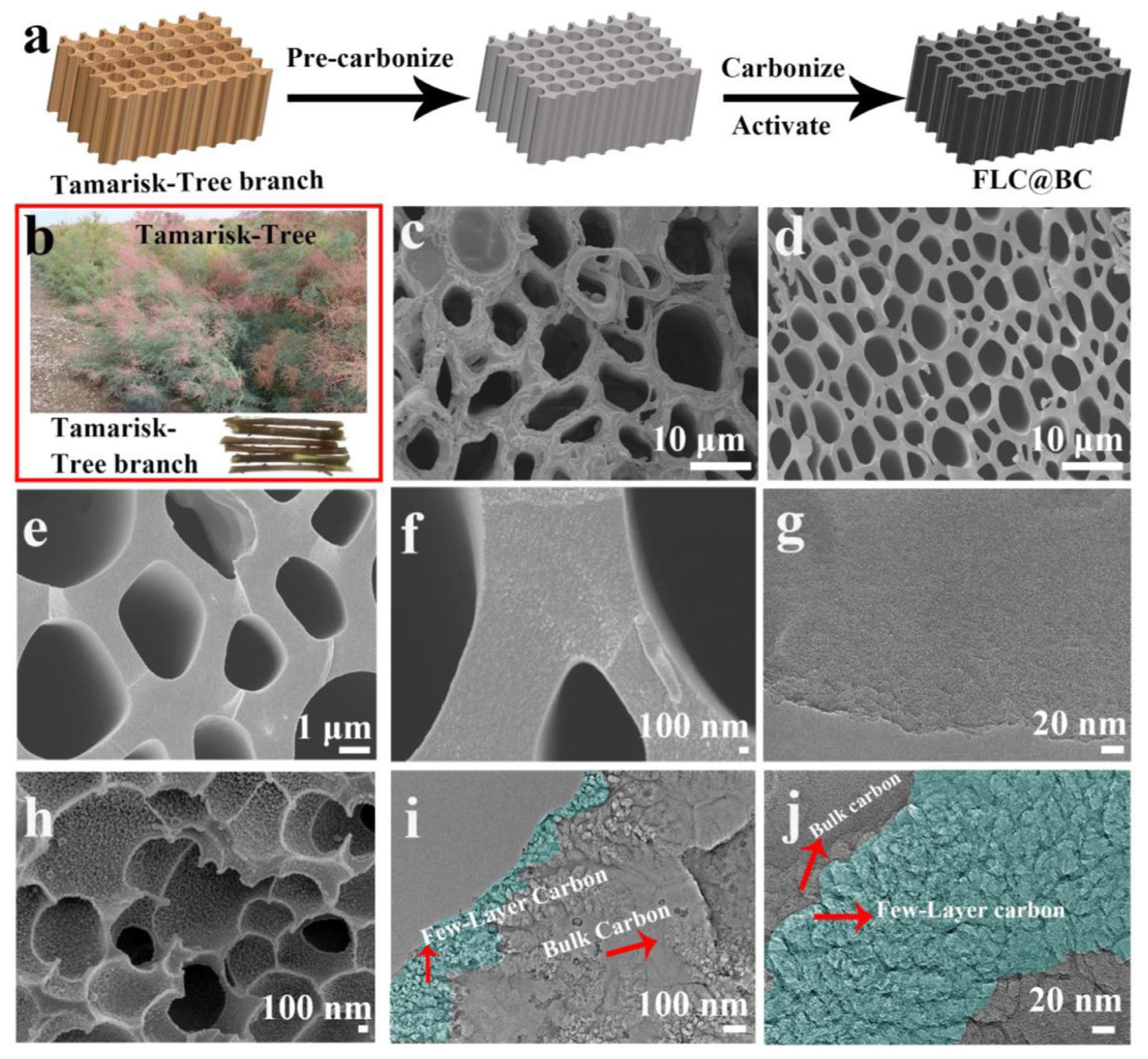
- Tan, Y.T.; Li, Y.; Wang, W.C.; Ran, F. High performance electrode of few-layer-carbon@bulk-carbon synthesized via controlling diffusion depth from liquid phase to solid phase for supercapacitors. J. Energy Storage 2020, 32, 101672. [Google Scholar] [CrossRef]
- Khorasgani, N.B.; Sengul, A.B.; Asmatulu, E. Briquetting grass and tree leaf biomass for sustainable production of future fuels. Biomass Conv. Bioref. 2020, 10, 915–924. [Google Scholar] [CrossRef]
- Vohra, M.; Al-Suwaiyan, M.; Hussaini, M. Gas phase toluene adsorption using date palm-tree branches based activated carbon. Int. J. Env. Res. Public Health 2020, 17, 9287. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.C.; Gao, J.; Yao, S.; Lan, X.Q.; Li, H.P.; Song, H. Modified ginkgo leaves for adsorption of methyl violet and malachite green dyes in their aqueous system. Desal. Water Treat. 2020, 206, 358–370. [Google Scholar] [CrossRef]
- Yargic, A.S. Evaluation of poplar tree-based sorbents in dye uptake via 2(5) full factorial experimental design and statistical analysis of %removal efficiency. J. Polytech. Politek. Derg. 2020, 23, 941–954. [Google Scholar]
- Saniya, A.; Sathya, K.; Nagarajan, K.; Yogesh, M.; Jayalakshmi, H.; Praveena, P.; Bharathi, S. Modelling of the removal of crystal violet dye from textile effluent using Murraya koenigii stem biochar. Desal. Water Treat. 2020, 203, 356–365. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, D.H.; Chen, Y.; Ran, M.J.; Gu, J.C. Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. In Proceedings of the 3rd International Conference on Advances in Energy, Environment and Chemical Engineering, Chengdu, China, 26–28 May 2017; p. 012051. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ouchi, K. Influence of alkali on the carbonization process-I: Carbonization of 3,5-dimethylphenol-formaldehyde resin with NaOH. Carbon 1982, 20, 41–45. [Google Scholar] [CrossRef]
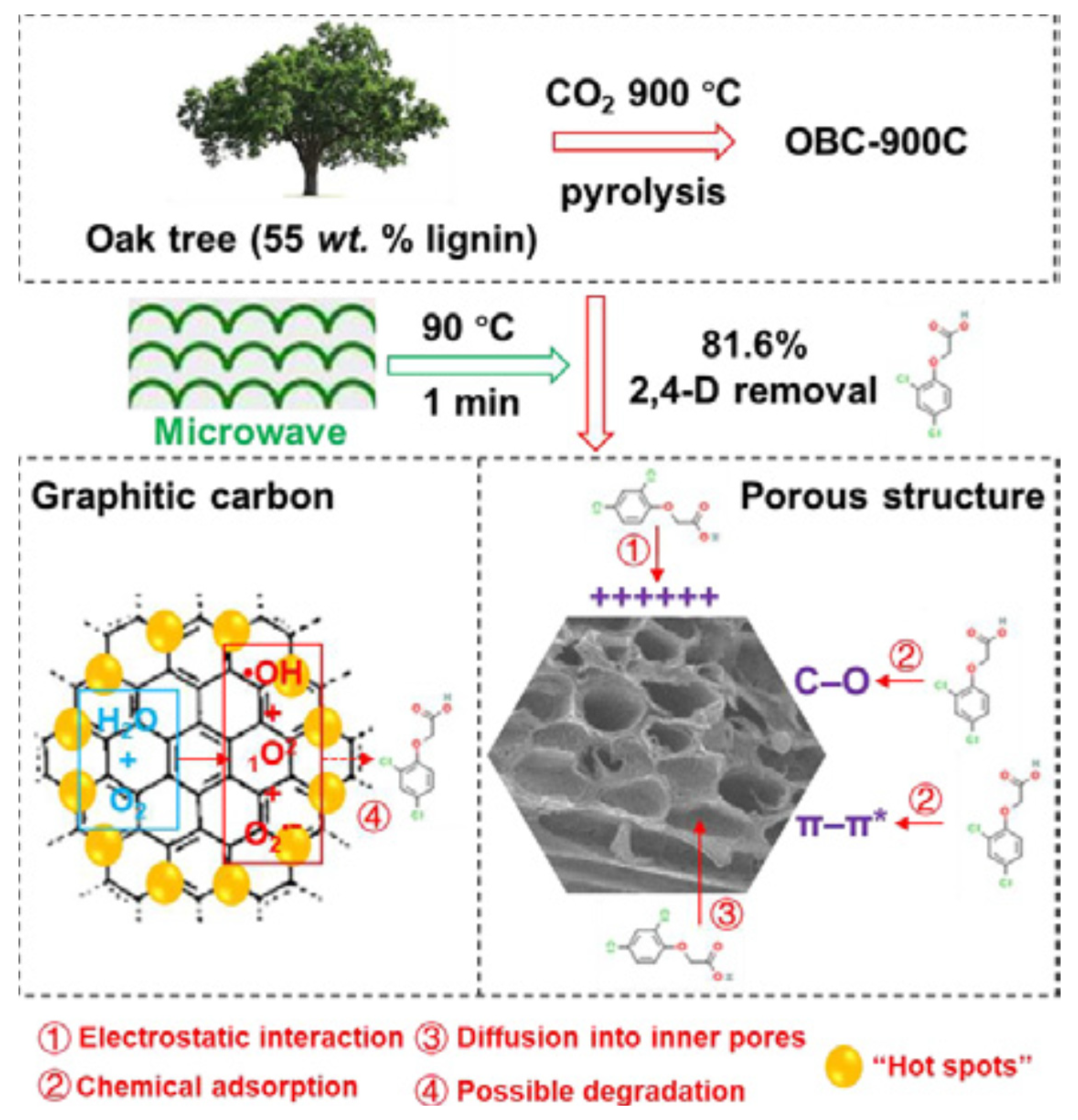
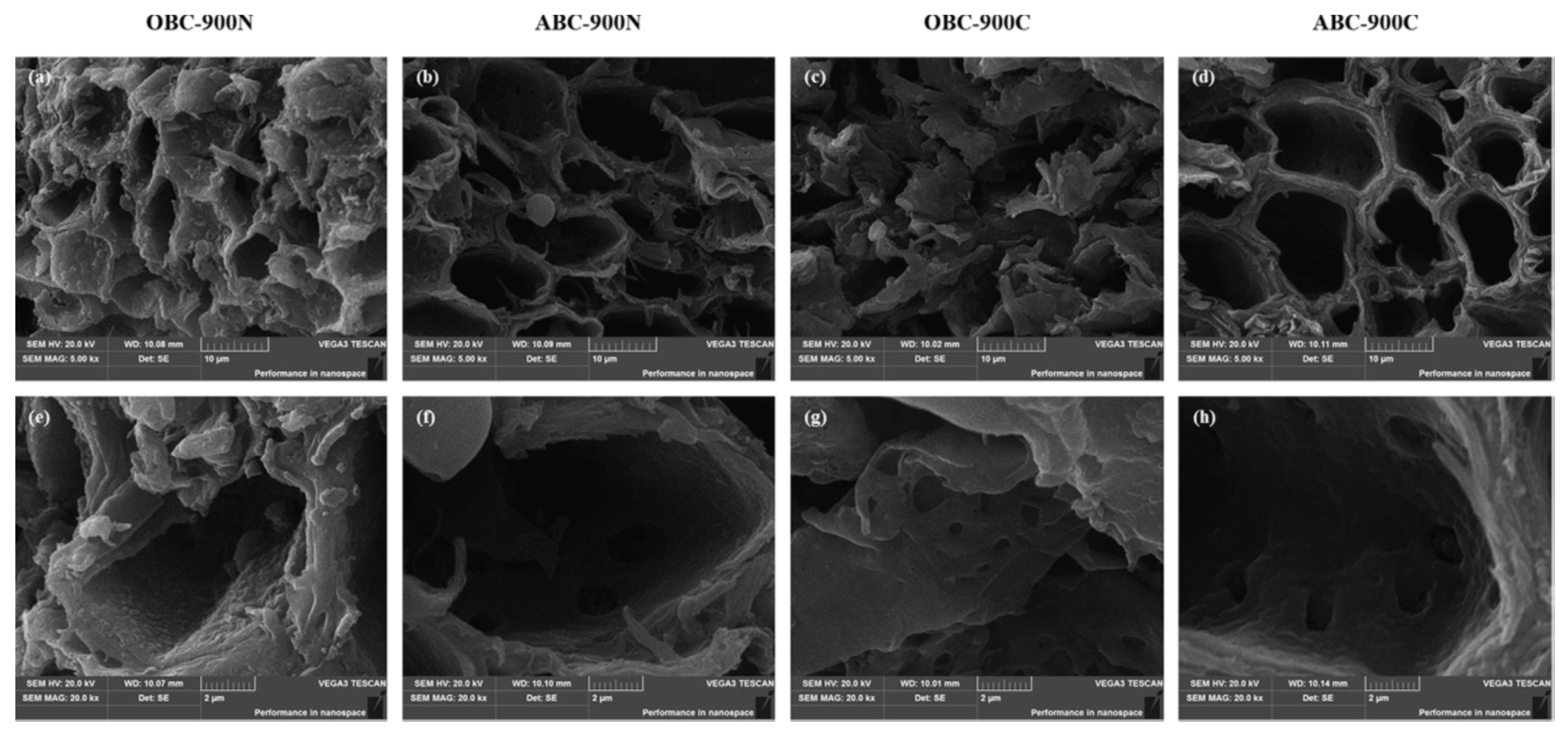
- Sun, Y.Q.; Yu, I.K.M.; Tsang, D.C.W.; Fan, J.J.; Clark, J.H.; Luo, G.; Zhang, S.C.; Khan, E.; Graham, N.J.D. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Wang, C.G.; Li, X.; Fan, W.Q.; Song, P.H.; Gu, Y.C.; Liu, F.Q.; Liu, G.Y. Preparation and electrochemical properties of S@C composite material with high capacity and ordered alignment of channels. Chem. J. Chin. Univ. 2020, 41, 1306–1312. [Google Scholar] [CrossRef]
- Hayashi, J.; Horikawa, T.; Takeda, I.; Muroyama, K.; Nasir Ani, F. Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Kim, J.-W.; Sohn, M.-H.; Kim, D.-S.; Sohn, S.-M.; Kwon, Y.-S. Production of granular activated carbon from waste walnut shell and its adsorption characteristics for Cu2+ ion. J. Hazard. Mater. 2001, 85, 301–315. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Rodriguez-Reinoso, F.; Caturla, F.; Selles, M.J. Porosity in granular carbons activated with phosphoric acid. Carbon 1995, 33, 1105–1113. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef]
- Nemati, F.; Jafari, D.; Esmaeili, H. Highly efficient removal of toxic ions by the activated carbon derived from citrus limon tree leaves. Carbon Lett. 2020. [Google Scholar] [CrossRef]
