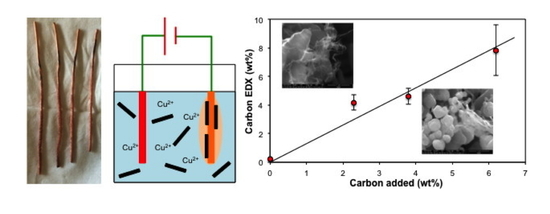
Electrodeposition of Cu–SWCNT Composites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
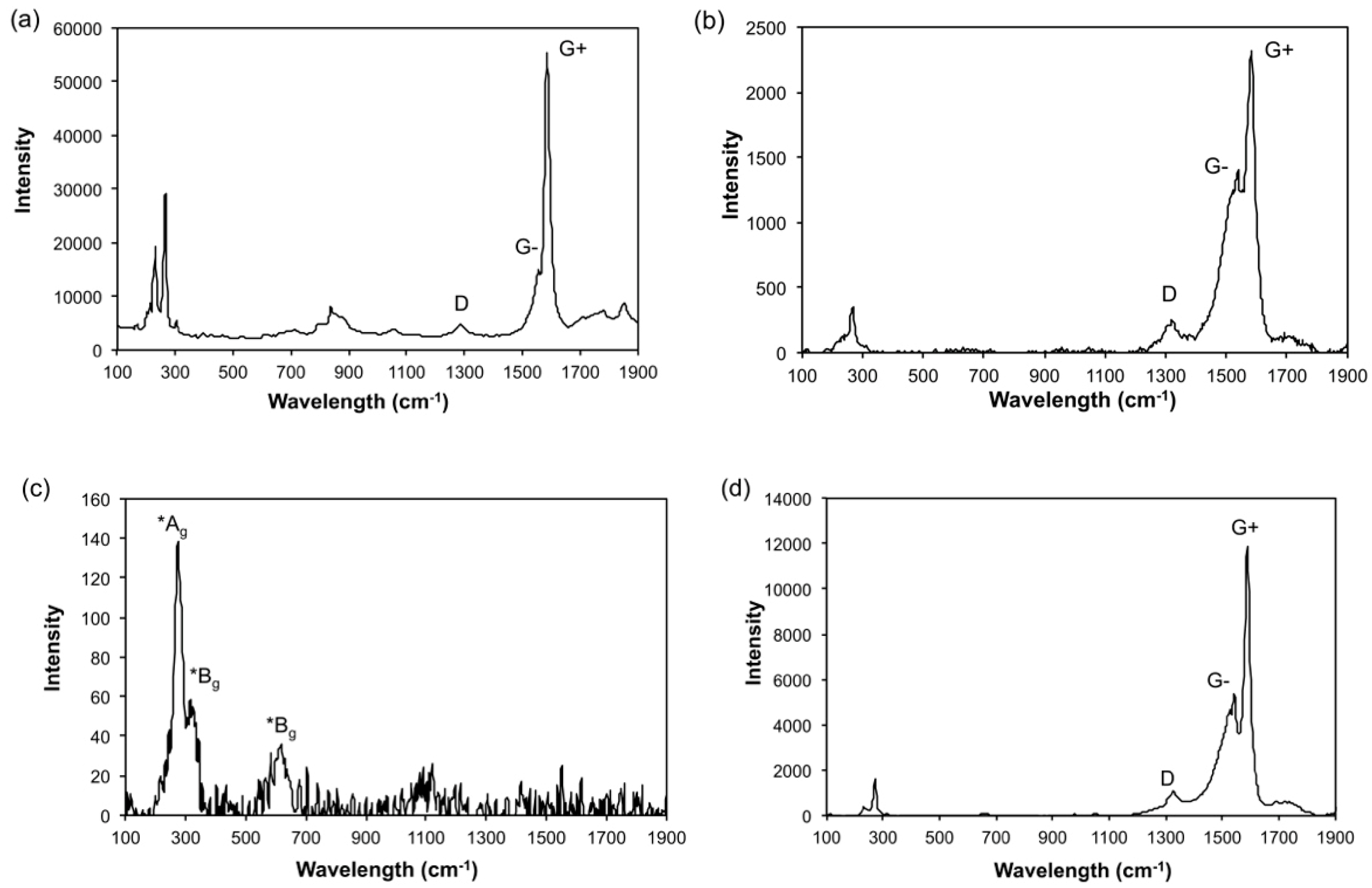
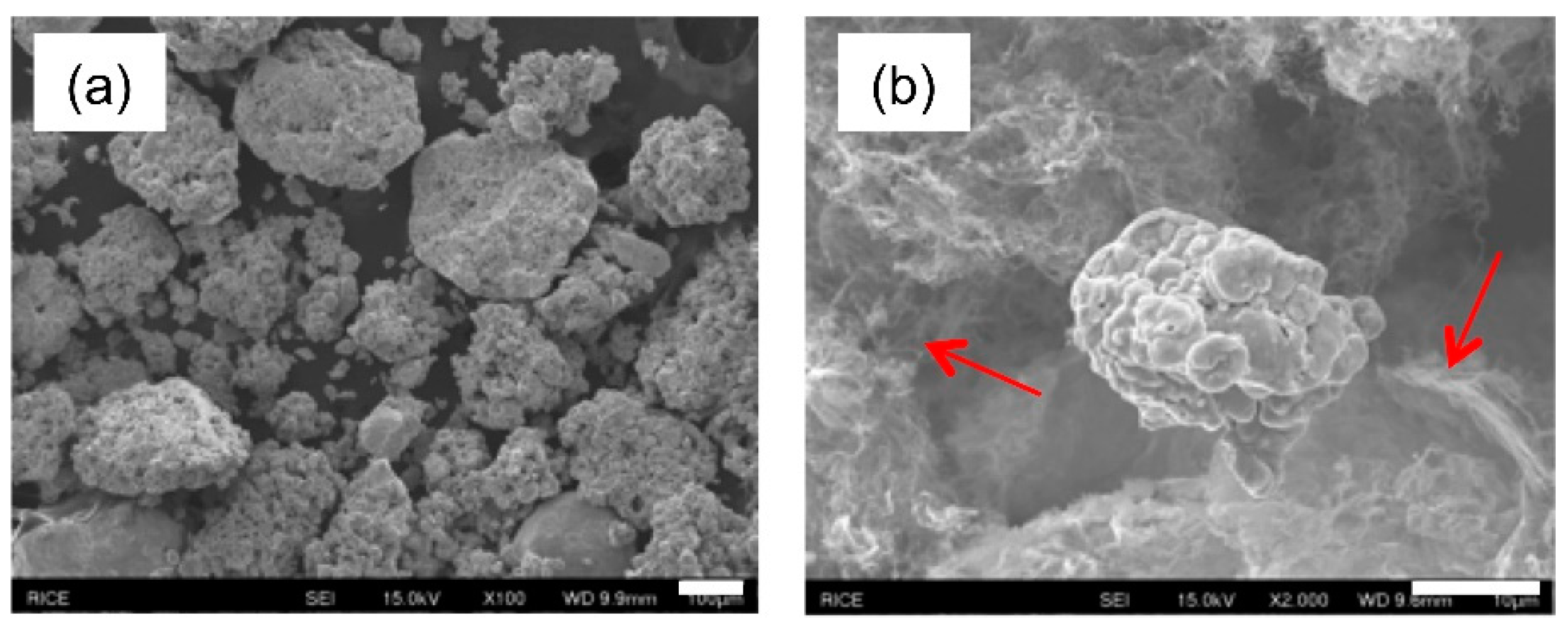
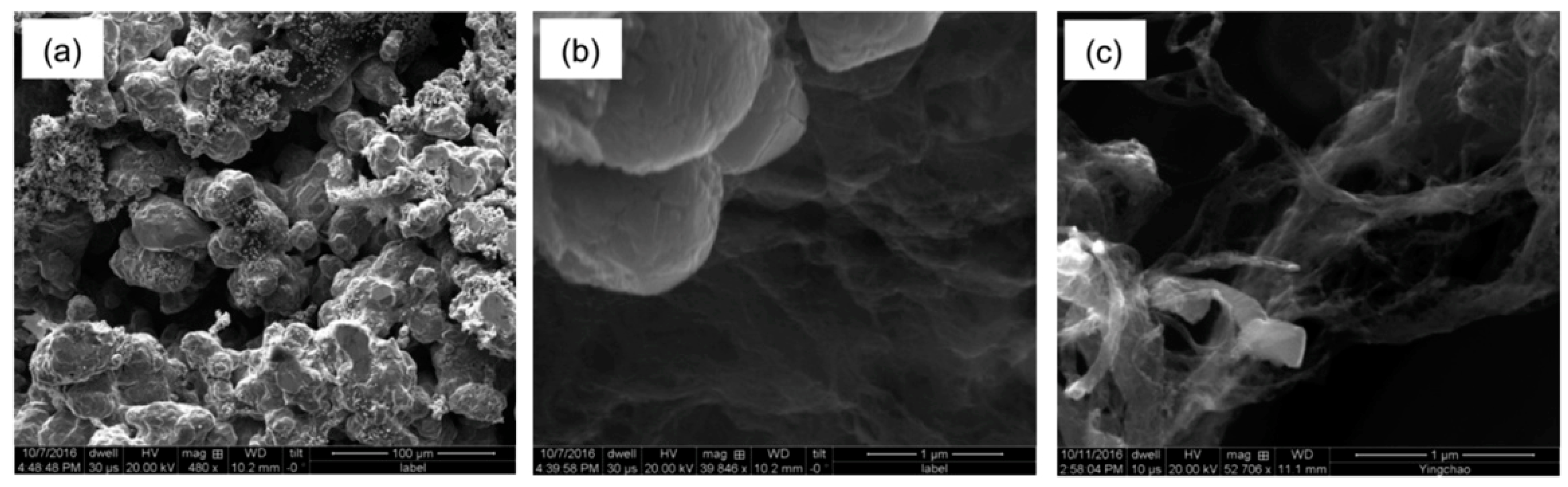
3.1. Electrolysis of SWCNTs in CuSO4 Solution
3.2. Electrolysis in the Presence of Cu Micropowder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jarosz, P.; Schauerman, C.; Alvarenga, J.; Moses, B.; Mastrangelo, T.; Raffaelle, R.; Ridgley, R.; Landi, B. Carbon nanotube wires and cables: Near-term applications and future. Nanoscale 2011, 3, 4542–4553. [Google Scholar] [CrossRef] [PubMed]
- Hjortstam, O.; Isberg, P.; Soderholm, S.; Dai, H. Can we achieve ultra-low resistivity in carbon nanotube-based metal composites? Appl. Phys. A Mater. Sci. Process 2004, 78, 1175–1179. [Google Scholar] [CrossRef]
- Janas, D.; Liszka, B. Copper matrix nanocomposites based on carbon nanotubes or graphene. Mater. Chem. Front. 2018, 2, 22–35. [Google Scholar] [CrossRef]
- Jordan, M.B.; Feng, Y.; Burkett, S.L. Development of seed layer for electrodeposition of copper on carbon nanotube bundles. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2015, 33, 02120201–02120208. [Google Scholar] [CrossRef]
- Subramaniam, C.; Sekiguchi, A.; Yamada, T.; Futaba, D.N.; Hata, K. Nano-scale, planar and multi-tiered current pathways from a carbon nanotube–copper composite with high conductivity, ampacity and stability. Nanoscale 2016, 8, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Sun, Y.; Sun, J.J.; Chen, Q. Mechanical properties of carbon nanotube–copper nanocomposites. J. Micromech. Microeng. 2008, 18, 0350131–0350134. [Google Scholar] [CrossRef]
