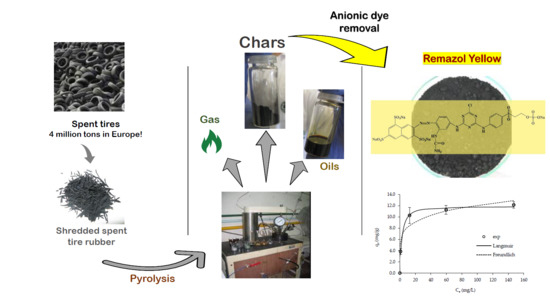
Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Spent Tire Rubber
- (i)
- Proximate analysis through a gravimetric method in order to determine the moisture content (105 °C), volatile matter (950 °C), and ashes (750 °C);
- (ii)
- Elemental analysis to quantify C, H, N, and S (Thermo Finnigan-CE Instruments Flash EA 1112 CHNS analyzer, Waltham, USA);
- (iii)
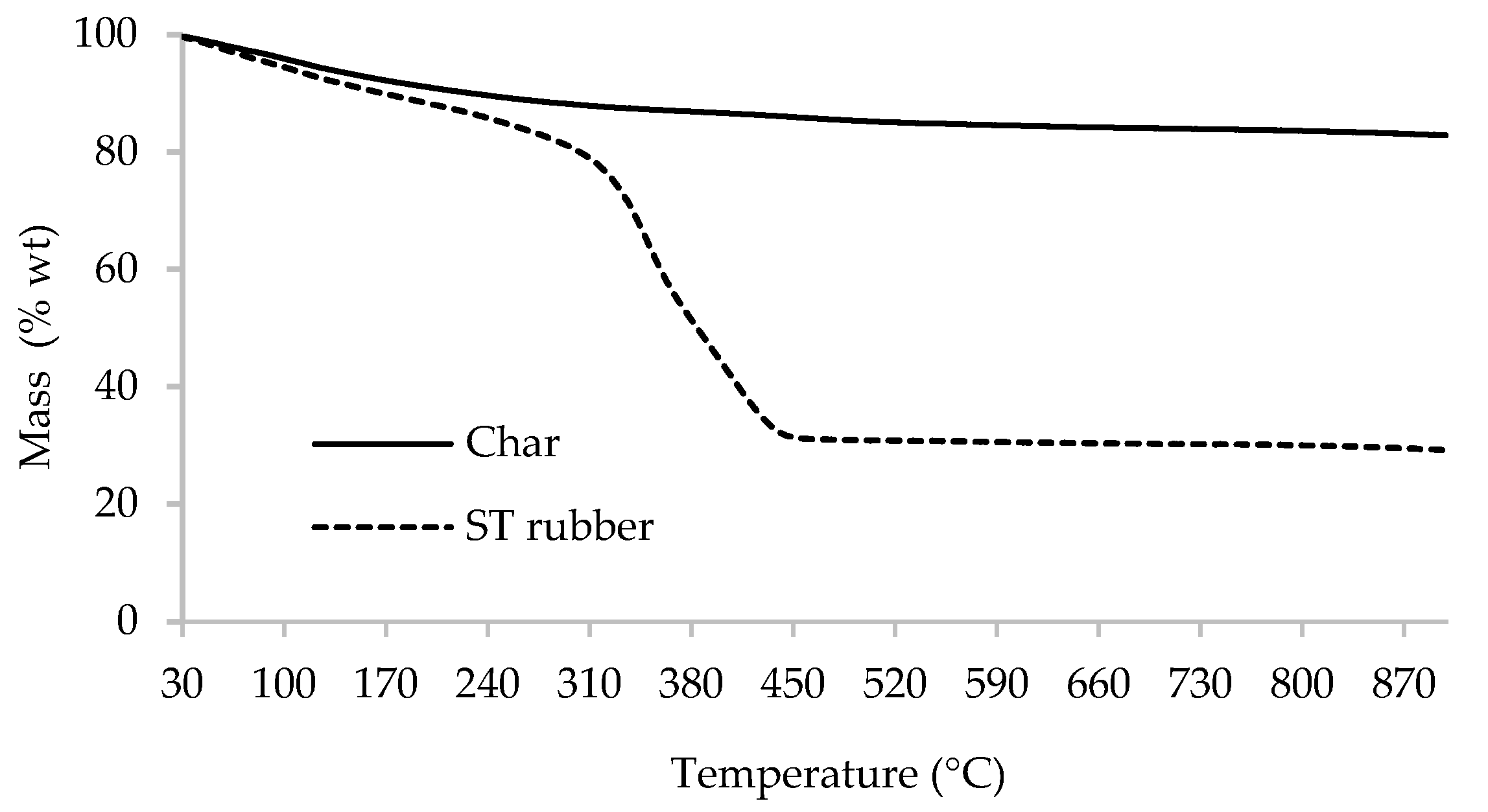
- Thermogravimetric analysis (TGA) between room temperature and 900 °C, with a heating rate of 5 °C/min, under an argon atmosphere (Setaram Labsys EVO equipment, France).