- Mansour, R.A.; El Shahawy, A.; Attia, A.; Beheary, M.S. Brilliant green dye biosorption using activated carbon derived from Guava tree wood. Int. J. Chem. Eng. 2020, 2020, 8053828. [Google Scholar] [CrossRef]
- Zhang, S.C.; Zang, L.L.; Dou, T.W.; Zou, J.L.; Zhang, Y.H.; Sun, L.G. Willow catkins-derived porous carbon membrane with hydrophilic property for efficient solar steam generation. ACS Omega 2020, 5, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, M.Q.; Chen, T.T.; Zhang, H.; Hu, D.F.; Wu, B.H.; Ji, J.; Xu, Z.K. Dopamine-triggered one-step polymerization and codeposition of acrylate monomers for functional coatings. ACS Appl. Mater. Interfaces 2017, 9, 34356–34366. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Mutuma, B.K.; Manyala, N. Onion-derived activated carbons with enhanced surface area for improved hydrogen storage and electrochemical energy application. RSC Adv. 2020, 10, 26928–26936. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisoption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sawood, G.M.; Gupta, S.K. Kinetic equilibrium and thermodynamic analyses of As (V) removal from aqueous solution using iron-impregnated Azadirachta indica carbon. Appl Water Sci. 2020, 10, 131. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Nguyen, T.V.; Nur, T.; Johir, M.A.; Nguyen, T.H.; Trinh, M.V.; Vigneswaran, S. Iron-impregnated granular activated carbon for arsenic removal: Application to practical column filters. J. Environ. Manag. 2019, 239, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Adnan, R.; Ngah, W.S.W.; Mohamed, N. Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: Regeneration and kinetics studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Kalantary, R.R.; Fard, E.D. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Alalm, G.M.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Daou, C.; Hamade, A.; El Mouchtari, E.; Rafqah, S.; Piram, A.; Wong-Wah-Chung, P.; Najjar, F. Zebrafish toxicity assessment of the photocatalysis-biodegradation of diclofenac using composites of TiO2 and activated carbon from Argania spinosa tree nutshells and Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2020, 27, 17258–17267. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.H.; Newman, A.P.; Al-Daffaee, H.; Phull, S.; Cresswell, N.; York, S. Deposition of anatase on the surface of activated carbon. Surf. Coat. Technol. 2004, 187, 284–292. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Hu, Z.; Chen, Y.; Liu, W.; Zhao, G. Degradation of methyl orange by composite photocatalysts nano-TiO2 immobilized on activated carbons of different porosities. J. Hazard. Mater. 2009, 169, 1061–1067. [Google Scholar] [CrossRef]
- Salih, H.H.; Sorial, G.A.; Patterson, C.L.; Sinha, R.; Krishnan, E.R. Removal of trichloroethylene by activated carbon in the presence and absence of TiO2 nanoparticles. Water Air Soil Pollut. 2012, 223, 2837–2847. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Parameter effect on photocatalytic degradation of phenol using TiO2-P25/activated carbon (AC). Korean J. Chem. Eng. 2010, 27, 1109–1116. [Google Scholar] [CrossRef]