- Arai, S.; Saito, T.; Endo, M. Cu–MWCNT composite films fabricated by electrodeposition. J. Electrochem. Soc. 2010, 157, D147–D153. [Google Scholar] [CrossRef]
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513, 247–253. [Google Scholar] [CrossRef]
- Wright, K.D.; Gowenlock, C.E.; Bear, J.C.; Barron, A.R. Understanding the effect of functional groups on the seeded growth of copper on carbon nanotubes for optimizing electrical transmission. ACS Appl. Mater. Interfaces 2017, 9, 27202–27212. [Google Scholar] [CrossRef]
- Uddin, S.M.; Mahmud, T.; Wolf, C.; Glanz, C.; Kolaric, I.; Volkmer, C.; Höller, H.; Wienecke, U.; Roth, S.; Fecht, H.-J. Effect of size and shape of metal particles to improve hardness and electrical properties of carbon nanotube reinforced copper and copper alloy composites. Compos. Sci. Technol. 2010, 70, 2253–2257. [Google Scholar] [CrossRef]
- Varentsova, V.I.; Varenstov, V.K.; Bataev, I.A.; Yusin, S.I. Effect of surface state of carbon fiber electrode on copper electroplating from sulfate solutions. Prot. Met. Phys. Chem. 2017, 47, 43–47. [Google Scholar] [CrossRef]
- Arai, S.; Endo, M. Carbon nanofiber-copper composites fabricated by electroplating. Electrochem. Solid-State Lett. 2004, 7, C25–C26. [Google Scholar] [CrossRef]
- Arai, S.; Endo, M. Various carbon nanofiber–copper composite films prepared by electrodeposition. Electrochem. Commun. 2005, 7, 19–22. [Google Scholar] [CrossRef]
- Arai, S.; Endo, M. Carbon nanofiber–copper composite powder prepared by electrodeposition. Electrochem. Commun. 2003, 5, 797–799. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, Y.D.; Ren, Y.; He, C.S.; Deng, J.N.; Nan, J.; Chen, J.G.; Zuo, L. Single-walled carbon nanotube-reinforced copper composite coatings prepared by electrodeposition under ultrasonic field. Mater. Lett. 2008, 62, 47–50. [Google Scholar] [CrossRef]