2.2. Pyrolysis Assays and Chars
- (i)
- Proximate analysis, elemental analysis, and TGA as described above;
- (ii)
- Mineral content, performed according to the European Standard EN 15290 in samples previously digested (3 cm3 H2O2 30% v/v + 8 cm3 HNO3 65% v/v + 2 cm3 HF 40% v/v) in a microwave station (Milestone Ethos 1600 Microwave Labstation, Italy) and neutralized (20 cm3 H3BO3 4% w/v). The acidic solutions were analyzed by inductively coupled plasma–atomic emission spectroscopy (ICP-AES) (Horiba Jobin-Yvon equipment, France) for the quantification of several chemical elements;
- (iii)
- pHPZC, for which 20 mL of NaCl 0.1 M were transferred to polyethylene bottles for pH adjustment with HCl 0.1 M or NaOH 0.1 M. The assay was performed with different initial pH values: 2, 4, 6, 8, 10, and 12. After pH adjustment, 0.1 g of char was added to each bottle. These mixtures were then agitated for 24 h in a roller-table device. After mixing, all samples were filtrated, and the final pH was then measured. The pHPZC value corresponds to the plateau of the curve pHfinal versus pHinitial;
- (iv)
- X-ray powder diffraction (XRPD), in which the diffractogram was obtained using a benchtop X-ray diffractometer (RIGAKU, model MiniFlex II, USA), with a Cu X-ray tube (30 kV/15 mA) by continuous scanning from 15° to 80° (2Ɵ) with a step size of 0.01° (2Ɵ) and scan speed of 2°/min;
- (v)
- Scanning electron microscopy with energy-dispersive X-ray spectrometry (SEM-EDS), for which the surface elemental composition and morphology of the char were analyzed using a JEOL 7001F analytical FEG-SEM with Oxford model INCA 250 PREMIUM EBSD (electron backscatter diffraction) and energy-dispersive X-ray spectrometer light element detector attachments (Japan);
- (vi)
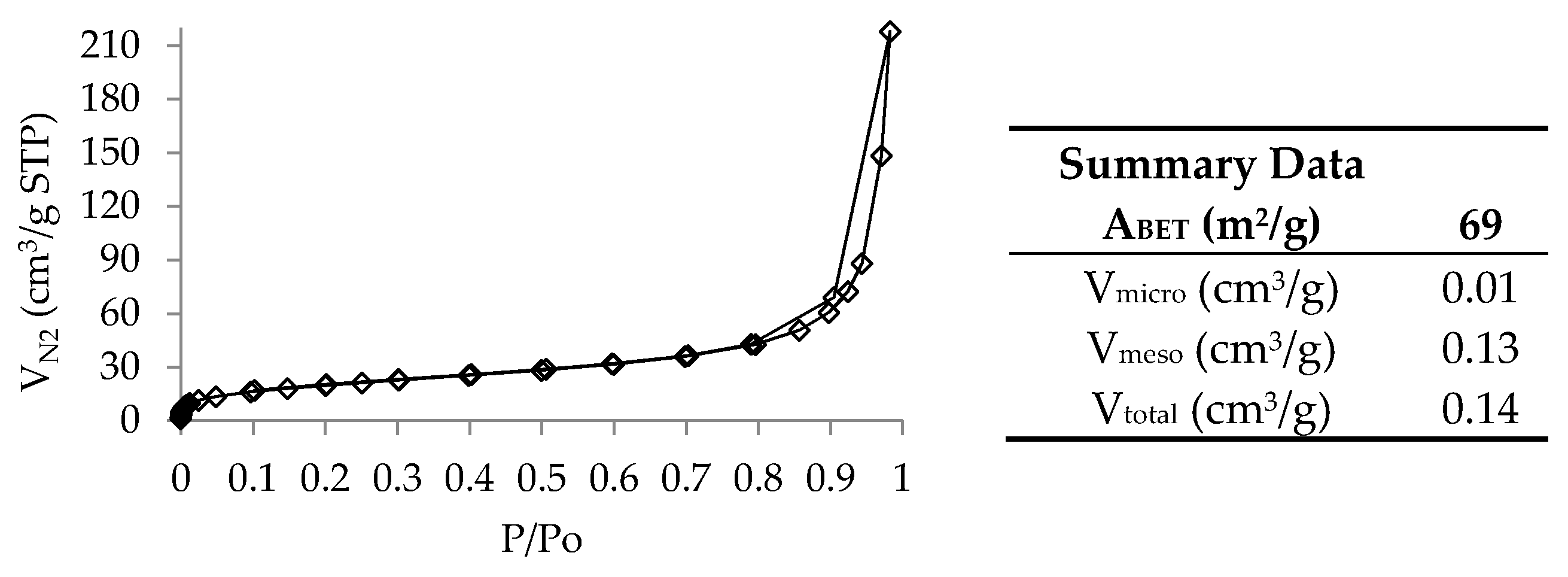
- N2 adsorption–desorption isotherms at 77 K, for which the isotherms were obtained in a ASAP 2010 Micromeritics equipment (USA) and allowed to calculate the following parameters: (1) apparent surface area using the BET (Brunauer–Emmett–Teller) equation (ABET); (2) total pore volume determined by the amount of nitrogen adsorbed at P/P0 = 0.95 (Vtotal); (3) micropore volume using the t-plot method (Vmicro); and (4) mesopore volume by the difference between Vtotal and Vmicro. Prior to this analysis, the samples were outgassed overnight, under vacuum, at 150 °C.