- Horikoshi, S.; Sakamoto, S.; Serpone, N. Formation and efficacy of TiO2/AC composites prepared under microwave irradiation in the photoinduced transformation of the 2-propanol VOC pollutant in air. Appl. Catal. B Environ. 2013, 140–141, 646–651. [Google Scholar] [CrossRef]
- Matos, J.; Garcia, A.; Cordero, T.; Chovelon, J.-M.; Ferronato, C. Eco-friendly TiO2-AC photocatalyst for the selective photooxidation of 4-chlorophenol. Catal. Lett. 2009, 130, 568–574. [Google Scholar] [CrossRef]
- Jamil, T.S.; Ghaly, M.Y.; Fathy, N.A.; Abd el-Halim, T.A.; Österlund, L. Enhancement of TiO2 behavior on photocatalytic oxidation of MO dye using TiO2/AC under visible irradiation and sunlight radiation. Sep. Purif. Technol. 2012, 98, 270–279. [Google Scholar] [CrossRef]
- Han, J.; Shi, L.; Yan, T.; Zhang, J.; Zhang, D. Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization. Environ. Sci. Nano 2018, 5, 2337–2345. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Wang, S.; Zhan, F.; Fu, Y.; Qiao, H.; Qiu, J. Highly stable hybrid capacitive deionization with a MnO2 anode and a positively charged cathode. Environ. Sci. Technol. Lett. 2018, 5, 98–102. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Justin, P.; Rao, G.R. Tuning the surface morphology and pseudocapacitance of MnO2 by a facile green method employing organic reducing sugars. ACS Appl. Energy Mater. 2018, 1, 3654–3664. [Google Scholar] [CrossRef]
- Qi, H.; Bo, Z.; Yang, S.; Duan, L.; Yang, H.; Yan, J.; Cen, K.; Ostrikov, K. Hierarchical nanocarbon-MnO2 electrodes for enhanced electrochemical capacitor performance. Energy Storage Mater. 2019, 16, 607–618. [Google Scholar] [CrossRef]
- Govindan, B.; Alhseinat, E.; Darawsheh, I.F.F.; Ismail, I.; Polychronopoulou, K.; Jaoude, M.A.; Arangadi, A.F.; Banat, F. Activated carbon derived from phoenix dactylifera (palm tree) and decorated with MnO2 nanoparticles for enhanced hybrid capacitive deionization electrodes. ChemistrySelect 2020, 5, 3248–3256. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M.H. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Liu, J.; Lu, M.; Yang, J.; Cheng, J.; Cai, W. Capacitive desalination of ZnO/activated carbon asymmetric capacitor and mechanism analysis. Electrochim. Acta 2015, 151, 312–318. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shen, J.; Qi, J.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Design of nitrogen-doped cluster-like porous carbons with hierarchical hollow nanoarchitecture and their enhanced performance in capacitive deionization. Desalination 2018, 430, 45–55. [Google Scholar] [CrossRef]
- Gu, X.; Yang, Y.; Hu, Y.; Hu, M.; Wang, C. Fabrication of graphene-based xerogels for removal of heavy metal ions and capacitive deionization. ACS Sustain. Chem. Eng. 2015, 3, 1056–1065. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Kim, H.Y. Graphene wrapped MnO2-nanostructures as effective and stable electrode materials for capacitive deionization desalination technology. Desalination 2014, 344, 289–298. [Google Scholar] [CrossRef]
- Min, B.H.; Choi, J.-H.; Jung, K.Y. Improved capacitive deionization of sulfonated carbon/titania hybrid electrode. Electrochim. Acta 2018, 270, 543–551. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Fang, J.; Shi, L.; Zhang, D. Nitrogen-doped porous carbon derived from a bimetallic metal-organic framework as highly efficient electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2016, 4, 10858–10868. [Google Scholar] [CrossRef]
- Sami, S.K.; Seo, J.Y.; Hyeon, S.-E.; Shershah, M.S.A.; Yoo, P.-J.; Chung, C.-H. Enhanced capacitive deionization performance by an rGO-SnO2 nanocomposite modified carbon felt electrode. RSC Adv. 2018, 8, 4182–4190. [Google Scholar] [CrossRef]
- Wei, K.J.; Zhang, Y.H.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Wang, L.J. A novel capacitive electrode based on TiO2-NTs array with carbon embedded for water deionization: Fabrication, characterization and application study. Desalination 2017, 420, 70–78. [Google Scholar] [CrossRef]
- Shi, W.H.; Li, H.B.; Cao, X.H.; Leong, Z.Y.; Zhang, J.; Chen, T.P.; Zhang, H.; Yang, H.Y. Ultrahigh performance of novel capacitive deionization electrodes based on a three-dimensional graphene architecture with nanopores. Sci. Rep. 2016, 6, 18966. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.S.; Mohamed, H.O.; Mohamed, I.M.A.; Mousa, H.M.; Barakat, N.A.M. Enhanced desalination performance of capacitive deionization using zirconium oxide nanoparticles-doped graphene oxide as a novel and effective electrode. Sep. Purif. Technol. 2016, 171, 34–43. [Google Scholar] [CrossRef]
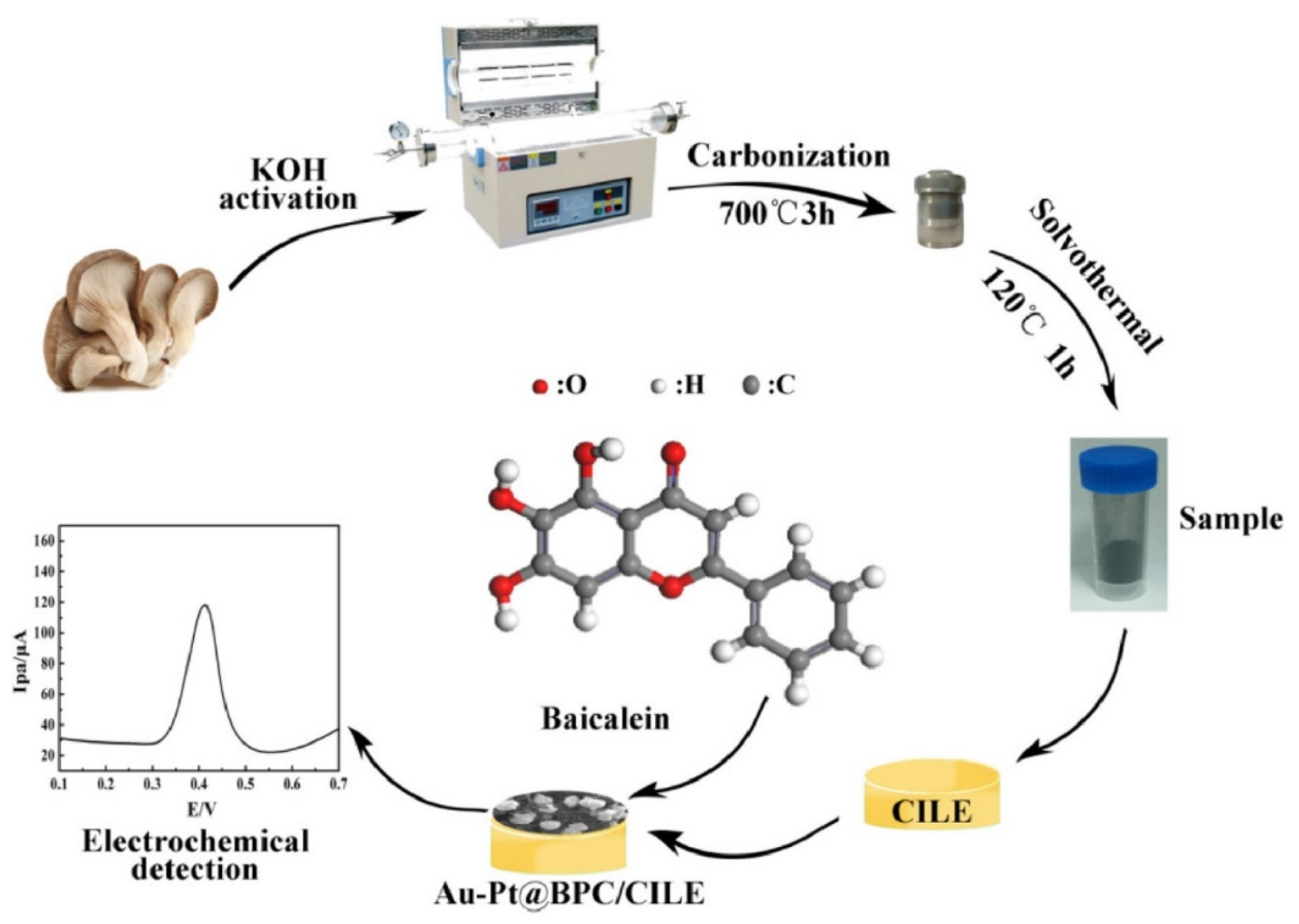
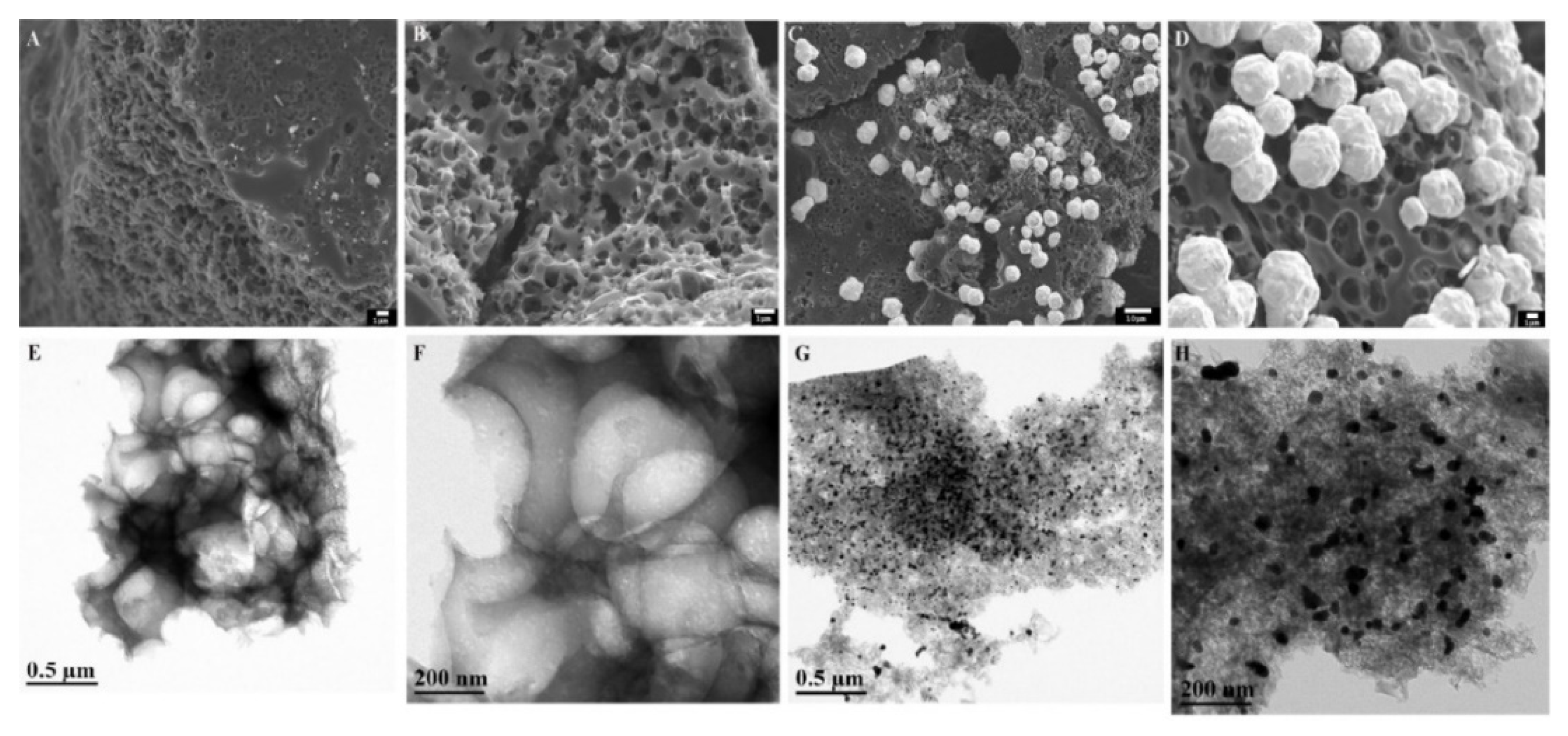
- Cheng, H.; Weng, W.J.; Xie, H.; Liu, J.; Luo, G.L.; Huang, S.M.; Sun, W.; Li, G.J. Au-Pt@Biomass porous carbon composite modified electrode for sensitive electrochemical detection of baicalein. Microchem. J. 2020, 154, 104602. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Xia, H.; Zhang, S.; Peng, J.; Wang, S. Crofton weed derived activated carbon by microwave-induced KOH activation and application to wastewater treatment. J. Porous Mat. 2016, 23, 1597–1607. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Weng, J.; Wang, X.; Zhou, Z.; Yang, K. Hydrothermal syntheses of gold nanocrystals: From icosahedral to its truncated form. Adv. Funct. Mater. 2008, 18, 277–284. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Figlarz, M. Preparing monodisperse metal powders in micrometer and submicrometer sizes by the polyol process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Chang, S.M.; Hu, S.C.; Shiue, A.; Lee, P.Y.; Leggett, G. Adsorption of silver nano-particles modified activated carbon filter media for indoor formaldehyde removal. Chem. Phys. Lett. 2020, 757, 137864. [Google Scholar] [CrossRef]
- Carlier, S.; Gripekoven, J.; Philippo, M.; Hermans, S. Ru on N-doped carbon supports for the direct hydrogenation of cellobiose into sorbitol. Appl. Catal. B Environ. 2021, 282, 119515. [Google Scholar] [CrossRef]
- Azar, F.Z.; Lillo-Rodenas, M.A.; Roman-Martinez, M.C. Mesoporous activated carbon supported Ru catalysts to efficiently convert cellulose into sorbitol by hydrolytic hydrogenation. Energies 2020, 13, 4394. [Google Scholar] [CrossRef]