- Arai, S.; Saito, T.; Endo, M. Metal-fixed multiwalled carbon nanotube patterned emitters using photolithography and electrodeposition technique. Electrochem. Solid-State Lett. 2008, 11, D72–D74. [Google Scholar] [CrossRef][Green Version]
- Arai, S.; Kirihata, K.; Shimizu, M.; Ueda, M.; Katada, A.; Uejima, M. Fabrication of Copper/Single-Walled Carbon Nanotube Composites by Electrodeposition Using Free-Standing Nanotube Film. J. Electrochem. Soc. 2017, 164, D922–D929. [Google Scholar] [CrossRef]
- Feng, Y.; McGuire, G.E.; Shenderova, O.A.; Ke, H.; Burkett, S.L. Fabrication of copper/carbon nanotube composite thin films by periodic pulse reverse electroplating using nanodiamond as a dispersing agent. Thin Solid Films 2016, 615, 116–121. [Google Scholar] [CrossRef]
- Raja, P.M.V.; Esquenazi, G.L.; Wright, K.D.; Gowenlock, C.E.; Brinson, B.E.; Alexander, S.; Jones, D.R.; Gangoli, V.S.; Barron, A.R. Aqueous electromigration of single-walled carbon nanotubes and co-electromigration with copper ions. Nanoscale 2018, 10, 19628–19637. [Google Scholar] [CrossRef]
- Kazimierska, E.; Andreoli, E.; Barron, A.R. Understanding the effect of carbon nanotube functionalization on copper electrodeposition. J. Appl. Electrochem. 2019, 49, 1–11. [Google Scholar] [CrossRef]
- Lee, S.M.; Raja, P.M.V.; Esquenazi, G.L.; Barron, A.R. Effect of raw and purified carbon nanotubes and iron oxide nanoparticles on the growth of wheatgrass prepared from the cotyledons of common wheat (triticum aestivum). Environ. Sci. Nano 2018, 5, 103–114. [Google Scholar] [CrossRef]