2.3. Adsorption Assays
3. Results and Discussion
3.1. ST Rubber and Char Characterization
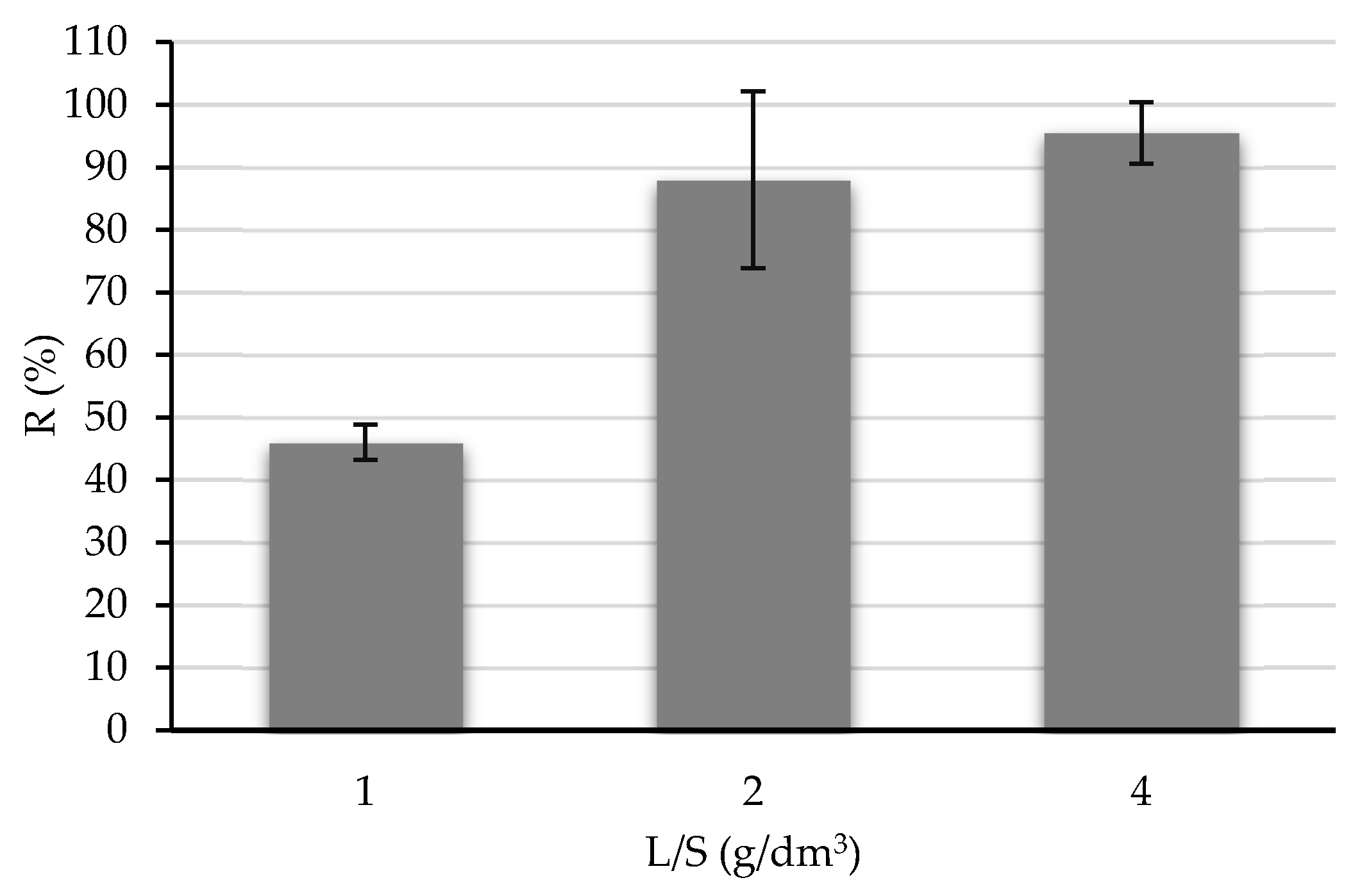
3.2. Adsorption Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valorpneu - Sociedade de Gestão de Pneus, LDA. Relatorio Anual e Contas 2017; Valorpneu - Sociedade de Gestão de Pneus, LDA: Lisboa, Portugal, 2017. [Google Scholar]
- ETRMA - European Tyre and Rubber Manufacturers Association. ELT Management Figures 2016; ETRMA - European Tyre and Rubber Manufacturers Association: Brussels, Belgium, 2018. [Google Scholar]
- Parthasarathy, P.; Choi, H.S.; Park, H.C.; Hwang, J.G.; Yoo, H.S.; Lee, B.-K.; Upadhyay, M. Influence of Process Conditions on Product Yield of Waste Tyre Pyrolysis- A Review. Korean J. Chem. Eng. 2016, 33, 2268–2286. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Karthikeyan, S. Recycling of Waste Tires and Its Energy Storage Application of By-Products – a Review. Sustain. Mater. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Processing Methods, Characteristics and Adsorption Behavior of Tire Derived Carbons: A Review. Adv. Colloid Interface Sci. 2014, 211, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, N.; Stavropoulos, G.; Zabaniotou, A. Activation of End of Life Tyres Pyrolytic Char for Enhancing Viability of Pyrolysis – Critical Review, Analysis and Recommendations for a Hybrid Dual System. Renew. Sustain. Energy Rev. 2014, 39, 1053–1073. [Google Scholar] [CrossRef]
- Makrigianni, V.; Giannakas, A.; Hela, D.; Papadaki, M.; Konstantinou, I. Adsorption of Methylene Blue Dye by Pyrolytic Tire Char in Fixed-Bed Column. Desalin. WATER Treat. 2017, 65, 346–358. [Google Scholar] [CrossRef]
- Makrigianni, V.; Giannakas, A.; Deligiannakis, Y.; Konstantinou, I. Adsorption of Phenol and Methylene Blue from Aqueous Solutions by Pyrolytic Tire Char: Equilibrium and Kinetic Studies. J. Environ. Chem. Eng. 2015, 3, 574–582. [Google Scholar] [CrossRef]
- Kiernan, J. Classification and Naming of Dyes, Stains and Fluorochromes. Biotech. Histochem. 2001, 76, 261–278. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO 2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Ratna, P.B. Pollution Due to Synthetic Dyes Toxicity & Carcinogenicity Studies and Remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of Azo Dyes in the Context of Environmental Processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Mondal, S.; Purkait, M.K.; De, S. Advances in Dye Removal Technologies; Green Chemistry and Sustainable Technology; Springer Singapore: Singapore, 2018. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Aftab, T.; Bashir, F.; Khan, R.A.; Iqbal, J. Treatment of Color through the Adsorption Efficiency of Waste Tire-Derived Char Using Response Surface Methodology. Desalin. Water Treat. 2016, 57, 10324–10332. [Google Scholar] [CrossRef]
- Chan, O.S.; Cheung, W.H.; McKay, G. Single and Multicomponent Acid Dye Adsorption Equilibrium Studies on Tyre Demineralised Activated Carbon. Chem. Eng. J. 2012, 191, 162–170. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Gupta, G.S.; Prasad, G.; Rupainwar, D.C. Use of Wollastonite in the Removal of Ni(II) from Aqueous Solutions. Water. Air. Soil Pollut. 1990, 49, 69–79. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 249–275. [Google Scholar] [CrossRef]