- Radlik, M.; Juszczyk, W.; Matus, K.; Rarog-Pilecka, W.; Karpinski, Z. Hydrodechlorination of CHClF2 (HCFC-22) over Pd-Pt catalysts supported on thermally modified activated carbon. Catalysts 2020, 10, 1291. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, G.X.; Jiang, H.; Liu, Y.F.; Chen, R.Z.; Xing, W.H. Phenol hydrogenation to cyclohexanone over palladium nanoparticles loaded on charming activated carbon adjusted by facile heat treatment. Chin. J. Chem. Eng. 2020, 28, 2600–2606. [Google Scholar] [CrossRef]
- Merodio-Morales, E.E.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Duran-Valle, C.J.; Bonilla-Petriciolet, A. Lanthanum- and cerium-based functionalization of chars and activated carbons for the adsorption of fluoride and arsenic ions. Int. J. Environ. Sci. Technol. 2020, 17, 115–128. [Google Scholar] [CrossRef]
- Korus, A.; Samson, A.; Szlek, A. Catalytic conversion of toluene over a biochar bed under an inert atmosphere—The comparison of chars from different types of wood and the role of selected metals. Fuel 2020, 279, 118468. [Google Scholar] [CrossRef]
- El Mouchtari, E.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-Wah-Chung, P. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112183. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three-dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interf. Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Nazir, R.; Khan, M.; Khan, W.; Shah, M.; Afridi, S.G.; Zada, A. The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells. Soil Water Res. 2020, 15, 30–37. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.Q.; Zhang, H.H.; Qiu, J.J.; Yu, W.H.; Li, M.Y.; Gan, Q.; Yu, W.B.; Xiao, K.K.; Liu, B.C.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, B.T.; Tran, H.N.; Chao, H.P. Efficient Removal of Cr(VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis: Adsorption-coupled reduction mechanism. Water 2019, 11, 1164. [Google Scholar] [CrossRef]
- Shi, S.Q.; Yang, J.K.; Liang, S.; Li, M.Y.; Gan, Q.; Xiao, K.K.; Hu, J.P. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Egirani, D.E.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for cadmium removal. Int. J. Environ. Sci. Technol. 2020, 17, 2443–2454. [Google Scholar] [CrossRef]
- Lupascu, T.; Petuhov, O.; Timbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B(12) and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [Google Scholar] [CrossRef]
- Chong, M.Y.; Tam, Y.J. Bioremediation of dyes using coconut parts via adsorption: A review. SN Appl. Sci. 2020, 2, 187. [Google Scholar] [CrossRef]
- Tolosa, N.C.; Mendoza, K.D.; Dumayas, D.L.P.; De Silva, J.M.D.F. Preparation and characterization of activated carbon derived from antidesma bunius L. in methylene blue removal from wastewater. J. Environ. Sci. Manag. 2020, 2020, 18–28. [Google Scholar]
- Ghaedi, A.M.; Baneshi, M.M.; Vafaei, A.; Nejad, A.R.S.; Tyagi, I.; Kumar, N.; Galunin, E.; Tkachev, A.G.; Agarwal, S.; Gupta, V.K. Comparison of multiple linear regression and group method of data handling models for predicting sunset yellow dye removal onto activated carbon from oak tree wood. Environ. Technol. Innov. 2018, 11, 262–275. [Google Scholar] [CrossRef]
