Near-Infrared, Light-Triggered, On-Demand Anti-Inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
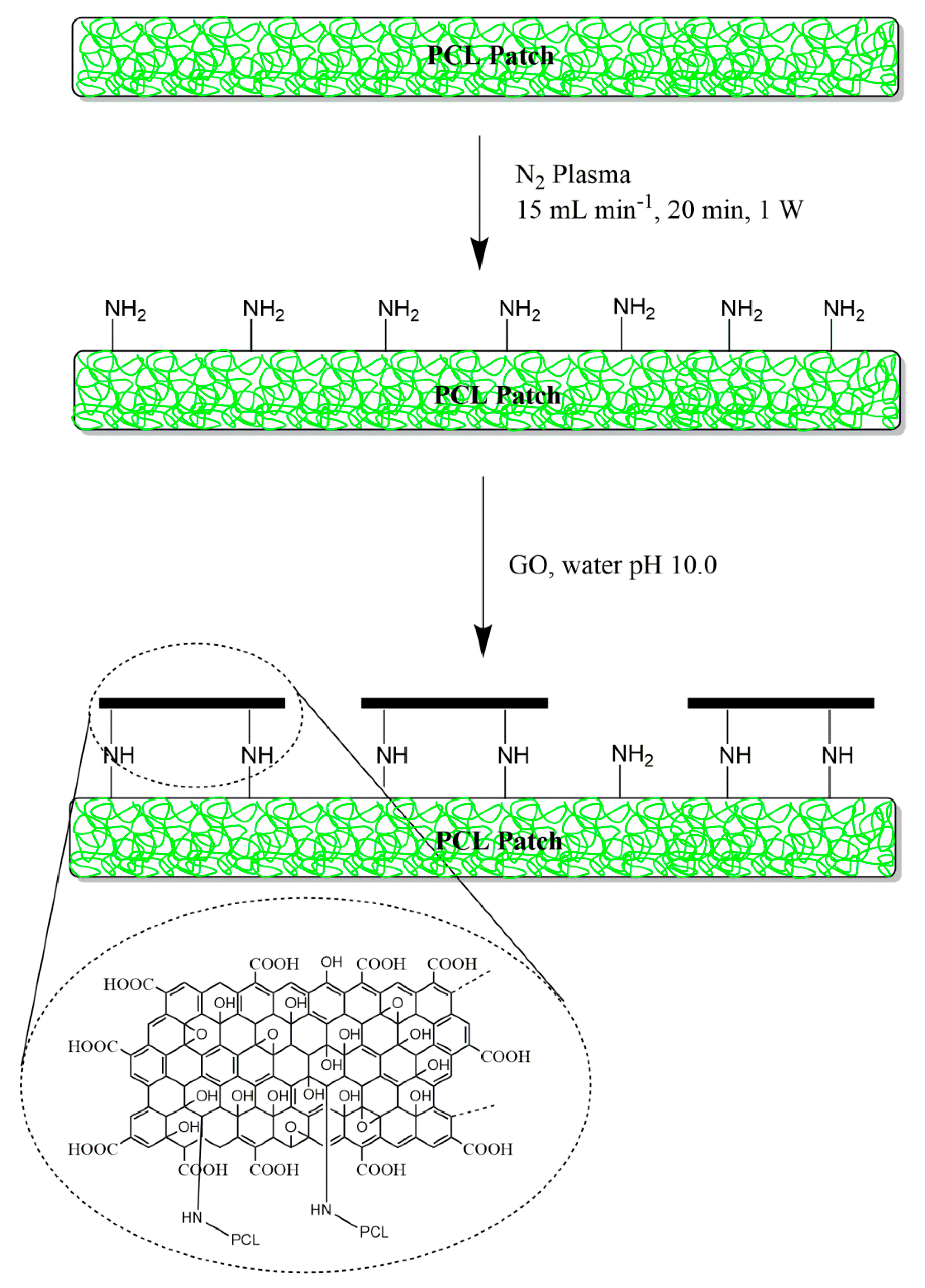
2.2. Scaffold Preparation
2.3. Attenuated Total Reflectance Infrared Spectroscopy (FTIR-ATR)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Scanning Electron Microscopy (SEM)
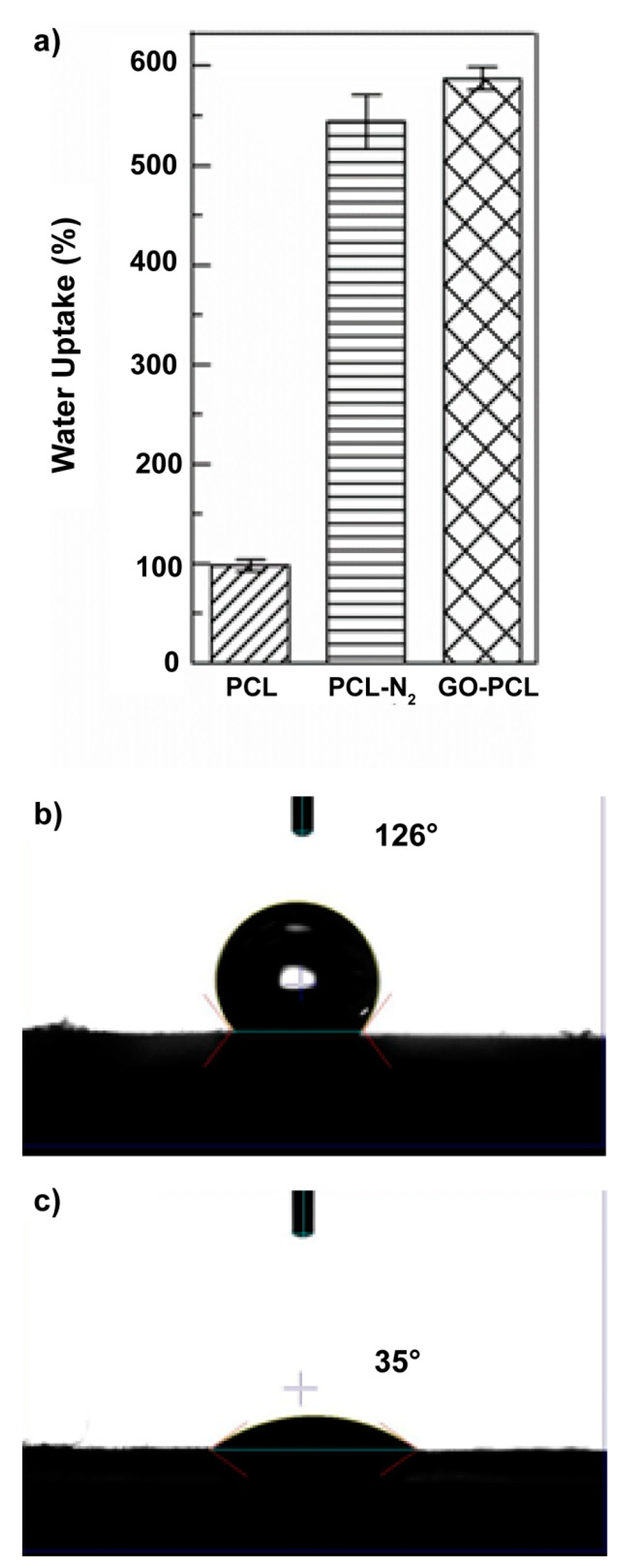
2.6. Water Uptake
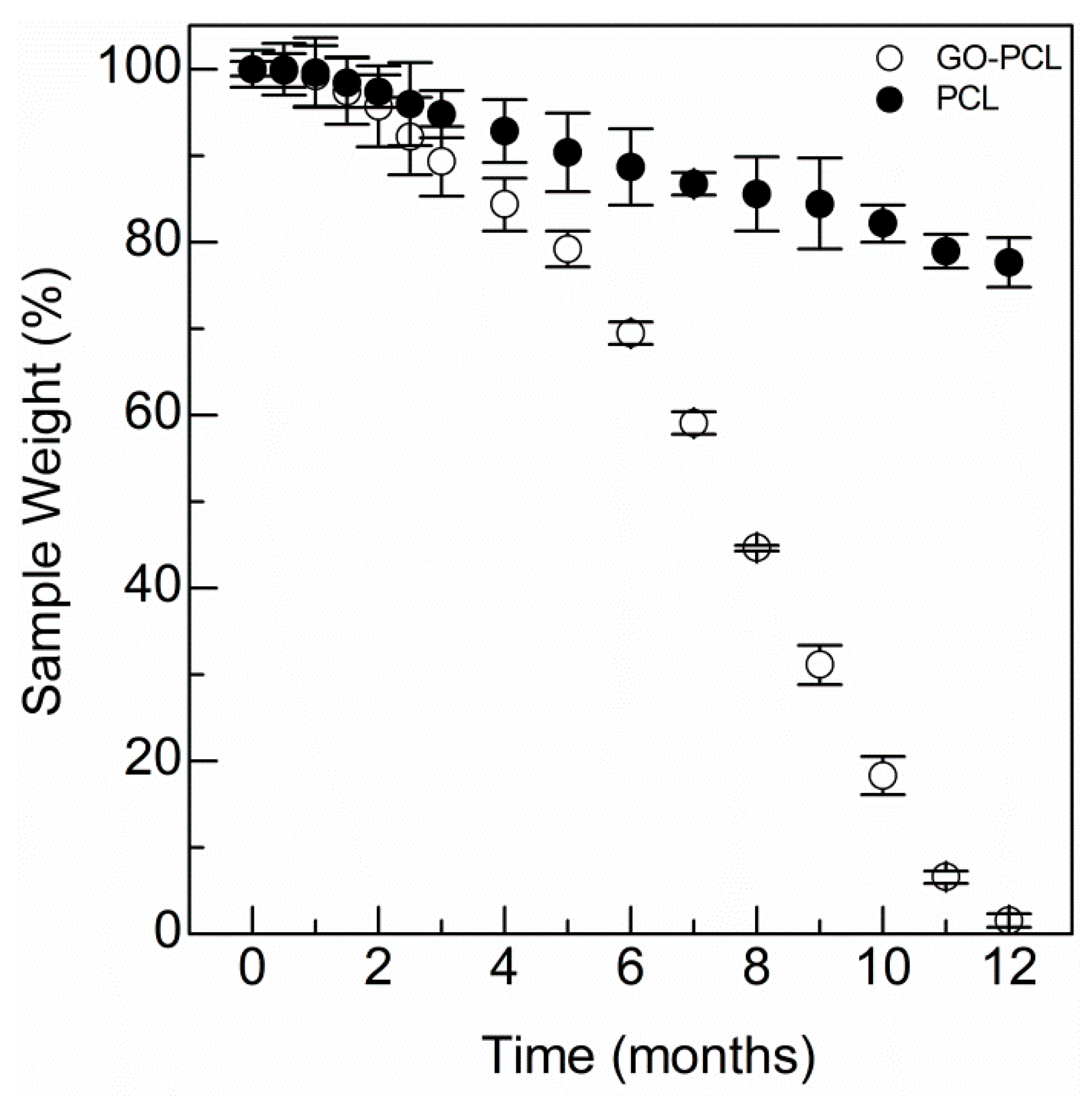
2.7. Degradation Time
2.8. Water Contact Angle Measurement
2.9. Drug Loading and Drug Release Kinetics
2.10. Cell Adhesion Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Surface Functionalization of PCL Patch with GO Via Nonthermal Low-Vacuum Nitrogen Plasma
3.2. GO Allows Water Uptake and Accelerates Degradation of the Scaffold
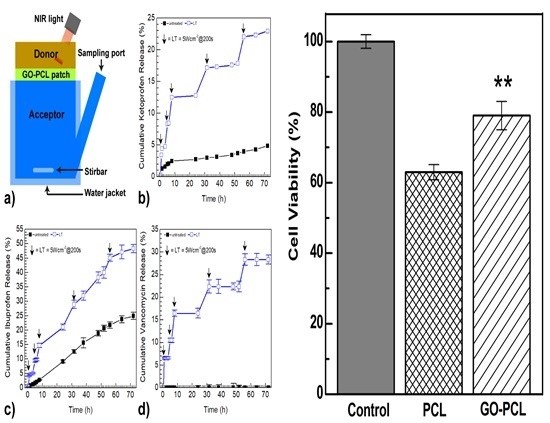
3.3. GO Coating Improves the Drug Loading and can Promote On-Demand Drug Release
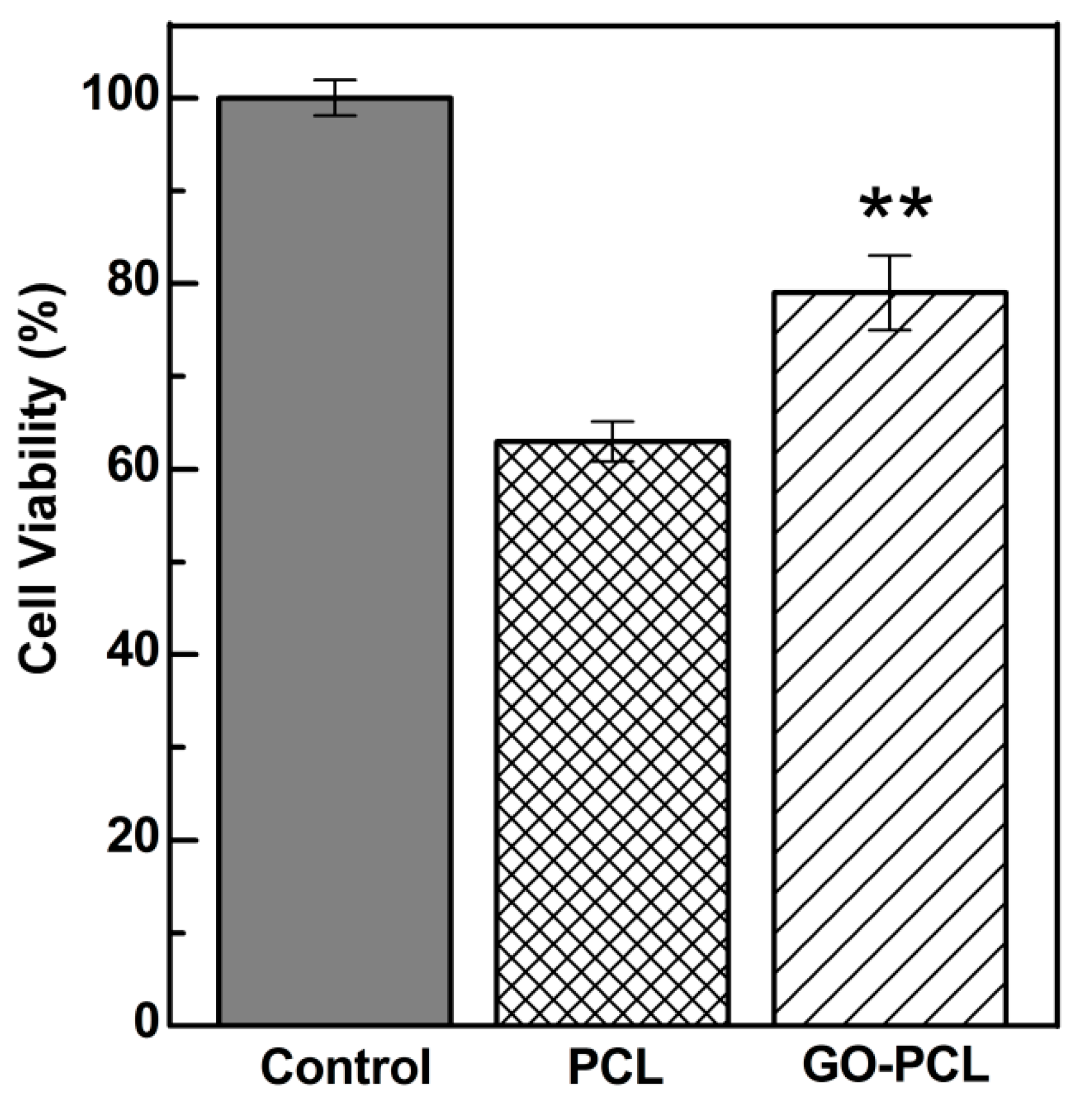
3.4. GO Can Foster Wound Healing by Improving Cell Adhesion and Proliferation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Vuerstaek, J.D.D.; Vainas, T.; Wuite, J.; Nelemans, P.; Neumann, M.H.A.; Veraart, J.C.J.M. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J. Vasc. Surg. 2006, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel Biodegradable Porous Scaffold Applied to Skin Regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 2018, 6, 4. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B. 2016, 4, 4801–4812. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Venugopal, J.; Sundarrajan, S.; Ramakrishna, S. Fabrication of a nanofibrous scaffold with improved bioactivity for culture of human dermal fibroblasts for skin regeneration. Biomed. Mater. 2011, 6, 015001. [Google Scholar] [CrossRef]
- Huang, C.H.; Chi, C.Y.; Chen, Y.S.; Chen, K.Y.; Chen, P.L.; Yao, C.H. Evaluation of proanthocyanidin-crosslinked electrospun gelatin nanofibers for drug delivering system. Mater. Sci. Eng. C 2012, 32, 2476–2483. [Google Scholar] [CrossRef]
- Ye, K.; You, M.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A Textile Dressing for Temporal and Dosage Controlled Drug Delivery. Adv. Funct. Mater. 2017, 27, 1702399. [Google Scholar] [CrossRef]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Mauro, N.; Li Volsi, A.; Scialabba, C.; Licciardi, M.; Cavallaro, G.; Giammona, G. Photothermal ablation of cancer cells using folate-coated gold/grapheme oxide composite. Curr. Drug Deliv. 2017, 14, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Scialabba, C.; Cavallaro, G.; Licciardi, M.; Giammona, G. Biotin-containing reduced graphene oxide-based nanosystem as a multieffect anticancer agent: Combining hyperthermia with targeted chemotherapy. Biomacromolecules 2015, 16, 2766–2775. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Jiang, W.; Liu, R.; Li, Z.; Luan, Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 2018, 89, 15–24. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.L.; Larosa, J.M.; Luo, X.; Cui, X.T. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef]
- Chang, S.-J.; Hyun, M.S.; Myung, S.; Kang, M.-A.; Yoo, J.H.; Lee, K.G.; Choi, B.G.; Cho, Y.; Park, G.L.J. Graphene growth from reduced graphene oxide by chemical vapour deposition: Seeded growth accompanied by restoration. Sci. Rep. 2016, 6, 22653. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Koepsell, L.; Zhang, L.; Neufeld, D.; Fong, H.; Deng, Y. Electrospun Nanofibrous Polycaprolactone Scaffolds for Tissue Engineering of Annulus Fibrosus. Macromol. Biosci. 2011, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, C.; Bloise, N.; Mauro, N.; Ferruti, P.; Manfredi, A.; Sampaolesi, M.; Liguori, A.; Laurita, R.; Gherardi, M.; Colombo, V.; et al. Poly-l-Lactic Acid Nanofiber-Polyamidoamine Hydrogel Composites: Preparation, Properties, and Preliminary Evaluation as Scaffolds for Human Pluripotent Stem Cell Culturing. Macromol. Biosci. 