- Zhang, K.S.; Pham, D.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Smalley, P.; Kennedy, K.; Brinson, B.E.; Billups, W.E.; Hauge, R.H.; et al. Overcoming Catalyst Residue Inhibition of the Functionalization of Single-Walled Carbon Nanotubes via the Billups–Birch Reduction. ACS Appl. Mater. Interfaces 2017, 9, 37972–37980. [Google Scholar] [CrossRef] [PubMed]
- Moradi, O.; Zare, K.; Yari, M. Interaction of some heavy metal ions with single walled carbon nanotube. Int. J. Nano Dimens. 2011, 1, 203–220. [Google Scholar]
- Peng, C.; Liu, Y.; Bi, J.; Xu, H.; Ahmed, A.S. Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. J. Haz. Mater. 2011, 189, 814–820. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, L.; Canepa, S.; Sun, H.; Yesibolati, M.N.; Sherburne, M.; Xu, R.; Sritharan, T.; Loo, J.S.C.; Ager, J.W., III; et al. Phosphate tuned copper electrodeposition and promoted formic acid selectivity for carbon dioxide reduction. J. Mater. Chem. A 2017, 5, 11905–11916. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, P.; Chen, X.; Li, W.; Liu, X. The influence of electro-deposition parameters on conductivity and morphology of structurally uniform MWCNTs/Cu composite films. Mater. Res. Express 2018, 5, 06560401–065660411. [Google Scholar] [CrossRef]
- Zhou, M.; Mai, Y.; Ling, H.; Chen, F.; Lian, W.; Jie, X. Electrodeposition of CNTs/copper composite coatings with enhanced tribological performance from a low concentration CNTs colloidal solution. Mat. Res. Bull. 2018, 97, 537–543. [Google Scholar] [CrossRef]
- Ogrin, D.; Chattopadhyay, J.; Sadana, A.K.; Billups, E.; Barron, A.R. Epoxidation and deoxygenation of single-walled carbon nanotubes: Quantification of epoxide defects. J. Am. Chem. Soc. 2006, 128, 11322–11323. [Google Scholar] [CrossRef]
- Nia, P.M.; Meng, W.P.; Lorestani, F.; Mahmoudian, M.R.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2015, 209, 100–108. [Google Scholar]