- Khafri, H.Z.; Ghaedi, M.; Asfaram, A.; Safarpoor, M. Synthesis and characterization of ZnS:Ni-NPs loaded on AC derived from apple tree wood and their applicability for the ultrasound assisted comparative adsorption of cationic dyes based on the experimental design. Ultrason. Sonochem. 2017, 38, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Song, S.; Li, J.; Tang, Z.H.; Ye, J.Y.; Yang, J.H. Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J. Mater. Sci. Technol. 2019, 35, 875–884. [Google Scholar] [CrossRef]
- Xu, J.G.; Shi, J.S.; Cui, H.M.; Yan, N.F.; Liu, Y.W. Preparation of nitrogen doped carbon from tree leaves as efficient CO2 adsorbent. Chem. Phys. Lett. 2018, 711, 107–112. [Google Scholar] [CrossRef]
- Dodevski, V.; Pagnacco, M.C.; Radovic, I.; Rosic, M.; Jankovic, B.; Stojmenovic, M.; Mitic, V.V. Characterization of silicon carbide ceramics obtained from porous carbon structure achieved by plant carbonization. Mater. Chem. Phys. 2020, 245, 122768. [Google Scholar] [CrossRef]
- Wood, A.R.; Garg, R.; Justus, K.; Cohen-Karni, T.; LeDuc, P.; Russell, A.J. Intact mangrove root electrodes for desalination. RSC Adv. 2019, 9, 4735–4743. [Google Scholar] [CrossRef]
- Farhan, S.; Wang, R.M.; Li, K.Z. Physical and electromagnetic shielding properties of green carbon foam prepared from biomaterials. Trans. Nonferr. Met. Soc. China 2018, 28, 103–113. [Google Scholar] [CrossRef]
- Momodu, D.; Sylla, N.F.; Mutuma, B.; Bello, A.; Masikhwa, T.; Lindberg, S.; Matic, A.; Manyala, N. Stable ionic-liquid-based symmetric supercapacitors from Capsicum seed-porous carbons. J. Electroanalyt. Chem. 2019, 838, 119–128. [Google Scholar] [CrossRef]
- He, D.; Huang, Z.H.; Wang, M.X. Porous nitrogen and oxygen co-doped carbon microtubes derived from plane tree fruit fluff for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 1468–1479. [Google Scholar] [CrossRef]
- Kumar, K.T.; Sundari, G.S.; Kumar, E.S.; Ashwini, A.; Ramya, M.; Varsha, P.; Kalaivani, R.; Andikkadu, M.S.; Kumaran, V.; Gnanamuthu, R.; et al. Synthesis of nanoporous carbon with new activating agent for high-performance supercapacitor. Mater. Lett. 2018, 218, 181–184. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Dangbegnon, J.K.; Manyala, N.; Xia, X.H. Asymmetric supercapacitor based on activated expanded graphite and pinecone tree activated carbon with excellent stability. Appl. Energy 2017, 207, 417–426. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.F.; Liu, H.; Wang, C.Y.; Sun, B.; Wang, G.X. Nitrogen-doped porous carbon nanosheets from eco-friendly eucalyptus leaves as high performance electrode materials for supercapacitors and lithium ion batteries. Chem. A Euro. J. 2017, 23, 3683–3690. [Google Scholar] [CrossRef]
- Zhang, M.Y.; You, X.L.; Liu, L.J.; Walle, M.D.; Li, Y.J.; Liu, Y.N. Biomass derived highly-ordered carbon tube as athode material for high performance lithium-sulfur batteries. Chin. J. Inorg. Chem. 2019, 35, 1493–1499. [Google Scholar] [CrossRef]