2016, 16, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Scialabba, C.; Pitarresi, G.; Giammona, G. Enhanced adhesion and in situ photothermal ablation of cancer cells in surface-functionalized electrospun microfiber scaffold with graphene oxide. Int. J. Pharm. 2017, 526, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Manfredi, A.; Ranucci, E.; Procacci, P.; Laus, M.; Antonioli, D.; Mantovani, C.; Magnaghi, V.; Ferruti, P. Degradable Poly(amidoamine) Hydrogels as Scaffolds for In Vitro Culturing of Peripheral Nervous System Cells. Macromol. Biosci. 2013, 13, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Tonna, N.; Bianco, F.; Matteoli, M.; Cagnoli, C.; Antonucci, F.; Manfredi, A.; Mauro, N.; Ranucci, E.; Ferruti, P. A soluble biocompatible guanidine-containing polyamidoamine as promoter of primary brain cell adhesion and in vitro cell culturing. Sci. Technol. Adv. Mater. 2014, 15, 045007. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, prevailingly cationic L-arginine polymers of poly(amidoamino acid) structure: Synthesis, acid/base properties and preliminary cytocompatibility and cell-permeating characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Bak, T.-Y.; Kook, M.-S.; Jung, S.-C.; Kim, B.-H. Biological Effect of Gas Plasma Treatment on CO2 Gas Foaming/Salt Leaching Fabricated Porous Polycaprolactone Scaffolds in Bone Tissue Engineering. J. Nanomater. 2014, 2014, 657542. [Google Scholar] [CrossRef]
- Tunma, S.; Inthanon, K.; Chaiwong, C.; Pumchusak, J.; Wongkham, W.; Boonyawan, D. Improving the attachment and proliferation of umbilical cord mesenchymal stem cells on modified polystyrene by nitrogen-containing plasma. Cytotechnology 2013, 65, 119–134. [Google Scholar] [CrossRef]
- Lopez, L.C.; Belviso, M.R.; Gristina, R.; Nardulli, M.; d’Agostino, R.; Favia, P. Plasma-Treated Nitrogen-Containing Surfaces for Cell Adhesion: The Role of the Polymeric Substrate. Plasma Process. Polym. 2007, 4, S402–S405. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr. Polym. 2018, 193, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide Reduct; Reduction Recipes, Spectroscopy, and Applications; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Zant, E.; Grijpma, D.W. Synthetic Biodegradable Hydrogels with Excellent Mechanical Properties and Good Cell Adhesion Characteristics Obtained by the Combinatorial Synthesis of Photo-Cross-Linked Networks. Biomacromolecules 2016, 17, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Peng, X.; Xu, Z. Understanding Water Permeation in Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2014, 6, 5877–5883. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the degradation of polylactic acid polymer in a solid substrate environment. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Pradhan, S.; Madke, B.; Kabra, P.; Singh, A. Anti-inflammatory and immunomodulatory effects of antibiotics and their use in dermatology. Indian J. Dermatol. 2016, 61, 469–481. [Google Scholar] [CrossRef]
- Ko, T.-J.; Kim, E.; Nagashima, S.; Oh, K.H.; Lee, K.-R.; Kim, S.; Moon, M. Adhesion behavior of mouse liver cancer cells on nanostructured superhydrophobic and superhydrophilic surfaces. Soft Matter 2013, 9, 8705–8711. [Google Scholar] [CrossRef]
- Jeong, J.-T.; Choi, M.-K.; Sim, Y.; Lim, J.-T.; Kim, G.-S.; Seong, M.-J.; Hyung, J.-H.; Kim, K.S.; Umar, A.; Lee, S.-K. Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate. Sci. Rep. 2016, 6, 33835. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.H.; Moon, J.H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]

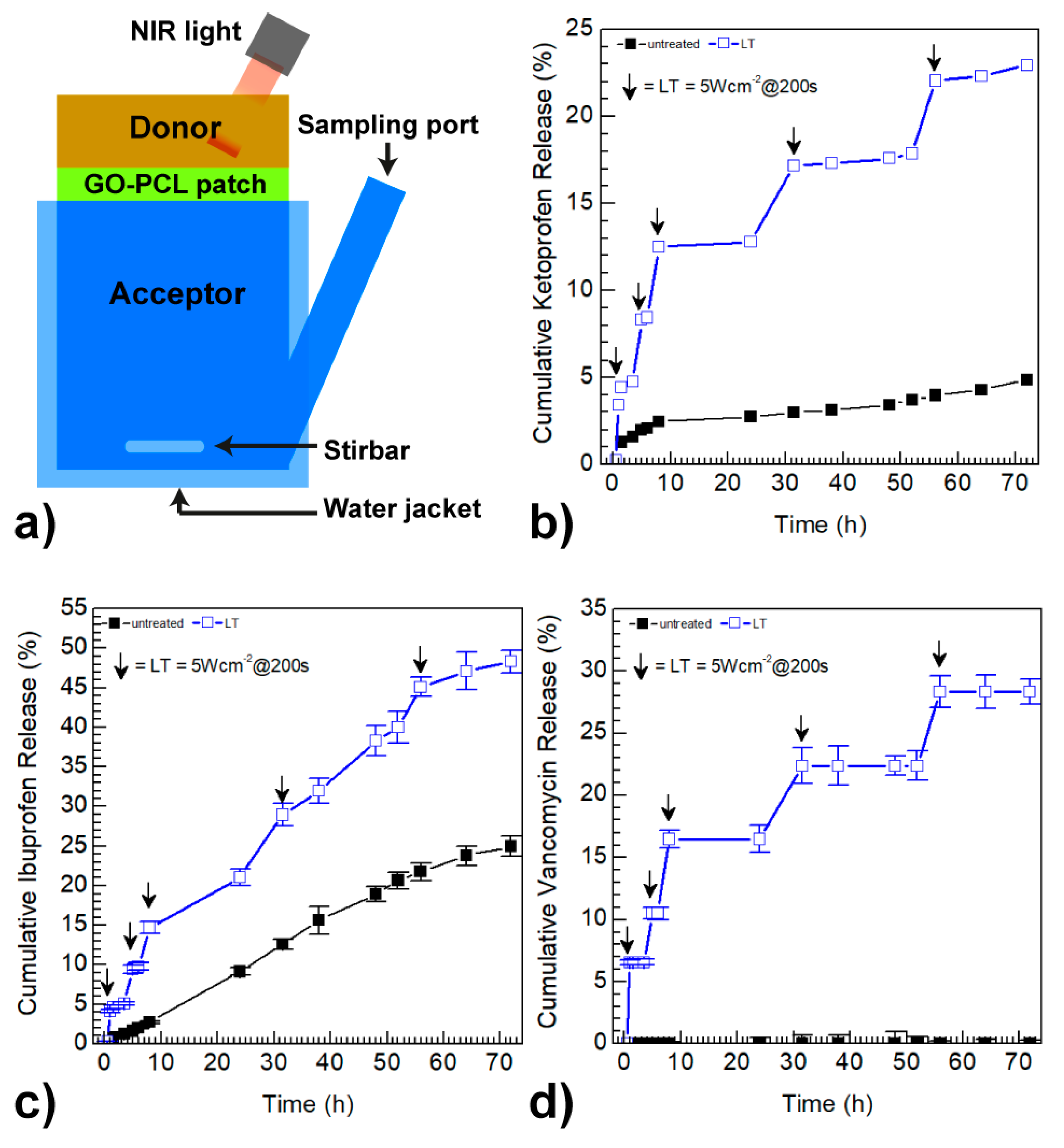
| Sample | Ketoprofen a (mg/cm2) | Ibuprofen a (mg/cm2) | Vancomycin a (mg/cm2) |
|---|---|---|---|
| GO–PCL | 0.86 ± 0.01 | 5.85 ± 0.13 | 0.95 ± 0.05 |
| Keplant® | 0.29 ± 0.02 | n.a. | n.a. |
| Ibupas® | n.a. | 0.89 ± 0.08 | n.a. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, N.; Drago, S.E.; Cavallaro, G.; Giammona, G. Near-Infrared, Light-Triggered, On-Demand Anti-Inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. C 2019, 5, 63. https://doi.org/10.3390/c5040063
Mauro N, Drago SE, Cavallaro G, Giammona G. Near-Infrared, Light-Triggered, On-Demand Anti-Inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. C. 2019; 5(4):63. https://doi.org/10.3390/c5040063
Chicago/Turabian StyleMauro, Nicolò, Salvatore Emanuele Drago, Gennara Cavallaro, and Gaetano Giammona. 2019. "Near-Infrared, Light-Triggered, On-Demand Anti-Inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing" C 5, no. 4: 63. https://doi.org/10.3390/c5040063
APA StyleMauro, N., Drago, S. E., Cavallaro, G., & Giammona, G. (2019). Near-Infrared, Light-Triggered, On-Demand Anti-Inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. C, 5(4), 63. https://doi.org/10.3390/c5040063