- Wang, L.C.; De Tacconi, N.R.; Chenthamarakshan, C.R.; Rajeshwar, K.; Tao, M. Electrodeposited copper oxide films: Effect of bath pH on grain orientation and orientation-dependent interfacial behavior. Thin Solid Film. 2007, 515, 3090–3095. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.-Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Cheng, F.Q.; Zhang, G.; Yi, G.J. Corrosion behavior of carbon nanotubes–Ni composite coating. Surf. Coat. Technol. 2005, 191, 351–356. [Google Scholar] [CrossRef]
- Rudd, J.A.; Gowenlock, C.E.; Gomez, V.; Kazimierska, E.; Al-Enizi, A.M.; Andreoli, E.; Barron, A.R. Solvent-free microwave-assisted synthesis of tenorite nanoparticle-decorated multi-walled carbon nanotubes. J. Mater. Sci. Technol. 2019, 35, 1121–1127. [Google Scholar] [CrossRef]
- Gomez, V.; Irusta, S.; Adams, W.W.; Hauge, R.H.; Dunnill, C.W.; Barron, A.R. Enhanced carbon nanotubes purification by physic-chemical treatment with microwave and Cl2. RSC Adv. 2016, 6, 11895–11902. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Galtayries, A.; Bonnelle, J.-P. XPS and ISS studies on the interaction of H2S with polycrystalline Cu, Cu2O and CuO surfaces. Surf. Interface Anal. 1995, 23, 171–179. [Google Scholar] [CrossRef]
- Skinner, W.M.; Prestidge, C.A.; Smart, R.S.C. Irradiation effects during XPS studies of Cu (II) activation of zinc sulphide. Surf. Interface Anal. 1996, 24, 620–626. [Google Scholar] [CrossRef]
- Zheng, M.; Diner, B.A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar] [CrossRef]
- Hsieh, H.-S. Carbon Nanotube Mediated Redox Reactions in Water. Ph.D. Thesis, Purdue University West Lafayette, West Lafayette, IN, USA, 2015. [Google Scholar]
- Liu, Y.; