Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries
Abstract
1. Introduction
- (i)
- Alloying materials such as Si, Ge, Sn, Al, Bi, etc.,
- (ii)
- Conversion materials like transition-metal oxides (MnxOy, NiO, FexOy, CuO, Cu2O, MoO2, etc.), metal sulfides, metal phosphides, and metal nitrides (MxXy; here X = S, P, N),
- (iii)
- Insertion materials, such as carbonaceous compounds (graphite, porous carbon, carbon nanotubes, graphene), TiO2, Li4Ti5O12, etc.
2. Graphene Oxide Materials
2.1. Properties of Graphene Oxides
2.2. Lithiation of rGO
2.3. rGO-Based Composites
3. Single Element/GO or rGO Composites
3.1. Silicon
3.2. Germanium
3.3. Tin
4. Metal Oxide/rGO Composites
4.1. Silicon-Based Oxide Composites
4.2. Tin-Based Oxide Composites
4.3. Manganese-Based Oxide Composites
4.3.1. MnO2-Based Composite
4.3.2. Mn3O4-Based Composite
4.3.3. MnO-Based Composites
4.4. Molybdenum-Based Oxide Composites
4.5. Titanium-Based Oxide Composites
4.5.1. TiO2-Based Composites
4.5.2. Li4Ti5O12-Based Composites
4.6. Iron-Based Oxide Composites
4.6.1. Fe3O4-Based Composites
4.6.2. Fe2O3-Based Composites
4.6.3. FeO-Based Composites
4.7. Vanadium-Based Oxide Composites
4.7.1. VO2-Based Composites
4.7.2. V2O3-Based Composites
4.7.3. V2O5-Based Composites
4.7.4. VPO4-Based Composites
4.8. Cobalt-Based Oxide Composites
4.8.1. Co3O4-Based Composites
4.8.2. CoO-Based Composites
4.9. Copper-Based Oxide Composites
4.10. Nickel-Based Oxide Composites
5. Anode Materials for Na-Ion Batteries
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Zaghib, K.; Mauger, A.; Julien, C. Rechargeable lithium batteries for energy storage in smart grids. Recharg. Lithium Batter. 2015, 81, 319–351. [Google Scholar]
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. Challenges and issues facing lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Hassoun, J.; Scrosati, B. Review—Advances in anode and electrolyte materials for the progress of lithium-ion and beyond lithium-ion batteries. J. Electrochem. Soc. 2015, 162, A2582–A2588. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Ivanovici, S.; Müllen, K. Fabrication of graphene-encapsulated oxide nanoparticles: Towards high-performance anode materials for lithium storage. Angew. Chem. Int. Ed. 2010, 49, 8408–8411. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y. Graphene-based nanocomposite anodes for lithium-ion batteries. Nanoscale 2014, 6, 11528–11552. [Google Scholar] [CrossRef]
- Mauger, A.; Xie, H.; Julien, C.M. Composite anodes for lithium-ion batteries: Status and trends. AIMS Mater. Sci. 2016, 3, 1054–1106. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2014, 2, 15–32. [Google Scholar] [CrossRef]
- Han, S.; Wang, J.; Li, S.; Wu, D.; Feng, X. Graphene aerogel supported Fe5(PO4)4(OH)3·2H2O microspheres as high performance cathode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 6174. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Javed, K.; Oolo, M.; Savest, N.; Krumme, A. A review on graphene-based electrospun conductive nanofibers, supercapacitors, anodes and cathodes for lithium-ion batteries. Crit. Rev. Solid State Mater. Sci. 2019, 44, 427–443. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2012, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shua, F.; Guo, X.; Xue, D. High performance porous MnO@C composite anode materials for lithium-ion batteries. Electrochim. Acta 2016, 188, 793–800. [Google Scholar] [CrossRef]
- Gao, F.; Qin, S.-H.; Zang, Y.-H.; Gu, J.-F.; Qu, J. Highly efficient formation of Mn3O4-graphene oxide hybrid aerogels for use as the cathode material of high performance lithium ion batteries. New Carbon Mater. 2020, 35, 121–130. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Yao, K.; Zhang, J.; Liu, Z. A SnO2/graphene composite as a high stability electrode for lithium ion batteries. Carbon 2011, 49, 133–139. [Google Scholar] [CrossRef]
- Deng, Y.; Fang, C.; Chen, G. The developments of SnO2/graphene nanocomposites as anode materials for high performance lithium ion batteries: A review. J. Power Sources 2016, 304, 81–101. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Self-assembled hierarchical MoO2/graphene nanoarchitectures and their application as a high-performance anode material for lithium-ion batteries. ACS Nano 2011, 5, 7100–7107. [Google Scholar] [CrossRef] [PubMed]
- Sennu, P.; Kim, H.S.; An, J.Y.; Aravindan, V.; Lee, Y.-S. Synthesis of 2D/2D structured mesoporous Co3O4 nanosheet/N-doped reduced graphene oxide composites as a highly stable negative electrode for lithium battery applications. Chem. Asian J. 2015, 10, 1776–1783. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Liu, D.; Wang, X.; Song, S.; Zhou, L.; Zhang, H. Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J. Phys. Chem. C 2011, 115, 21567–21573. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Du, M.; Sun, J.; Gao, L.; Zhang, P.; Yao, H.; Lin, C. Solvothermal-induced self-assembly of Fe2O3/GS aerogels for high Li-storage and excellent stability. Small 2014, 10, 2260–2269. [Google Scholar] [CrossRef]
- Wu, P.; Wang, H.; Tanga, Y.; Zhou, Y.; Lu, T. Three-dimensional interconnected network of graphene-wrapped porous silicon spheres: In situ magnesiothermic-reduction synthesis and enhanced lithium-storage capabilities. ACS Appl. Mater. Interfaces 2014, 6, 3546–3552. [Google Scholar] [CrossRef]
- Ren, J.-G.; Wu, Q.-H.; Tang, H.; Hong, G.; Zhang, W.; Lee, S.-T. Germanium–graphene composite anode for high-energy lithium batteries with long cycle life. J. Mater. Chem. A 2012, 1, 1821–1826. [Google Scholar] [CrossRef]
- Chockla, A.M.; Panthani, M.G.; Holmberg, V.C.; Hessel, C.M.; Reid, D.K.; Bogart, T.D.; Harris, J.T.; Mullins, C.B.; Korgel, B.A. Electrochemical lithiation of graphene-supported silicon and germanium for rechargeable batteries. J. Phys. Chem. C 2012, 116, 11917–11923. [Google Scholar] [CrossRef]
- Yue, W.; Yang, S.; Liu, Y.; Yang, X. A facile synthesis of mesoporous graphene-tin composites as high-performance anodes for lithium-ion batteries. Mater. Res. Bull. 2013, 48, 1575–1580. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Jana, A.; Scheer, E.; Polarz, S. Synthesis of graphene–transition metal oxide hybrid nanoparticles and their application in various fields. Beilstein J. Nanotechnol. 2017, 8, 688–714. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Harrison, I.; Andou, Y. Flexible graphene-based supercapacitors: A review. J. Phys. Chem. C 2016, 120, 4153–4172. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Brodie, B.C. Sur le poids atomique du graphite. Ann. Chem. Phys. 1860, 59, 466–472. [Google Scholar]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, Y.; Wang, L.; Fan, L.-Z. Reverse microemulsion synthesis of nickel-cobalt hexacyanoferrate/reduced graphene oxide nanocomposites for high-performance supercapacitors and sodium ion batteries. Appl. Surf. Sci. 2018, 434, 1285–1292. [Google Scholar] [CrossRef]
- Wang, M.-S.; Song, W.-L.; Fan, L.-Z. Three-dimensional interconnected network of graphene-wrapped silicon/carbon nanofiber hybrids for binder-free anodes in lithium-ion batteries. ChemElectroChem 2015, 2, 1699–1706. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Rao, S.; Upadhyay, J.; Polychronopoulou, K.; Umer, R.; Das, R. Reduced graphene oxide: Effect of reduction on electrical conductivity. J. Compos. Sci. 2018, 2, 25. [Google Scholar] [CrossRef]
- Wang, H.; Tian, H.-W.; Wang, X.-W.; Qiao, L.; Wang, S.-M.; Wang, X.-L.; Zheng, W.-T.; Liu, Y.-C. Electrical conductivity of alkaline-reduced graphene oxide. Chem. Res. Chinese Univ. 2011, 27, 857–861. [Google Scholar]
- Tokarczyk, M.; Kowalski, G.; Witowski, A.; Kozinski, R.; Librant, K.; Aksienionek, M.; Lipińska, L.; Ciepielewski, P. Structural and electronic properties of graphene oxide and reduced graphene oxide papers prepared by high pressure and high temperature treatment. Acta Phys. Pol. A 2014, 126, 1190–1194. [Google Scholar] [CrossRef]
- Marquez, C.; Rodríguez, N.; Ruiz, R.; Gamiz, F. Electrical characterization and conductivity optimization of laser reduced graphene oxide on insulator using point-contact methods. RSC Adv. 2016, 6, 46231–46237. [Google Scholar] [CrossRef]
- Shabir, A.; Sehrawat, P.; Julien, C.M.; Islam, S.S. Reversible synthesis of GO: Role of differential bond structure transformation in fine-tuning photodetector response. Nanotechnology 2020, 32, 045601. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nat. Cell Biol. 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Reduction of graphene oxide with substituted borohydrides. J. Mater. Chem. A 2012, 1, 1892–1898. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ma, G.; Wang, Z.; Liu, K.; Liu, H. Ethylene glycol reduced graphene oxide/polypyrrole composite for supercapacitor. Electrochim. Acta 2013, 88, 519–525. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, K.; Wei, T.; Yan, J.; Song, L.; Shao, B. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon 2010, 48, 1686–1689. [Google Scholar] [CrossRef]
- Maddineni, S.B.; Mandal, B.K. Biofabrication of reduction of graphene oxide nanosheets using terminalia Bellirica fruit extract. Current Nanosci. 2016, 12, 94–102. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Wang, J.; Chan-Park, M.B. Reducing graphene oxide with a modified Birch reaction. RSC Adv. 2015, 5, 11124–11127. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Qu, M. Enhanced anode performances of the Fe3O4–carbon–rGO three dimensional composite in lithium ion batteries. Chem. Commun. 2011, 47, 10374–10376. [Google Scholar] [CrossRef]
- Mei, L.; Xu, C.; Yang, T.; Ma, J.; Chen, L.; Li, Q.; Wang, T. Superior electrochemical performance of ultrasmall SnS2 nanocrystals decorated on flexible RGO in lithium-ion batteries. J. Mater. Chem. A 2013, 1, 8658–8664. [Google Scholar] [CrossRef]
- Patil, S.B.; Nagaraju, G.; Kishore, B.; Nagaraju, G.; Dupont, J.; Nagaraju, G. High capacity MoO3/rGO nanocomposite anode for lithium ion batteries: An intuition into the conversion mechanism of MoO3. New J. Chem. 2018, 42, 18569–18577. [Google Scholar] [CrossRef]
- Paek, S.-M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q. (Max); Cheng, H.-M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Ma, C.; Xu, J.; Alvarado, J.; Qu, B.; Somerville, J.; Lee, J.Y.; Meng, Y.S. Investigating the energy storage mechanism of SnS2-rGO composite anode for advanced Na-ion batteries. Chem. Mater. 2015, 27, 5633–5640. [Google Scholar] [CrossRef]
- Zhang, Q.; Karthick, R.; Zhao, X.; Zhang, L.-G.; Shi, Y.; Sun, L.; Su, C.-Y.; Chen, F. Sb nanoparticle decorated rGO as a new anode material in aqueous chloride ion batteries. Nanoscale 2020, 12, 12268–12274. [Google Scholar] [CrossRef]
- Zensich, M.; Jaumann, T.; Morales, G.M.; Giebeler, L.; Barbero, C.; Balach, J. A top-down approach to build Li2S@rGO cathode composites for high-loading lithium–sulfur batteries in carbonate-based electrolyte. Electrochim. Acta 2018, 296, 243–250. [Google Scholar] [CrossRef]
- Abalonyx. Available online: https://www.abalonyx.no/?cat=42023. (accessed on 17 September 2020).
- Kimiagar, S.; Rashidi, N.; Ghadim, E.E. Investigation of the effects of temperature and time on reduction of graphene oxide by microwave hydrothermal reactor. Bull. Mater. Sci. 2015, 38, 1699–1704. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Tian, L.; Zhuang, Q.; Yueli, S.; Shigang, S.; Chen, J.; Lu, F.; Sun, S. Mechanism of intercalation and deintercalation of lithium ions in graphene nanosheets. Chin. Sci. Bull. 2011, 56, 3204–3212. [Google Scholar] [CrossRef]
- Datta, D.; Li, J.; Koratkar, N.; Shenoy, V.B. Enhanced lithiation in defective graphene. Carbon 2014, 80, 305–310. [Google Scholar] [CrossRef]
- Lin, K.-H.; Kuo, C.-L. Lithiation mechanisms and lithium storage capacity of reduced graphene oxide nanoribbons: A first-principles study. J. Mater. Chem. A 2017, 5, 4912–4922. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li Storage properties of disordered graphene nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Robledo, C.; Otero, M.; Luque, G.L.; Camara, O.; Barraco, D.; Rojas, M.; Leiva, E. First-principles studies of lithium storage in reduced graphite oxide. Electrochim. Acta 2014, 140, 232–237. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Graphene and Graphene Oxide for Energy Storage; Wiley: Hoboken, NJ, USA, 2017; pp. 725–744. [Google Scholar]
- Kühne, M. Lithium Intercalation in Bilayer Graphene Devices; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 1–92. [Google Scholar]
- Matsuo, Y.; Sasaki, T.; Maruyama, S.; Inamoto, J.; Okamoto, Y.; Tamura, N. Electrochemical intercalation behaviors of lithium ions into graphene-like graphite. J. Electrochem. Soc. 2018, 165, A2409–A2414. [Google Scholar] [CrossRef]
- Ji, K.; Han, J.; Hirata, A.; Fujita, T.; Shen, Y.; Ning, S.; Liu, P.; Kashani, H.; Tian, Y.; Ito, Y.; et al. Lithium intercalation into bilayer graphene. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Kheirabadi, N.; Shafiekhani, A. Graphene/Li-ion battery. J. Appl. Phys. 2012, 112, 124323. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Too, C.O.; Wallace, G.G. Electrochemical properties of graphene paper electrodes used in lithium batteries. Chem. Mater. 2009, 21, 2604–2606. [Google Scholar] [CrossRef]
- Gedela, V.; Puttapati, S.K.; Nagavolu, C.; Srikanth, V.V.S.S. A unique solar radiation exfoliated reduced graphene oxide/polyaniline nanofibers composite electrode material for supercapacitors. Mater. Lett. 2015, 152, 177–180. [Google Scholar] [CrossRef]
- Puttapati, S.K.; Gedela, V.; Srikanth, V.V.S.S.; Reddy, M.V.; Adams, S.; Chowdari, B.V.R. Unique reduced graphene oxide as efficient anode material in Li ion battery. Bull. Mater. Sci. 2018, 41, 53. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Li, G.; Qu, M.; Yin, G. Co3O4@graphene Composites as Anode Materials for High-Performance Lithium Ion Batteries. Inorg. Chem. 2011, 50, 1628–1632. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Xie, Y.; Yi, N.; Huang, Y.; Chen, Y. Pyrolytic carbon-coated Si nanoparticles on elastic graphene framework as anode materials for high-performance lithium-ion batteries. Carbon 2015, 82, 161–167. [Google Scholar] [CrossRef]
- Ji, J.; Ji, H.; Zhang, L.L.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Graphene-encapsulated Si on ultrathin-graphite foam as anode for high capacity lithium-ion batteries. Adv. Mater. 2013, 25, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Zhou, J.; Zhu, Y.; Qian, Y. Embedding silicon nanoparticles in graphene based 3D framework by cross-linking reaction for high performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 19604–19608. [Google Scholar] [CrossRef]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Mao, S.; Chen, J. Three-dimensional carbon-coated Si/rGO nanostructures anchored by nickel foam with carbon nanotubes for Li-ion battery applications. Nano Energy 2015, 15, 679–687. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable silicon–carbon nanocables sandwiched between reduced graphene oxide sheets as lithium ion battery anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, B.; Zhang, J.; Lian, F.; Kong, X.; Qu, M. Synthesis and superior anode performance of TiO2@reduced graphene oxide nanocomposites for lithium ion batteries. J. Mater. Chem. 2012, 22, 9759–9766. [Google Scholar] [CrossRef]
- Ji, L.; Zheng, H.; Ismach, A.; Tan, Z.; Xun, S.; Lin, E.; Battaglia, V.; Srinivasan, V.; Zhang, Y. Graphene/Si multilayer structure anodes for advanced half and full lithium-ion cells. Nano Energy 2012, 1, 164–171. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Yang, J.; Cheng, M.M.-C.; Salley, S.O.; Ng, K.Y.S. Effects of graphene and carbon coating modifications on electrochemical performance of silicon nanoparticle/graphene composite anode. J. Power Sources 2014, 246, 335–345. [Google Scholar] [CrossRef]
- Wong, D.P.; Tseng, H.-P.; Chen, Y.-T.; Hwang, B.-J.; Chen, L.-C.; Chen, K.-H. A stable silicon/graphene composite using solvent exchange method as anode material for lithium ion batteries. Carbon 2013, 63, 397–403. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles–graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016; pp. 323–429. [Google Scholar]
- Chou, S.-L.; Wang, J.-Z.; Choucair, M.; Liu, H.-K.; Stride, J.A.; Dou, S.-X. Enhanced reversible lithium storage in a nanosize silicon/graphene composite. Electrochem. Commun. 2010, 12, 303–306. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, D. Solutions for the problems of silicon–carbon anode materials for lithium-ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, A.; Umesh, B.; Chen, F.; Chang, J.-K.; Su, C.-Y. Facile synthesis of core–shell structured Si@graphene balls as a high-performance anode for lithium-ion batteries. Nanoscale 2020, 12, 9616–9627. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, Z.; Chen, H.; Qiao-Bao, Z.; Chen, S.; Yang, Y. On the interface design of Si and multilayer graphene for a high-performance Li-ion battery anode. ACS Appl. Mater. Interfaces 2020, 12, 44840–44849. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, J.; Luo, J.; Li, W.; Wu, Z.; Shi, M.; Li, X.; Li, N.; Chang, B.; Wang, X.; et al. Spherical Gr/Si/GO/C composite as high-performance anode material for lithium-ion batteries. Energy Fuels 2020, 34, 7639–7647. [Google Scholar] [CrossRef]
- Kim, D.; Luo, Y.; Tiwari, A.P.; Hwang, H.M.; Oh, S.; Lee, K.; Lee, H. Highly stable multi-layered silicon-intercalated graphene anodes for lithium-ion batteries. MRS Commun. 2020, 10, 25–31. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.-K.; Ahn, D.-J.; Lee, S.-I.; Roh, K.C.; Kim, K.-B. Self-assembly of Si entrapped graphene architecture for high-performance Li-ion batteries. Electrochem. Commun. 2013, 34, 117–120. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Xie, X.; Rick, J.; Chang, F.-C.; Hwang, B.-J. Improved anode materials for lithium-ion batteries comprise non-covalently bonded graphene and silicon nanoparticles. J. Power Sources 2014, 247, 991–998. [Google Scholar] [CrossRef]
- Li, Q.; Chen, D.; Li, K.; Wang, J.; Zhao, J. Electrostatic self-assembly bmSi@C/rGO composite as anode material for lithium ion battery. Electrochim. Acta 2016, 202, 140–146. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Zhang, G.; Zhang, Z.; Fang, C.; Ma, H.; Luo, W.; Liu, Z. Enhanced stability lithium-ion battery based on optimized graphene/Si nanocomposites by templated assembly. ACS Omega 2019, 4, 18195–18202. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Hallac, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Multilayered Si nanoparticle/reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Adv. Mater. 2013, 26, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Zhou, J.; Wang, L.; Zhu, Y.; Qian, Y. Polyaniline-assisted synthesis of Si@C/RGO as anode material for rechargeable lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 7, 409–414. [Google Scholar] [CrossRef]
- Wang, S.; Liao, J.; Wu, M.; Xu, Z.; Gong, F.; Chen, C.; Wang, Y.; Yan, X. High rate and long cycle life of a CNT/rGO/Si nanoparticle composite anode for lithium-ion batteries. Part. Part. Syst. Charact. 2017, 34, 1700141. [Google Scholar] [CrossRef]
- Kumar, K.T.; Reddy, M.J.K.; Sundari, G.S.; Raghu, S.; Kalaivani, R.; Ryu, S.H.; Shanmugharaj, A. Synthesis of graphene-siloxene nanosheet based layered composite materials by tuning its interface chemistry: An efficient anode with overwhelming electrochemical performances for lithium-ion batteries. J. Power Sources 2020, 450, 227618. [Google Scholar] [CrossRef]
- Dai, J.; Liao, J.; He, M.; Yang, M.; Wu, K.; Yao, W. Si@SnS2-reduced graphene oxide composite anodes for high-capacity Li-ion batteries. ChemSusChem 2019, 12, 5092–5098. [Google Scholar] [CrossRef]
- Deng, B.; Xu, R.; Wang, X.; An, L.; Zhao, K.; Cheng, G.J. Roll to roll manufacturing of fast charging, mechanically robust 0D/2D nanolayered Si-graphene anode with well-interfaced and defect engineered structures. Energy Storage Mater. 2019, 22, 450–460. [Google Scholar] [CrossRef]
- Bian, F.; Yu, J.; Song, W.; Huang, H.; Liang, C.; Gan, Y.; Xia, Y.; Zhang, J.; He, X.; Zhang, W. A new magnesium hydride route to synthesize morphology-controlled Si/rGO nanocomposite towards high-performance lithium storage. Electrochim. Acta 2020, 330, 135248. [Google Scholar] [CrossRef]
- Zhaoa, H.; Xuab, X.; Yaoa, Y.; Lic, Y. Si nanoparticles veiled with ultrathin rGO film reduced directly by precoated Ni template: Fabrication and electrochemical performance. Appl. Surf. Sci. 2020, 528, 146993. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Zhang, H.; He, Y.-B.; Li, B.; Ke, L.; Lv, W.; Du, H.; Yang, Q.-H.; Kang, F. Multilayred silicon embedded porous carbon/graphene hybrid as a high performance anode. Carbon 2015, 84, 434–443. [Google Scholar] [CrossRef]
- Majeed, M.K.; Saleem, A.; Ma, X.; Ma, W. Clay-derived mesoporous Si/rGO for anode material of lithium-ion batteries. J. Alloys Compd. 2020, 848, 156590. [Google Scholar] [CrossRef]
- Fang, R.; Xiao, W.; Miao, C.; Mei, P.; Yan, W.; Zhang, Y.; Jiang, Y. Improved lithium storage performance of pomegranate-like Si@NC/rGO composite anodes by facile in-situ nitrogen doped carbon coating and freeze drying processes. J. Alloys Compd. 2020, 834, 155230. [Google Scholar] [CrossRef]
- Zhang, K.; Xia, Y.; Yang, Z.; Fu, R.; Shen, C.; Liu, Z. Structure-preserved 3D porous silicon/reduced graphene oxide materials as anodes for Li-ion batteries. RSC Adv. 2017, 7, 24305–24311. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.; Qi, H.; Liang, J.-C.; Liang, C. High performance of porous silicon/carbon/RGO network derived from rice husks as anodes for lithium-ion batteries. New J. Chem. 2018, 42, 19811–19817. [Google Scholar] [CrossRef]
- Chen, S.; Bao, P.; Huang, X.; Sun, B.; Wang, G. Hierarchical 3D mesoporous silicon-graphene nanoarchitectures for lithium ion batteries with superior performance. Nano Res. 2014, 7, 85–94. [Google Scholar] [CrossRef]
- Men, X.; Kong, X.; Yang, X.; Wang, B.; Wang, Y.; Liu, Y.; Yu, L.; Li, H.; Xu, B. Synthesis of a pomegranate shaped reduced graphene oxide stabilized secondary Si nanoparticles composite anode for lithium ion batteries. Int. J. Hydrogen Energy 2020, 45, 29492–29504. [Google Scholar] [CrossRef]
- Benzait, Z.; Yuca, N. Synergistic effect of carbon nanomaterials on a cost-effective coral-like Si/rGO composite for lithium ion battery application. Electrochim. Acta 2020, 339, 135917. [Google Scholar] [CrossRef]
- Tang, X.; Wen, G.; Zhang, Y.; Wang, D.; Song, Y. Novel silicon nanoparticles with nitrogen-doped carbon shell dispersed in nitrogen-doped graphene and CNTs hybrid electrode for lithium ion battery. Appl. Surf. Sci. 2017, 425, 742–749. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Y.; Li, H. Engineering of a bowl-like Si@rGO architecture for an improved lithium ion battery via synergistic effect. Nanotechnology 2020, 31, 095402. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shen, Z.; Chen, R.; He, X.; Zhang, X.; Zhang, Y.; Wu, K. Sandwich structure of graphene-protected silicon/carbon nanofibers for lithium-ion battery anodes. Electrochim. Acta 2016, 210, 53–60. [Google Scholar] [CrossRef]
- Pan, Q.; Zuo, P.; Lou, S.; Mu, T.; Du, C.; Cheng, X.; Ma, Y.; Gao, Y.; Yin, G. Micro-sized spherical silicon@carbon@graphene prepared by spray drying as anode material for lithium-ion batteries. J. Alloys Compd. 2017, 723, 434–440. [Google Scholar] [CrossRef]
- He, Z.; Wu, X.; Yi, Z.; Wang, X.; Xiang, Y. Silicon/graphene/carbon hierarchical structure nanofibers for high performance lithium ion batteries. Mater. Lett. 2017, 200, 128–131. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, Y.-J.; Nam, Y.-S.; Park, S.-H.; Lee, H.; Hyun, Y.; Lee, C.-S. Synthesis and characterization of silicon/reduced graphene oxide composites as anodes for Lithium secondary batteries. J. Nanosci. Nanotechnol. 2018, 18, 5026–5032. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Xiong, L.; Zhu, S.; Zhang, L.; Yang, X. Porous Si/C/reduced graphene oxide microspheres by spray drying as anode for Li-ion batteries. J. Electroanal. Chem. 2017, 797, 16–22. [Google Scholar] [CrossRef]
- Qin, J.; Wu, M.; Feng, T.; Chen, C.; Tu, C.; Li, X.; Duan, C.; Xia, D.; Wang, D. High rate capability and long cycling life of graphene-coated silicon composite anodes for lithium ion batteries. Electrochim. Acta 2017, 256, 259–266. [Google Scholar] [CrossRef]
- Pan, Q.; Zhao, J.; Qu, W.; Liu, R.; Li, N.; Xing, B.; Jiang, S.; Pang, M.; Zhao, L.; Zhang, Y.; et al. Facile synthesis of the 3D framework Si@N-doped C/Reduced graphene oxide composite by polymer network method for highly stable lithium storage. J. Phys. Chem. Solids 2019, 133, 92–99. [Google Scholar] [CrossRef]
- Toçoğlua, U.; Alaf, M.; Akbulut, H. Towards high cycle stability yolk-shell structured silicon/rGO/MWCNT hybrid composites for Li-ion battery negative electrodes. Mater. Chem. Phys. 2020, 240, 122160. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Chenab, R.; Baiab, Y.; Liab, T.; Jinab, H.; Wanga, J.; Xiaa, H. A green-synthetic spiderweb-like Si@Graphene-oxide anode material with multifunctional citric acid binder for high energy-density Li-ion batteries. Carbon 2020, 157, 330–339. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Yang, C.-C.; Wu, S.; Wu, Z.; Wei, C.; Yang, M.; Lue, S.J. Preparation of ternary hierarchical silicon/reduced graphene oxide/carbon composites as anodes for lithium–ion batteries. J. Alloys Compd. 2019, 793, 433–445. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; He, S.; Huang, C.; Gong, Z.; Gan, L.; Long, M. N-doped rGO/C@Si composites using sustainable chitosan as the carbon source for lithium-ion batteries. Appl. Surf. Sci. 2020, 501, 144136. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Ma, Z.; Wang, H.; Sly, G.L. Yolk-void-shell Si-C nano-particles with tunable void size for high-performance anode of lithium ion batteries. Nanotechnology 2020. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.; Notten, P.H.N. Lithium ion (de)insertion reaction of germanium thin-film electrodes and electrochemical and in-situ XRD study batteries and energy storage. J. Electrochem. Soc. 2009, 156, A169–A175. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Wu, X.; Chang, B.; Wang, K.-X. Recent progress on germanium-based anodes for lithium ion batteries: Efficient lithiation strategies and mechanisms. Energy Storage Mater. 2020, 30, 146–169. [Google Scholar] [CrossRef]
- Liang, W.; Yang, H.; Fan, F.; Liu, Y.; Liu, X.H.; Huang, J.Y.; Zhu, T.; Zhang, S. Tough germanium nanoparticles under electrochemical cycling. ACS Nano 2013, 7, 3427–3433. [Google Scholar] [CrossRef]
- Panayotov, V.; Panayotova, M.; Chukharev, S. Recent studies on germanium-nanomaterials for LIBs anodes. E3S Web Conf. 2020, 166, 06012. [Google Scholar] [CrossRef]
- Loaiza, L.C.; Montconduit, L.; Seznzc, V. Si and Ge-based anode materials for Li-, Na-, and K-ion batteries: A perspective from structure to electrochemical mechanism. Small 2020, 16, 1905260. [Google Scholar] [CrossRef]
- Xue, D.-J.; Xin, S.; Yan, Y.; Jiang, K.-C.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.-J. Improving the electrode performance of Ge through Ge@C core–shell nanoparticles and graphene networks. J. Am. Chem. Soc. 2012, 134, 2512–2515. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.; Zhou, X.; Liu, X.; Liu, Y.; Dai, Z.; Bao, J. Ge nanoparticles encapsulated in nitrogen-doped reduced graphene oxide as an advanced anode material for lithium-ion batteries. J. Phys. Chem. C 2014, 118, 28502–28508. [Google Scholar] [CrossRef]
- Wang, B.; Wen, Z.; Jin, J.; Hong, X.; Zhang, S.; Rui, K. A novel strategy to prepare Ge@C/rGO hybrids as high-rate anode materials for lithium ion batteries. J. Power Sources 2017, 342, 521–528. [Google Scholar] [CrossRef]
- Wang, B.; Jin, J.; Hong, X.; Gu, S.; Guo, J.; Wen, Z. Facile synthesis of the sandwich-structured germanium/reduced graphene oxide hybrid: An advanced anode material for high-performance lithium ion batteries. J. Mater. Chem. A 2017, 5, 13430–13438. [Google Scholar] [CrossRef]
- Fang, S.; Shen, L.; Zheng, H.; Zhang, X. Ge–graphene–carbon nanotube composite anode for high performance lithium-ion batteries. J. Mater. Chem. A 2014, 3, 1498–1503. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Shen, X.; Ji, Z.; Yuan, A.; Xu, K.; Shah, S.A. In-situ synthesis of Ge/reduced graphene oxide composites as ultrahigh rate anode for lithium-ion battery. J. Alloys Compd. 2019, 801, 90–98. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, D.-L.; Yang, H.-X.; Han, X.-Y.; Duan, Y.-J.; Tian, X.-M.; Meng, W.-J. Graphene-supported cubic hollow carbon shell-coated germanium particles as high-performance anode for lithium-ion batteries. Ceram. Int. 2019, 45, 13210–13218. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, X.; Liang, X.; Zhang, F.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon 2020, 161, 287–298. [Google Scholar] [CrossRef]
- Meng, W.-J.; Zhao, M.; Yang, H.-X.; Wu, Y.-Q.; Pu, H.; Gao, R.-Z.; Yang, Y.; Zhao, D.-L. Synthesis of CuGeO3/reduced graphene oxide nanocomposite by hydrothermal reduction for high performance Li-ion battery anodes. Ceram. Int. 2020, 46, 9249–9255. [Google Scholar] [CrossRef]
- Wen, C.J.; Huggins, R.A. Thermodynamic study of the lithium-tin system. J. Electrochem. Soc. 1981, 128, 1181–1187. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.S.; Jiang, W.; Wang, Y.; Pan, Y.; Lu, C. Mechanical properties of Li–Sn alloys for Li-ion battery anodes: A first-principles perspective. AIP Adv. 2016, 6, 015107. [Google Scholar] [CrossRef]
- Li, H. Nano-alloy anode for lithium ion batteries. Solid State Ionics 2002, 148, 247–258. [Google Scholar] [CrossRef]
- Wang, B.; Luo, B.; Li, X.; Zhi, L. The dimensionality of Sn anodes in Li-ion batteries. Mater. Today 2012, 15, 544–552. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Wang, X.; Park, J.; Dou, S.; Ahn, H.; Kim, K. Sn/graphene nanocomposite with 3D architecture for enhanced reversible lithium storage in lithium ion batteries. J. Mater. Chem. 2009, 19, 8378–8384. [Google Scholar] [CrossRef]
- Luo, B.; Wang, B.; Liang, M.; Ning, J.; Li, X.; Zhi, L. Reduced graphene oxide-mediated growth of uniform tin-core/carbon-sheath coaxial nanocables with enhanced lithium ion storage properties. Adv. Mater. 2012, 24, 1405–1409. [Google Scholar] [CrossRef]
- Wang, H.; Xing, Z.; Hu, Z.; Zhang, Y.; Hu, Y.; Sun, Y.; Ju, Z.; Zhuang, Q. Sn-based submicron-particles encapsulated in porous reduced graphene oxide network: Advanced anodes for high-rate and long life potassium-ion batteries. Appl. Mater. Today 2019, 15, 58–66. [Google Scholar] [CrossRef]
- Botas, C.; Carriazo, D.; Singh, G.; Rojo, T. Sn—And SnO2–graphene flexible foams suitable as binder-free anodes for lithium ion batteries. J. Mater. Chem. A 2015, 3, 13402–13410. [Google Scholar] [CrossRef]
- Soler-Piña, F.J.; Hernández-Rentero, C.; Caballero, A.; Morales, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Highly graphitized carbon nanosheets with embedded Ni nanocrystals as anode for Li-ion batteries. Nano Res. 2020, 13, 86–94. [Google Scholar] [CrossRef]
- Dashairya, L.; Das, D.; Saha, P. Electrophoretic deposition of antimony/reduced graphite oxide hybrid nanostructure: A stable anode for lithium-ion batteries. Mater. Today Commun. 2020, 24, 101189. [Google Scholar] [CrossRef]
- Yang, J.; Takeda, Y.; Imanishi, N.; Capiglia, C.; Xie, J.Y.; Yamamoto, O. SiOx-based anodes for secondary lithium batteries. Solid State Ion. 2002, 152-153, 125–129. [Google Scholar] [CrossRef]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Yamada, Y.; Iriyama, Y.; Abe, T.; Ogumi, Z. Kinetics of electrochemical insertion and extraction of lithium ion at SiO. J. Electrochem. Soc. 2010, 157, A26. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Chen, Y.; Li, N.; Li, Y.; Liu, L. SiO2@SnO2/graphene composite with a coating and hierarchical structure as high performance anode material for lithium ion battery. J. Alloys Compd. 2016, 677, 237–244. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, L.; Wu, H.; Chen, H.; Su, L.; Wang, L.; Wang, Y.; Ren, M. Co2SiO4/SiO2/RGO nanosheets: Boosting the lithium storage capability of tetravalent Si by using highly-dispersed Co element. Electrochim. Acta 2018, 282, 609–617. [Google Scholar] [CrossRef]
- Hu, Z.; Cui, H.; Li, J.; Lei, G.; Li, Z. Constructing three-dimensional Li-transport channels within the Fe3O4@SiO2@RGO composite to improve its electrochemical performance in Li-ion batteries. Ceram. Int. 2020, 46, 18868–18877. [Google Scholar] [CrossRef]
- Haeri, S.; Ramezanzadeh, B.; Asghari, M. A novel fabrication of a high performance SiO2-graphene oxide (GO) nanohybrids: Characterization of thermal properties of epoxy nanocomposites filled with SiO2-GO nanohybrids. J. Colloid Interface Sci. 2017, 493, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kasimayan, U.; Nadarajan, A.; Singaravelu, C.M.; Pan, G.-T.; Kandasamy, J.; Yang, T.C.-K.; Lin, J.-H. In-situ DRIFT investigation of photocatalytic reduction and oxidation properties of SiO2@α-Fe2O3 core-shell decorated RGO nanocomposite. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.; Hou, Z.; Zhang, X.; Li, C. One-step synthesis of SiOx@graphene composite material by a hydrothermal method lithium-ion batteries anodes. Energy Fuels 2020, 34, 3895–3900. [Google Scholar] [CrossRef]