- Russo, V.; Trifuoggi, M.; Di Serio, M.; Tesser, R. Fluid-Solid Adsorption in Batch and Continuous Processing: A Review and Insights into Modeling. Chem. Eng. Technol. 2017, 40, 799–820. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. In The Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 2013; pp. 337–381. [Google Scholar] [CrossRef]
- Zhou, Q.; Zarei, A.; De Girolamo, A.; Yan, Y.; Zhang, L. Catalytic Performance of Scrap Tyre Char for the Upgrading of Eucalyptus Pyrolysis Derived Bio-Oil via Cracking and Deoxygenation. J. Anal. Appl. Pyrolysis 2019, 139, 167–176. [Google Scholar] [CrossRef]
- Preciado-Hernandez, J.; Zhang, J.; Zhu, M.; Zhang, Z.; Zhang, D. An Experimental Study of CO2 Gasification Kinetics during Activation of a Spent Tyre Pyrolysis Char. Chem. Eng. Res. Des. 2019, 149, 129–137. [Google Scholar] [CrossRef]
- Chan, O.S.; Cheung, W.H.; McKay, G. Preparation and Characterisation of Demineralised Tyre Derived Activated Carbon. Carbon N. Y. 2011, 49, 4674–4687. [Google Scholar] [CrossRef]
- Husár, J.; Haydary, J.; Šuhaj, P.; Steltenpohl, P. Potential of Tire Pyrolysis Char as Tar-Cracking Catalyst in Solid Waste and Biomass Gasification. Chem. Pap. 2019, 73, 2091–2101. [Google Scholar] [CrossRef]
- Han, J.; Li, W.; Liu, D.; Qin, L.; Chen, W.; Xing, F. Pyrolysis Characteristic and Mechanism of Waste Tyre: A Thermogravimetry-Mass Spectrometry Analysis. J. Anal. Appl. Pyrolysis 2018, 129, 1–5. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of Waste Tyres: A Review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Passaponti, M.; Rosi, L.; Savastano, M.; Giurlani, W.; Miller, H.A.; Lavacchi, A.; Filippi, J.; Zangari, G.; Vizza, F.; Innocenti, M. Recycling of Waste Automobile Tires: Transforming Char in Oxygen Reduction Reaction Catalysts for Alkaline Fuel Cells. J. Power Sources 2019, 427, 85–90. [Google Scholar] [CrossRef]
- Seng-eiad, S.; Jitkarnka, S. Untreated and HNO 3 -Treated Pyrolysis Char as Catalysts for Pyrolysis of Waste Tire: In-Depth Analysis of Tire-Derived Products and Char Characterization. J. Anal. Appl. Pyrolysis 2016, 122, 151–159. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, W.-C.; Yuan, C.-S.; Hung, C.-H. Surface Functional Characteristics (C, O, S) of Waste Tire-Derived Carbon Black before and after Steam Activation. J. Air Waste Manage. Assoc. 2008, 58, 78–84. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Rodríguez, O.; Alguacil, F.J. Preparation and Characterization of Activated Carbon from the Char Produced in the Thermolysis of Granulated Scrap Tyres. J. Air Waste Manage. Assoc. 2013, 63, 534–544. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Li, A.; Irfan, M.; Du, Y.; Di, W. Comparative Pyrolysis Behaviors of Tire Tread and Side Wall from Waste Tire and Characterization of the Resulting Chars. J. Environ. Manage. 2019, 232, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K. Reactive and Basic Dyes Removal by Sorption onto Chitosan Derivatives. J. Colloid Interface Sci. 2009, 331, 32–39. [Google Scholar] [CrossRef]
- Bernardo, M.; Mendes, S.; Lapa, N.; Gonçalves, M.; Mendes, B.; Pinto, F.; Lopes, H.; Fonseca, I. Removal of Lead (Pb2+) from Aqueous Medium by Using Chars from Co-Pyrolysis. J. Colloid Interface Sci. 2013, 409, 158–165. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A Comparative Investigation on Adsorption Performances of Mesoporous Activated Carbon Prepared from Waste Rubber Tire and Activated Carbon for a Hazardous Azo Dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef]
