Abstract
We report a rational investigation of the selective synthesis of poly(cyclohexene carbonate) from CO2 and cyclohexene oxide by using commercially available Lewis acids with nontoxic metal centers. After a preliminary screening, we focused on the use of zinc salts, and the effect of the pressure, the temperature, the catalyst loading, and the presence of cocatalyst or a solvent on the reaction yields, selectivity, and molar masses was evaluated for selected catalytic platforms. Thus, we found that ZnTosylate in catalytic amounts under solvent- and cocatalyst-free conditions enables the selective synthesis of poly(cyclohexene carbonate) with a molecular weight of about 62.1 kg/mol with about 70% yields at 343 K and 4 MPa. To the best of our knowledge, this is a rare example of high molar mass polycyclohexene carbonates that are moreover obtained under solvent- and cocatalyst-free conditions. The high selectivity of ZnTos towards the formation of poly(cyclohexene carbonate) was interpreted, thanks to in situ FTIR spectroscopy and DFT calculations, as resulting from its ability to coactivate CO2.
1. Introduction
The valorization of carbon dioxide (CO2) as a renewable C1 feedstock to produce fine chemicals or polymers is of growing interest in academic laboratories and industries in the context of sustainable chemistry [1,2,3,4,5,6,7,8,9,10,11]. In this context, the coupling of CO2 with epoxides that leads to the formation of cyclic carbonates or polycarbonates is a highly attractive 100% atom-economic reaction. Both products are valuable chemicals that should be selectively produced in order to minimize the separation and purification steps. The selectivity of this reaction is dependent on the nature of the substrate and the formation of either cyclic carbonates [12,13,14,15], polycarbonates [1,2,16,17,18], or polyethers is strongly related to the choice of an appropriate catalyst (Scheme 1).
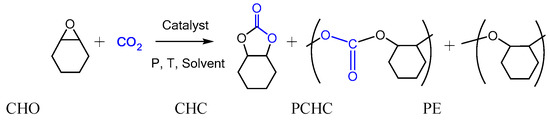
Scheme 1.
The CO2/cyclohexene oxide (CHO) coupling leading to various possible products: cyclic cyclohexene carbonate (CHC), poly(cyclohexene carbonate) (PCHC), and poly(cyclohexene ether) (PE).
The selectivity is also influenced by the reaction conditions (temperature, CO2 pressure) as the activation barrier for the formation of polycarbonates is generally lower and cyclic carbonates are the thermodynamically favored products [4].
The common point between the most efficient catalysts is that they act as a Lewis acid/nucleophile couple, where the Lewis acid activates the epoxide for the ring opening of the epoxide by the nucleophile that allows the insertion of CO2. Then, this intermediate can undergo a ring closure to produce the cyclic carbonate or undergo a nucleophilic attack towards another epoxide leading to the polycarbonate [7]. Thus, a nucleophilic species with a poor leaving ability will favor the growth of the polymer chain, whereas a nucleophile with a good leaving ability will promote the ring closure and formation of the cyclic carbonate product. By the same token, the molar ratio between the nucleophile and the Lewis acid site is expected to play a significant role on the selectivity [19]. Thus, for the selective and efficient synthesis of polycarbonates (PCs) from the copolymerization of CO2 and epoxides, many Lewis acid catalysts such as organometallic complexes usually used in combination with a nucleophile have been developed. In particular, the important characteristics of metal-based catalysts (zinc [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], aluminum [38,39], chromium [40,41,42,43], cobalt [44,45,46,47], magnesium [48,49], iron [19,50,51,52,53,54], titanium [55,56], copper [57], ytterbium [58],…) with a variety of ligands have been thoroughly discussed in recent reviews [59,60,61]. However, many highly active complexes including the types mentioned above are air- and moisture-sensitive, which is a serious limitation to their possible industrial application [62]. In addition, most of these systems require halogen-containing compounds as cocatalyst, such as ammonium and phosphonium salts. More recently, polycarbonates were successfully synthesized for the first time through the anionic copolymerization of epoxides with CO2 under metal-free conditions using an organic Lewis acid (Triethyl borane) in combination with an organic initiator (onium halides or onium alkoxides) [63]. Although very attractive, this protocol still requires a combination of catalysts and the use of a solvent to reach relatively low molar masses (around 30 kg/mol). In the context of green chemistry, some recent investigations were devoted to the copolymerization and terpolymerisation of CO2 with biobased epoxides precursors [64,65,66].
A recent review by Pescarmona et al. [17] offers an updated overview of the catalytic synthesis of polycarbonates, their physicochemical properties, and the growing applications of this class of green polymers. Therefore, this research field is still very active as further improvements are still needed in order to develop robust, active, and selective catalysts while respecting environmental and economic standards and improving the material properties of PCs that generally display low molar masses, hence severely limiting their usefulness. In this context, we have performed a rational investigation of the copolymerization of cyclohexene oxide (CHO) with CO2 in the presence of commercially available Lewis acids under solvent-free conditions (Scheme 2). After a preliminary screening with metal center such as Zn, Mg, Al, Ni, Sc, Y, we focused on the use of commercially available Lewis acids with Zn as a nontoxic metal center. The influence of the pressure, the temperature, the catalyst loading, and the presence of a cocatalyst or a solvent on the reaction yields, selectivity, and molar masses has been evaluated for selected catalytic platforms. Interestingly, we have shown that the presence of ZnTosylate in catalytic amounts under solvent- and cocatalyst-free conditions enables the selective synthesis of poly(cyclohexene carbonate) (PCHC).
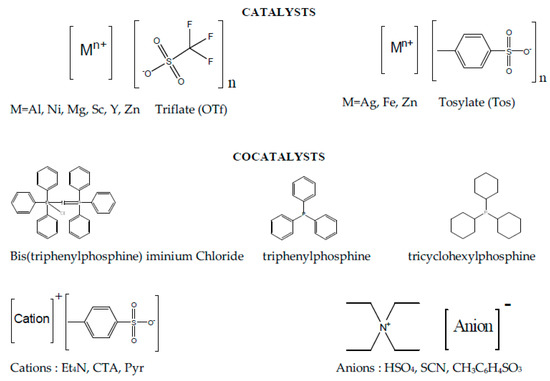
Scheme 2.