- Huang, J.; Fam, D.; He, Q.; Chen, H.; Zhan, D.; Faulkner, S.H.; Nimmo, M.; Tok, A.I.Y. The mechanism of graphene oxide as a growth template for complete reduced graphene oxide coverage on an SiO2 substrate. J. Mater. Chem. C 2014, 2, 109–114. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Song, X.; Zhu, Y.; Jiang, S. Layer-by-layer self-assembled graphene oxide/silica microsphere composites as stationary phase for high performance liquid chromatography. Analyst 2012, 137, 5237–5244. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.R.; Zhu, W.X.; Su, D.; Sang, Z.Y.; Yan, X.; Li, S.; Liang, J.; Dou, S.X. A multifunctional hierarchical porous SiO2/GO membrane for high efficiency oil/water separation and dye removal. Carbon 2020, 160, 88–97. [Google Scholar] [CrossRef]
- Guo, C.; Wang, D.; Liu, T.; Zhu, J.X.; Lang, X. A three dimensional SiOx/C@RGO nanocomposite as a high energy anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 3521–3527. [Google Scholar] [CrossRef]
- Guo, X. Reduced Graphene oxide -coated 3D interconnected SiO2 Nanoparticles with Enhanced Lithium Storage Performance. Int. J. Electrochem. Sci. 2018, 13, 5645–5653. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Lin, S.; Khan, S.; Huang, J.; Liu, S.; Chen, Z.; Wu, D.; Fu, R. Synthesis of SiOx/C composite nanosheets as high-rate and stable anode materials for lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 3562–3568. [Google Scholar] [CrossRef]
- Idota, Y.; Kubota, T.; Matsufuji, A.; Maekawa, Y.; Miyasaka, T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material. Science 1997, 276, 1395–1397. [Google Scholar] [CrossRef]
- Zoller, F.; Böhm, D.; Bein, T.; Fattakhova-Rohlfing, D. Tin oxide based nanomaterials and their application as anodes in lithium-ion batteries and beyond. ChemSusChem 2019, 12, 4140–4159. [Google Scholar] [CrossRef]
- Li, Y.; Lv, X.; Lu, J.; Li, J. Preparation of SnO2-nanocrystal/graphene-nanosheets composites and their lithium storage ability. J. Phys. Chem. C 2010, 114, 21770–21774. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, G.; Song, J.; Jiang, Y.; Zhuang, H.; Liu, P.; Fang, T. Bivalent tin ion assisted reduction for preparing graphene/SnO2 composite with good cyclic performance and lithium storage capacity. Electrochim. Acta 2011, 56, 7340–7346. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2011, 56, 4532–4539. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Zhou, L.; Qian, K.; Wang, Y.; Liu, Z.; Yu, C. A facile one-step solvothermal synthesis of SnO2/graphene nanocomposite and its application as an anode material for lithium-ion batteries. ChemPhysChem 2010, 12, 278–281. [Google Scholar] [CrossRef]
- Xie, J.; Liu, S.Y.; Chen, X.F.; Zhang, Y.X.; Song, W.T.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Nanocrystal-SnO2-loaded graphene with improved Li-storage properties prepared by a facile one-pot hydrothermal route. Int. J. Electrochem. Sci. 2011, 6, 5539–5549. [Google Scholar]
- Lim, S.P.; Huang, N.M.; Lim, H.N. Solvothermal synthesis of SnO2/graphene nanocomposites for supercapacitor application. Cream. Int. 2013, 39, 6647–6655. [Google Scholar] [CrossRef]
- Ding, S.; Luan, D.; Boey, F.Y.C.; Chen, J.S.; Lou, X.W.D. SnO2 nanosheets grown on graphene sheets with enhanced lithium storage properties. Chem. Commun. 2011, 47, 7155–7157. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Huang, K.; Qi, X.; Li, H.; Yang, L.; Zhong, J. Free-standing SnO2 nanoparticles@graphene hybrid paper for advanced lithium-ion batteries. Ceram. Int. 2014, 40, 6891–6897. [Google Scholar] [CrossRef]
- Cui, D.; Zheng, Z.; Peng, X.; Li, T.; Sun, T.; Yuan, L. Fluorine-doped SnO2 nanoparticles anchored on reduced graphene oxide as a high-performance lithium ion battery anode. J. Power Sources 2017, 362, 20–26. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Q.; Wu, Q.; Zhang, X.; Huang, Y.-G.; Lushington, A.; Li, Q.; Sun, X. Ultrasmall MoS2 embedded in carbon nanosheets-coated Sn/SnOx as anode material for high-rate and long life Li-ion batteries. J. Mater. Chem. A 2017, 5, 4576–4582. [Google Scholar] [CrossRef]
- Tang, J.; Yang, J.; Zhou, L.; Xie, J.; Chen, G.; Zhou, X. Layer-by-layer self-assembly of a sandwich-like graphene wrapped SnOx@graphene composite as an anode material for lithium ion batteries. J. Mater. Chem. A 2014, 2, 6292–6295. [Google Scholar] [CrossRef]
- Cai, D.; Yang, T.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, Q.; Wang, T. A nanocomposite of tin dioxide octahedral nanocrystals exposed to high-energy facets anchored onto graphene sheets for high performance lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13990. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, J.; Han, S.; Lv, L.; Zhang, F.; Feng, X. Amphiphilic polymer promoted assembly of macroporous graphene/SnO2 frameworks with tunable porosity for high-performance lithium storage. Small 2014, 10, 2226–2232. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Binding SnO2 Nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, G.; Chen, J.; Lu, C.; Wen, Z. 3D graphene network encapsulating SnO2 hollow spheres as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4535–4542. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Chen, G.; Ba, C.; Wang, Z.; Zhu, J.; Zhao, Y.; Zhang, M.; Yuan, S. In situ synthesis of tungsten-doped SnO2 and graphene nanocomposites for high-performance anode materials of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 17163–17171. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Wang, J.; Ynag, J.; Geng, D.; Li, R.; Cai, M.; Sham, T.-K.; Sun, X. defect-rich crystalline SnO2 immobilized on graphene nanosheets with enhanced cycle performance for Li ion batteries. J. Phys. Chem. C 2012, 116, 22149–22156. [Google Scholar] [CrossRef]
- Shao, Q.; Tang, J.; Sun, Y.; Li, J.; Zhang, K.; Yuan, J.; Zhu, D.-M.; Qin, L.-C. Unique interconnected graphene/SnO2 nanoparticle spherical multilayers for lithium-ion battery applications. Nanoscale 2017, 9, 4439–4444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, P.; Ni, Y.; Geng, H.; Zheng, G.; Dong, B.; Jiao, Z. In situ chemical synthesis of SnO2/reduced graphene oxide nanocomposites as anode materials for lithium-ion batteries. J. Mater. Res. 2014, 29, 617–624. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Wang, M.; Chen, C.; Ge, X. Hierarchical porous SnO2/reduced graphene oxide composites for high-performance lithium-ion battery anodes. Electrochim. Acta 2016, 215, 42–49. [Google Scholar] [CrossRef]
- Hou, C.-C.; Brahma, S.; Weng, S.-C.; Chang, C.-C.; Huang, J.-L. Facile, low temperature synthesis of SnO2/reduced graphene oxide nanocomposite as anode material for lithium-ion batteries. Appl. Surf. Sci. 2017, 413, 160–168. [Google Scholar] [CrossRef]
- Tan, Q.; Kong, Z.; Chen, X.; Zhang, L.; Hu, X.; Mu, M.; Sun, H.; Shao, X.; Guan, X.; Gao, M.; et al. Synthesis of SnO2/graphene composite anode materials for lithium-ion batteries. Appl. Surf. Sci. 2019, 485, 314–322. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, N.; Wang, X.; Yang, C.; Cheng, H.; Zhao, H. SnO2 nanoparticles anchored on graphene oxide as advanced anode materials for high-performance lithium-ion batteries. Front. Mater. Sci. 2019, 13, 186–192. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, X.; Ma, Z.S.; Lin, J.; Lu, C. SnO2/reduced graphene oxide nanocomposite as anode material for lithium-ion batteries with enhanced cyclability. J. Nanosci. Nanotechnol. 2016, 16, 4136–4140. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, R.; Zhu, W.; Li, X.; Zhao, Y.; Gao, Z.; Gao, L.; Zhao, J. Free-standing SnO2@rGO hybrid via the anti-solvent-assisted precipitation for superior lithium storage performance. Front. Chem. 2019, 7, 878. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Brahma, S.; Weng, S.-C.; Chang, C.-C.; Huang, J.-L. Reduced graphene oxide (RGO)-SnOx (x = 0,1,2) nanocomposite as high performance anode material for lithium-ion batteries. J. Alloys Compd. 2020, 818, 152889. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhang, S.; Liang, S.; Liang, X.; Huang, H.; Zhou, W.; Guo, J. Facile synthesis of iron-doped SnO2/reduced graphene oxide composite as high-performance anode material for lithium-ion batteries. J. Alloys Compd. 2018, 748, 1013–1021. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Ding, S.; Chen, C.; Zhang, M. Enhanced electrochemical properties of SnO2–graphene–carbon nanofibers tuned by phosphoric acid for potassium storage. Nanotechnology 2018, 29, 375702. [Google Scholar] [CrossRef] [PubMed]
- Zoller, F.; Peters, K.; Zehetmaier, P.M.; Zeller, P.; Dçblinger, M.; Bein, T.; Sofer, Z.; Fattakhova-Rohlfing, D. Making ultrafast high-capacity anodes for lithium-ion batteries via antimony doping of nanosized tin oxide/graphene composites. Adv. Funct. Mater. 2018, 28, 1706529. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, L.; Jiang, S.; Li, G.; Huang, Y.; Geng, J. Fluorine-doped SnO2@graphene porous composite for high capacity lithium-ion batteries. Chem. Mater. 2015, 27, 4594–4603. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Zheng, Z.; Li, T.; Tong, Z.; Ai, C. Fluorine-doped SnO2/reduced graphene oxide-artificial graphite hybrids as lithium-ion battery anodes with stable capacity. Ionics 2020, 26, 2835–2843. [Google Scholar] [CrossRef]
- Dong, W.; Xu, J.; Wang, C.; Lu, Y.; Liu, X.; Wang, X.; Yuan, X.; Wang, Z.; Lin, T.; Sui, M.; et al. A robust and conductive black tin oxide nanostructure makes efficient lithium-ion batteries possible. Adv. Mater. 2017, 29, 1700136. [Google Scholar] [CrossRef]
- Dou, P.; Cao, Z.; Wang, C.; Zheng, J.; Xu, X. Multilayer Zn-doped SnO2 hollow nanospheres encapsulated in covalently interconnected three-dimensional graphene foams for high performance lithium-ion batteries. Chem. Eng. J. 2017, 320, 405–415. [Google Scholar] [CrossRef]
- Kokubu, T.; Oaki, Y.; Hosono, E.; Zhou, H.; Imai, H. Biomimetic solid-solution precursors of metal carbonate for nanostructured metal oxides: MnO/Co and MnO-CoO nanostructures and their electrochemical properties. Adv. Funct. Mater. 2011, 21, 3673–3680. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Wang, H.; Wang, X.; Zhang, X.; Jin, G. A novel solvothermal synthesis of Mn3O4/graphene composites for supercapacitors. Electrochim. Acta 2013, 90, 210–218. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, G.-L.; Yan, K.; Sun, H.; Xiao, J.; Yang, S.; Sun, S.; Jin, L.; Deng, H. Morphology-conserved transformation: Synthesis of hierarchical mesoporous nanostructures of Mn2O3 and the nanostructural effects on Li-ion insertion/deinsertion properties. J. Mater. Chem. 2011, 21, 6346–6353. [Google Scholar] [CrossRef]
- Li, L.; Raji, A.-R.O.; Tour, J.M. Graphene-wrapped MnO2-graphene nanoribbons as anode materials for high-performance lithium ion batteries. Adv. Mater. 2013, 25, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, T.; Golikand, A.N.; Mashhadizadeh, M.H.; Aghazadeh, M. Facile synthesis of α-MnO2 one-dimensional (1D) nanostructure and energy storage ability studies. J. Solid State Chem. 2012, 190, 202–207. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, D.; Li, Y.; Wu, Z.; Zhuo, R.; Li, S.; Feng, J.; Wang, J.; Yan, P.; Geng, Z. Manganese dioxide nanosheet arrays grown on graphene oxide as an advanced electrode material for supercapacitors. Electrochim. Acta 2014, 117, 528–533. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, G.; Huang, J.; Nan, J.; Zhen, S.; Wang, Y.; Li, A. Hollow MnO2 spheres/porous reduced graphene oxide as a cathode host for high-performance lithium-sulfur batteries. J. Solid State Chem. 2020, 286, 121297. [Google Scholar] [CrossRef]
- Majid, S.R. Green synthesis of in situ electrodeposited rGO/MnO2 nanocomposite for high energy density supercapacitors. Sci. Rep. 2015, 5, 16195. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Krittayavathananon, A.; Luanwuthi, S.; Limtrakul, J. High-performance supercapacitor of manganese oxide/reduced graphene oxide nanocomposite coated on flexible carbon fiber paper. Carbon 2013, 60, 109–116. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Patil, B.H.; Shinde, S.S.; Lokhande, C.D. Enhanced activity of chemically synthesized hybrid graphene oxide/Mn3O4 composite for high performance supercapacitors. Electrochim. Acta 2013, 92, 205–215. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.-L. Preparation and electrochemical characterization of MnOOH nanowire–graphene oxide. Electrochim. Acta 2011, 56, 5010–5015. [Google Scholar] [CrossRef]
- Chen, T.; Zhiu, Y.; Zhang, J.; Cao, Y. Two-dimensional MnO2/reduced graphene oxide nanosheet as a high-capacity and high-rate cathode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2018, 13, 8575–8588. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, C.-W.; Yoon, S.-B.; Kim, M.-S.; Jeong, J.H.; Nam, K.-W.; Roh, K.C.; Kim, K.-B. Superior electrochemical properties of manganese dioxide/reduced graphene oxide nanocomposites as anode materials for high-performance lithium ion batteries. J. Power Sources 2016, 312, 207–215. [Google Scholar] [CrossRef]
- Devi, P.; Bansod, B.; Kaur, M.; Bagchi, S.; Nayak, M.K. Co-electrodeposited rGO/MnO2 nanohybrid for arsenite detection in water by stripping voltammetry. Sensors Actuators B Chem. 2016, 237, 652–659. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Ruan, H.; Su, Y.; Hu, R.; Tian, L.; Hu, Z.; Li, J. Fabrication of β-MnO2/rGO composite and its electrochemical properties. Int. J. Electrochem. Sci. 2016, 11, 10815–10826. [Google Scholar] [CrossRef]
- Liu, H. Preparation of (MnO2/RGO)/Li7La3Zr2O12/LiCoO2 Solid State Lithium Ion Batteries and Theirs Electrochemical Performance. Int. J. Electrochem. Sci. 2017, 12, 11479–11486. [Google Scholar] [CrossRef]
- Ali, G.A.; Yusoff, M.; Algarni, H.; Chong, K.F. One-step electrosynthesis of MnO2/rGO nanocomposite and its enhanced electrochemical performance. Ceram. Int. 2018, 44, 7799–7807. [Google Scholar] [CrossRef]
- Liu, H.D.; Hu, Z.L.; Su, Y.Y.; Ruan, H.B.; Hu, R.; Zhang, L. MnO2 nanorods/3D-rGO composite as high performance anode materials for Li-ion batteries. Appl. Surf. Sci. 2017, 392, 777–784. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, X.; Zhang, C.; Yang, C.; Zhang, Q.; Hu, W.; Zheng, M.; Dong, Q. MnO2 nanoflakes anchored on reduced graphene oxide nanosheets as high performance anode materials for lithium-ion batteries. RSC Adv. 2014, 4, 30150–30155. [Google Scholar] [CrossRef]
- Chae, C.; Kim, K.W.; Yun, Y.J.; Lee, D.; Moon, J.; Choi, Y.; Lee, S.S.; Choi, S.; Jeong, S. Polyethylenimine-mediated electrostatic assembly of MnO2 nanorods on graphene oxides for use as anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 11499–11506. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Zhu, Z.; Wong, K.; Mi, R.; Mei, J.; Lau, W. A green hydrothermal approach for the preparation of graphene/α-MnO2 3D network as anode for lithium ion battery. Electrochim. Acta 2013, 108, 465–471. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, T. Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle Lithium ion batteries. Electrochim. Acta 2016, 201, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Liu, W.; Shi, B.; Guo, R.; Pei, H.; Xie, J. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO2/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interface Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y.; Liu, Z.; Yang, L.; Zhang, J.; Wang, M.; Che, R. Flexible graphene-wrapped carbon nanotube/graphene@MnO2 3D multilevel porous film for high-performance lithium-ion batteries. Small 2018, 14, 1801007. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yun, Y.J.; Kim, K.W.; Chae, C.; Jeong, S.; Kang, Y.; Choi, S.-Y.; Lee, S.S.; Choi, S. Superior lithium storage performance using sequentially stacked MnO2/reduced graphene oxide composite electrodes. ChemSusChem 2015, 8, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Shi, B.; Liu, W.; Guo, R.; Pei, H.; Xie, J. Free-standing reduced graphene oxide/MnO2–reduced graphene oxide–carbon nanotube nanocomposite flexible membrane as an anode for improving lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 7498–7505. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Xia, F.; Bu, L.; Qiu, H.; Chen, M.; Zhang, L.; Gao, J. Electrochemically active MnO2/RGO nanocomposites using Mn powder as the reducing agent of GO and the MnO2 precursor. Electrochim. Acta 2014, 130, 305–313. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Sci. Rep. 2015, 4, 4824. [Google Scholar] [CrossRef]
- Chan, P.Y.; Rusi, R.; Majid, S. RGO-wrapped MnO2 composite electrode for supercapacitor application. Solid State Ionics 2014, 262, 226–229. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, H.; Chen, P.; Nie, L.H.; Li, C.-H.; Li, S.K. Self-assembled three-dimensional hierarchical graphene hybrid hydrogels with ultrathin β-MnO2 nanobelts for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 1540–1548. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.W.; Wu, X.D.; Han, Q.F.; Wang, X. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Lu, L.; Xu, S.; An, J.; Yan, S. Electrochemical performance of CNTs/RGO/MnO2 composite material for supercapacitor. Nanomater. Nanotechnol. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Hareesh, K.; Shateesh, B.; Joshi, R.P.; Williams, J.F.; Phase, D.M.; Haram, S.K.; Dhole, S.D. Ultra high stable supercapacitance performance of conducting polymer coated MnO2 nanorods/rGO nanocomposites. RSC Adv. 2017, 7, 20027–20036. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Evaluation of GO/MnO2 composites as supercapacitors in neutral electrolytes: Role of graphite oxide oxidation level. J. Mater. Chem. 2012, 22, 23525–23533. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y. Rapid synthesis of graphene/amorphous α-MnO2 composite with enhanced electrochemical performance for electrochemical capacitor. Mater. Sci. Eng. B 2015, 194, 41–47. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 2017, 240, 53–62. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Ouyang, J.-H.; Jia, D.; Zhou, Y. Flexible and solid-state asymmetric supercapacitor based on ternary graphene/MnO2/carbon black hybrid film with high power performance. Electrochim. Acta 2015, 182, 861–870. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Flexible asymmetric supercapacitors based on CuO@MnO2-rGO and MoS2-rGO with ultrahigh energy density. J. Electroanal. Chem. 2018, 827, 221–229. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Xue, W.; Zhou, S.; Lan, Y.; Zhang, H.; Sui, Z. Electrodeposition synthesis of PANI/MnO2/graphene composite materials and its electrochemical performance. Int. J. Electrochem. Sci. 2017, 12, 8306–8314. [Google Scholar]
- Dong, W.; Meng, L.; Hong, X.; Liu, S.; Shen, D.; Xia, Y.; Yang, S. MnO2/rGO/CNTs framework as a sulfur host for high-performance Li-S batteries. Molecules 2020, 25, 1989. [Google Scholar] [CrossRef]
- Jie, G.; Michael, A.L.; Hector, D.A. Sponge like nanosized Mn3O4 as a high-capacity anode material for rechargeable lithium batteries. Chem. Mater. 2011, 23, 3223–3227. [Google Scholar]
- Su, Q.; Wang, S.; Du, G.; Xu, B.; Ma, S.; Shang, L. Microstructure evolution and conversion mechanism of Mn3O4 under electrochemical cyclings. J. Phys. Chem. C 2018, 122, 2475–2480. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.-F.; Yang, Y.; Casalongue, H.S.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4−graphene hybrid as a high-capacity anode material for lithium-ion batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, L.; Shi, W.; Zou, X.; Xiang, B.; Xing, S. Rapid production of Mn3O4/rGO as an efficient electrode material for supercapacitor by flame plasma. Materials 2018, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Coprecipitated 3D nanostructured graphene oxide-Mn3O4 hybrid as anode for lithium-ion batteries. J. Mater. Res. 2015, 30, 484–492. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Fulari, A.; Ghodake, G.; Kim, D.; Lohar, G.M. Performance of chemically synthesized Mn3O4/rGO nanocomposite for electrochemical supercapacitor: A cost-effective high-performance electrode. Nanotechnology 2020, 31, 415403. [Google Scholar] [CrossRef]
- Wang, L.; Ouyang, Y.; Jiao, X.; Xia, X.; Lei, W.; Hao, Q. Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chem. Eng. J. 2018, 334, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Shi, M.; Lu, W.; Zhu, D.; Li, L.; Gan, L. Core–shell reduced graphene oxide/MnOx@carbon hollow nanospheres for high performance supercapacitor electrodes. Chem. Eng. J. 2017, 313, 518–526. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.H.; Wang, N. A reduced graphene oxide/mixed-valent manganese oxides composite electrode for tailorable and surface mountable supercapacitors with high capacitance and super-long life. Energy Environ. Sci. 2017, 10, 941–949. [Google Scholar] [CrossRef]
- Seong, C.-Y.; Park, S.-K.; Bae, Y.; Yoo, S.; Piao, Y. An acid-treated reduced graphene oxide/Mn3O4 nanorod nanocomposite as an enhanced anode material for lithium ion batteries. RSC Adv. 2017, 7, 37502–37507. [Google Scholar] [CrossRef]
- Wang, J.; Guan, C.; Feng, G.; Ke, Q.; Huang, X.; Wang, J. Flexible asymmetric supercapacitor based on structure-optimized Mn3O4/reduced Graphene oxide nanohybrid paper with high energy and power density. Adv. Funct. Mater. 2015, 25, 7291–7299. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, J.; Wang, X.; Liu, W.; Yan, S. Producing symbiotic reduced graphene oxide/Mn3O4 nanocoposites directly from converting graphite for high-performance supercapacitor electrodes. ACS Omega 2020, 5, 18975–18986. [Google Scholar] [CrossRef]
- Li, Y.; Gai, T.; Shao, L.; Tang, H.; Li, R.; Yang, S.; Wang, S.; Wu, Q.; Ren, Y. Synthesis of sandwich-like Mn3O4@reduced graphene oxide nano-composites via modified Hummers’ method and its application as uranyl adsorbents. Heliyon 2019, 5, e01972. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.G.S.; Ramprasad, R.N.R.; Asiri, A.M.; Wu, J.J.; Anandan, S. Ultrasound assisted synthesis of Mn3O4 nanoparticles anchored graphene nanosheets for supercapacitor applications. Electrochim. Acta 2015, 156, 127–137. [Google Scholar] [CrossRef]
- Park, G.; Bartolome, L.; Lee, K.G.; Lee, S.J.; Kim, D.H.; Park, T.J. One-step sonochemical synthesis of a graphene oxide-manganese oxide nanocomposite for catalytic glycolysis of polu(ethylene terephthalate). Nanoscale 2012, 4, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, I.A.; Meduri, P.; Oh, H.; Bhimanapati, G.R.; Li, Q.; Yang, G.; Robinson, J.A.; Wang, Q. Effect of Mn3O4 nanoparticle composition and distribution on graphene as a potential hybrid anode material for lithium-ion batteries. RSC Adv. 2016, 6, 33022–33030. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, W.-J. Hierarchically mesoporous CuO/carbon nanofiber coaxial shell-core nanowires for lithium ion batteries. Sci. Rep. 2015, 5, 9754. [Google Scholar] [CrossRef] [PubMed]
- Rosaiah, P.; Zhu, J.; Shaik, D.P.M.D.; Hussain, O.M.; Qiu, Y.; Zhao, L. Reduced graphene oxide/Mn3O4 nanocomposite electrodes with enhanced electrochemical performance for energy storage applications. J. Electroanal. Chem. 2017, 794, 78–85. [Google Scholar] [CrossRef]
- Nithya, C.; Vishnuprakash, P.; Gopukumar, S. A Mn3O4 nanospheres@rGO architecture with capacitive effects on high potassium storage capability. Nanoscale Adv. 2019, 1, 4347–4358. [Google Scholar] [CrossRef]
- Nam, I.; Kim, N.D.; Kim, G.-P.; Park, J.; Yi, J. One step preparation of Mn3O4/graphene composites for use as an anode in Li ion batteries. J. Power Sources 2013, 244, 56–62. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, S.; Hao, N.; Zhong, S.; Liu, W. An ultrasound-assisted approach to synthesize Mn3O4/RGO hybrids with high capability for lithium ion batteries. Dalton Trans. 2014, 43, 15317–15320. [Google Scholar] [CrossRef]
- Li, S.; Yu, L.-L.; Shi, Y.-T.; Fan, J.; Li, R.-B.; Fan, G.-D.; Xu, W.-L.; Zhao, J.-T. Greatly Enhanced Faradic Capacities of 3D Porous Mn3O4/G Composites as Lithium-Ion Anodes and Supercapacitors by C–O–Mn Bonding. ACS Appl. Mater. Interfaces 2019, 11, 10178–10188. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, D.; Qin, L.-C.; Wen, G.; Pan, H.; Zhang, Y.; Tian, N.; Zhou, Y.; Huang, X. MnCO3/Mn3O4/reduced graphene oxide ternary anode materials for lithium-ion batteries: Facile green synthesis and enhanced electrochemical performance. J. Mater. Chem. A 2017, 5, 17001–17011. [Google Scholar] [CrossRef]
- Shah, H.U.; Wang, F.; Javed, M.S.; Shaheen, N.; Saleem, M.; Li, Y. Hydrothermal synthesis of reduced graphene oxide – Mn3O4 nanocomposite as an efficient electrode material for supercapacitors. Ceram. Int. 2018, 44, 3580–3584. [Google Scholar] [CrossRef]
- Varghese, S.P.; Babu, B.; Prasannachandran, R.; Antony, R.; Shaijumon, M.M. Enhanced electrochemical properties of Mn3O4/graphene nanocomposite as efficient anode material for lithium ion batteries. J. Alloys Compd. 2019, 780, 588–596. [Google Scholar] [CrossRef]
- Petnikota, S.; Srikanth, V.V.S.S.; Nithyadharseni, P.; Reddy, M.V.; Adams, S.; Chowdari, B.V.R. Sustainable graphenothermal reduction chemistry to obtain MnO nanonetwork supported exfoliated graphene oxide composite and its electrochemical characteristics. ACS Sustain. Chem. Eng. 2015, 3, 3205–3213. [Google Scholar] [CrossRef]
- Yuan, T.; Xu, B.; Sun, W.; Xiang, B.; Li, Y.; Yan, M.; Xu, B.; Dou, S. Ever-Increasing Pseudocapacitance in RGO-MnO-RGO Sandwich Nanostructures for Ultrahigh-Rate Lithium Storage. Adv. Funct. Mater. 2016, 26, 2198–2206. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, X.; Yang, J.; Sun, A.; Wang, H.; Tang, J. MnO nanoparticles sandwiched within 3D graphene-based hierarchical architecture for efficient lithium storage. Inorg. Chem. 2019, 58, 3329–3337. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Liu, Z.; Zhao, H.; Liu, H.; Zhang, Y. Bottom-up construction of reduced-graphene-oxide-anchored MnO with an nitrogen-doped carbon coating for synergistically improving lithium-ion storage. Inorg. Chem. 2018, 57, 13693–13701. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lin, C.-Y.; Lin, J.-Y. High reversibility of Li intercalation and de-intercalation in MnO-attached graphene anodes for Li-ion batteries. Electrochim. Acta 2011, 56, 8861–8867. [Google Scholar] [CrossRef]
- Zhang, K.; Han, P.; Gu, L.; Zhang, L.; Liu, Z.; Kong, Q.; Zhang, C.; Dong, S.; Zhang, Z.; Yao, J.; et al. Synthesis of nitrogen-doped MnO/graphene nanosheets hybrid material for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 658–664. [Google Scholar] [CrossRef]
- Qiu, D.; Ma, L.; Zheng, M.; Lin, Z.; Zhao, B.; Wen, Z.; Hu, Z.; Pu, L.; Shi, Y. MnO nanoparticles anchored on graphene nanosheets via in situ carbothermal reduction as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2012, 84, 9–12. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Xie, J.; Zheng, Y.-X.; Cao, G.-S.; Zhu, T.-J.; Zhao, X. Nanocrystal manganese oxide (Mn3O4, MnO) anchored on graphite nanosheet with improved electrochemical Li-storage properties. Electrochim. Acta 2012, 66, 271–278. [Google Scholar] [CrossRef]
- Mai, Y.; Zhang, D.; Qiao, Y.; Gu, C.; Wang, X.; Tu, J. MnO/reduced graphene oxide sheet hybrid as an anode for Li-ion batteries with enhanced lithium storage performance. J. Power Sources 2012, 216, 201–207. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Song, H.; Chen, X.; Zhou, J. Enhanced electrochemical performance of MnO nanowire/graphene composite during cycling as the anode material for lithium-ion batteries. Nano Energy 2014, 10, 172–180. [Google Scholar] [CrossRef]
- Wu, T.; Tu, F.; Liu, S.; Zhuang, S.; Jin, G.; Pan, C. MnO nanorods on graphene as an anode material for high capacity lithium ion batteries. J. Mater. Sci. 2013, 49, 1861–1867. [Google Scholar] [CrossRef]
- Zang, J.; Qian, H.; Wei, Z.; Cao, Y.; Zheng, M.; Dong, Q. Reduced graphene oxide supported MnO nanoparticles with excellent lithium storage performance. Electrochim. Acta 2014, 118, 112–117. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, X.; Liu, X.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Nanospherical-like manganese monoxide/reduced graphene oxide composite Synthesized by rlectron beam radiation as anode Material for high-performance lithium-ion batteries. Electrochim. Acta 2016, 196, 431–439. [Google Scholar] [CrossRef]
- Auborn, J.J.; Barberio, Y.L. Lithium intercalation cells without metallic lithium: MoO2/LiCoO2 and WO2/LiCoO2. J. Electrochem. Soc. 1987, 134, 638–640. [Google Scholar] [CrossRef]
- Rogers, D.B.; Shannon, R.D.; Sleight, A.W.; Gillson, J.L. Crystal chemistry of metal dioxides with rutile-related structures. Inorg. Chem. 1969, 8, 841–849. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, B.; Corr, S.A.; Shi, Q.; Hu, Y.-S.; Heier, K.R.; Chen, L.; Seshadri, R.; Stucky, G.D. Ordered mesoporous metallic MoO2 materials with highly reversible lithium storage capacity. Nano Lett. 2009, 9, 4215–4220. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z. A new approach to synthesize MoO2@C for high-rate lithium ion batteries. J. Mater. Chem. A 2015, 3, 21314–21320. [Google Scholar] [CrossRef]
- Sen, U.K.; Shaligram, A.; Mitra, S. Intercalation anode material for lithium ion battery based on molybdenum dioxide. ACS Appl. Mater. Interfaces 2014, 6, 14311–14319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Wu, T.-H.; Wang, K.-X.; Wu, X.-Y.; Chen, X.-T.; Jiang, Y.-M.; Wei, X.; Chen, J.-S. Uniform hierarchical MoO2/carbon spheres with high cycling performance for lithium ion batteries. J. Mater. Chem. A 2013, 1, 12038–12043. [Google Scholar] [CrossRef]
- Petnikota, S.; Teo, K.W.; Chen, L.; Sim, A.; Marka, S.K.; Reddy, M.V.; Srikanth, V.V.S.S.; Adams, S.; Chowdari, B. Exfoliated graphene oxide/MoO2 composites as anode materials in Lithium-ion batteries: An insight into intercalation of Li and conversion mechanism of MoO2. ACS Appl. Mater. Interfaces 2016, 8, 10884–10896. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yin, F.; Uchaker, E.; Chen, W.; Zhang, M.; Zhou, J.; Qi, Y.; Cao, G. Facile and green preparation for the formation of MoO2-GO composites as anode material for lithium-ion batteries. J. Phys. Chem. C 2014, 118, 24890–24897. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, S.; Gao, F. Preparation of MoO2/graphene composite as electrode material for supercapacitors. J. Mater. Sci. 2011, 46, 3517–3522. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Z.; Cao, L.; Zhang, Q.; Ouyang, H.; Li, J. Tailoring MoO2/graphene oxide nanostructures for stable, high-density sodium-ion battery anodes. Energy Technol. 2015, 3, 1108–1114. [Google Scholar] [CrossRef]
- Tang, W.; Peng, C.X.; Nai, C.T.; Su, J.; Liu, Y.P.; Reddy, M.V.V.; Lin, M.; Loh, K.P. Ultrahigh capacity due to multi-electron conversion reaction in reduced graphene oxide-wrapped MoO2 porous nanobelts. Small 2015, 11, 2446–2453. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Chen, G.; Zhong, Y.; Cai, R.; Li, L.; Shao, Z. Surfactant-free self-assembly of reduced graphite oxide-MoO2 nanobelt composites used as electrode for lithium-ion batteries. Electrochim. Acta 2016, 211, 972–981. [Google Scholar] [CrossRef]
- Ju, P.; Zhu, Z.; Shao, X.; Wang, S.; Zhao, C.; Qian, X.; Zhao, C. 3D walnut-shaped TiO2/RGO/MoO2@Mo electrode exhibiting extraordinary supercapacitor performance. J. Mater. Chem. A 2017, 5, 18777–18785. [Google Scholar] [CrossRef]
- Hwang, J.; Yoon, D.; Kweon, B.; Chang, W.; Kim, J. A simple, one-pot synthesis of molybdenum oxide-reduced graphene oxide composites in supercritical methanol and their electrochemical performance. RSC Adv. 2016, 6, 108298–108309. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, K.; Fu, H.; Shen, X.; Duan, X.; Cao, L.; Huang, J.; Wang, H. Constructing MoO2 porous architectures using graphene oxide flexible supports for lithium io battery anodes. Glob. Chall. 2017, 1, 7. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhi, G.; Xu, G.; Wang, Q.; Zhang, J. 2D layered mesoporous MoO2/rGO composites for high performance anode materials in lithium-ion battery. Microporous Mesoporous Mater. 2017, 246, 14–23. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Zeng, L.; Huang, X.; Fang, Y.; Liu, J.; Xu, Y.; Chen, Q.; Wei, M.; Qian, Q. Preparation of hierarchical MoO2@RGO composite and its application for high rate performance lithium-ion batteries. Mater. Lett. 2018, 212, 198–201. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Wang, W.; Gao, J. A new facile approach to prepare reduced graphene oxide and MoO2/reduced graphene oxide as electrode materials for oxygen reduction reactions. J. Colloid Interface Sci. 2018, 519, 194–202. [Google Scholar] [CrossRef]
- Liu, C.-L.; Luo, S.-H.; Huang, H.-B.; Zhai, Y.-C.; Wang, Z.-W. Direct growth of MoO2/reduced graphene oxide hollow sphere composites as advanced anode materials for potassium-ion batteries. ChemSusChem 2019, 12, 873–880. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Ji, X.; Cheng, S. Facile synthesis of MoO2/Mo-GO with high initial columbic efficiency and enhanced lithiation ability. Mater. Lett. 2019, 254, 332–335. [Google Scholar] [CrossRef]