- Selva, R.K.; Zhu, P.; Yan, C.I.; Zhu, J.D.; Dirican, M.; Shanmugavani, A.; Lee, Y.S.; Zhang, X.W. Biomass-derived porous carbon modified glass fiber separator as polysulfide reservoir for Li-S batteries. J. Colloid Interf. Sci. 2018, 513, 231–239. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, R.; Chen, Y.L.; Xiao, X.F.; Zhao, G.M.; Yang, H.J.; Li, J.H.; Xu, W.L.; Wang, X.B. Banyan-inspired hierarchical evaporators for efficient solar photothermal conversion. Appl. Energy 2020, 276, 115545. [Google Scholar] [CrossRef]
- Narowska, B.E.; Kulaznski, M.; Lukaszewicz, M. Application of activated carbon to obtain biodiesel from vegetable oils. Catalysis 2020, 10, 1049. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Adsorption of pollutants by plant bark derived adsorbents: An empirical review. J. Water Proc. Eng. 2020, 35, 101228. [Google Scholar] [CrossRef]
- Heidari, A.; Khaki, E.; Younesi, H.; Lu, H.Y.R. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach. J. Clean. Prod. 2020, 241, 118394. [Google Scholar] [CrossRef]
- Ozbay, N.; Yargic, A.S. Carbon foam production from bio-based polyols of liquefied spruce tree sawdust: Effects of biomass/solvent mass ratio and pyrolytic oil addition. J. Appl. Polym. Sci. 2019, 136, 47185. [Google Scholar] [CrossRef]
- Mamani, A.; Sardella, M.F.; Gimenez, M.; Deiana, C. Highly microporous carbons from olive tree pruning: Optimization of chemical activation conditions. J. Environ. Chem. Eng. 2019, 7, 102830. [Google Scholar] [CrossRef]
- Rimoli, M.F.D.; Nogueira, R.M.; Ferrarini, S.R.; de Castro, P.M.; Pires, E.M. Preparation and characterization of carbon from the fruit of Brazil nut tree activated by physical process. Rev. Arvore 2019, 43, E430206. [Google Scholar] [CrossRef]
- Marques, S.C.R.; Mestre, A.S.; Machuqueiro, M.; Gotvajn, A.Z.; Marinsek, M.; Carvalho, A.P. Apple tree branches derived activated carbons for the removal of beta-blocker atenolol. Chem. Eng. J. 2018, 345, 669–678. [Google Scholar] [CrossRef]
- Ardekani, P.S.; Karimi, H.; Ghaedi, M.; Asfaram, A.; Purkait, M.K. Ultrasonic assisted removal of methylene blue on ultrasonically synthesized zinc hydroxide nanoparticles on activated carbon prepared from wood of cherry tree: Experimental design methodology and artificial neural network. J. Mol. Liq. 2017, 229, 114–124. [Google Scholar] [CrossRef]
- Sanni, E.S.; Emetere, M.E.; Odigure, J.O.; Efeovbokhan, V.E.; Agboola, O.; Sadiku, E.R. Determination of optimum conditions for the production of activated carbon derived from separate varieties of coconut shells. Int. J. Chem. Eng. 2017, 2017, 2801359. [Google Scholar] [CrossRef]
- Momodu, D.; Bello, A.; Oyedotun, K.; Ochai-Ejeh, F.; Dangbegnon, J.; Madito, M.; Manyala, N. Enhanced electrochemical response of activated carbon nanostructures from tree-bark biomass waste in polymer-gel active electrolytes. RSC Adv. 2017, 7, 37286–37295. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zuo, J.; Jiang, Y.S.; Zhang, J.Y.; Zhang, J.P.; Wei, C.D. Carbon-silica mesoporous composite in situ prepared from coal gasification fine slag by acid leaching method and its application in nitrate removing. Sci. Total Environ. 2020, 707, 136102. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F.C.; de Leon, C.P. Progress in electrochemical flow reactors for laboratory and pilot scale processing. Electrochim. Acta 2018, 280, 121–148. [Google Scholar] [CrossRef]