Structures of the catalysts and cocatalysts investigated in this study.
2. Materials and Methods
2.1. Materials
Metal triflates complexes, Sc(OTf)3 (Sigma-Aldrich, St. Louis, MO, USA, 99%), Y(OTf)3 (Sigma-Aldrich, 98%), Ni(OTf)2 (Sigma-Aldrich, 96%), Mg(OTf)2 (Sigma-Aldrich, 97%), Al(OTf)3 (Sigma-Aldrich, 99%), and Zn(OTf)2 (Sigma-Aldrich, 98%), were used as received and stored under Argon. Organic cocatalysts, Bis(triphenylphosphine)iminium chloride (PPNCl, Sigma-Aldrich, 97%), Triphenylphosphine (P(Ph)3, AlfaAesar, Haverhil, MA, USA, 99%), Tricyclohexylphosphine (P(Cy)3, AlfaAesar, 96%), Tetraethylammonium hydrogensulfate (Et4NHSO4, Fluka, Charlotte, NC, USA, 99%), Tetraethylammonium thiocyanate (Et4NSCN, Fluka, 99%), Tetraethylammonium p-toluenesulfonate (Et4NTos, Fluka, 99%), Cetyltrimethylammonium p-toluenesulfonate (CTATos, Sigma-Aldrich, 99%), and Pyridinium p-toluenesulfonate (PyrTos, AlfaAesar, 98%), were used as received and stored under Argon. Metal tosylates complexes, Zincp-toluenesulfonate hydrate (ZnTos xH2O, AlfaAesar), Silver p-toluenesulfonate (AgTos, AlfaAesar, 98%), and Iron p-toluenesulfonate hexahydrate (FeTos 6H2O, Sigma-Aldrich), were stored under Argon. AgTos was used as received and anhydrous ZnTos and FeTos were obtained after drying for 1 h under vacuum at 160 °C using the protocol described in reference [67]. Carbon dioxide with a purity of 99.95% was purchased from Air Liquide and used as received.
2.2. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
Fourier transform infrared spectra were recorded using a Nicolet iS50 spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a transmission or with a diamond attenuated transmission reflectance (ATR) device. Spectra were obtained in ATR mode as a result of 200 spectra in the range of 4000–400 cm−1 with a nominal resolution of 4 cm−1.
2.3. High-Pressure Transmission Infrared Spectroscopy
In order to measure the spectrum of CO2 in interaction with selected catalysts investigated in this study, we dispersed the solid catalyst in a KBr pellet (10 wt % of the catalyst, typically 0.012 g in 0.11 g of KBr). Then, the pellet was “stuck up” between the two ZnSe windows of a high-pressure transmission homemade cell (see ref [68] for details). A spectrum of the catalysts-loaded KBr pellet under atmospheric pressure was taken using the FTIR spectrometer described above (32 scans, 4 cm−1 resolution, in transmission mode) after which CO2 was introduced into the cell at a pressure of 4 MPa.
2.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
NMR samples were prepared by dissolving about 5 mg of product in 600 µL of deuterated chloroform (CDCl3, D = 99.96%, Euriso-top, Saint-Aubin, France). The NMR experiment was recorded on Brüker Avance 600 (Billerica, MA, USA) equipped with 5 mm BBI probe. 1H NMR spectra was obtained at 600.16 MHz and chemical shifts from proton NMR spectra are reported in ppm relative to the CDCl3 peak at 7.26 ppm. 1H NMR experiment was recorded using pulse sequences available from the Bruker sequence library. All NMR spectra were phased and baseline-corrected.
2.5. Steric Extrusion Chromatography (SEC)
SEC analyses were performed in Tetrahydrofuran (THF) (25 °C) on an Agilent PL GPC50 (Santa Clara, CA, USA)with four TSK columns: HXL-L (guard column), G4000HXL (particles of 5 mm, pore size of 200 A, and exclusion limit of 400,000 g/mol), G3000HXL (particles of 5 mm, pore size of 75 A, and exclusion limit of 60,000 g/mol), G2000HXL (particles of 5 mm, pore size of 20 A, and exclusion limit of 10,000 g/mol) at an elution rate of 1 mL/min. The elution times of the filtered samples were monitored using UV and RI detectors and SEC was calibrated using polystyrene standards.
2.6. Differential Scanning Calorimetry (DSC)
DSC thermograms were measured using a DSC Q100 apparatus from TA instruments (New Castle, UK). For each sample, two cycles from –50 to 200 °C at 10 °C∙min−1 were performed and then the glass transition and melting temperatures were calculated from the second heating run. Thermogravimetric (TGA) analyses were performed on TGA-Q50 system from TA instruments at a heating rate of 10 °C∙min−1 under nitrogen atmosphere from room temperature to 600 °C.
2.7. Coupling of Cyclohexene Oxide with CO2: Catalysts Screening
In a representative experiment, a stainless-steel autoclave with a nominal volume of 2 mL, equipped with a magnetic rod, a manometer, and a gas inlet/outlet, was charged with 0.5 mL (0.0049 mol) of cyclohexene oxide and 1 mol % of catalyst. The autoclave was equilibrated at 70 °C for 30 min. Then, the reaction was allowed to run during 20 h at constant CO2 pressure of 4 MPa. The crude reaction mixture was analyzed by the ATR-IR set-up described above in paragraph 2.2. The conversion and the selectivity towards the formation of the end-products reported in the tables below have been determined by ATR-IR. Indeed, as reported previously, ATR-IR spectroscopy is better adapted than 1H NMR to determine the selectivity in particular between the cyclic carbonate and the polycarbonate [17]. Nevertheless, we checked for a few samples that the conversions and yields determined by ATR-IR were in accordance with the NMR results. Figure 1 illustrates the ATR-IR spectra of CHO, CHC, PCHC, PE.
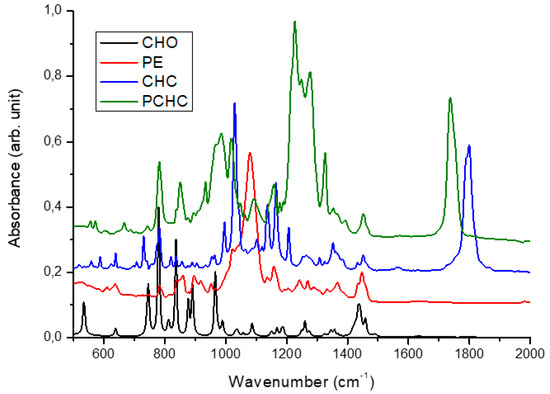
Figure 1.
ATR-IR spectra of cyclohexene oxide (CHO), cyclic cyclohexene carbonate (CHC), poly(cyclohexene carbonate) (PCHC), and poly(cyclohexene ether) (PE).
The conversion of cyclohexene oxide was highlighted by the decrease of the CHO peak at 534 cm−1. Meanwhile, formation of PCHC and PE were attested by the appearance of peaks at 1740 and 1080 cm−1, respectively. The presence of CHC in the IR spectrum of the crude sample was revealed by a peak at 1803 cm−1 [69]. Then, the conversion of cyclohexene oxide and the selectivity towards the formation of the cyclic carbonate, the polycarbonate, and the polyether were determined by comparing the relative intensities of the characteristic peaks of each species defined above (see ESI 1 in Supplementary Materials). The purification of the polycarbonate was carried out by precipitation from a dichloromethane solution of the crude mixture with acidic methanol, followed by repeated washing steps with methanol and drying under vacuum to obtain the purified polymer as a white powder. The 1H NMR spectra of the purified sample is characteristic of the polycarbonate (PCHC) with almost exclusively carbonate linkages and traces of ether linkages (see ESI 2, Figure S1 in Supplementary Materials). In other words, the polyether segments obtained during the CHO/CO2 copolymerization were produced as contaminant and were not along the polycarbonate chain. All the samples for SEC and DSC analyses were purified along the same protocol and were controlled by 1H NMR.
3. Results and Discussion
3.1. Catalytic Studies Performed with Metal Triflates Complexes
Series of metal triflates complexes catalysts were screened in combination with nucleophilic cocatalysts for the solvent-free coupling of CO2 with cyclohexene oxide which can lead to the formation of cyclic carbonate, polycarbonate, and polyether products (Scheme 1). This epoxide was chosen as its structure containing a six-membered ring is known to favor the production of polycarbonate over cyclic carbonate. The coupling reactions were performed for 20 h using 1 mol % of catalyst at different temperatures and pressures ranging between 60 and 90 °C and 2 and 7 MPa, respectively.
3.1.1. Influence of the Nature of the Metal
The influence of the metal cations of the Lewis acid catalysts with Triflate anion on their activity and selectivity was first evaluated (Table 1, entries 1–6). Interestingly, the cyclohexene oxide conversion was found to be higher than 85% whatever the nature of the metal cation with a general trend of high selectivity towards the formation of PE due to epoxide homopolymerization. In particular, for the Al, Ni, and Mg complexes (Table 1, entries 1–3), the selectivity towards the formation of PE was higher than 99%. However, we observed that for the Sc, Y, and Zn complexes (Table 1, entries 4–6), a small amount of polycarbonate could be obtained with a slightly better selectivity towards PCHC found for the Zn salt. Finally, we did not observe any formation of cyclic carbonates in any case. Thus, it appears clearly that these strong Lewis acid species that activate the epoxide towards ring opening allow mainly the consecutive insertion of two epoxides leading to the formation of polyethers. In order to favor the alternating copolymerization of CO2 and cyclohexene oxide and thus improve the selectivity of the Sc, Y, and Zn complexes towards the formation of PCHC, we investigated various cocatalysts of different types with different relative catalyst/cocatalyst ratios.

Table 1.
Catalytic studies performed with metal triflates complexes a.
3.1.2. Influence of the Nature of the Cocatalyst
Preliminary investigations of the influence of a cocatalyst on the selectivity of the Sc and Y complexes towards the formation of PCHC were performed. The results are reported in the ESI 3, Table S1 (Supplementary Materials) and put in evidence only a slight improvement of their selectivity towards the formation of PCHC. The influence of different types of cocatalysts on the selectivity of the Zn complex towards the formation of PCHC was then evaluated (Table 2). PPNCl, P(Ph)3, and P(Cy)3, which have been reported previously as possible efficient cocatalysts for the PCHC synthesis, were tested (Table 2, entries 1–11).

Table 2.
Catalytic studies performed with Zn(OTf)2 catalyst using various cocatalysts a.
PPNCl at a catalyst/cocatalyst ratio of 1:1 allowed only a slight improvement of the selectivity towards PCHC at the expenses of a lower conversion (69% instead of 94%)(Table 2, entry 1). A similar conclusion is drawn from the results obtained with P(Ph)3 (Table 2, entry 2). Higher catalyst/cocatalyst ratio between 1:2 and 1:3 slightly improved the selectivity towards the formation of PCHC from 12% to 15% and 14%, respectively (Table 2, entries 3–4), while a higher conversion between 74% and 79% was obtained in comparison with PPNCl. Finally, the use of P(Cy)3 at 1:2 and 1:3 catalyst/cocatalyst ratio significantly improved the selectivity towards the formation of PCHC to 20% and 36%, respectively (Table 2, entries 5–6). However, at the 1:3 catalyst/cocatalyst ratio, the conversion was decreased to 63% and a non-negligible contribution of 6% of cyclohexene carbonate was detected. Interestingly, increasing the temperature to 70 °C led to an increase of the conversion to 82% and improved the selectivity towards the formation of PCHC to 54% (Table 2, entry 7). A further increase of the temperature to 80 °C slightly improved the selectivity towards the formation of PCHC to 58% (Table 2, entry 8) at the expense of a significant increase of the production of cyclic carbonate to 10%. So, we do consider that the temperature of 70 °C is optimum for this catalytic platform. Finally, at 70 °C, when the pressure was changed between 3 and 7 MPa (Table 2, entries 9–11), it appears that the higher conversion and selectivity towards the formation of PCHC were obtained at P = 4 MPa. From these encouraging results, we investigated other types of cocatalysts in order to favor the CO2-epoxide copolymerization. Firstly, we investigated different tetraethyl ammonium (Et4N) salts with different counter-anion (HSO4−, SCN−, CH₃C₆H₄SO3− (Tos)) as potential efficient cocatalysts for this reaction under the optimized conditions obtained with P(Cy)3 as cocatalyst. Et4NHSO4 displayed a good cocatalytic activity with a conversion of 87%, but at the expense of a low selectivity towards the formation of PCHC of about 11% (Table 2, entry 12). A low conversion of only 50% and selectivity towards the formation of PCHC of about 52% were observed with Et4NSCN (Table 2, entry 13). Interestingly, Et4NTos displayed a very good selectivity towards the formation of PCHC of about 83% with a good conversion (74%), the contribution of cyclohexene carbonate staying below 6% in both cases (Table 2, entry 14). As Et4NTos was found to be the most effective cocatalyst, we then tested other tosylate-based salts with different cations, namely cetyltrimethylammonium tosylate (CTATos) and pyridinium tosylate (PyrTos). Although PyrTos was found to be an ineffective cocatalyst with a low conversion (48%) and low selectivity (14%) (Table 2, entry 16), CTATos displayed a good selectivity towards the formation of PCHC of about 55% with a good conversion (73%), the contribution of cyclohexene carbonate staying below 6% (Table 2, entry 15). The lower efficiency of PyrTos versus CTATos and Et4NTos was probably due to a higher stabilization of the ion pair versus the “free” ions that might hamper the nucleophilicity of the tosylate anion. Then, in order to find the optimal catalytic performance of the two most efficient catalytic platforms reported above, namely (Zn(OTf)2/Et4NTos and Zn(OTf)2/CTATos), we investigated the effect of the catalyst/cocatalyst ratio on their catalytic activity and selectivity for the synthesis of PCHC (see Table 3).

Table 3.
Catalytic studies performed with Zn(OTf)2/Et4NTos and Zn(OTf)2/CTATos catalytic platforms using various catalyst:cocatalyst ratios a.
The catalyst/cocatalyst ratio was varied from 1:1 to 1:4 for both systems. Whatever the nature of the cocatalyst, an increase of the cocatalyst content led to an increase of the cyclic cyclohexene carbonate production, probably due to an excess of nucleophile in the medium. Interestingly, an overall increase of the selectivity towards the formation of PCHC was observed, although this tendency was only clearly observed in the case of Zn(OTf)2/Et4NTos. Thus, from these results, we consider that for both catalytic platforms, the best compromise between the conversion, the selectivity towards the formation of PCHC, and the quantity of cocatalyst is obtained for a catalyst/cocatalyst ratio of 1:2.
Finally, the synthesis of PCHC using Zn(OTf)2/Et4NTos as the catalytic platform was performed in solvents of different polarity that have been used previously in the literature, namely, toluene, tetrahydrofuran, and dichloromethane (see ESI 4, Table S2 in Supplementary Materials). It was found that the conversion and selectivity towards the formation of PCHC remained relatively comparable to the one reported under neat conditions.
3.2. Catalytic Studies Performed with Metal Tosylates Complexes
The good results obtained above with the Zn(OTf)2/Et4NTos catalytic platform prompted us to investigate the catalytic activity of a series of commercially available metal tosylates complexes for the synthesis of poly(cyclohexene carbonate) without the use of any cocatalysts. Unless mentioned otherwise, the coupling reactions were performed at 70 °C and 4 MPa for 20 h using 1 mol % of catalyst (Table 4). AgTos was found to be an ineffective catalyst with a low conversion (24%) and low selectivity (16%) towards the formation of PCHC (Table 4, entry 1). Although FeTos displayed a good catalytic activity with a conversion of 91%, a rather low selectivity towards the formation of PCHC of about 18% was obtained (Table 4, entry 2). Finally, it is noteworthy that ZnTos afforded a good conversion of 67% with a very high selectivity towards the formation of PCHC of about 97% (Table 4, entry 3). Thus, we emphasize that the performance of ZnTos in terms of selectivity towards the formation of PCHC was higher than that obtained with the Zn(OTf)2/Et4NTos catalytic platform. Increasing the temperature to 80 °C slightly improved the conversion (72%) together with a high selectivity towards the formation of PCHC (95%) (Table 4, entry 4). A further increase to 90 °C did not improve the selectivity towards the formation of PCHC (94%) (Table 4, entry 5). At 80 °C, an increase of the catalyst concentration to 2 mol % allowed reaching an almost full conversion (98%) of cyclohexene oxide but at the expense of the selectivity towards the formation of PCHC (66%) (Table 4, entry 6). Interestingly, at 70 °C and decreasing the pressure to 2 MPa, a good conversion of 65% was observed together with a high selectivity towards the formation of PCHC (95%) (Table 4, entry 7). Finally, performing the reaction in toluene at different concentrations did not improve the conversion and had a detrimental effect on the selectivity towards the formation of PCHC in comparison to the one reported under neat conditions (Table 4, entries 8–10). The most promising poly(cyclohexene carbonate) samples in terms of yield and selectivity obtained in selected sets of experiments reported above were characterized by steric extrusion chromatography (SEC) (Table 5). The PCHC sample obtained with the Zn(OTf)2/P(Cy)3 catalytic platform displayed a molar mass of about 12 kg/mol (Table 5, entry 2/7). Interestingly, the PCHC sample prepared using Zn(OTf)2/Et4NTos and Zn(OTf)2/CTATos led to a significant increase of the Mn from 12 to about 25 kg/mol with a dispersity of about 5 (Table 5, entries 3/4, 3/9). Thus, even if the selectivity toward PCHC of these three catalytic platforms is not optimal, they allow the synthesis of PCHC with molecular weights comparable to those obtained with other catalytic systems [7,17,19,51]. Then, the performance of ZnTos without the use of any cocatalysts was much better than that of the Zn(OTf)2/Et4NTos catalytic platform as the obtained PCHC displayed a molar mass Mn of 62 kg/mol while the observed dispersity was maintained at about 4 (Table 5, entry 4/3). Increasing the temperature to 80 and 90 °C led to a decrease of the Mn to 42 and 39 kg/mol, respectively (Table 5, entries 4/4, 4/5), in accordance with the results previously reported by Werner et al. [21]. By the same token, at 70 °C and decreasing the pressure to 2 MPa led to a product with a lower molecular weight of 33 kg/mol and high dispersity of about 10 (Table 5, entry 4/7). Finally, even though the use of increasing concentration of toluene led to a product with higher molecular weight, the yield and the incorporation of CO2 in the polymer chain decreased significantly (Table 5, entries 4/8, 4/9, 4/10). Thus, as a general comment regarding the observed broad dispersity reported in Table 5, this could be due to the presence of a small amount of water in the reaction medium that can deactivate our catalyst during the reaction and/or act as a chain-transfer agent by reacting with the growing polymer chain to yield hydroxyl-terminated polycarbonates of various chain length, thus preventing the chain growth. Indeed, although our catalysts were handled and stored under argon, the CO2/CHO coupling experiments were not performed under strict avoidance of water. In addition, as it is often observed for the copolymerization of cyclohexene oxide with CO2, a bimodal Mn distribution was observed that has been also attributed to the presence of small amounts of water. The glass transition temperature (Tg) values determined by DSC and reported in Table 5 for four selected samples are in the range 120–125 °C. These values are comparable to the highest one reported in the literature for PCHC [17]. On the other hand, the fact that they were higher compared to previously reported Tg for poly(cyclohexene carbonate) obtained from the copolymerization of CO2 and cyclohexene oxide mediated by zinc organyls [21] is ascribed to the higher molecular weight of our polymers. To conclude, under optimized conditions at 343 K and 4 MPa, we found that ZnTosylate in catalytic amounts (1% mol) under solvent- and cocatalyst-free conditions enables the selective synthesis of poly(cyclohexene carbonate) with a molecular weight of about 62.1 kg/mol (Tg = 124 °C) with about 70% yields.

Table 4.
Catalytic studies performed with metal tosylates complexes a.

Table 5.
GPC data for the PCHC samples prepared using catalysts based on Zn(OTf)2/cocatalysts a.
Finally, in order to evaluate the performance of ZnTos with another monomer, we performed a few experiments using propylene oxide (PO) as a substrate. Under the optimized conditions found for CHO (5 mmoles PO, 1 mol % catalyst, 70 °C, 20 h, 4 MPa CO2), a high conversion of PO of about 90% was obtained with a high selectivity of about 70% towards the formation of cyclic propylene carbonate and a low selectivity of about 30% towards the formation of poly(propylene carbonate) PPC. To try to improve the selectivity towards the formation of PPC, the temperature was decreased to 40 °C. Although the selectivity towards the formation of PPC was increased to about 70% at the expense of the formation of cyclic propylene carbonate (30%), there was a strong decrease of the conversion of PO to about 30%.
3.3. Infrared Absorption Experiments
In order to put in evidence any specific interaction between CO2 and the catalysts investigated in this study, we performed an infrared investigation of pressurized gaseous CO2 in contact with some selected catalysts. In particular, we investigated the CO2 bending mode in the spectral region of about 670 cm−1 that is known to be very sensitive to any specific solute–CO2 interactions. Indeed, for isolated CO2 molecules, the out-of-plane and in-plane bending modes are degenerated and display the same vibrational frequency. When CO2 interacts with a solute, there is a removal of the degeneracy and the out-of-plane and in-plane bending modes display two distinct vibrational bands. For example, such splitting of the bending mode has already been reported to put in evidence CO2–polymer [70], CO2–ionic liquids [71], and CO2–metal isopropoxide interactions [68]. Thus, we have reported in Figure 2 the infrared spectra of three selected catalysts dispersed in a KBr pellet, namely, ZnTos, Zn(OTf)2, and Mg(OTf)2 under a CO2 pressure of 4 MPa. In order to reveal the spectrum of CO2 interacting with each catalyst under a CO2 pressure of 4 MPa, each spectrum was corrected from the contribution of the catalysts-loaded KBr pellet recorded under atmospheric pressure.
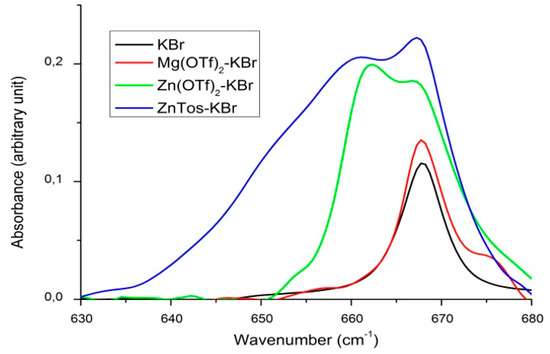
Figure 2.
Infrared spectra of three selected catalysts dispersed in a KBr pellet, namely, ZnTos, Zn(OTf)2, and Mg(OTf)2 under a CO2 pressure of 4 MPa corrected from the contribution of the catalysts-loaded KBr pellet recorded under atmospheric pressure. The spectrum of a KBr pellet under CO2 pressure at 4 MPa is reported for comparison.
The spectrum of a KBr pellet under CO2 pressure at 4 MPa reported for comparison displays a single narrow band at 668 cm−1 that is associated to the degenerated bending mode of CO2, thus revealing that there is no specific interaction between KBr and CO2. The same conclusion can be done in the case of Mg(OTf)2-loaded KBr pellet. A different behavior is observed in the case of Zn(OTf)2 and ZnTos. Indeed, an additional component is observed at about 662 and 660 cm−1 for Zn(OTf)2 and ZnTos, respectively. Thus, from these observations, we can conclude that there is a specific interaction between CO2 and both Zn(OTf)2 and ZnTos. The higher shift, width, and intensity of the broad component observed for ZnTos can be interpreted as a signature of the stronger activation of CO2 by ZnTos.
3.4. DFT Calculations
In order to support this experimental evidence, we performed DFT (Density Functional Theory) calculations on the 1:1 complex of CO2 with ZnTos and Zn(OTf)2. For the minimum energy structures of both complexes that are displayed in Figure 3, we observed that the CO2 molecule acts simultaneously as electron donor towards the Zn atom and as an electron acceptor towards an O atom of the SO3− group. Although the stabilization energy of both complexes were nearly equal at –3.26 (ZnTos) and –3.36 kcal/mol (Zn(OTf)2), the structures of both complexes were different as we observed that the interaction of CO2 with the SO3− group was favored with ZnTos as shown by a shorter C(CO2)–O(SO3−) distance of about 3 Å instead of 3.5 Å in the Zn(OTf)2 complex. This specific interaction led to an intramolecular OCO angle of CO2 that was lower (178°) than that calculated for CO2 in the Zn(OTf)2 complex (179°), thus putting in evidence a stronger activation of CO2 by ZnTos. Finally, from the calculated frequencies of the out-of-plane (νO) and in-plane (νi) bending modes of CO2 in the ZnTos and Zn(OTf)2 complexes, we found that the frequency gaps (νO-νi) were of about 12 and 4 cm−1 for the ZnTos and Zn(OTf)2 complexes, respectively, which are consistent with the experimental infrared spectra reported above. Thus, these experimental and theoretical results are in line with the higher selectivity of ZnTos towards the formation of polycarbonate in comparison with Zn(OTf)2 and Mg(OTf)2, thus revealing that the ability of ZnTos to coactivate CO2 is a key factor that governs its selectivity towards the formation of polycarbonates. In other words, although ZnTos is expected to activate the epoxide towards ring opening, the coactivation of CO2 by ZnTos should favor the alternating copolymerization of CO2 and cyclohexene oxide instead of the consecutive insertion of two epoxides that leads to the formation of polyethers.
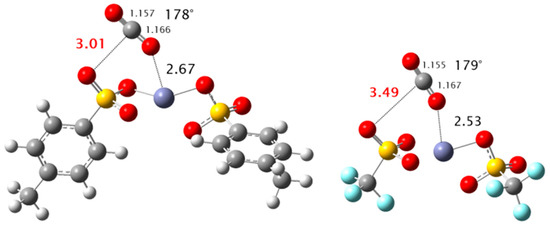
Figure 3.
Optimized geometries (CAM-B3LYP/aug-cc-pVDZ) of the structures of the ZnTos-CO2 (left) and Zn(OTf)2-CO2 (right) complexes. Bond lengths of interest are displayed in Ångström (dashed lines). The intramolecular C–O distances and OCO angle of CO2 are also displayed.
4. Conclusions
The aim of this study was to investigate the copolymerization of cyclohexene oxide (CHO) with CO2 in the presence of commercially available Lewis acids with metal centers such as Zn, Mg, Fe, Al, Ni, Sc, and Y. The influence of the pressure, the temperature, the catalyst loading, and the presence of a cocatalyst or a solvent on the reaction yields, selectivity, and molar masses has been evaluated for selected catalytic platforms. Zn(OTf)2 in the presence of a tosylate-based cocatalyst has allowed the synthesis of PCHC in good to high yields. Then, the commercially available Zinc tosylate salt investigated under various thermodynamic conditions appears to be the most attractive simple Lewis catalyst that enables the selective synthesis of poly(cyclohexene carbonate) under solvent- and cocatalyst-free conditions. Thanks to in situ FTIR spectroscopy and DFT calculations, we have put in evidence a specific interaction between ZnTos and CO2. Thus, we propose that, although ZnTos is expected to activate the epoxide towards ring opening, such catalytic performance is related to the coactivation of CO2 by ZnTos. This rational investigation is expected to help researchers to identify suitable catalysts that offer the best compromise in terms of activity, toxicity, and cost.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-5629/5/3/39/s1, ESI 1: Method for determination of conversion and selectivity, ESI 2-Figure S1: 1H NMR spectra of polymer sample, ESI 3-Table S1: Sc and Y complexes investigation, ESI 4-Table S2: Solvent effect.
Author Contributions
Conceptualization, J.G. and T.T.; Data curation, J.G.; Investigation, J.G. and C.A.; Methodology, J.G. and T.T.; Supervision, T.T.; Writing—original draft, T.T.; writing—review and editing, J.G. and T.T.
Acknowledgments
The authors acknowledge the “Conseil Régional Nouvelle Aquitaine” for financial support to the infrared equipment. The authors are also indebted to Amelie VAX and Etienne GRAU (LCPO, University Bordeaux) for providing facilities and help in the SEC and DSC analyses. We are also pleased to acknowledge Cybille ROSSY (Cesamo, ISM) for her assistance in the NMR measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coates, G.W.; Moore, D.R. Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Macdowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Tech. 2012, 2, 2169–2187. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Lang, X.-D.; He, X.; Li, Z.-M.; He, L.-N. New routes for CO2 activation and subsequent conversion. Curr. Opin. Green Sustain. Chem. 2017, 7, 31–38. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalton Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Rintjema, J.; Kleij, A.W. Substrate-assisted carbon dioxide activation as a versatile approach for heterocyclic synthesis. Synthesis 2016, 48, 3863–3878. [Google Scholar]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Tech. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Gennen, S.; Mereau, R.; Detrembleur, C.; Jerome, C.; Tassaing, T. Organocatalytic promoted coupling of carbon dioxide with epoxides: A rational investigation of the cocatalytic activity of various hydrogen bond donors. Catal. Sci. Tech. 2015, 5, 4636–4643. [Google Scholar] [CrossRef]
- Lu, X.B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef] [PubMed]
- Taherimehr, M.; Pescarmona, P.P. Green polycarbonates prepared by the copolymerization of CO2 with epoxides. J. Appl. Polym. Sci. 2014, 131, 41141. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Comm. 2015, 51, 6459–6479. [Google Scholar] [CrossRef]