- Devina, W.; Hwang, J.; Kim, J. Synthesis of MoO2/Mo2C/rGO composite in supercritical fluid and its enhanced cycling stability in Li-ion batteries. Chem. Eng. J. 2018, 345, 1–12. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, Z.; Wang, H.; Sun, D.; Tang, Y.; Xie, Z. The fabrication of hierarchical MoO2@MoS2/rGO composite as high reversible anode material for lithium ion batteries. Electrochim. Acta 2020, 364, 136996. [Google Scholar] [CrossRef]
- Xia, F.; Hu, X.; Sun, Y.; Luo, W.; Han, J. Layer-by-layer assembled MoO2–graphene thin film as a high-capacity and binder-free anode for lithium-ion batteries. Nanoscale 2012, 4, 4707. [Google Scholar] [CrossRef]
- Xu, Y.; Yi, R.; Yuan, B.; Wu, X.; Dunwell, M.; Lin, Q.; Fei, L.; Deng, S.; Andersen, P.; Wang, D.; et al. High capacity MoO2/graphite oxide composite anode for lithium-ion batteries. J. Phys. Chem. Lett. 2012, 3, 309–314. [Google Scholar] [CrossRef]
- Tang, Q.; Shan, Z.; Wang, L.; Qin, X. MoO2–graphene nanocomposite as anode material for lithium-ion batteries. Electrochim. Acta 2012, 79, 148–153. [Google Scholar] [CrossRef]
- Seng, K.H.; Du, G.D.; Li, L.; Chen, Z.X.; Liu, H.K.; Guo, Z. Facile synthesis of graphene–molybdenum dioxide and its lithium storage properties. J. Mater. Chem. 2012, 22, 16072. [Google Scholar] [CrossRef][Green Version]
- Guo, L.; Wang, Y. Standing carbon-coated molybdenum dioxide nanosheets on graphene: Morphology evolution and lithium ion storage properties. J. Mater. Chem. A 2015, 3, 4706–4715. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Rao, T.; Varadaraju, U. Enhanced nanoscale conduction capability of a MoO2/graphene composite for high performance anodes in lithium ion batteries. J. Power Sources 2012, 216, 169–178. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Wang, J.; Sun, L.; Cao, M. Facile fabrication of molybdenum dioxide/nitrogen-doped graphene hybrid as high performance anode material for lithium ion batteries. J. Power Sources 2015, 274, 142–148. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H. One-dimensional architecture with reduced graphene oxide supporting ultrathin MoO2 nanosheets as high performance anodes for lithium-ion batteries. Nanotechnology 2019, 30, 315602. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, J.; Hu, X.; Wang, X.; Liang, J.-C.; Yu, K. Fabrication of TiO2 hollow nanostructures and their application in lithium ion batteries. J. Alloys Compd. 2015, 651, 685–689. [Google Scholar] [CrossRef]
- Zhang, X.; Kumar, P.S.; Aravindan, V.; Liu, H.H.; Sundaramurthy, J.; Mhaisalkar, S.G.; Duong, H.M.; Ramakrishna, S.; Madhavi, S. Electrospun TiO2-graphene composite nanofibers as a highly durable insertion anode for lithium ion batteries. J. Phys. Chem. C 2012, 116, 14780–14788. [Google Scholar] [CrossRef]
- El-Deen, S.S.; Hashem, A.M.; Ghany, A.E.A.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Anatase TiO2 nanoparticles for lithium-ion batteries. Ionics 2018, 24, 2925–2934. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Guo, H. Simple preparation of petal-like TiO2 nanosheets as anode materials for lithium-ion batteries. Ceram. Int. 2014, 40, 16805–16810. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Li, B.; Yuan, Z.; Xu, Y.; Liu, J. N-doped graphene as an efficient electrocatalyst for lithium-thionyl chloride batteries. Appl. Catal. A Gen. 2016, 523, 241–246. [Google Scholar] [CrossRef]
- Zhen, M.; Zhu, X.; Zhang, X.; Zhou, Z.; Liu, L. Reduced graphene oxide-supported TiO2 fiber bundles with mesostructures as anode materials for lithium-ion batteries. Chem. A Eur. J. 2015, 21, 14454–14459. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ma, R.; Du, N.; Ren, J.; Wong, T.; Li, Y.Y.; Lee, S.T. Growth of TiO2 nanorod arrays on reduced graphene oxide with enhanced lithium-ion storage. J. Mater. Chem. 2012, 22, 19061–19066. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Li, J.; Cao, L.; Qi, H.; Cheng, Y.; Xi, Q.; Dang, H. Improved Li-ion diffusion process in TiO2/rGO anode for lithium-ion battery. J. Alloys Compd. 2017, 727, 998–1005. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Li, W.; Yu, A.; Wu, P. Carbon-coated mesoporous TiO2 nanocrystals grown on graphene for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10395–10400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y. Recent progress of TiO2-based anodes for Li ion batteries. J. Nanomater. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Etacheri, V.; Yourey, J.E.; Bartlett, B.M. Chemically bonded TiO2–bronze nanosheet/reduced graphene oxide hybrid for high-power lithium-ion batteries. ACS Nano 2014, 8, 1491–1499. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Ding, M.; Feng, Y. In-situ constructing of hollow TiO2@rGO hybrid spheres as high-rate and long-life anode materials for lithium-ion batteries. Ceram. Int. 2019, 45, 12476–12483. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Zhao, M.; Feng, J.; Han, Y.; Deng, J.; Li, X.; Li, D.; Sun, X. Hydrothermal synthesis of mixed crystal phases TiO2–reduced graphene oxide nanocomposites with small particle size for lithium ion batteries. Int. J. Hydrogen Energy 2014, 39, 16116–16122. [Google Scholar] [CrossRef]
- Yang, M.-C.; Lee, Y.-Y.; Xu, B.; Powers, K.; Meng, Y.S. TiO2 flakes as anode materials for Li-ion-batteries. J. Power Sources 2012, 207, 166–172. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Dai, Y.; Pei, X.-Y.; Lyu, S.-S.; Heng, Y.; Mo, D.-C. TiO2 nanorods anchor on reduced graphene oxide (R-TiO2/rGO) composite as anode for high performance lithium-ion batteries. Appl. Surf. Sci. 2019, 497, 143553. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Liu, Z.; Liu, H.K.; Guo, Z. TiO2 nanoparticles on nitrogen-doped graphene as anode material for lithium ion batteries. J. Nanopart. Res. 2013, 15, 1674. [Google Scholar] [CrossRef]
- Geng, C.; Yu, J.; Shi, F. Synthesis and electrochemical performance of TiO2/rGO composites with different layers of graphene oxide as anode materials for lithium-ion battery. IOP Conf. Ser. Mater. Sci. Eng. 2019, 585, 012090. [Google Scholar] [CrossRef]
- Mondal, A.; Maiti, S.; Singha, K.; Mahanty, S.; Panda, A.B. TiO2-rGO nanocomposite hollow spheres: Large scale synthesis and application as an efficient anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 23853–23862. [Google Scholar] [CrossRef]
- Shen, T.; Zhou, X.; Cao, H.; Zheng, C.; Liu, Z. TiO2(B)–CNT–graphene ternary composite anode material for lithium ion batteries. RSC Adv. 2015, 5, 22449–22454. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, R.; Liu, B.; Zhang, Y.; Zhu, K.; Yan, J.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries. J. Mater. Chem. A 2019, 7, 5363–5372. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, F.; Pervez, S.A.; Rehman, S.; Pope, M.A.; Fichtner, M.; Roberts, E.P. A stable TiO2–graphene nanocomposite anode with high rate capability for lithium-ion batteries. RSC Adv. 2020, 10, 29975–29982. [Google Scholar] [CrossRef]
- Subaşı, Y.; Somer, M.; Yağcı, M.B.; Slabon, A.; Afyon, S. Surface modified TiO2/reduced graphite oxide nanocomposite anodes for lithium ion batteries. J. Solid State Electrochem. 2020, 24, 1085–1093. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, P.; Ling, M.; Li, S.; Liu, P.; Zhao, H.; Zhang, S. Photocatalytic synthesis of TiO2 and reduced graphene oxide nanocomposite for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 3636–3642. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zeng, M.; Li, J.; Li, F. UV-assisted synthesis of surface modified mesoporous TiO2/G microspheres and its electrochemical performances in lithium ion batteries. Appl. Surf. Sci. 2017, 392, 897–903. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, X.; Li, B. Improving the anode performances of TiO2–carbon–rGO composites in lithium ion batteries by UV irradiation. New J. Chem. 2015, 39, 9345–9350. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J.; Liu, Y.; Li, H.; Wei, H.; Li, B.; Wang, X. Synthesis and superior anode performances of TiO2-carbon-rGO composites in lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4776–4780. [Google Scholar] [CrossRef]
- Madhusanka, S.A. TiO2 microparticles/reduced graphene oxide composite as anode material for lithium-ion battery. Int. J. Electrochem. Sci. 2020, 15, 2792–2805. [Google Scholar] [CrossRef]
- Bai, L.; Fang, F.; Zhao, Y.; Liu, Y.; Li, J.; Huang, G.; Sun, H. A sandwich structure of mesoporous anatase TiO2 sheets and reduced graphene oxide and its application as lithium-ion battery electrodes. RSC Adv. 2014, 4, 43039–43046. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, G.; Choi, J. RGO-coated TiO2 microcones for high-rate lithium-ion batteries. ACS Omega 2018, 3, 10205–10210. [Google Scholar] [CrossRef]
- Yu, W.-B.; Hu, Z.-Y.; Jin, J.; Yi, M.; Yan, M.; Li, Y.; Wang, H.-E.; Gao, H.-X.; Mai, L.-Q.; Hasan, T.; et al. Unprecedented and highly stable lithium storage capacity of (001) faceted nanosheet-constructed hierarchically porous TiO2/rGO hybrid architecture for high-performance Li-ion batteries. Natl. Sci. Rev. 2020, 7, 1046–1058. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, X.; Xu, Z.; Xia, Q.; Wan, L.; Liu, R.; Chen, G.; Chou, S. Ultrathin 2D mesoporous TiO2/rGO heterostructure for high-performance lithium storage. Small 2020, 16, 2000030. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Li, H.; Wang, X.; Nie, P.; Ding, B.; Xu, G.; Dou, H.; Zhang, X. A facile one-pot synthesis of TiO2/nitrogen-doped reduced graphene oxide nanocomposite as anode materials for high-rate lithium-ion batteries. Electrochim. Acta 2014, 133, 209–216. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Li, J.; Cao, L.; Qi, H.; Cheng, Y. N-doped TiO2/rGO hybrids as superior Li-ion battery anodes with enhanced Li-ions storage capacity. J. Alloys Compd. 2019, 784, 165–172. [Google Scholar] [CrossRef]
- Fang, R.; Miao, C.; Mou, H.; Xiao, W. Facile synthesis of Si@TiO2@rGO composite with sandwich-like nanostructure as superior performance anodes for lithium ion batteries. J. Alloys Compd. 2020, 818, 152884. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Ri, S.G.; Zhan, L.; Wang, Y.; Zhou, L.; Hu, J.; Liu, H. Li4Ti5O12/graphene nanostructure for lithium storage with high-rate performance. Electrochim. Acta 2013, 109, 389–394. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Yang, S.; Lu, X. In situ synthesis of high-loading Li4Ti5O12–graphene hybrid nanostructures for high rate lithium ion batteries. Nanoscale 2011, 3, 572–574. [Google Scholar] [CrossRef]
- Kim, H.-K.; Jegal, J.-P.; Kim, J.-Y.; Yoon, S.-B.; Roh, K.C.; Kim, K.-B. In situ fabrication of lithium titanium oxide by microwave-assisted alkalization for high-rate lithium-ion batteries. J. Mater. Chem. A 2013, 1, 14849. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, H.; Hu, Y.; Dai, Y.; Li, C. Mesoporous single crystals Li4Ti5O12 grown on rGO as high-rate anode materials for lithium-ion batteries. Chem. Commun. 2014, 50, 8856–8859. [Google Scholar] [CrossRef]
- Ni, H.; Song, W.-L.; Fan, L.-Z. A strategy for scalable synthesis of Li4Ti5O12/reduced graphene oxide toward high rate lithium-ion batteries. Electrochem. Commun. 2014, 40, 1–4. [Google Scholar] [CrossRef]
- Di, S.; Li, J.; Zhao, Y.; Hou, L.; Ma, Z.; Qin, X.; Shao, G. Hierarchical microspheres assembled from Li4Ti5O12-TiO2 nanosheets with advanced lithium ion storage. Ionics 2020, 26, 2763–2772. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Zhang, H.; Wang, X.; Li, G.; Wang, Y.; Jiao, L.; Yuan, H. Small amount of reduce graphene oxide modified Li4Ti5O12 nanoparticles for ultrafast high-power lithium ion battery. J. Power Sources 2015, 278, 693–702. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Wu, J.; Yao, J. Graphene oxide-confined synthesis of Li4Ti5O12 microspheres as high-performance anodes for lithium ion batteries. Electrochim. Acta 2015, 165, 422–429. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, M.-S.; Kim, Y.-H.; Roh, K.C.; Kim, K.-B. High-rate Li4Ti5O12/N-doped reduced graphene oxide composite using cyanamide both as nanospacer and a nitrogen doping source. J. Power Sources 2016, 336, 376–384. [Google Scholar] [CrossRef]
- Cao, N.; Wen, L.; Song, Z.; Meng, W.; Qin, X. Li4Ti5O12/reduced graphene oxide composite as a high-rate anode material for lithium ion batteries. Electrochim. Acta 2016, 209, 235–243. [Google Scholar] [CrossRef]
- Ogechi, O.; Hao, T.; Osgood, H.; Zhang, B.; Chen, L.; Cui, L.; Song, X.-M.; Ogoke, O.; Wu, G. Advanced mesoporous spinel Li4Ti5O12/rGO composites with increased surface lithium storage capability for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 9162–9169. [Google Scholar] [CrossRef]
- Meng, T.; Yi, F.; Cheng, H.; Hao, J.; Shuab, D.; Zhao, S.; He, C.; Song, X.; Zhang, F. Preparation of lithium titanate/reduced graphene oxide composites with three-dimensional “fishnet-like” conductive Structure via a gas-foaming method for high-rate lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 42883–42892. [Google Scholar] [CrossRef]
- Xiao, H.; Pender, J.P.; Meece-Rayle, M.A.; De Souza, J.P.; Klavetter, K.C.; Ha, H.; Lin, J.; Heller, A.; Ellison, C.J.; Mullins, C.B. Reduced-graphene oxide/poly(acrylic acid) aerogels as a three-dimensional replacement for metal-foil current collectors in lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 22641–22651. [Google Scholar] [CrossRef]
- Pender, J.P.; Xiao, H.; Dong, Z.; Cavallaro, K.A.; Weeks, J.A.; Heller, A.; Ellison, C.J.; Mullins, C.B. Compact lithium-ion battery electrodes with lightweight reduced graphene oxide/poly(acrylic acid) current collectors. ACS Appl. Energy Mater. 2018, 2, 905–912. [Google Scholar] [CrossRef]
- Ha, H.; Shanmuganathan, K.; Ellison, C.J. Mechanically stable thermally crosslinked poly(acrylic acid)/reduced graphene oxide aerogels. ACS Appl. Mater. Interfaces 2015, 7, 6220–6229. [Google Scholar] [CrossRef]
- Zhang, F.; Yiab, F.; Meng, T.; Gao, A.; Shuab, D.; Chen, H.; Cheng, H.; Zhou, X. In situ supramolecular self-assembly assisted synthesis of Li4Ti5O12–carbon-reduced graphene oxide microspheres for lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 7, 916–924. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Li, Y.; Chen, Y.; Luo, T. Electrochemical performance of Li4Ti5O12/carbon nanotubes/graphene composite s an anode material in lithium-ion batteries. Int. J. Hydrogen Energy 2017, 42, 7195–7201. [Google Scholar] [CrossRef]
- Roh, H.-K.; Lee, G.-W.; Haghighat-Shishavan, S.; Chung, K.Y.; Kim, K.-B. Polyol-mediated carbon-coated Li4Ti5O12 nanoparticle/graphene composites with long-term cycling stability for lithium and sodium ion storages. Chem. Eng. J. 2020, 385, 123984. [Google Scholar] [CrossRef]
- Uceda, M.; Chiu, H.-C.; Gauvin, R.; Zaghib, K.; Demopoulos, G.P. Electrophoretically co-deposited Li4Ti5O12/reduced graphene oxide nanolayered composites for high-performance battery application. Energy Storage Mater. 2020, 26, 560–569. [Google Scholar] [CrossRef]
- Mitra, S.; Poizot, P.; Finke, A.; Tarascon, J.-M. Growth and electrochemical characterization versus lithium of Fe3O4 electrodes made by electrodeposition. Adv. Funct. Mater. 2006, 16, 2281–2287. [Google Scholar] [CrossRef]
- Zhu, S.P.; Fan, L.; Lu, Y.Y. Highly uniform Fe3O4 nanoparticle-rGO composites as anode materials for high performance lithium-ion batteries. RSC Adv. 2017, 7, 54939–54946. [Google Scholar] [CrossRef]
- Su, Q.; Xie, D.; Zhang, J.; Du, G.; Xu, B. In situ transmission electron microscopy observation of the coversion mechanism of Fe2O3/graphene anode during lithiation-delithiation processes. ACS Nano 2013, 7, 9115–9121. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Wang, K.; Li, H.-H.; Wang, H.-F.; Li, X.-Y.; Wu, X.-L.; Zhang, J.-P.; Xie, H.-M.; Su, Z.-M.; Wang, J.; et al. Fe3O4 nanoflakes-RGO composites: A high rate anode material for lithium-ion batteries. Appl. Surf. Sci. 2020, 511, 145465. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Xiang, H.; Li, Z.; Yang, W.; Wang, H. Enhanced cycling performance of Fe3O4-graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Zhu, S.-P.; Wu, T.; Su, H.-M.; Qu, S.-S.; Xie, Y.-J.; Chen, M.; Diao, G. Hydrothermal synthesis of Fe3O4/rGO nanocomposites as anode materials for lithium ion batteries. Acta Physico Chimica Sin. 2016, 32, 2737–2744. [Google Scholar] [CrossRef]
- Ji, L.; Tan, Z.; Kuykendall, T.R.; Aloni, S.; Xun, S.; Lin, E.; Battaglia, V.; Zhang, Y. Fe3O4 nanoparticle-integrated graphene sheets for high-performance half and full lithium ion cells. Phys. Chem. Chem. Phys. 2011, 13, 7170–7177. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Tan, W.K.; Asami, K.; Maegawa, K.; Kumar, R.; Kawamura, G.; Muto, H.; Matsuda, A. Fe3O4-embedded rGO composites as anode for rechargeable FeOx-air batteries. Mater. Today Commun. 2020, 25, 101540. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-assembled and one-step synthesis of interconnected 3D network of Fe3O4/reduced graphene oxide nanosheets hybrid for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Chen, D.; Zhao, J. A facile electrophoretic deposition route to the Fe3O4/CNTs/rGO composite electrode as a binder-free anode for lithium ion battery. ACS Appl. Mater. Interfaces 2016, 8, 26730–26739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Wang, Y.; Tian, W.; Liu, J.; Zang, J.; Ning, H.; Yang, C.; Wu, M. Novel in-situ redox synthesis of Fe3O4/rGO composites with superior electrochemical performance for lithium-ion batteries. Electrochim. Acta 2018, 262, 233–240. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Pratheeksha, P.M.; Anandan, S.; Rangappa, D.; Gopalan, R.; Rao, T.N. Efficient reduced graphene oxide grafted porous Fe3O4 composite as a high performance anode material for Li-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 5284–5294. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-L.; Liu, Y.; Bao, R.-Y.; Luo, Y.; Yangb, W.; Xie, B.; Yang, M.-B. Effects of Fe3O4 loading on the cycling performance of Fe3O4/rGO composite anode material for lithium ion batteries. J. Alloys Compd. 2016, 678, 80–86. [Google Scholar] [CrossRef]
- Wang, H.; Kalubowilage, M.; Bossmann, S.H.; Amama, P.B. Design of highly porous Fe3O4@reduced graphene oxide via a facile PMAA-induced assembly. RSC Adv. 2019, 9, 27927–27936. [Google Scholar] [CrossRef]
- Xiao, Z.C.; Li, Y.; Liang, C.L.; Bao, R.Y.; Yang, M.B.; Yang, W. Scalable synthesis of an artificial polydopamine solid-electrolyte-interface-assisted 3D rGO/Fe3O4@PDA hydrogel for a highly stable anode with enhanced lithium-ion-storage properties. ChemElectroChem 2019, 6, 1069–1077. [Google Scholar] [CrossRef]
- Qi, H.; Cao, L.; Li, J.; Huang, J.; Ma, M.; Cheng, Y.; Wang, C.; Dang, H. Rice crust-like Fe3O4@C/rGO with improved extrinsic pseudocapacitance for high-rate and long-life Li-ion anodes. J. Alloys Compd. 2019, 804, 57–64. [Google Scholar] [CrossRef]
- Khan, A.J.; Javed, M.S.; Hanif, M.; Abbas, Y.; Liao, X.; Ahmed, G.; Saleem, M.; Yun, S.; Liu, Z. Facile synthesis of a novel Fe3O4-rGO-MoO3 ternary nano-composite for high-performance hybrid energy storage applications. Ceram. Int. 2020, 46, 3124–3131. [Google Scholar] [CrossRef]
- Pei, M.; Wu, Y.; Qi, Z.; Mei, D. Synthesis and electrochemical performance of NiO/Fe3O4/rGO as anode material for lithium ion battery. Ionics 2020, 26, 3831–3840. [Google Scholar] [CrossRef]
- Xiao, Z.-C.; Li, Y.; Liang, C.-L.; Bao, R.-Y.; Yang, M.-B.; Yangb, W. Driven by electricity: Multilayered GO- Fe3O4@PDA-PAM flake assembled micro flower-like anode for high-performance lithium ion batteries. Appl. Surf. Sci. 2020, 499, 143934. [Google Scholar] [CrossRef]
- Gao, R.; Wang, S.; Xu, Z.; Li, H.; Chen, S.; Hou, H. High-performance anode materials with superior structure of Fe3O4/FeS/rGO composite for lithium ion batteries. Nano 2020, 15, 2050128. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, R.; Liu, H. Carbon layer encapsulated Fe3O4@reduced graphene oxide lithium battery anodes with long cycle performance. Ceram. Int. 2020, 46, 12732–12739. [Google Scholar] [CrossRef]
- Liang, Y.; Lu, W. Gamma-irradiation synthesis of Fe3O4/rGO nanocomposites as lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2020, 31, 17075–17083. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Q.; Qinglin, D.; Jiang, K.; Zhang, J.; Hu, Z.; Chu, J.; Wang, J.; Deng, Q.; Qinglin, D.; et al. Copper ferrites@reduced graphene oxide anode materials for advanced lithium storage applications. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Gao, T.; Xu, C.; Li, R.; Zhang, R.; Wang, B.; Jiang, X.; Hu, M.; Bando, Y.; Kong, D.; Dai, P.; et al. Biomass-derived carbon paper to sandwich magnetite anode for long-life Li-ion battery. ACS Nano 2019, 13, 11901–11911. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, H.; Dong, T.; Shen, P.; Cai, Y.; Zhang, H.; Zhang, S. Improved electrochemical performance in nanoengineered pomegranate-shaped Fe3O4/RGO nanohybrids anode material. J. Mater. Sci. 2016, 52, 3233–3243. [Google Scholar] [CrossRef]
- Lee, K.K.; Deng, S.; Fan, H.M.; Mhaisalkar, S.; Tan, H.R.; Tok, E.S.; Loh, K.P.; Chin, W.S.; Sow, C.H. α-Fe2O3 nanotubes-reduced graphene oxide composites as synergistic electrochemical capacitor materials. Nanoscale 2012, 4, 2958–2961. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Wang, Y.-X.; Chou, S.-L.; Li, H.-J.; Liu, H.-K.; Wang, J.-Z. Rapid synthesis of α-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Padhi, D.K.; Parida, K.M. Fabrication of α-Fe2O3 nanorod/RGO composite: A novel hybrid photocatalyst for phenol degradation. ACS Appl. Mater. Interfaces 2013, 5, 9101–9110. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yin, Y.-X.; Wang, Y.-Q.; Yan, Y.; Guo, Y.-G.; Song, W. Layer structured α-Fe2O3 nanodisk/reduced graphene oxide composites as high-performance anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3932–3936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, T.; Chu, H.; Niu, L.; Sun, Z.; Pan, L.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Wang, G.; Liu, T.; Luo, Y.; Zhao, Y.; Ren, Z.; Bai, J.; Wang, H. Preparation of Fe2O3/graphene composite and its electrochemical performance as an anode material for lithium ion batteries. J. Alloys Compd. 2011, 509, L216–L220. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Nanda, J.; Braun, P.V. Three-dimensionally mesostructured Fe2O3 electrodes with good rate performance and reduced voltage hysteresis. Chem. Mater. 2015, 27, 2803–2811. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Xie, K.; Li, Y.; Xu, J.; Zheng, C. A facile one-step hydrothermal synthesis of α-Fe2O3 nanoplates imbedded in graphene networks with high-rate lithium storage and long cycle life. J. Mater. Chem. A 2014, 2, 13942–13948. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, S.; Lee, W.; Yoon, Y.S. Hollow nanobarrels of α-Fe2O3 on reduced graphene oxide as high-performance anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 2027–2034. [Google Scholar] [CrossRef]
- Xu, B.; Guan, X.; Zhang, L.Y.; Liu, X.; Jiao, Z.; Liu, X.; Hu, X.; Zhao, X.S. A simple route to preparing γ-Fe2O3/RGO composite electrode materials for lithium ion batteries. J. Mater. Chem. A 2018, 6, 4048–4054. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, H.; Gao, B.; Wang, Y.; Li, K.; Sun, Y.; Yin, J.; Kan, J. In-situ synthesis of Fe2O3/rGO using different hydrothermal methods as anode materials for lithium-ion batteries. Rev. Adv. Mater. Sci. 2020, 59, 477–487. [Google Scholar] [CrossRef]
- Petnikota, S.; Marka, S.; Banerjee, A.; Reddy, M.; Srikanth, V.; Chowdari, B.; Reddy, M.V. Graphenothermal reduction synthesis of ‘exfoliated graphene oxide/iron (II) oxide’ composite for anode application in lithium ion batteries. J. Power Sources 2015, 293, 253–263. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358. [Google Scholar] [CrossRef]
- Qi, H.; Cao, L.; Li, J.; Huang, J.; Xu, Z.; Cheng, Y.; Kong, X.; Yanagisawa, K. High Pseudocapacitance in FeOOH/rGO composites with superior performance for high rate anode in Li-ion battery. ACS Appl. Mater. Interfaces 2016, 8, 35253–35263. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xie, Y. Promising vanadium oxide and hydroxide nanostructures: From energy storage to energy saving. Energy Environ. Sci. 2010, 3, 1191–1206. [Google Scholar] [CrossRef]
- Murphy, D.W.; Christian, P.A.; Disalvo, F.J.; Carides, J.N.; Waszczak, J.V. Lithium Incorporation by V6O13 and Related Vanadium (+4, +5) Oxide Cathode Materials. J. Electrochem. Soc. 1981, 128, 2053–2060. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Bai, Z.; Lin, L.; Chen, G.; Song, X.; Xiong, D.; Li, D.; Sun, X. Superior sodium storage of novel VO2 nano-microspheres encapsulated into crumpled reduced graphene oxide. J. Mater. Chem. A 2017, 5, 4850–4860. [Google Scholar] [CrossRef]
- Li, S.; Wei, Z.; Tang, Y.; Qian, K.; Li, Y.; Ji, S.; Luo, H.; Gao, Y.; Jin, P. Synthesis of reduced graphene oxide carried VO2(B) Nanorods and their application in lithium-ion batteries. Sci. Adv. Mater. 2014, 6, 1293–1297. [Google Scholar] [CrossRef]
- He, G.; Li, L.; Manthiram, A. VO2/rGO nanorods as a potential anode for sodium- and lithium-ion batteries. J. Mater. Chem. A 2015, 3, 14750–14758. [Google Scholar] [CrossRef]
- Nethravathi, C.; Viswanath, B.; Michael, J.; Rajamath, M. Hydrothermal synthesis of a monoclinic VO2 nanotube–graphene hybrid for use as cathode material in lithium ion batteries. Carbon 2012, 50, 4839–4846. [Google Scholar] [CrossRef]
- Song, H.J.; Choi, M.; Kim, J.-C.; Park, S.; Lee, C.W.; Hong, S.-H.; Kim, B.-K.; Kim, D.-W. Enhanced lithium storage in reduced graphene oxide-supported M-phase vanadium(IV) dioxide nanoparticles. Sci. Rep. 2016, 6, 30202. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Uniform decoration of vanadium oxide nanocrystals on reduced graphene-oxide balls by an aerosol process for lithium-ion battery cathode material. Chem. A Eur. J. 2014, 20, 6294–6299. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, S.-B.; Lv, L.-P.; Lieberwirth, I.; Zhang, L.-C.; Ding, C.-X.; Chen, C.-H. A composite film of reduced graphene oxide modified vanadium oxide nanoribbons as a free standing cathode material for rechargeable lithium batteries. J. Power Sources 2013, 241, 168–172. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, Z.; Wang, H.; Tang, Y. Synthesis and electrochemical properties of VO2/rGO composite for high-performance anode materials for Li-ion batteries. J. Funct. Mater. 2017, 48, 9011–9017. [Google Scholar]
- Shi, Y.; Chou, S.L.; Wang, J.Z.; Li, H.J.; Liu, H.K.; Wu, Y.P. In situ hydrothermal synthesis of graphene woven VO2 nanoribbons with improved cycling performance. J. Power Sources 2013, 244, 684–689. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, J.; Zhang, D.; Fang, Y.; Hu, F.; Zhu, K. VO2(B) nanobelts and reduced graphene oxides composites as cathode materials for low-cost rechargeable aqueous zinc ion batteries. Chem. Eng. J. 2020, 390, 124118. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, L.; Xing, S.; Luo, J.; Xu, J. Vanadium oxides–reduced graphene oxide composite for lithium-ion batteries and supercapacitors with improved electrochemical performance. J. Power Sources 2013, 222, 21–31. [Google Scholar] [CrossRef]
- Lee, M.; Wee, B.-H.; Hong, J.-D. High Performance flexible supercapacitor electrodes composed of ultralarge graphene sheets and vanadium dioxide. Adv. Energy Mater. 2015, 5, 1401890. [Google Scholar] [CrossRef]
- Xiao, X.; Li, S.; Wei, H.; Sun, D.; Wu, Y.; Jin, G.; Wang, F.; Zou, Y. Synthesis and characterization of VO2(B)/graphene nanocomposite for supercapacitors. J. Mater. Sci. Mater. Electron. 2015, 26, 4226–4233. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Wang, Y.; Cai, S.; Yang, H.; Yu, H.; Shi, X.; Huang, C.; Liu, X.; Peng, Y. VO₂(B)/graphene composite-based symmetrical supercapacitor electrode via screen printing for intelligent packaging. Nanomaterials 2018, 8, 1020. [Google Scholar] [CrossRef]
- Sahoo, R.; Lee, T.H.; Pham, D.T.; Luu, T.H.T.; Lee, Y.H. Fast-charging high-energy battery–supercapacitor hybrid: Anodic reduced graphene oxide–vanadium(IV) oxide sheet-on-sheet heterostructure. ACS Nano 2019, 13, 10776–10786. [Google Scholar] [CrossRef]
- Lv, W.; Yang, C.; Meng, G.; Zhao, R.; Han, A.; Wang, R.; Liu, J. VO2(B) nanobelts/reduced graphene oxide composites for high-performance flexible all-solid-state supercapacitors. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, C.; Rajamathi, C.R.; Rajamathi, M.; Gautam, U.K.; Wang, X.; Golberg, D.; Bando, Y. N-Doped graphene–VO2(B) nanosheet-built 3D flower hybrid for lithium ion battery. ACS Appl. Mater. Interfaces 2013, 5, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.S.; Ravichandran, D.; Rambabu, B.; Holze, R. Carbon and functionalized graphene oxide coated vanadium oxide electrodes for lithium ion batteries. Appl. Surf. Sci. 2014, 305, 596–602. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, S.K.; Nakhanivej, P.; Shin, K.H.; Yeon, J.S.; Park, H.S. Mesoporous VO2(B) nanorods deposited onto graphene architectures for enhanced rate capability and cycle life of Li ion battery cathodes. J. Alloys Compd. 2020, 855, 157361. [Google Scholar] [CrossRef]
- Song, Z.; Lu, X.; Hu, Q.; Lin, D.; Zheng, Q. Construction of reduced graphene oxide wrapped yolk–shell vanadium dioxide sphere hybrid host for high-performance lithium–sulfur batteries. Dalton Trans. 2020, 49, 14921–14930. [Google Scholar] [CrossRef] [PubMed]
- Mahadi, N.B.; Park, J.-S.; Park, J.-H.; Chung, K.Y.; Yi, S.Y.; Sun, Y.-K.; Myung, S.-T. Vanadium dioxide−reduced graphene oxide composite as cathode materials for rechargeable Li and Na batteries. J. Power Sources 2016, 326, 522–532. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, Y.; Ren, Z.; Yu, P.; Zhao, D.; Zhou, W.; Wang, L.; Fu, H. In situ carbon-coated yolk–shell V2O3 microspheres for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 1595–1601. [Google Scholar] [CrossRef]
- Song, H.J.; Choi, M.; Kim, J.-C.; Park, S.; Lee, C.W.; Hong, S.-H.; Kim, D.-W. Li-electroactivity of thermally-reduced V2O3 nanoparticles. Mater. Lett. 2016, 180, 243–246. [Google Scholar] [CrossRef]
- Leng, J.; Mei, H.; Zhan, L.; Wang, Y.; Yang, S.; Song, Y. V2O3 nanoparticles anchored onto the reduced graphene oxide for superior lithium storage. Electrochim. Acta 2017, 231, 732–738. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, B.; Tang, L.-B.; An, C.-S.; He, Z.-J.; Tong, H.; Yu, W.-J.; Zheng, J. V2O3/rGO composite as a potential anode material for lithium ion batteries. Ceram. Int. 2018, 44, 15044–15049. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Liang, S.; Chen, T.; Tang, Y.; Tan, X. Reduced graphene oxide modified V2O3 with enhanced performance for lithium-ion battery. Mater. Lett. 2014, 137, 174–177. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Li, G.; Xue, C.; Ding, J.; Li, B.; Chen, D.; Li, L. In situ synthesis of V2O3 nanorods anchored on reduced graphene oxide as high-performance lithium ion battery anode. ChemistrySelect 2018, 3, 12108–12112. [Google Scholar] [CrossRef]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Massot, M.; Julien, C.M. Surface analysis of pulsed laser-deposited V2O5 thin films and their lithium intercalated products studied by Raman spectroscopy. Surf. Interface Anal. 2005, 37, 406–411. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, G.; Zhang, Y.; Zhou, J.; Pan, A. Controllable preparation of V2O5/graphene nanocomposites as cathode materials for lithium-ion batteries. Nanoscale Res. Lett. 2016, 11, 549. [Google Scholar] [CrossRef]
- Pandey, G.P.; Liu, T.; Brown, E.; Yang, Y.; Li, Y.; Sun, X.S.; Fang, Y.; Li, J.; Brown, J.E. Mesoporous hybrids of reduced graphene oxide and vanadium pentoxide for enhanced performance in lithium-ion batteries and electrochemical capacitors. ACS Appl. Mater. Interfaces 2016, 8, 9200–9210. [Google Scholar] [CrossRef]
- Luo, W.; Zheng, B. Improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by double-layer coating with graphene oxide and V2O5 for lithium-ion batteries. Appl. Surf. Sci. 2017, 404, 310–317. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.-F.; Liu, Y.; Zhang, H.; Ren, Y.; Sun, C.-J.; Lu, W.; Zhou, Y.; Stanciu, L.; Stach, E.A.; et al. Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries. Nat. Commun. 2015, 6, 6127. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, B.; Xin, H.L.; Yang, G.; Cai, H.-Q.; Nie, F.; Huang, H. Self-assembled V2O5 nanosheets/reduced graphene oxide hierarchical nanocomposite as a high-performance cathode material for lithium ion batteries. J. Mater. Chem. A 2013, 1, 10814–10820. [Google Scholar] [CrossRef]
- Rui, X.; Zhu, J.; Sim, D.; Xu, C.; Zeng, Y.; Hng, H.H.; Lim, T.M.; Yan, Q. Reduced graphene oxide supported highly porous V2O5 spheres as a high-power cathode material for lithium ion batteries. Nanoscale 2011, 3, 4752–4758. [Google Scholar] [CrossRef]
- Hu, B.; Xiang, Q.; Cen, Y.; Li, S.; Liu, L.; Yu, D.; Chen, C. In situ constructing flexible V2O5@GO composite thin film electrode for superior electrochemical energy storage. J. Electrochem. Soc. 2018, 165, A3738–A3747. [Google Scholar] [CrossRef]
- Pham-Cong, D.; Ahn, K.; Hong, S.W.; Jeong, S.; Kim, D.-H.; Doh, C.; Jin, J.; Jeong, E.; Cho, C.R. Cathodic performance of V2O5 nanowires and reduced graphene oxide composites for lithium ion batteries. Curr. Appl. Phys. 2014, 14, 215–221. [Google Scholar] [CrossRef]
- Kurc, B.; Wysokowski, M.; Rymaniak, L.; Lijewski, P.; Piasecki, A.; Fuc, P. The Impact of the vanadium oxide addition on the physicochemical performance stability and intercalation of lithium ions of the TiO2-rGO-electrode in lithium ion batteries. Materials 2020, 13, 1018. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Quan, H.; Luo, S.; Luo, X.; Luoab, X.; Jiang, H. Reduced graphene oxide enwrapped vanadium pentoxide nanorods as cathode materials for lithium-ion batteries. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 231–237. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Bai, Z.; Zhao, Y.; Dong, L.; Song, X.; Li, D.; Langford, C.; Sun, X. Crumpled reduced graphene oxide conformally encapsulated hollow V2O5 nano/microsphere achieving brilliant lithium storage performance. Nano Energy 2016, 24, 32–44. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, K.; Yang, L.; Li, H.; Wang, S.; Tang, S.; Zhang, M.; Abliz, A.; Zhao, F. Synthesis and study of V2O5/rGO nanocomposite as a cathode material for aqueous zinc ion battery. Ionics 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Du, X.; Huang, G.; Qin, Y.-L.; Wang, L. Solvothermal synthesis of GO/V2O5 composites as a cathode material for rechargeable magnesium batteries. RSC Adv. 2015, 5, 76352–76355. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Zhang, X.; Huang, S.; Jiang, S.; Zhu, Q.; Sun, H.; Zakharova, G.S. Self-assembled V3O7/graphene oxide nanocomposites as cathode material for lithium-ion batteries. Int. J. Nanotechnol. 2014, 11, 808. [Google Scholar] [CrossRef]
- Ramadoss, A.; Saravanakumar, B.; Kim, S.-J. Vanadium pentoxide/reduced graphene oxide composite as an efficient electrode material for high-performance supercapacitors and self-powered systems. Energy Technol. 2015, 3, 913–924. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, A.; Wang, C.; Wang, H.; Shen, Y.; Tian, X. Bifunctional reduced graphene oxide/V2O5 composite hydrogel: Fabrication, high performance as electromagnetic wave absorbent and supercapacitor. ChemPhysChem 2013, 15, 366–373. [Google Scholar] [CrossRef]
- Foo, C.Y.; Sumboja, A.; Tan, D.J.H.; Wang, J.; Lee, P.S. Flexible and highly scalable V2O5-rGO electrodes in an organic electrolyte for supercapacitor devices. Adv. Energy Mater. 2014, 4, 1400236. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xiong, Z.; Hu, Y.; Song, W.; Huang, Q.-A.; Cheng, X.; Chen, L.-Q.; Sun, C.; Gu, H. V2O5 nanowire composite paper as a high-performance lithium-ion battery cathode. ACS Omega 2017, 2, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, G.; Yin, P.; Ruan, C.; Ai, K. Controlling the formation of rodlike V2O5 nanocrystals on reduced graphene oxide for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 11462–11470. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, G.; Kang, L.; Lei, Z.; Liu, C.; Liu, Z. Graphene/VO2 hybrid material for high performance electrochemical capacitor. Electrochim. Acta 2013, 112, 448–457. [Google Scholar] [CrossRef]
- Fu, M.; Ge, C.; Hou, Z.; Cao, J.; He, B.; Zeng, F.; Kuang, Y. Graphene/vanadium oxide nanotubes composite as electrode material for electrochemical capacitors. Phys. B Condens. Matter 2013, 421, 77–82. [Google Scholar] [CrossRef]
- Wang, H.; Yi, H.; Chen, X.; Wang, X. One-step strategy to three-dimensional graphene/VO2 nanobelt composite hydrogels for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 1165–1173. [Google Scholar] [CrossRef]
- Perera, S.D.; Liyanage, A.D.; Nijem, N.; Ferraris, J.P.; Chabal, Y.J.; Balkus, J.K.J. Vanadium oxide nanowire—Graphene binder free nanocomposite paper electrodes for supercapacitors: A facile green approach. J. Power Sources 2013, 230, 130–137. [Google Scholar] [CrossRef]
- Li, H.; Wei, J.; Qian, Y.; Zhang, J.; Yu, J.; Wang, G.; Xu, G. Effects of the graphene content and the treatment temperature on the supercapacitive properties of VOx/graphene nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2014, 449, 148–156. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. Fabrication of two-dimensional reduced graphene oxide supported V2O5 networks and their application in supercapacitors. Mater. Chem. Phys. 2016, 170, 266–275. [Google Scholar] [CrossRef]
- Govindarajan, D.; Shankar, V.U.; Gopalakrishnan, R. Supercapacitor behavior and characterization of RGO anchored V2O5 nanorods. J. Mater. Sci. Mater. Electron. 2019, 30, 16142–16155. [Google Scholar] [CrossRef]
- Zhang, B.; Han, Y.-D.; Zheng, J.; Zhang, J.-F.; Shen, C.; Ming, L.; Yuan, X.-B.; Li, H. VOPO4 nanosheets as anode materials for lithium-ion batteries. Chem. Commun. 2014, 50, 11132–11134. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Bai, Y.; Zhang, J.; Kang, L.; Lei, Z.; Liu, Z. Vanadyl phosphate/reduced graphene oxide nanosheet hybrid material and its capacitance. Electrochim. Acta 2015, 178, 312–320. [Google Scholar] [CrossRef]
- Lu, W.; Cong, L.; Liu, Y.; Liu, J.; Mauger, A.; Julien, C.M.; Sun, L.; Xie, H. Pseudocapacitance controlled fast-charging and long-life lithium ion battery achieved via a 3D mutually embedded VPO4/rGO electrode. J. Alloys Compd. 2020, 812, 152135. [Google Scholar] [CrossRef]
- Yao, Z.; Kim, S.; Aykol, M.; Li, Q.; Wu, J.; He, J.; Wolverton, C. Revealing the conversion mechanism of transition metal oxide electrodes during lithiation from first-principles. Chem. Mater. 2017, 29, 9011–9022. [Google Scholar] [CrossRef]
- Kumar, N.; Huang, C.-W.; Yen, P.-J.; Wu, W.-W.; Wei, K.-H.; Tseng, T.Y. Probing the electrochemical properties of an electrophoretically deposited Co3O4/rGO/CNTs nanocomposite for supercapacitor applications. RSC Adv. 2016, 6, 60578–60586. [Google Scholar] [CrossRef]
- Bankole, O.M.; Olaseni, S.E.; Adeyemo, M.A.; Ogunlaja, A.S. Microwave-assisted synthesis of cobalt oxide/reduced graphene oxide (Co3O4–rGo) composite and its sulfite enhanced photocatalytic degradation of organic Dyes. Z. Phys. Chem. 2020, 234, 1681–1708. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Y. Microwave-assisted synthesis of a Co3O4–graphene sheet-on-sheet nanocomposite as a superior anode material for Li-ion batteries. J. Mater. Chem. 2010, 20, 9735–9739. [Google Scholar] [CrossRef]
- Yang, X.; Fan, K.; Zhu, Y.; Shen, J.; Jiang, X.; Zhao, P.; Li, C. Tailored graphene-encapsulated mesoporous Co3O4 composite microspheres for high-performance lithium ion batteries. J. Mater. Chem. 2012, 22, 17278–17283. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Y.; Xiao, N.; Hng, H.H.; Yan, Q. One-step electrochemical preparation of graphene-based heterostructures for Li storage. J. Mater. Chem. 2012, 22, 8455–8461. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, H.; Shen, W.; Dong, X.; Xu, J. Surfactant-assisted synthesis of a Co3O4/reduced graphene oxide composite as a superior anode material for Li-ion batteries. J. Mater. Chem. A 2013, 1, 7159–7166. [Google Scholar] [CrossRef]
- Lou, Y.; Liang, J.; Peng, Y.; Chen, J. Ultra-small Co3O4 nanoparticles–reduced graphene oxide nanocomposite as superior anodes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 8885–8893. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Jung, J.-W.; Kim, C.; Kim, I.-D. Rational design of 1-D Co3O4 nanofibers@low content graphene composite anode for high performance Li-ion batteries. Sci. Rep. 2017, 7, srep45105. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, T.; Gunawardhana, N.; Senthil, C.; Kundu, M.; Maduraiveeran, G.; Yoshio, M.; Sasidharan, M. Fabrication of hollow Co3O4 nanospheres and their nanocomposites of CNT and rGO as high-performance anodes for lithium-ion batteries. ChemistrySelect 2018, 3, 5502–5511. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.-K.; Roh, K.C.; Kim, K.-B. Co3O4-reduced graphene oxide nanocomposite synthesized by microwave-assisted hydrothermal process for Li-ion batteries. Electron. Mater. Lett. 2015, 11, 282–287. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, T.H.; Cha, J.H.; Jang, H.W.; Choi, J.-W.; Mahmoudi, M.; Shokouhimehr, M. Metal-organic framework-derived metal oxide nanoparticles@reduced graphene oxide composites as cathode materialf for rechargeable aluminum-ion batteries. Sci. Rep. 2019, 9, 13739. [Google Scholar] [CrossRef]
- Mussa, Y.; Ahmed, F.; Arsalan, M.; Alsharaeh, E.H. Two dimensional (2D) reduced graphene oxide (RGO)/hexagonal boron nitride (h-BN) based nanocomposites as anodes for high temperature rechargeable lithium-ion batteries. Sci. Rep. 2020, 10, 1882. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co₃O₄ nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Chen, Z.; Liu, H.K.; Jia, D.; Guo, Z. Enhanced rate performance of cobalt oxide/nitrogen doped graphene composite for lithium ion batteries. RSC Adv. 2013, 3, 5003–5008. [Google Scholar] [CrossRef][Green Version]
- Lee, J.S.; Jo, M.S.; Saroha, R.; Jung, D.S.; Seon, Y.H.; Lee, J.S.; Kang, Y.C.; Kang, D.-W.; Cho, J.S. Hierarchically well-developed porous graphene nanofibers comprising N-doped graphitic C-coated cobalt oxide hollow nanospheres as anodes for high-rate Li-ion batteries. Small 2020, 16, 2002213. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Kang, Y.C. Novel cobalt oxide-nanobubble-decorated reduced graphene oxide sphere with superior electrochemical properties prepared by nanoscale Kirkendall diffusion process. Nano Energy 2015, 17, 17–26. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Ultrathin CoO/graphene hybrid nanosheets: A highly stable anode material for lithium-ion batteries. J. Phys. Chem. C 2012, 116, 20794–20799. [Google Scholar] [CrossRef]
- Fu, G.; Chang, K.; Shangguan, E.; Tang, H.; Li, B.; Chang, Z.; Yuan, X.-Z.; Wang, H. Synthesis of CoO/reduced graphene oxide composite as an alternative additive for the nickel electrode in alkaline secondary batteries. Electrochim. Acta 2015, 180, 373–381. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Yeh, M.-H.; Chou, T.-C.; Chang, P.-Y.; Andoongc, R.-. Ultrafine CoO Embedded reduced graphene oxide nanocomposites: A high rate anode for Li-ion battery. ChemistrySelect 2016, 1, 5758–5767. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Chang, P.-Y.; Yeh, M.-H.; Andoongc, R.-. Ultra-small CoO nanocrystals anchored on reduced graphene oxide for enhanced lithium storage in lithium ion batteries. MRS Commun. 2017, 7, 236–244. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, D.; Liu, X.; Yu, P.; Fu, H. Hydrothermal for synthesis of CoO nanoparticles/graphene composite as Li-ion battery anodes. Acta Chim. Sin. 2017, 75, 231. [Google Scholar] [CrossRef][Green Version]
- Sun, L.; Deng, Q.; Li, Y.; Mi, H.; Wang, S.; Deng, L.; Ren, X.; Zhang, P. CoO-Co3O4 heterostructure nanoribbon/RGO sandwich-like composites as anode materials for high performance lithium-ion batteries. Electrochim. Acta 2017, 241, 252–260. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Q.; Li, J.; Huang, J.; Cheng, Y. Assembly control of CoO/reduced graphene oxide composites for their enhanced lithium storage behavior. Appl. Surf. Sci. 2018, 455, 96–105. [Google Scholar] [CrossRef]
- Leng, X.; Ding, X.; Hu, J.; Wei, S.; Jiang, Z.; Lian, J.; Wang, G.; Jiang, Q.; Liu, J. In situ prepared reduced graphene oxide/CoO nanowires mutually-supporting porous structure with enhanced lithium storage performance. Electrochim. Acta 2016, 190, 276–284. [Google Scholar] [CrossRef]
- Yin, J.; Sun, P.; Qu, G.; Xiang, G.; Hou, P.; Xu, X. A new CoO/Co2B/rGO nanocomposite anode with large capacitivecontribution for high-efficiency and durable lithium storage. Appl. Surf. Sci. 2020, 508, 144698. [Google Scholar] [CrossRef]
- Guan, X.; Nai, J.; Zhang, Y.; Wang, P.; Yang, J.; Zheng, L.; Zhang, J.; Guo, L. CoO hollow cube/reduced graphene oxide composites with enhanced lithium storage capability. Chem. Mater. 2014, 26, 5958–5964. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Gan, Y.; Tao, X.; Xia, Y.; Zhang, W. Mesoporous cobalt monoxide nanorods grown on reduced graphene oxide nanosheets with high lithium storage performance. Electrochim. Acta 2014, 138, 376–382. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Jia, M. Three-dimensional reduced graphene oxides hydrogel anchored with ultrafine CoO nanoparticles as anode for lithium ion batteries. Electrochim. Acta 2014, 129, 425–432. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, M.; Jin, Y.; Shi, X. Synthesis and electrochemical performance of CoO/graphene nanocomposite as anode for lithium ion batteries. Appl. Surf. Sci. 2012, 263, 573–578. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.-F.; Liu, W.-L.; Kong, F.; Ren, M.; Wang, S.-J.; Wang, X.-Q.; Duan, X.-L.; Peng, D. Designed synthesis of CoO/CuO/rGO ternary nanocomposites as high-performance anodes for lithium-ion batteries. JOM 2018, 70, 1793–1799. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Sun, N.; Wei, W.; Zhang, Z.; Liu, H.; Liu, G.; Zhao, Z. One-step self-assembly fabrication of three-dimensional copper oxide/graphene oxide aerogel composite material for supercapacitors. Solid State Commun. 2019, 287, 27–30. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, X.; Shi, W. Free-standing graphene/carbon nanotubes/CuO aerogel paper anode for lithium ion batteries. Mater. Lett. 2016, 172, 72–75. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, M.; Ge, M.-H.; Zhang, H.; Lia, S.; Li, C.-H. Design and construction of three-dimensional CuO/polyaniline/rGO ternary hierarchical architectures for high performance supercapacitors. J. Power Sources 2016, 306, 593–601. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Song, Q.; Yin, Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm 2012, 14, 6710–6719. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, G.; Nie, F.; Xu, X.; Chen, S.; Han, Q.; Wang, X. Decorating graphene oxide with CuO nanoparticles in a water–isopropanol system. Nanoscale 2010, 2, 988–994. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kang, J.; Paul, B.J.; Singh, N.K.; Song, J.; Kim, J. Facile approach to synthesize CuO/reduced graphene oxide nanocomposite as anode materials for lithium-ion battery. J. Power Sources 2013, 244, 435–441. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Guo, Y.; Li, C.; Zhou, X.-Y.; Tucker, M.C.; Chen, J.; Sun, R.; Wong, C.-P. Graphene oxide nano-sheets wrapped Cu2O microspheres as improved performance anode materials for lithium ion batteries. Nano Energy 2015, 11, 38–47. [Google Scholar] [CrossRef]
- Sun, L.; Deng, Q.; Li, Y.; Deng, L.; Wang, Y.; Ren, X.; Zhang, P. Solvothermal synthesis of ternary Cu2O-CuO-RGO composites as anode materials for high performance lithium-ion batteries. Electrochim. Acta 2016, 222, 1650–1659. [Google Scholar] [CrossRef]
- Sheikhzadeh, M.; Sanjabi, S.; Gorji, M.; Khabazian, S. Nano composite foam layer of CuO/graphene oxide (GO) for high performance supercapacitor. Synth. Met. 2018, 244, 10–14. [Google Scholar] [CrossRef]
- Mirzaiey, V.; Dehghanian, C.; Bokati, K.S. One-step electrodeposition of reduced graphene oxide on three-dimensional porous nano nickel-copper foam electrode and its use in supercapacitor. J. Electroanal. Chem. 2018, 813, 152–162. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, X.; Shan, H.; Chen, C.; Yan, B.; Xiong, D.; Li, D. Enhanced anode performance of flower-like NiO/RGO nanocomposites for lithium-ion batteries. Mater. Chem. Phys. 2018, 217, 547–552. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Wang, G.; Zhang, J.; Zhang, S. Sandwich-like NiO/rGO nanoarchitectures for 4 V solid-state asymmetric-supercapacitors with high energy density. Electrochim. Acta 2018, 283, 1401–1410. [Google Scholar] [CrossRef]
- Tian, S. Preparation of RGO/NiO Anode for Lithium-ion Batteries. Int. J. Electrochem. Sci. 2019, 14, 9459–9467. [Google Scholar] [CrossRef]
- Ma, L.; Pei, X.-Y.; Mo, D.-C.; Heng, Y.; Lyu, S.-S.; Fu, Y.-X. Facile fabrication of NiO flakes and reduced graphene oxide (NiO/RGO) composite as anode material for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2019, 30, 5874–5880. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, S.-S.; Dai, Y.; Pei, X.-Y.; Mo, D.-C.; Fu, Y.-X. Lithium storage properties of NiO/reduced graphene oxide composites derived from different oxidation degrees of graphite oxide. J. Alloys Compd. 2019, 810, 151954. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Q.; Xie, L.; Chen, C.; Lu, C.; Su, F.; Zhou, P. Layered NiO/reduced graphene oxide composites by heterogeneous assembly with enhanced performance as high-performance asymmetric supercapacitor cathode. RSC Adv. 2016, 6, 46548–46557. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Ding, J. The synthesis and lithium storage performance of NiO/rGO/PPy anode materials. J. Changzhou Univ. (Nat. Sci.) 2015, 27, 18–23. [Google Scholar]