- Korhonen, K.; Kristensen, T.B.; Falk, J.; Lindgren, R.; Andersen, C.; Carvalho, R.L.; Malmborg, V.; Eriksson, A.; Boman, C.; Pagels, J.; et al. Ice-nucleating ability of particulate emissions from solid-biomass-fired cookstoves: An experimental study. Atmos. Chem. Phys. 2020, 20, 4951–4968. [Google Scholar] [CrossRef]
- Kharrazi, S.M.; Mirghaffari, N.; Dastgerdi, M.M.; Soleimani, M. A novel post-modification of powdered activated carbon prepared from lignocellulosic waste through thermal tension treatment to enhance the porosity and heavy metals adsorption. Powder Technol. 2020, 366, 358–368. [Google Scholar] [CrossRef]
- Gu, H.M.; Bergman, R.; Anderson, N.; Alanya-Rosenbaum, S. Life cycle assessment of activated carbon from woody biomass. Wood Fiber Sci. 2018, 50, 229–243. [Google Scholar] [CrossRef]
- Roman, S.; Ledesma, B.; Alvarez-Murillo, A.; Al-Kassir, A.; Yusaf, T. Dependence of the microporosity of activated carbons on the lignocellulosic composition of the precursors. Energies 2017, 10, 542. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Mendez, A.; El Azzouzi, M.; Romero, E. New insights into the efficient removal of emerging contaminants by biochars and hydrochars derived from olive oil wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef]







| Sample Name | BET Surface Area (m2/g) | Micropore Area (m2/g) | Total Pore vol. (cm3/g) | Micropore vol. (cm3/g) | H2 Uptake (wt%) |
|---|---|---|---|---|---|
| OP AC-600 °C | 2241 | 2220 | 0.94 | 0.89 | 3.08 |
| OP AC-700 °C | 2706 | 2651 | 1.28 | 1.10 | 3.29 |
| OP AC-800 °C | 3150 | 3080 | 1.64 | 1.39 | 3.67 |
| Electrodes and Materials | Specific Capacitance (F/g) | Applied Voltage (V) | NaCl Concentration (ppm) | Desalination Capacity (mg/g) | Source |
|---|---|---|---|---|---|
| Anode: α-MnO2/f-AC Cathode: f-AC | 388 | 1.2 | 600 | 17.8 | [43] |
| ZnO/activated carbon | 66 | 1.2 | 500 | 9.4 | [45] |
| N-doped cluster-like porous C | 199 | 1.2 | 100 | 11.98 | [46] |
| Graphene–chitosan–Mn3O4 | 190 | 1.6 | 300 | 12.7 | [47] |
| MnO2-nanorods@graphene | 292 | 1.2 | --- | 5.01 | [48] |
| Sulfonated carbon/TiO2 | 238 | 1.2 | 500 | 10.0 | [49] |
| N-doped porous C | 292 | 1.4 | 500 | 16.63 | [50] |
| rGO–SnO2 nanocomposite | 142 | 1.2 | 400 | 17.62 | [51] |
| TiO2-nanotube array with carbon embedded electrode | 238 | 1.2 | 500 | 13.11 | [52] |
| Nanoporous 3D Graphene | 200 | 1.6 | 500 | 17.1 | [53] |
| GO/ZrO210% | 452 | 1.2 | 50 | 4.76 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. https://doi.org/10.3390/c7020039
Gan YX. Activated Carbon from Biomass Sustainable Sources. C. 2021; 7(2):39. https://doi.org/10.3390/c7020039
Chicago/Turabian StyleGan, Yong X. 2021. "Activated Carbon from Biomass Sustainable Sources" C 7, no. 2: 39. https://doi.org/10.3390/c7020039
APA StyleGan, Y. X. (2021). Activated Carbon from Biomass Sustainable Sources. C, 7(2), 39. https://doi.org/10.3390/c7020039