- Taherimehr, M.; Al-Amsyar, S.M.; Whiteoak, C.J.; Kleij, A.W.; Pescarmona, P.P. High activity and switchable selectivity in the synthesis of cyclic and polymeric cyclohexene carbonates with iron amino triphenolate catalysts. Green Chem. 2013, 15, 3083–3090. [Google Scholar] [CrossRef]
- Ang, R.-R.; Sin, L.T.; Bee, S.-T.; Tee, T.-T.; Kadhum, A.a.H.; Rahmat, A.R.; Wasmi, B.A. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Wulf, C.; Doering, U.; Werner, T. Copolymerization of CO2 and epoxides mediated by zinc organyls. RSC Adv. 2018, 8, 3673–3679. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; He, C.-T.; Qin, J.; Li, Z.; Wang, S.; Xiao, M.; Meng, Y. Kinetic and mechanistic investigation for the copolymerization of CO2 and cyclohexene oxide catalyzed by trizinc complexes. Polym. Chem. 2017, 8, 3632–3640. [Google Scholar] [CrossRef]
- Reiter, M.; Vagin, S.; Kronast, A.; Jandl, C.; Rieger, B. A Lewis acid [small beta]-diiminato-zinc-complex as all-rounder for co- and terpolymerisation of various epoxides with carbon dioxide. Chem. Sci. 2017, 8, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Marbach, J.; Nornberg, B.; Rahlf, A.F.; Luinstra, G.A. Zinc glutarate-mediated copolymerization of CO2 and PO - parameter studies using design of experiments. Catal. Sci. Tech. 2017, 7, 2897–2905. [Google Scholar] [CrossRef]
- Sudakar, P.; Sivanesan, D.; Yoon, S. Copolymerization of Epichlorohydrin and CO2 Using Zinc Glutarate: An Additional Application of ZnGA in Polycarbonate Synthesis. Macromol. Rapid Commun. 2016, 37, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xu, B.; Zhang, Y.; Yuan, D.; Yao, Y. Cooperative rare earth metal-zinc based heterometallic catalysts for copolymerization of CO2 and cyclohexene oxide. Green Chem. 2016, 18, 4270–4275. [Google Scholar] [CrossRef]
- Martinez, J.; Castro-Osma, J.A.; Lara-Sanchez, A.; Otero, A.; Fernandez-Baeza, J.; Tejeda, J.; Sanchez-Barba, L.F.; Rodriguez-Dieguez, A. Ring-opening copolymerisation of cyclohexene oxide and carbon dioxide catalysed by scorpionate zinc complexes. Polym. Chem. 2016, 7, 6475–6484. [Google Scholar] [CrossRef]
- Kissling, S.; Lehenmeier, M.W.; Altenbuchner, P.T.; Kronast, A.; Reiter, M.; Deglmann, P.; Seemann, U.B.; Rieger, B. Dinuclear zinc catalysts with unprecedented activities for the copolymerization of cyclohexene oxide and CO2. Chem. Comm. 2015, 51, 4579–4582. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, M.; Wang, S.; Pan, M.; Meng, Y. Activities comparison of Schiff base zinc and tri-zinc complexes for alternating copolymerization of CO2 and epoxides. Polym. Chem. 2014, 5, 3838–3846. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, M.; Xu, Y.; Wang, S.; Meng, Y. Zinc adipate/tertiary amine catalytic system: Efficient synthesis of high molecular weight poly(propylene carbonate). J. Polym. Res. 2013, 20, 190. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Z.; Cheng, R.; Yang, Y.; Zhong, L.; He, X.; Liu, B. Mechanism for alternating copolymerization of CO2 and propylene oxide in diethylzinc–water catalytic system: A DFT study. J. CO2 Util. 2013, 2, 39–48. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Herdtweck, E.; Deglmann, P.; Ott, A.K.; Rieger, B. Mechanistic Insights into Heterogeneous Zinc Dicarboxylates and Theoretical Considerations for CO2–Epoxide Copolymerization. J. Am. Chem. Soc. 2011, 133, 13151–13161. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Rainey, P.; Yarbrough, J.C. Bis-salicylaldiminato complexes of zinc. Examination of the catalyzed epoxide/CO2 copolymerization. Inorg. Chem. 2001, 40, 986–993. [Google Scholar] [CrossRef]
- Cheng, M.; Moore, D.R.; Reczek, J.J.; Chamberlain, B.M.; Lobkovsky, E.B.; Coates, G.W. Single-site β-diiminate zinc catalysts for the alternating copolymerization of CO2 and epoxides: Catalyst synthesis and unprecedented polymerization activity. J. Am. Chem. Soc. 2001, 123, 8738–8749. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kwon, H.Y.; Lee, S.Y.; Na, S.J.; Han, S.-I.; Yun, H.; Lee, H.; Park, Y.-W. Bimetallic Anilido-Aldimine Zinc Complexes for Epoxide/CO2 Copolymerization. J. Am. Chem. Soc. 2005, 127, 3031–3037. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, H.; Ullah, A. Rapid copolymerization of canola oil derived epoxide monomers with anhydrides and carbon dioxide (CO2). Polym. Chem. 2017, 8, 6431–6442. [Google Scholar] [CrossRef]
- Deacy, A.C.; Durr, C.B.; Garden, J.A.; White, A.J.P.; Williams, C.K. Groups 1, 2 and Zn(II) Heterodinuclear Catalysts for Epoxide/CO2 Ring-Opening Copolymerization. Inorg. Chem. 2018, 57, 15575–15583. [Google Scholar] [CrossRef] [PubMed]
- Ikpo, N.; Flogeras, J.C.; Kerton, F.M. Aluminium coordination complexes in copolymerization reactions of carbon dioxide and epoxides. Dalton Trans. 2013, 42, 8998–9006. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wu, W.; Qin, Y.; Wang, X.; Wang, F. Efficient synthesis and stabilization of poly(propylene carbonate) from delicately designed bifunctional aluminum porphyrin complexes. Polym. Chem. 2015, 6, 4719–4724. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yarbrough, J.C.; Ortiz, C.; Fang, C.C. Comparative kinetic studies of the copolymerization of cyclohexene oxide and propylene oxide with carbon dioxide in the presence of chromium salen derivatives. in situ ftir measurements of copolymer vs cyclic carbonate production. J. Am. Chem. Soc. 2003, 125, 7586–7591. [Google Scholar] [CrossRef]
- Devaine-Pressing, K.; Dawe, L.N.; Kozak, C.M. Cyclohexene oxide/carbon dioxide copolymerization by chromium(iii) amino-bis(phenolato) complexes and MALDI-TOF MS analysis of the polycarbonates. Polym. Chem. 2015, 6, 6305–6315. [Google Scholar] [CrossRef]