- Ma, L.; Pei, X.-Y.; Mo, D.-C.; Lyu, S.-S.; Fu, Y.-X. Fabrication of NiO-ZnO/rGO composite as an anode material for lithium-ion batteries. Ceram. Int. 2018, 44, 22664–22670. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Kang, Y.C. Multiphase and double-layer NiFe2O4@NiO-hollow-nanosphere-decorated reduced graphene oxide composite powders prepared by spray pyrolysis applying nanoscale Kirkendall diffusion. ACS Appl. Mater. Interfaces 2015, 7, 16842–16849. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Li, S.; Li, Q.; Xu, J.; Liu, X.; Liu, C.; Xu, Y.; Liu, J.; Li, H.; et al. Optimization of NiFe2O4/rGO composite electrode for lithium-ion batteries. Appl. Surf. Sci. 2017, 416, 308–317. [Google Scholar] [CrossRef]
- Jo, M.S.; Lee, J.S.; Jeong, S.Y.; Kim, J.K.; Kang, Y.C.; Kang, D.-W.; Jeong, S.M.; Cho, J.S. Golden bristlegrass-like hierarchical graphene nanofibers entangled with N-doped CNTs containing CoSe2 nanocrystals at each node for high-performance anodes in sodium-ion batteries. Small 2020, 16, 2003391. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Park, S.-K.; Jeon, K.M.; Piao, Y.; Cho, J.S. Mesoporous reduced graphene oxide/WSe2 composite particles for efficient sodium-ion batteries and hydrogen evolution reactions. Appl. Surf. Sci. 2018, 459, 309–317. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Lee, J.-K.; Kang, Y.C. Iron telluride-decorated reduced graphene oxide hybrid microspheres as anode materials with improved Na-ion storage properties. ACS Appl. Mater. Interfaces 2016, 8, 21343–21349. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First introduction of NiSe2 to anode material for sodium-ion batteries: A hybrid of graphene-wrapped NiSe2/C porous nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, J.-K.; Kang, Y.C. Graphitic carbon-coated FeSe2 hollow nanosphere-decorated reduced graphene oxide hybrid nanofibers as an efficient anode material for sodium ion batteries. Sci. Rep. 2016, 6, 23699. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Lee, J.-K.; Kang, Y. Na-ion storage performances of FeSex and Fe2O3 hollow nanoparticles-decorated reduced graphene oxide balls prepared by nanoscale Kirkendall diffusion Process. Sci. Rep. 2016, 6, 22432. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Kvarnström, C. Graphene vs. reduced graphene oxide: A comparative study of graphene-based nanoplatforms on electrochromic switching kinetics. Carbon 2016, 96, 377–381. [Google Scholar] [CrossRef]
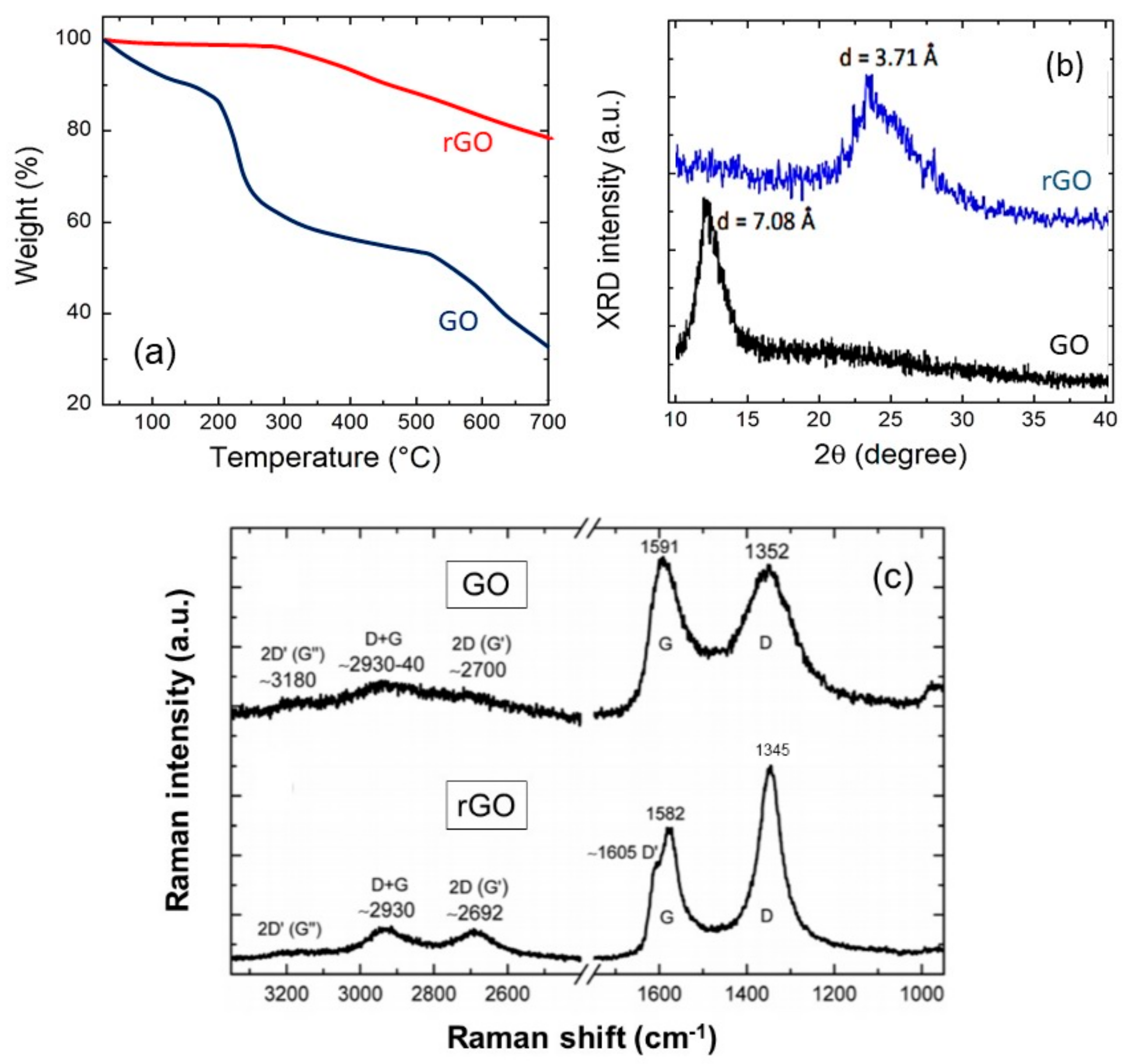
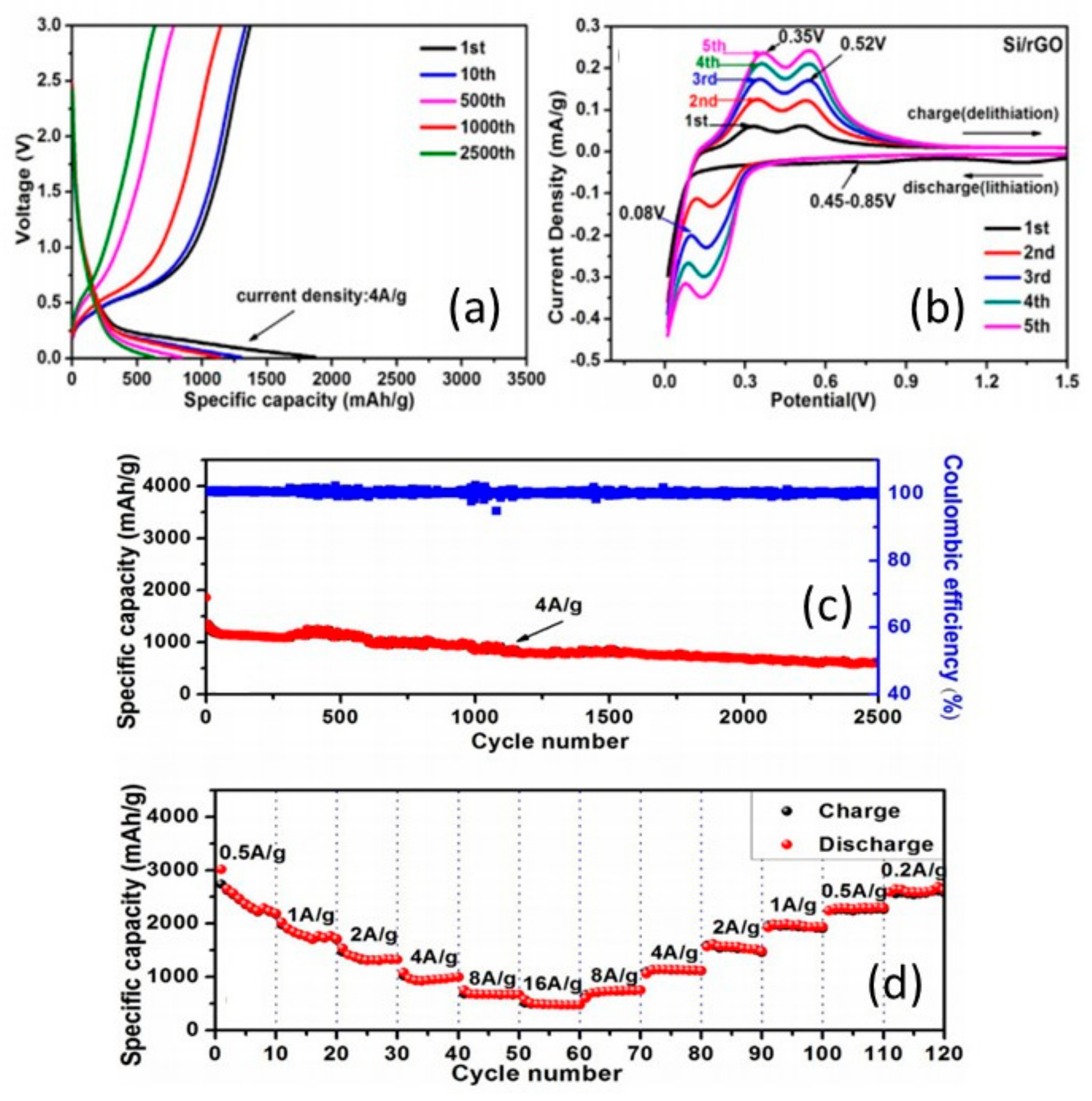
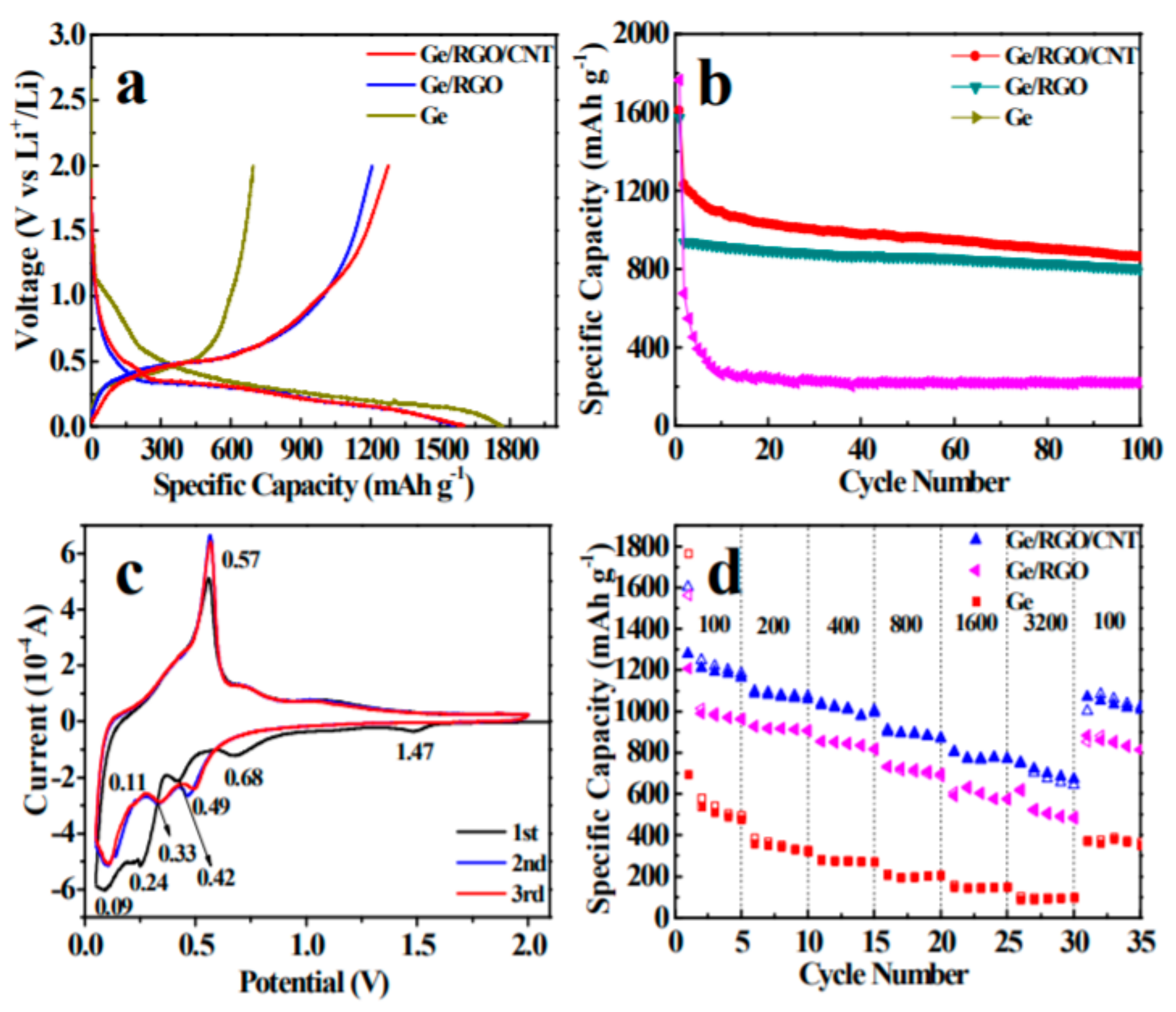

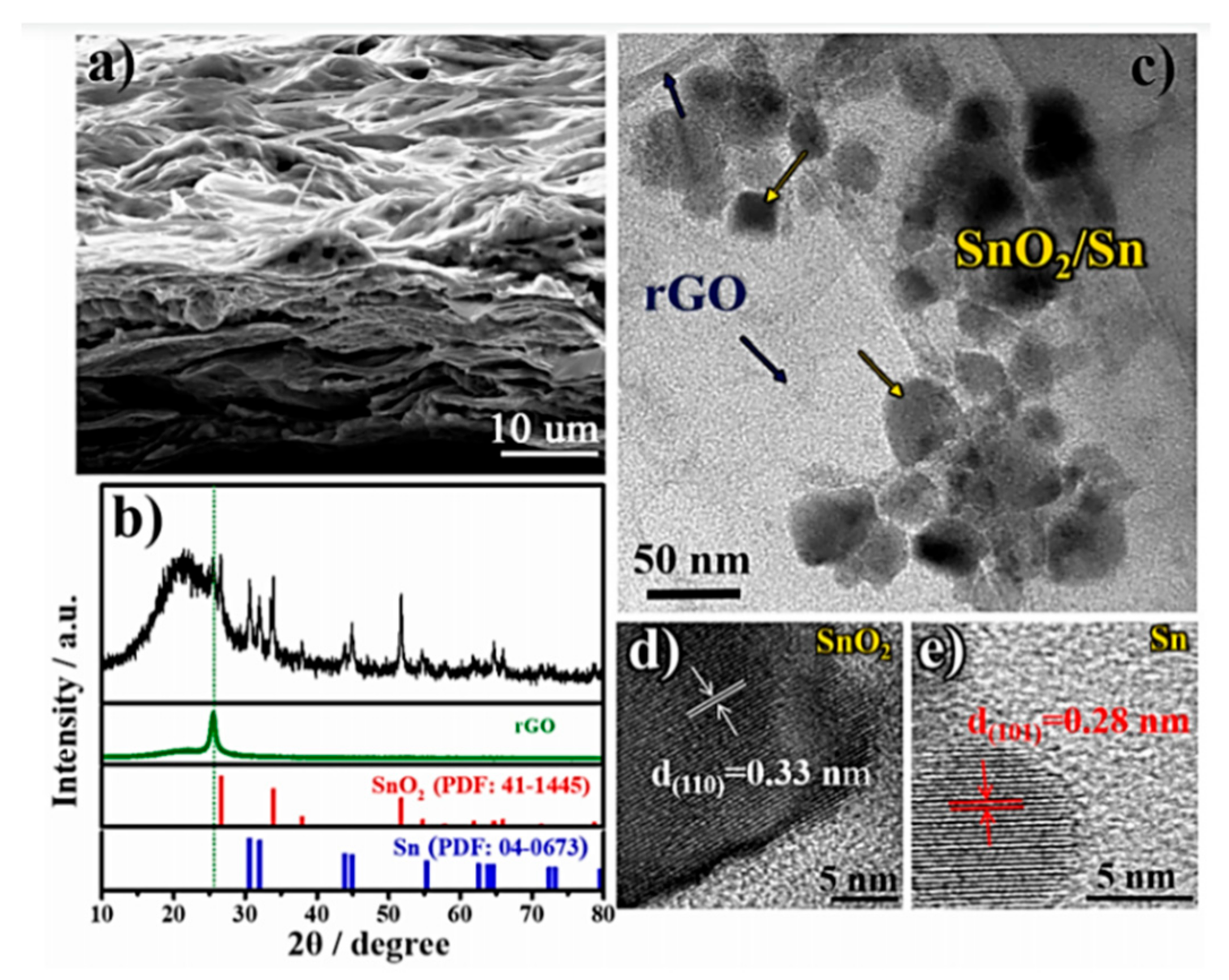
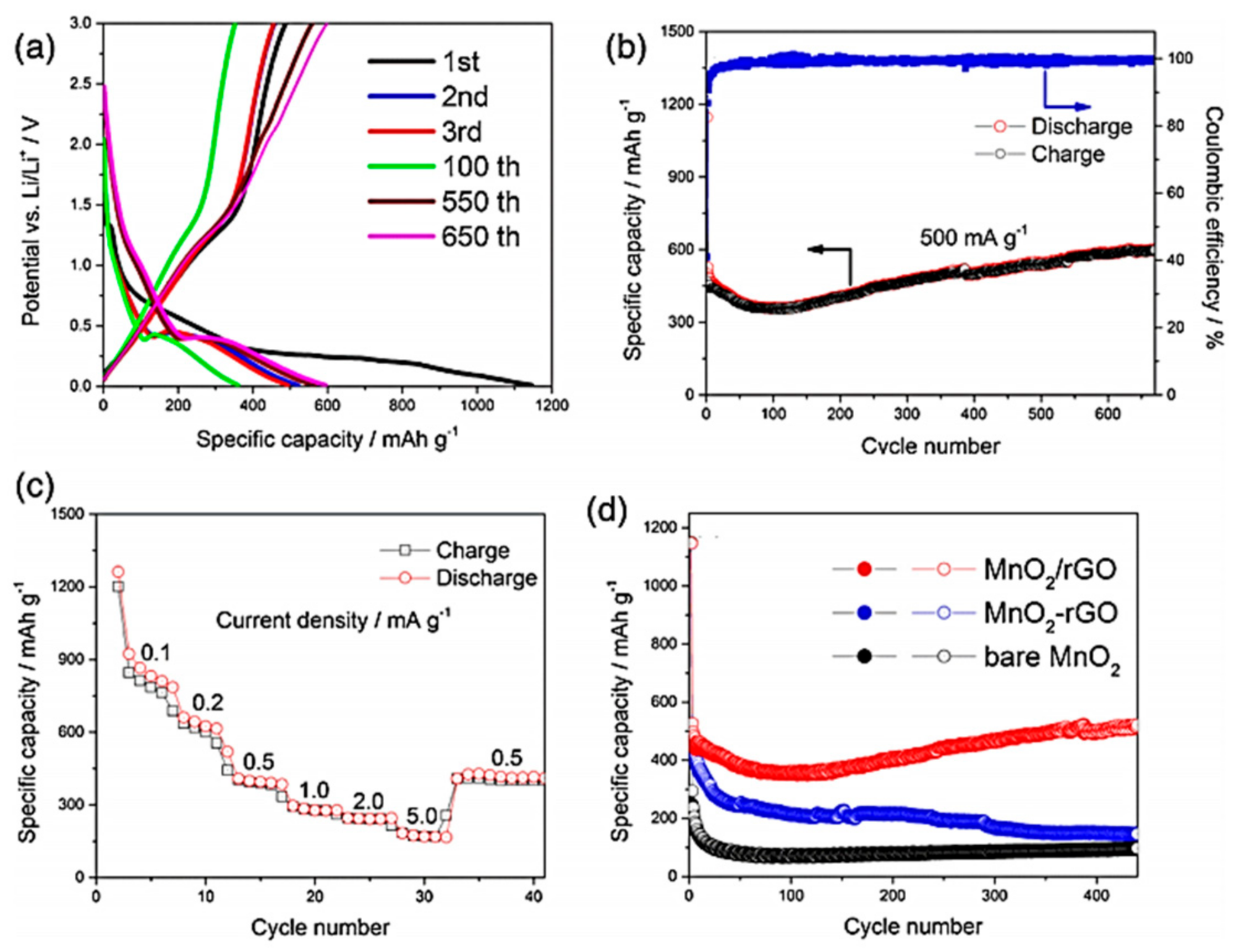
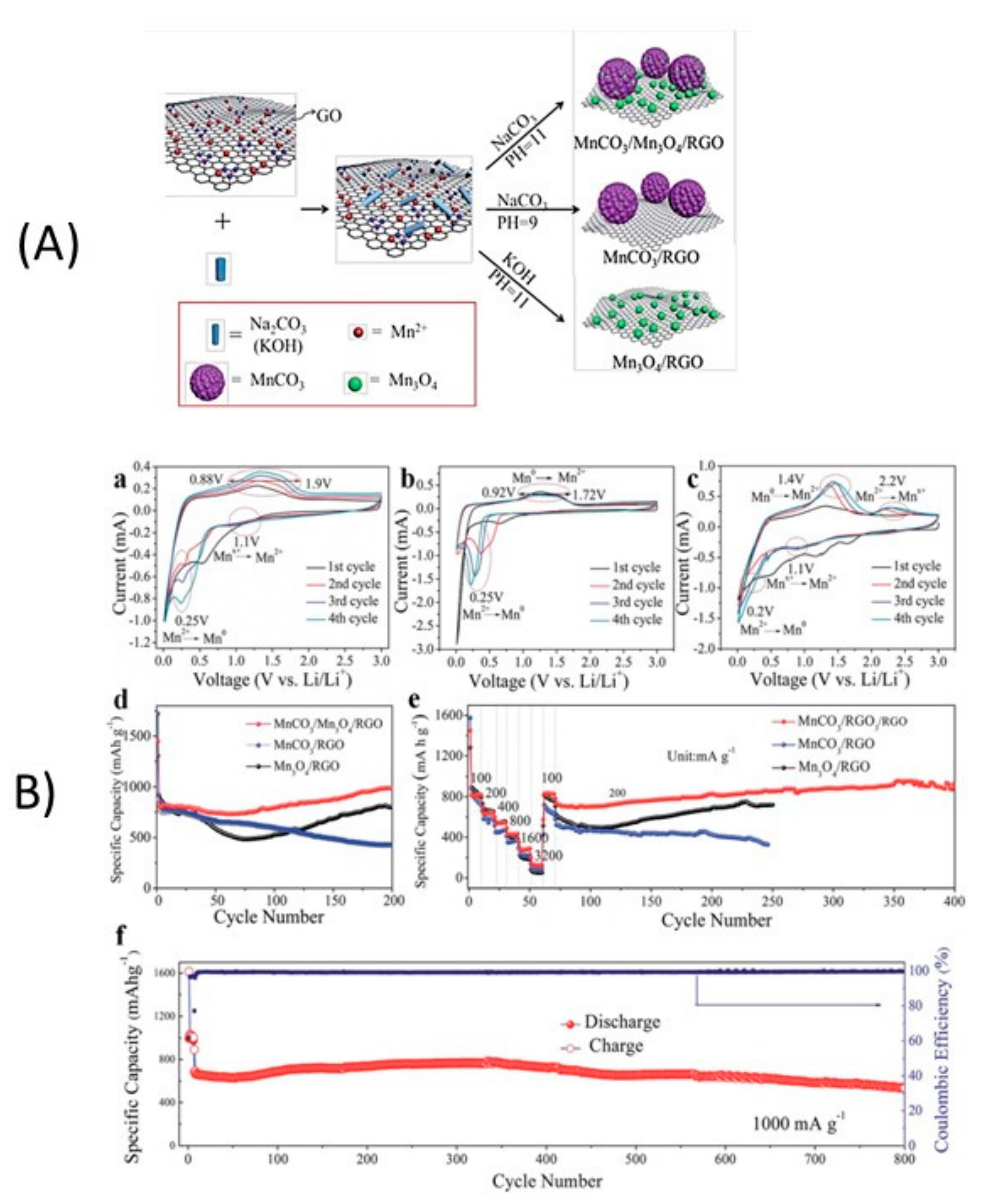
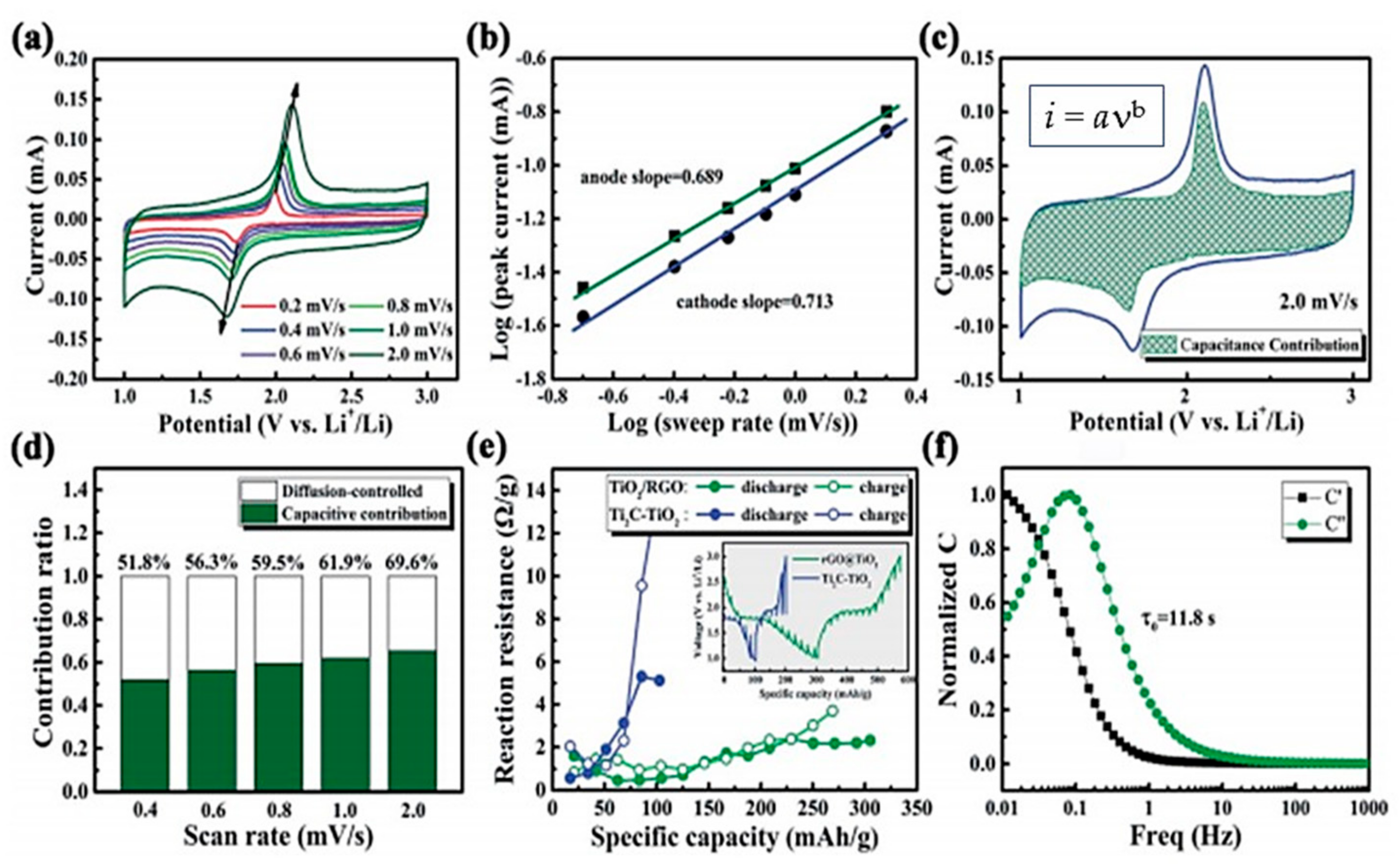
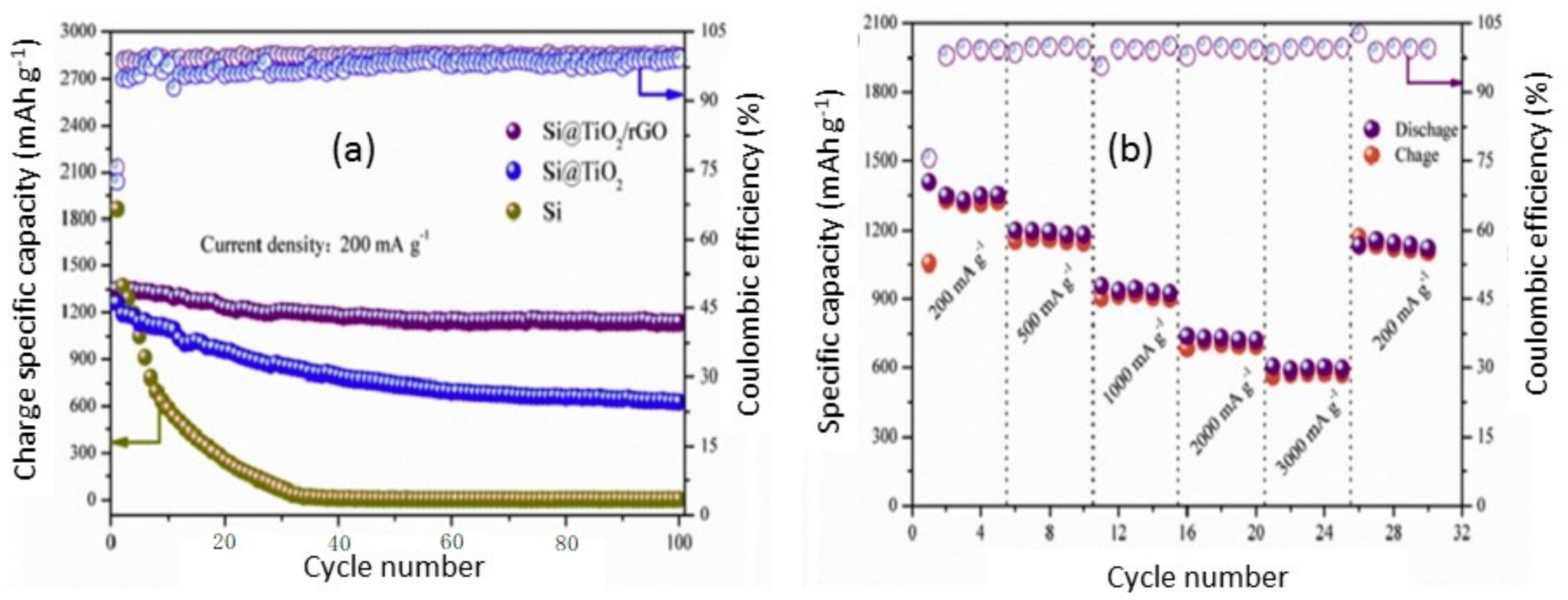
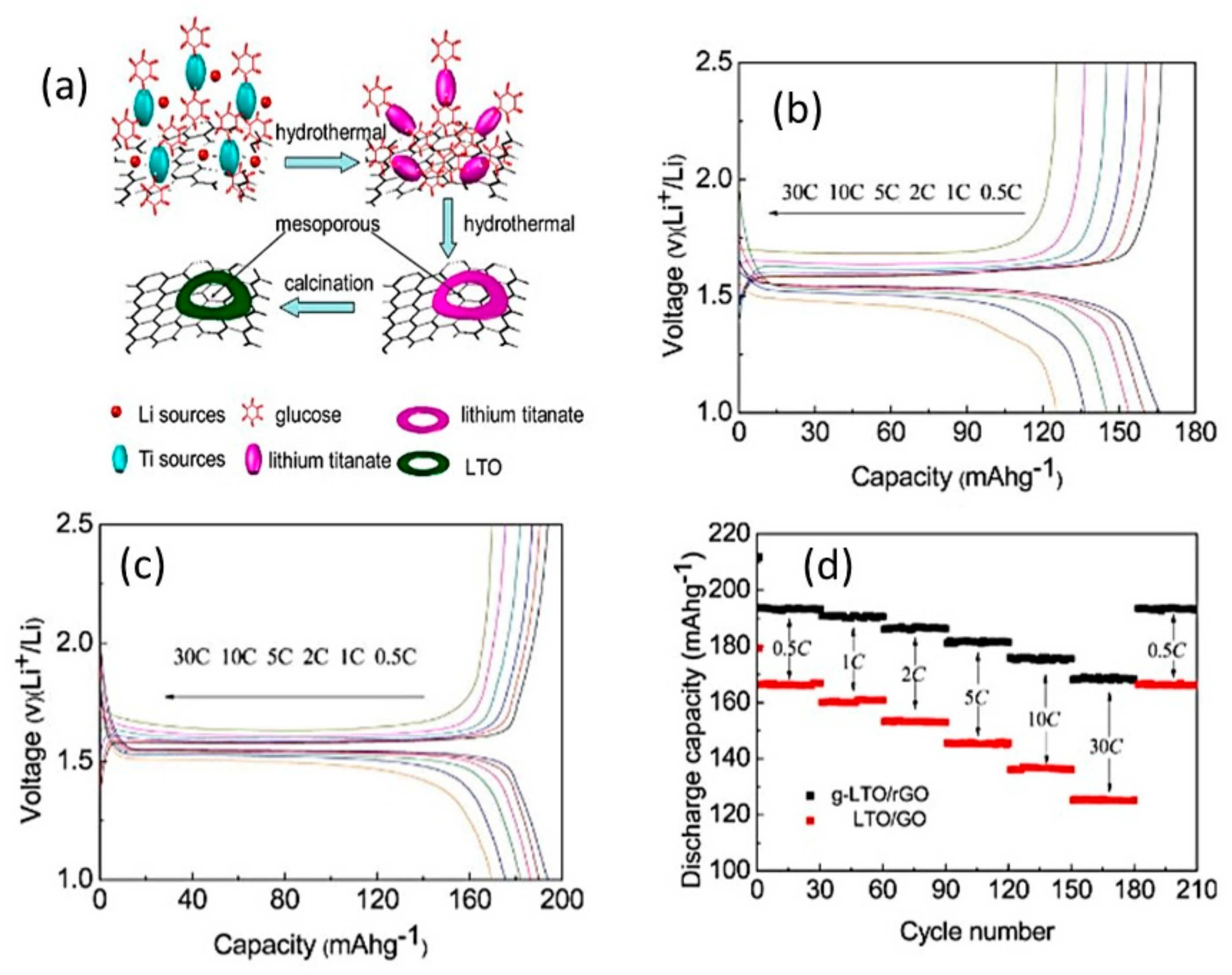
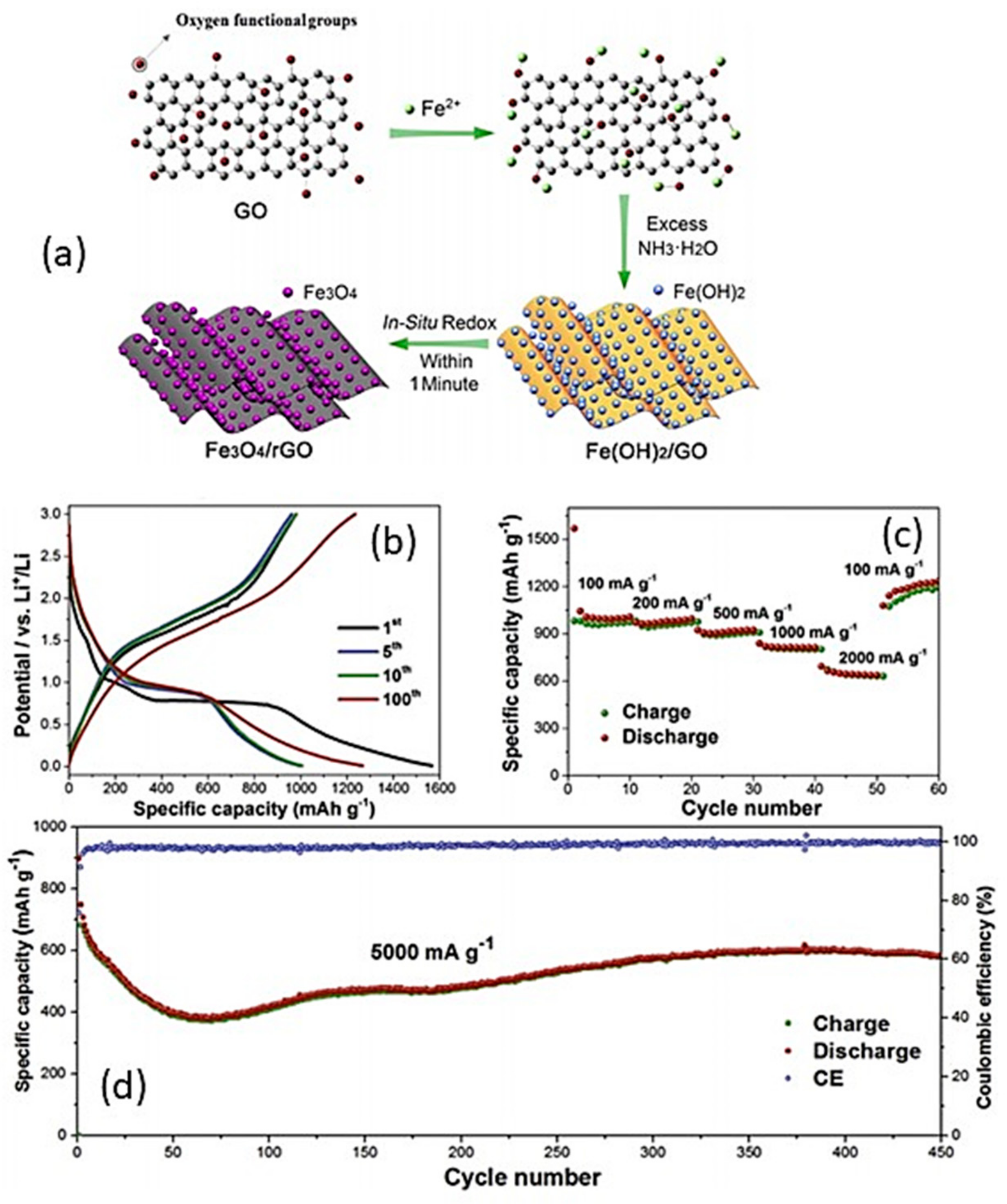
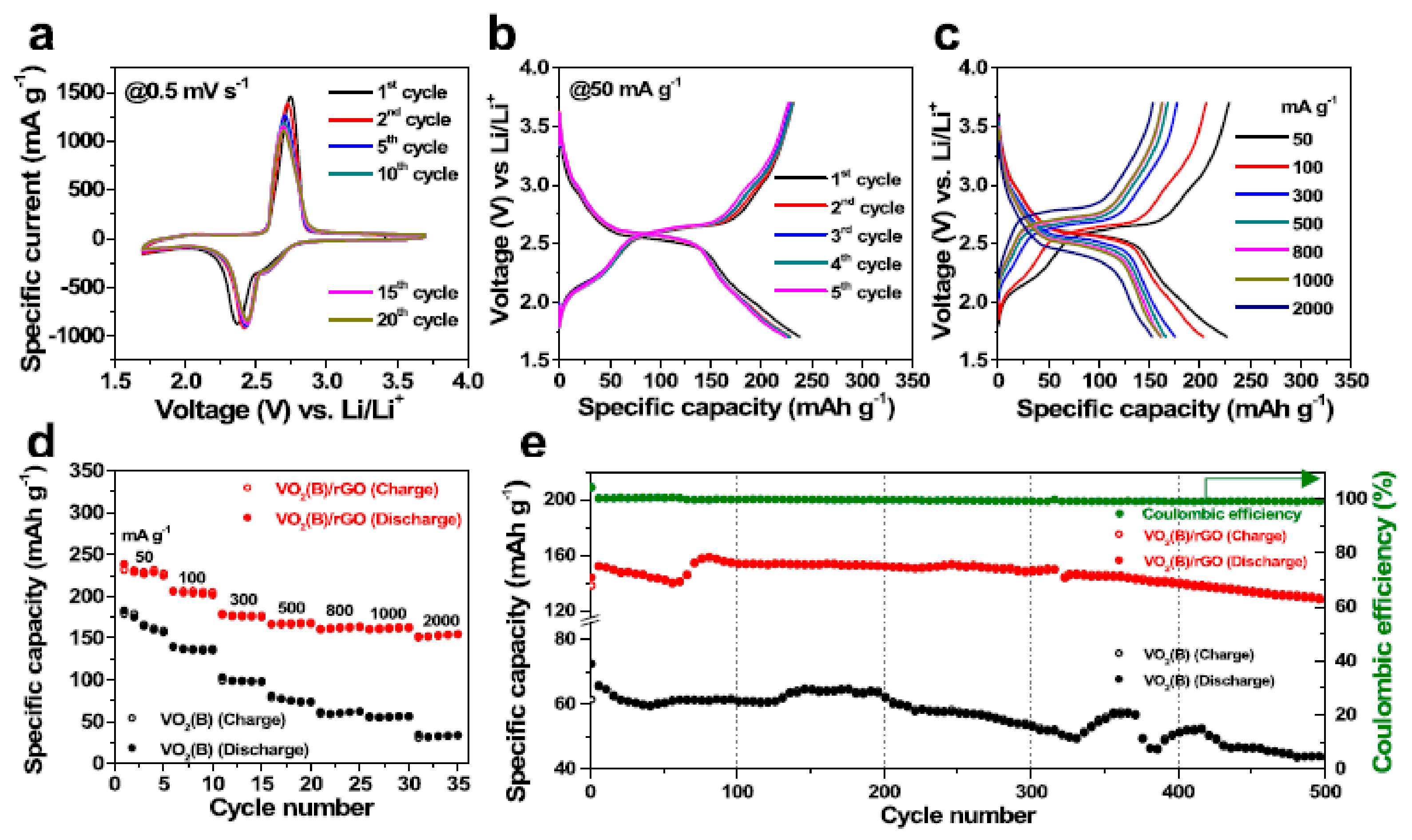
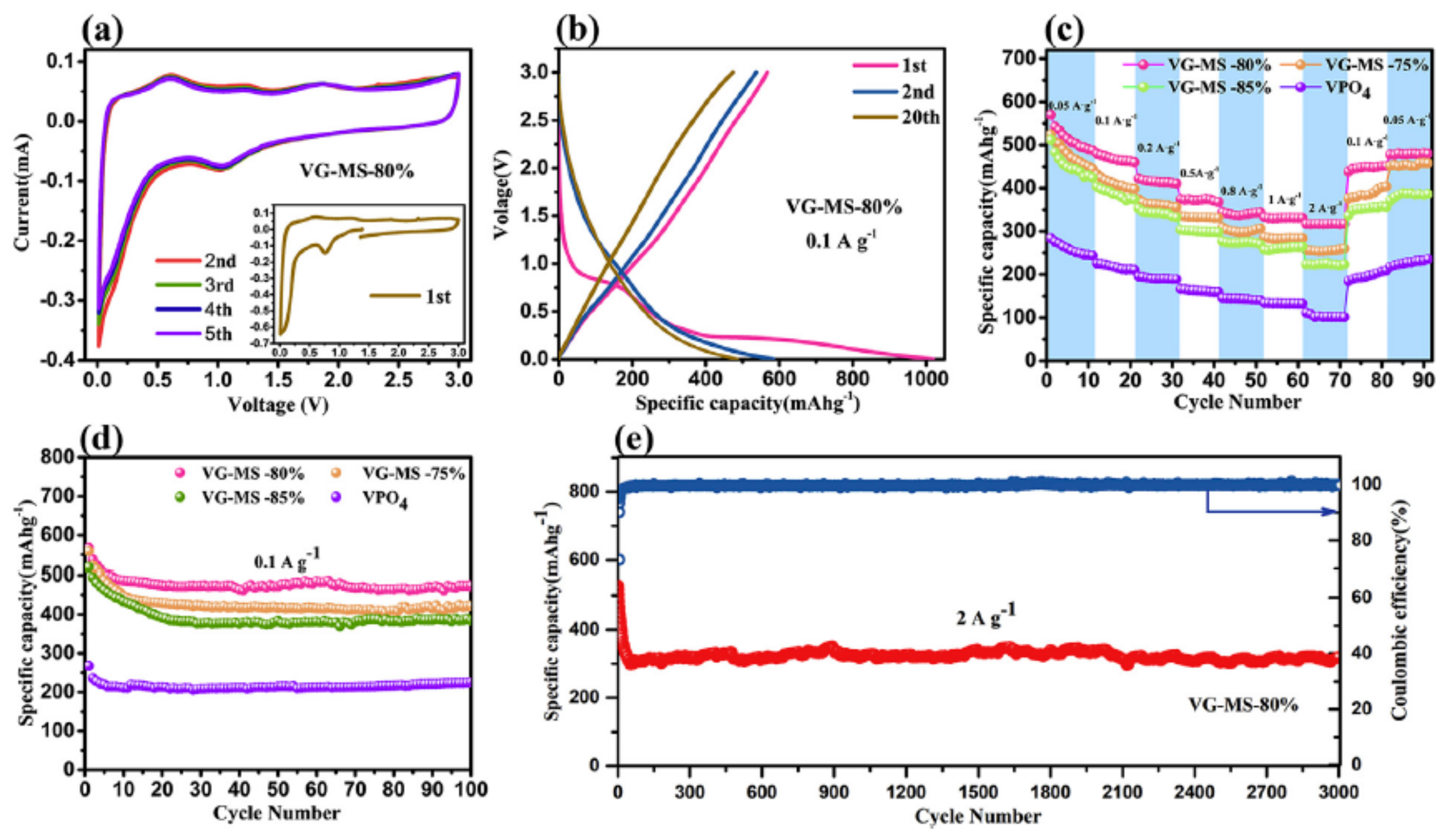
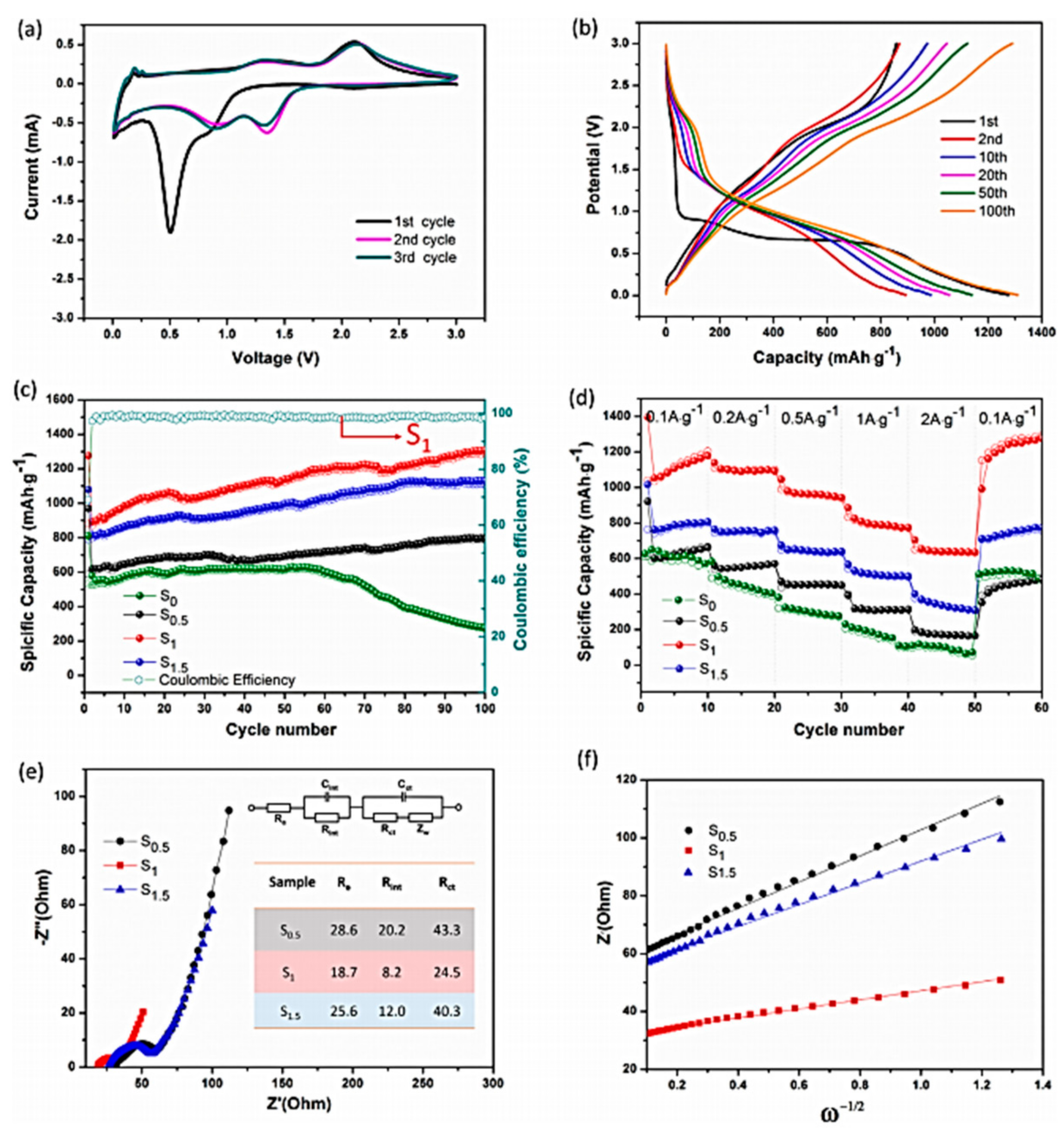

| Material | Synthesis | Specific Capacity (mAh g−1) | Current Density (A g−1) | Ref. |
|---|---|---|---|---|
| SiNWs@G/rGO | sandwich procedure | 1280 | 2.1 (100) | [88] |
| Si NPs/rGO | polymer-assisted | 1311 | 2.6 (900) | [87] |
| Si/CNFs@rGO | layer-by-layer assembly | 455 | 15 (2000) | [109] |
| CNT/rGO/Si-NPs | electrostatic method | 1480 | 0.2 (200) | [111] |
| Si@C/rGO | sonication + calcination | 780 | 1.0 (80) | [119] |
| Si NPS/rGO | roll-to-roll deposition | 1394 | 15 (1000) | [112] |
| Si/rGO | self-assembly | 1481 | 0.5 (50) | [102] |
| 3D Si/rGO | evaporation | 1406 | 0.05 (100) | [117] |
| Si@SnS2/rGO | electrostatic self-assembly | 1055 | 0.1 (130) | [125] |
| Si@C@rGO | spray drying + calcination | 1567 | 0.2 (100) | [126] |
| Si@N-doped C/rGO | polymer network method | 479 | 2.0 (200) | [131] |
| Si/C/rGO | spray-drying + carbonization | 928 | 0.1 (70) | [129] |
| Si/rGO/C NFs | electrospinning | 884 | 0.8 (100) | [127] |
| bmSi@C/rGO | ball-milling + pyrolysis | 935 | 0.2 (100) | [104] |
| Si@N-doped C/rGO | freeze drying | 709 | 1.0 (400) | [117] |
| N-doped rGO/C@Si | freeze-drying + pyrolysis | 1115 | 0.42 (150) | [135] |
| Si/rGO/C | self-assembling + spray-drying | 602 | 0.4 (500) | [134] |
| rGO/MWCNT/Si@void@C | dispersion + ultrasonication | 951 | 0.2 (500) | [116] |
| rGO/Si@void@C | dispersion + ultrasonication | 500 | 0.2 (500) | [132] |
| SiNPs@CA@rGO | suspension + freeze drying | 2566 | 1.0 (100) | [133] |
| Si@rGO (bowl-like) | templating | 450 | 0.1 (100) | [124] |
| Si/rGO @ Ni foam | hydrothermal | 1500 | 2 (2500) | [130] |
| Si@Void@C/rGO | surface carbonization + etching | 1294 | 0.5 (100) | [136] |
| Material | Synthesis | Specific Capacity (mAh/g−1) | Current Density (A/g−1) | Ref. |
|---|---|---|---|---|
| Ge@C/rGO | polymerization with PDA | 1074 | 3.2 (600) | [144] |
| Ge@C/rGO | dispersion + thermal reduction | 380 | 0.1 (50) | [142] |
| Ge@C/rGO | low-pressure thermal deposition | 940 | 0.05 (50) | [31] |
| Ge-rGO-CNTs | dispersion + thermal reduction | 863 | 0.1 (100) | [146] |
| Ge/rGO | PVP-assisted hydrolysis | 700 | 0.5 (200) | [143] |
| rGO/Ge/rGO | carbothermal reduction | 1085 | 1.6 (500) | [145] |
| Ge@C/rGO | carbothermal reduction | 710 | 1.0 (200) | [148] |
| Ge/rGO | polymeric in-situ reduction | 960 | 1.0 (100) | [147] |
| GeCH3/rGO | ultrasonic wet dispersion | 1058 | 0.2 (100) | [149] |
| CuGeO3/rGO (30 wt%) | hydrothermal reduction | 909 | 0.1 (200) | [150] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Density (A g−1) | Ref. |
|---|---|---|---|---|
| SnO2/rGO | in-situ hydrolysis | 600 | 0.05 (20) | [198] |
| H-SnO2/rGO | hydrothermal reduction | 552 | 1.0 (500) | [194] |
| PS@GO/SnO2 | hydrolysis | 781 | 0.1 (100) | [197] |
| SnO2/rGO | spray drying + thermal treatment | 708 | 0.5 (150) | [201] |
| SnO2-x/rGO | precipitation | 950 | 0.1 (1100) | [211] |
| F–SnO2/rGO | hydrothermal @180 °C/12 h | 1037 | 0.1 (150) | [188] |
| F–SnO2/rGO | hydrothermal | 440 | 0.5 (500) | [210] |
| HP-SnO2/rGO | hydrolysis + calcination | 850 | 0.3C (100) | [199] |
| W–SnO2/rGO | hydrothermal reduction | 1100 | 0.1 (100) | [187] |
| Zn–SnO2/rGO | sol-gel + freeze-drying | 628 | 0.1 (200) | [212] |
| SnO2/GO | ultrasonication + calcination | 492 | 2C (100) | [202] |
| SnOx/rGO | chemical reduction | 767 | 0.1 (100) | [205] |
| Fe@SnO2/rGO | wet chemistry | 1353 | 0.1 (100) | [206] |
| Sb-SnO2/rGO | in-situ microwave-assisted | 577 | 60C | [211] |
| F-SnO2 NCs/rGO | hydrothermal | 1277 | 0.1 (100) | [209] |
| SnO2/rGO | precipitation + calcination | 522 | 0.03 (50) | [200] |
| Material (a) | Synthesis | Specific Capacity (mAh g−1) | Current Density (b) (A g−1) | Ref. |
|---|---|---|---|---|
| b-MnO2/rGO | hydrothermal @160 °C/6 h | 448 | 0.1 (50) | [227] |
| a-MnO2/3D-rGO | in situ hydrothermal @ 60 °C/6 h | 595 | 0.1 (60) | [230] |
| d-MnO2 NFs/rGO | redox reaction with KMnO4 | 1000 | 1 (200) | [231] |
| a-MnO2/rGO | solution method with CTAB | 222 | 5 (200) | [225] |
| a-MnO2/rGO | hydrothermal @150 °C/15 h | 590 | 12 (30) | [233] |
| MnO2 NRs/rGO | hydrothermal @160 °C/18 h | 600 | 0.5 (650) | [234] |
| d-MnO2 NRs/GO | sonication | 460 | 5 (80) | [232] |
| CNT/rGO@MnO2 | vacuum freeze-drying | 608 | 7.5 (1000) | [236] |
| PPy/MnO2/rGO-CNTs | vacuum filtration | 941 | 1 (1200) | [235] |
| rGO/MnO2/CNTS | vacuum filtration + thermal annealing | 1172 | 0.1 (100) | [238] |
| Mn3O4/rGO (10) | two-step solution-phase reaction | 900 | 0.04 (5) | [255] |
| MnO/GO (9.17) | impregnation + thermal reduction | 500 | 0.2C (32) | [282] |
| N-MnO/rGO (8.9) | hydrothermal + ammonia annealing | 772 | 0.1 (90) | [283] |
| MnO/rGO (75) | in-situ carbothermal reduction | 782 | 0.1 (60) | [284] |
| MnCO3/Mn3O4/rGO | sonication + alkaline solution | 998 | 0.2 (100) | [275] |
| Mn3O4-MnO/rGO (12) | one-pot solvothermal | 825 | 0.05 (30) | [285] |
| MnO/rGO (72.5) | two-step liquid phase deposition | 665 | 0.1 (50) | [286] |
| MnO NWs/GO | dialysis | 930 | 0.1 (470) | [287] |
| MnO NRs/GO (7.58) | in-situ reduction | 705 | 0.05 (100) | [288] |
| MnO/rGO (6) | pyrolysis | 950 | 0.1 (5) | [289] |
| MnO NSs/rGO | hydrolysis + calcination | 648 | 0.75 (50) | [290] |
| Material | Synthesis | Reversible Capacity (mAh g−1) | Current Rate (A g−1) | Ref. |
|---|---|---|---|---|
| MoO2/Gr | layer-by-layer assembly | 676 | 48 (100) | [313] |
| MoO2/Gr | sonication in water (11.2) | 597 | 1000 (70) | [26] |
| MoO2/rGO | solvothermal in ethanol (10.0) | 714 | 100 (30) | [314] |
| MoO2/rGO | hydrothermal @200 °C/24 h | 503 | 100 (30) | [298] |
| MoO2/rGO | hydrothermal @400 °C/3 h (10) | 1009 | 100 (60) | [315] |
| MoO2/rGO | solid state reaction (22.0) | 640 | 200 (50) | [316] |
| MoO2/GO | thermal reduction @550 °C/2 h | 752 | 100 (100) | [317] |
| MoO2/Gr | hydrothermal @180 °C/26 h (33) | 769 | 540 (83) | [318] |
| MoO2/N-rGO | hydrothermal @180 °C/24 h | 400 | 1000 (5) | [319] |
| MoO2/exfol-rGO | solid-state graphenothermal (46) | 878 | 100 (100) | [297] |
| MoO2 NSs/rGO | in situ reduction of MoO3 | 1003 | 100 (100) | [320] |
| MoO2/GO | solvothermal @160 °C/16 h (10) | 500 | 800 (30) | [299] |
| MoO2/rGO | solid-state reaction @500 °C (15) | 276 | 100 (1000) | [300] |
| MoO2/rGO | hydrothermal @200 °C/2 days (21) | 708 | 500 (50) | [307] |
| MoO2/rGO | thermal reduction @550 °C (19) | 584 | 1000 (100) | [308] |
| MoO2/rGO/NBs | surfactant-free self-assembly | 420 | 5000 (1900) | [302] |
| MoO2 NBs/rGO | supercritical methanol route | 793 | 50 (50) | [304] |
| MoO2/Mo-GO | freeze-drying (5) | 550 | 100 (150) | [310] |
| MoO2/rGO | solid state reaction @500 °C (15) | 1127 | 100 (150) | [305] |
| MoO2 NBs/rGO | freeze-drying (8) | 420 | 5000 (1900) | [301] |
| m-MoO2/rGO | nanocasting (50) | 801 | 100 (100) | [306] |
| MoO2@MoS2/rGO | hydrothermal @180 °C/4h | 733 | 200 (80) | [312] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Rate (mA g−1) | Ref. |
|---|---|---|---|---|
| TiO2/rGO | hydrothermal 160 °C/12 h | 112 | 1700 (100) | [334] |
| TiO2/GO | UV photocatalysis | 130 | 200 (100) | [345] |
| TiO2/rGO | UV photocatalysis | 215 | 200 (100) | [345] |
| TiO2/N-doped rGO | hydrothermal 200 °C/20 h | 187 | - | [337] |
| TiO2/rGO | solvothermal with Ti(OBu)4 | 153 | 1000 (100) | [89] |
| TiO2/rGO | hydrothermal 150 °C/14 h | 236 | 100 (100) | [338] |
| TiO2/rGO | hydrothermal 180 °C/20 h | 235 | 200 (1000) | [327] |
| TiO2-carbon-rGO | hydrothermal 160 °C/24 h | 158 | 1000 (100) | [348] |
| TiO2/rGO | sonication for 5 min | 143 | 100 (100) | [349] |
| R-TiO2/rGO | hydrothermal 180 °C/12 h | 90 | 1680 (1000) | [336] |
| TiO2/rGO | hydrothermal 160 °C/16 h | 130 | 1680 (1000) | [341] |
| TiO2/rGO | hydrothermal 180 °C/12 h | 180 | 500 (2000) | [329] |
| TiO2-carbon-rGO | UV photocatalysis (254 nm) | 191 | 40 (100) | [347] |
| TiO2/rGO | thermal reduction | 161 | 170 (50) | [350] |
| TiO2(B)–CNTs–GO | hydrolysis + hydrothermal | 190 | 1C (200) | [340] |
| TiO2/rGO (9.9% rGO) | hydrothermal + annealing | 254 | 168 (600) | [333] |
| TiO2/rGO (10% rGO) | aerosol-assisted spray-drying | 174 | 1C (200) | [339] |
| N-doped TiO2/rGO | hydrothermal + annealing | 210 | 100 (2000) | [355] |
| Si@TiO2@rGO | sol-gel | 1135 | 200 (100) | [356] |
| TiO2/rGO | solvothermal alcoholysis | 176 | 1675 (1000) | [352] |
| TiO2/rGO | microwave hydrothermal process | 250 | 0.2C (200) | [342] |
| TiO2/rGO | coprecipitation Ti3O72− + GO | 245 | 1000 (1000) | [353] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Density (A g−1) | Ref. |
|---|---|---|---|---|
| Fe3O4/rGO | co-precipitation + annealing | 200 | 9.26 | [383] |
| Fe3O4/rGO | microwave-assisted combustion | 446 | 5C (50) | [389] |
| Fe3O4/rGO | coprecipitation | 300 | 1.0 (100) | [390] |
| Fe3O4/rGO | hydrothermal + heating | 593 | 5.0 (400) | [378] |
| Fe3O4/rGO | redox reaction | 584 | 5.0 (450) | [388] |
| Fe3O4/CNTs/rGO | electrophoretic deposition | 1080 | 1.0 (450) | [387] |
| rGO/Fe3O4@PDA | hydrothermal + calcination solvothermal | 712 | 3.0 (2000) | [392] |
| Fe3O4@C/rGO | hydrothermal + calcination | 594 | 5.0 (1000) | [393] |
| Fe3O4/FeS/rGO | hydrolysis | 744 | 1C (50) | [397] |
| Fe3O4 NFs/rGO | gamma-irradiation method | 636 | 0.5 (1000) | [380] |
| 78.8 wt%Fe3O4/rGO | gamma-irradiation method | 568 | 0.05 (100) | [399] |
| 74.7 wt%Fe3O4/rGO | solvothermal | 738 | 0.5 (100) | [399] |
| NiO/Fe3O4/rGO | electrostatic assembly | 580 | 1.0 (200) | [395] |
| CP/Fe3O4/rGO | solvothermal | 1160 | 0.5 (1000) | [401] |
| Fe3O4/rGO | PMAA-induced self-assembly | 992 | 0.15 (100) | [402] |
| Fe3O4/rGO | hydrothermal + calcination | 740 | 0.5 (200) | [391] |
| CuFeO2/rGO | one-pot solvothermal | 587 | 0.2 (100) | [400] |
| C/Fe3O4/rGO | 844 | 0.2 (300) | [398] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Density (mA g−1) | Ref. |
|---|---|---|---|---|
| Cathode | ||||
| VO2(B)/rGO | hydrothermal 150 °C/12 h | 250 | 50 (50) | [440] |
| VO2(B)/GO | sonication + hydrothermal | 350 | 40 (20) | [436] |
| VO2.07/rGO | hydrothermal 180 °C/20 h | 133 | 70 (200) | [426] |
| VO2(R)rGO | hydrothermal 190 °C/48 h | 85 | 20 (100) | [430] |
| VO2(B)/rGO | hydrothermal 180 °C/24 h | 130 | 1000 (500) | [438] |
| VO2(B)/rGO | single-step hydrothermal | 250 | 5000 (1600) | [429] |
| Anode | ||||
| VO2(B)/rGO | microwave-assisted solvothermal | 400 | 200 (400) | [422] |
| VO2(M)/rGO | sol-gel assisted hydrothermal | 283 | 60 (200) | [424] |
| GO-coated VO2(B) | sol-gel + calcination | 220 | 100 (20) | [437] |
| VO2(B)/rGO | hydrothermal 180 °C/24 h | 1214 | 100 (120) | [434] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Rate (mA g−1) | Ref. |
|---|---|---|---|---|
| V2O5/GO/NCM | wet chemistry | 125 | 55 | [450] |
| V2O5/rGO | solvothermal solution | 122 | 64C (500) | [448] |
| V2O5/rGO | two-step solvothermal | 205 | 1C (120) | [449] |
| V2O5/GO (2 wt%) | sol-gel | 240 | 1C (200) | [451] |
| V2O5/rGO (3.2%) | hydrothermal | 175 | 20 (100) | [430] |
| V2O5 NSs/rGO | solvothermal | 141 | 600 (160) | [452] |
| V2O5/rGO | solvothermal | 102 | 5700 (200) | [453] |
| V2O5 NRs/rGO | hydrothermal + reflux | 140 | 150 (100) | [457] |
| V2O5/rGO | post-annealing of VO2/rGO | 214 | 1000 (100) | [425] |
| V2O5/rGO | electrospinning | 125 | C/5 (60) | [455] |
| VxOy-TiO2/rGO | solvothermal | 180 | 50 (20) | [456] |
| Material | Synthesis | Capacitance (F g−1) | Rate (mA g−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| V2O5/rGO | solvothermal | 537 | 1000 | 8 M LiCl | [466] |
| VO2/rGO | hydrothermal | 225 | 250 | 0.5M K2SO4 | [467] |
| VOx NTs/rGO | hydrothermal | 210 | 1000 | 1M Na2SO4 | [468] |
| VO2 NBs/rGO | hydrothermal | 426 | 1000 | 0.5M K2SO4 | [469] |
| V2O5 NWs/rGO | hydrothermal | 80 | 500 | 1M LiTFSI | [470] |
| VOx/rGO | solvothermal | 183 | 500 | 0.5M Na2SO4 | [471] |
| V2O5/rGO | hydrothermal | 130 | 100 | 1M LiClO4 | [464] |
| V2O5/rGO | coprecipitation | 484 | 500 | 0.5M K2SO4 | [472] |
| V2O5/rGO | solvothermal | 450 | 500 | 1M Na2SO4 | [449] |
| V2O5 NRs/rGO | sol-gel | 218 | 5000 | 1M Na2SO4 | [473] |
| Material | Synthesis | Specific Capacity (mAh g−1) | Current Density (mA g−1) | Ref. |
|---|---|---|---|---|
| Co hollow cube/rGO | sacrificial-template + annealing | 1170 | 150 (60) | [504] |
| m-CoO NRs/rGO | hydrothermal + calcination | 960 | 100 (50) | [505] |
| CoO/3D rGO hydrogel | hydrothermal + calcination | 962 | 200 (80) | [506] |
| Co/rGO | simultaneous reduction | 690 | 600 (60) | [497] |
| CoO/rGO flakes | simultaneous reduction | 900 | 150 (60) | [498] |
| CoO-Co3O4/rGO | solvothermal + sintering | 994 | 100 (200) | [500] |
| CoO/Co2B/rGO | precipitation + pyrolysis | 276 | 10C | [503] |
| CoO/rGO | one-pot in situ solution | 577 | 100 (435) | [507] |
| CoO/CuO/rGO | hydrothermal | 1364 | 200 (100) | [508] |
| CoO/rGO | hydrothermal + calcination | 557 | 10000 (300) | [499] |
| CoO/rGO | hydrothermal (oleic acid) | 1039 | 100 (100) | [501] |
| CoO/rGO NWs | in situ self-assembly | 520 | 3000 (750) | [502] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehrawat, P.; Abid, A.; Islam, S.S.; Mauger, A.; Julien, C.M. Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries. C 2020, 6, 81. https://doi.org/10.3390/c6040081
Sehrawat P, Abid A, Islam SS, Mauger A, Julien CM. Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries. C. 2020; 6(4):81. https://doi.org/10.3390/c6040081
Chicago/Turabian StyleSehrawat, Poonam, Abid Abid, Saikh S. Islam, Alain Mauger, and Christian M. Julien. 2020. "Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries" C 6, no. 4: 81. https://doi.org/10.3390/c6040081
APA StyleSehrawat, P., Abid, A., Islam, S. S., Mauger, A., & Julien, C. M. (2020). Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries. C, 6(4), 81. https://doi.org/10.3390/c6040081