- Karadag, D.; Akgul, E.; Tok, S.; Erturk, F.; Kaya, M.A.; Turan, M. Basic and Reactive Dye Removal Using Natural and Modified Zeolites. J. Chem. Eng. Data 2007, 52, 2436–2441. [Google Scholar] [CrossRef]
- Ozdemir, O.; Armagan, B.; Turan, M.; Çelik, M.S. Comparison of the Adsorption Characteristics of Azo-Reactive Dyes on Mezoporous Minerals. Dye. Pigment. 2004, 62, 49–60. [Google Scholar] [CrossRef]
- Pengthamkeerati, P.; Satapanajaru, T.; Singchan, O. Sorption of Reactive Dye from Aqueous Solution on Biomass Fly Ash. J. Hazard. Mater. 2008, 153, 1149–1156. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; Valix, M.; McKay, G. Dye Adsorption onto Activated Carbons from Tyre Rubber Waste Using Surface Coverage Analysis. J. Colloid Interface Sci. 2010, 347, 290–300. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; Valix, M.; McKay, G. Mesoporous Activated Carbon from Waste Tyre Rubber for Dye Removal from Effluents. Microporous Mesoporous Mater. 2010, 130, 287–294. [Google Scholar] [CrossRef]
- Órfão, J.J.M.; Silva, A.I.M.; Pereira, J.C.V.; Barata, S.A.; Fonseca, I.M.; Faria, P.C.C.; Pereira, M.F.R. Adsorption of a Reactive Dye on Chemically Modified Activated Carbons—Influence of PH. J. Colloid Interface Sci. 2006, 296, 480–489. [Google Scholar] [CrossRef] [PubMed]

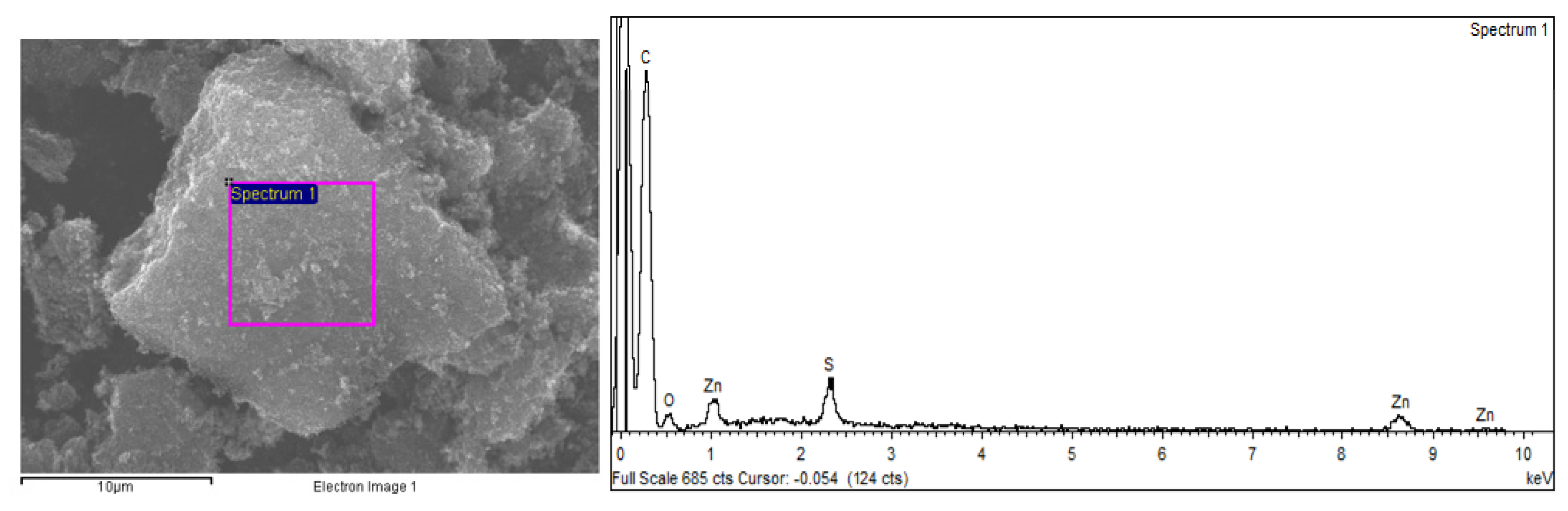
| Parameter | Moisture | Volatile Matter | Fixed Carbon b | Ashes | C | H | N | S | pHPZC |
|---|---|---|---|---|---|---|---|---|---|
| ST rubber | 0.43 | 68.9 | 27.7 | 2.96 | 86.8 | 7.36 | 0.00 | 1.23 | n.d. |
| Char | 0.70 | 16.7 | 69.7 | 12.9 | 86.7 | 0.83 | 0.00 | 2.67 | 6.80 |