- Devaine-Pressing, K.; Kozak, C.M. Mechanistic Studies of Cyclohexene Oxide/CO2 Copolymerization by a Chromium(III) Pyridylamine-Bis(Phenolate) Complex. ChemSuschem 2017, 10, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Ulusoy, M.; Karroonnirum, O.; Poland, R.R.; Reibenspies, J.H.; Çetinkaya, B. Highly selective and reactive (salan)crcl catalyst for the copolymerization and block copolymerization of epoxides with carbon dioxide. Macromolecules 2009, 42, 6992–6998. [Google Scholar] [CrossRef]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A Highly Active and Recyclable Catalytic System for CO2/Propylene Oxide Copolymerization. Angew. Chem. Int. Ed. 2008, 47, 7306–7309. [Google Scholar]
- Li, C.-H.; Chuang, H.-J.; Li, C.-Y.; Ko, B.-T.; Lin, C.-H. Bimetallic nickel and cobalt complexes as high-performance catalysts for copolymerization of carbon dioxide with cyclohexene oxide. Polym. Chem. 2014, 5, 4875–4878. [Google Scholar] [CrossRef]
- Xia, W.; Sun, X.Y. Copolymerization of Epoxides and CO2 by Cobalt(II) oxaporphyrins with mechanistic explorations on poly(propylene carbonate) formation. Macromol. Chem. Phys. 2018, 219, 1700478. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt(III) catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef] [PubMed]
- Kember, M.R.; Williams, C.K. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J Am Chem Soc. 2012, 134, 15676–15679. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Ding, K. intramolecularly dinuclear magnesium complex catalyzed copolymerization of cyclohexene oxide with CO2 under ambient CO2 pressure: Kinetics and mechanism. Macromolecules 2006, 39, 128–137. [Google Scholar] [CrossRef]
- Taherimehr, M.; Sertã, J.P.C.C.; Kleij, A.W.; Whiteoak, C.J.; Pescarmona, P.P. New iron pyridylamino-bis(phenolate) catalyst for converting CO2 into cyclic carbonates and cross-linked polycarbonates. ChemSuschem 2015, 8, 1034–1042. [Google Scholar] [CrossRef]
- Buchard, A.; Kember, M.R.; Sandeman, K.G.; Williams, C.K. A bimetallic iron(III) catalyst for CO2/epoxide coupling. Chem. Comm. 2011, 47, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-type iron(III) complexes for the low-pressure reaction of carbon dioxide with epoxides: Catalytic activity, reaction kinetics, and computational study. ACS Catal. 2018, 8, 6882–6893. [Google Scholar] [CrossRef]
- Nakano, K.; Kobayashi, K.; Ohkawara, T.; Imoto, H.; Nozaki, K. Copolymerization of epoxides with carbon dioxide catalyzed by iron–corrole complexes: Synthesis of a crystalline copolymer. J. Am. Chem. Soc. 2013, 135, 8456–8459. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Capacchione, C. Homogeneous iron catalysts in the reaction of epoxides with carbon dioxide. Adv. Synth. Catal. 2019, 361, 265–282. [Google Scholar] [CrossRef]
- Quadri, C.C.; Le Roux, E. Copolymerization of cyclohexene oxide with CO2 catalyzed by tridentate N-heterocyclic carbene titanium(IV) complexes. Dalton Trans. 2014, 43, 4242–4246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling reaction between CO2 and cyclohexene oxide: Selective control from cyclic carbonate to polycarbonate by ligand design of salen/salalen titanium complexes. Catal. Sci. Tech. 2014, 4, 3964–3972. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Huang, B.-H.; Hsiao, M.-W.; Lin, C.-C.; Ko, B.-T. Structurally diverse copper complexes bearing nno-tridentate schiff-base derivatives as efficient catalysts for copolymerization of carbon dioxide and cyclohexene oxide. Inorg. Chem. 2014, 53, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Decortes, A.; Haak, R.M.; Martín, C.; Belmonte, M.M.; Martin, E.; Benet-Buchholz, J.; Kleij, A.W. Copolymerization of CO2 and cyclohexene oxide mediated by yb(salen)-based complexes. Macromolecules 2015, 48, 8197–8207. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A 2016, 374. [Google Scholar] [CrossRef]
- Ang, R.-R.; Tin Sin, L.; Bee, S.-T.; Tee, T.-T.; Kadhum, A.a.H.; Rahmat, A.R.; Wasmi, B.A. A review of copolymerization of green house gas carbon dioxide and oxiranes to produce polycarbonate. J. Cleaner Prod. 2015, 102, 1–17. [Google Scholar] [CrossRef]
- Qin, Y.; Sheng, X.; Liu, S.; Ren, G.; Wang, X.; Wang, F. Recent advances in carbon dioxide based copolymers. J. CO2 Util. 2015, 11, 3–9. [Google Scholar] [CrossRef]
- Chapman, A.M.; Keyworth, C.; Kember, M.R.; Lennox, A.J.J.; Williams, C.K. Adding value to power station captured CO2: Tolerant zn and mg homogeneous catalysts for polycarbonate polyol production. ACS Catal. 2015, 5, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, D.; Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Metal-free alternating copolymerization of CO2 with epoxides: Fulfilling “green” synthesis and activity. J. Am. Chem. Soc. 2016, 138, 11117–11120. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Bou, J.J. Synthesis of vegetable-oil based polymer by terpolymerization of epoxidized soybean oil, propylene oxide and carbon dioxide. Sci. Total Environ. 2017, 598, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Stößer, T.; Li, C.; Unruangsri, J.; Saini, P.K.; Sablong, R.J.; Meier, M.A.R.; Williams, C.K.; Koning, C. Bio-derived polymers for coating applications: Comparing poly(limonene carbonate) and poly(cyclohexadiene carbonate). Polym. Chem. 2017, 8, 6099–6105. [Google Scholar]
- Holmes, S.M.; Mckinley, S.G.; Girolami, G.S. Transition metal p-toluenesulfonates. Inorg. Synth. 2002, 33, 91–103. [Google Scholar]
- Bergeot, V.; Tassaing, T.; Besnard, M.; Cansell, F.; Mingotaud, A.-F. Anionic ring-opening polymerization of ε-caprolactone in supercritical carbon dioxide: Parameters influencing the reactivity. J. Supercrit. Fluids 2004, 28, 249–261. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Rodgers, J.L.; Mackiewicz, R.M.; Phelps, A.L. Probing the mechanistic aspects of the chromium salen catalyzed carbon dioxide/epoxide copolymerization process using in situ ATR/FTIR. Catal. Today 2004, 98, 485–492. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific intermolecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Jutz, F.; Baiker, A. Supercritical CO2/Ionic Liquid Systems: What Can We Extract from Infrared and Raman Spectra? J. Phys. Chem. B 2009, 113, 10249–10254. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).