| Element | mg/kg db (X ± σ) |
|---|---|
| Zn | 37,543 ± 5251 |
| Si | 11,212 ± 1011 |
| Al | 2800 ± 335 |
| Na | 2534 ± 765 |
| Ba | 1882 ± 195 |
| K | 1796 ± 477 |
| Ca | 1588 ± 495 |
| Mg | 576 ± 55.5 |
| P | 501 ± 53.2 |
| Ni | 123 ± 28.6 |
| Ti | 110 ± 16 |
| Pb | 42.4 ± 5.33 |
| Sb | 10.1 ± 1.45 |
| Mo | 7.33 ± 1.24 |
| As | 5.99 ± 1.46 |
| Cd | 3.53 ± 0.441 |
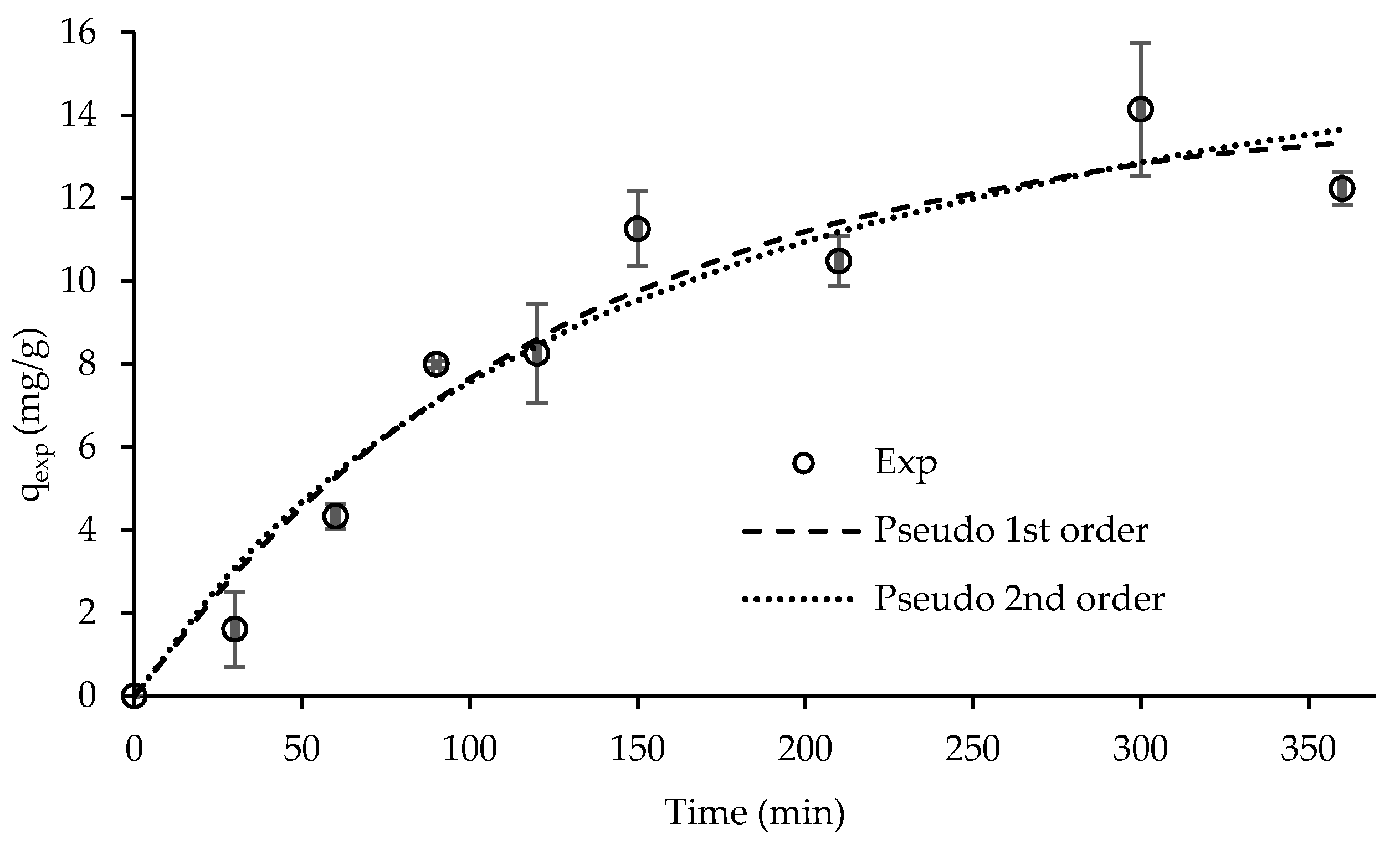
| Pseudo-First Order Kinetic Model | Pseudo-Second Order Kinetic Model | ||||
| R2 | qe, calc (mg/g) | k1 (1/min) | R2 | qe, calc (mg/g) | k2 (g/(mg.min)) |
| 0.952 | 14.2 | 0.008 | 0.942 | 19.8 | 0.0003 |
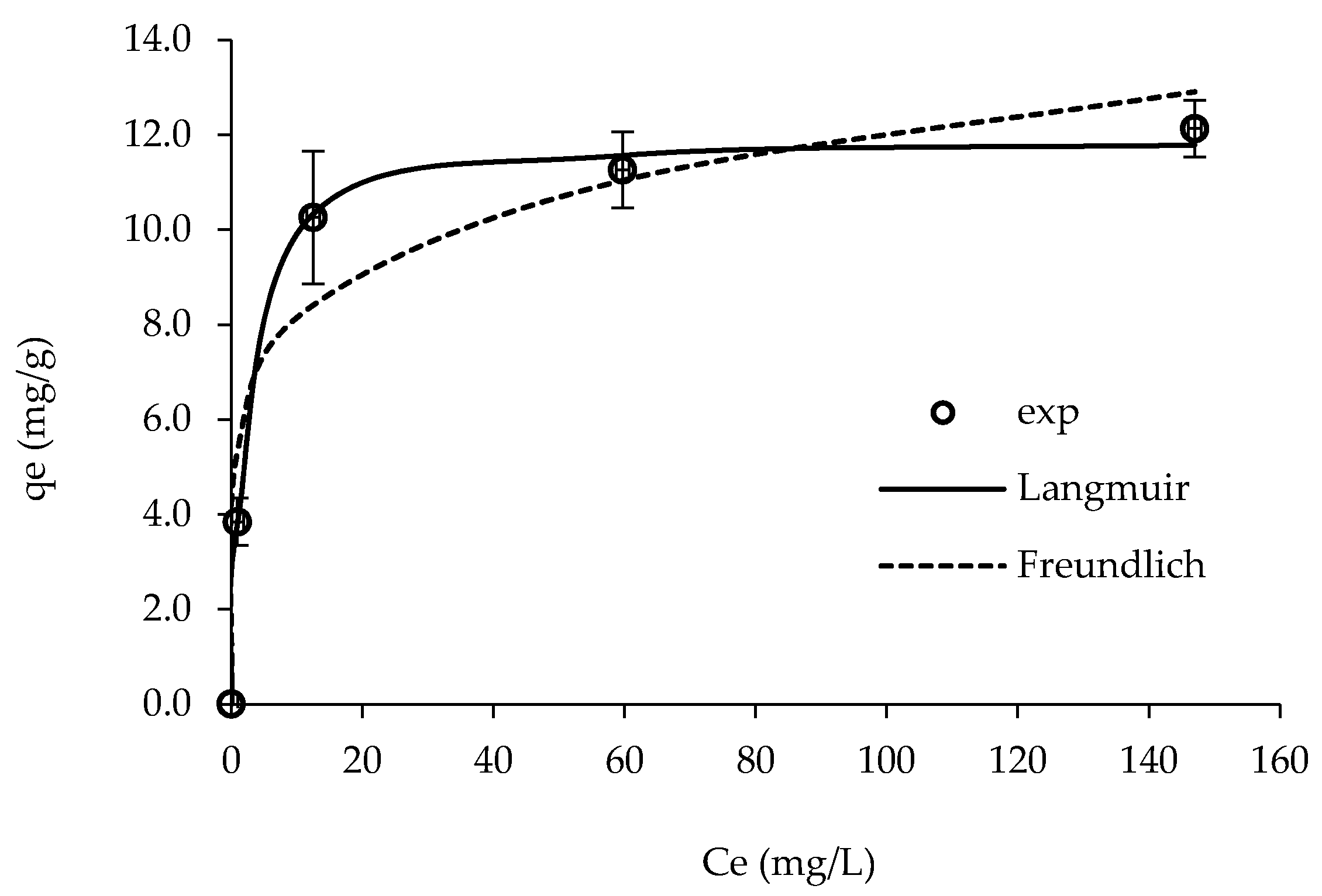
| Langmuir | Freundlich | ||||
| R2 | qmax (mg/g) | KL (L/mg) | R2 | KF (mg (1−1/n) L1/n/g) | 1/n |
| 0.998 | 11.9 | 0.514 | 0.945 | 5.43 | 0.174 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, M.; Matos, I.; Bernardo, M.; Pinto, F.; Lapa, N.; Surra, E.; Fonseca, I. Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye. C 2019, 5, 76. https://doi.org/10.3390/c5040076
Nogueira M, Matos I, Bernardo M, Pinto F, Lapa N, Surra E, Fonseca I. Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye. C. 2019; 5(4):76. https://doi.org/10.3390/c5040076
Chicago/Turabian StyleNogueira, Miguel, Inês Matos, Maria Bernardo, Filomena Pinto, Nuno Lapa, Elena Surra, and Isabel Fonseca. 2019. "Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye" C 5, no. 4: 76. https://doi.org/10.3390/c5040076
APA StyleNogueira, M., Matos, I., Bernardo, M., Pinto, F., Lapa, N., Surra, E., & Fonseca, I. (2019). Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye. C, 5(4), 76. https://doi.org/10.3390/c5040076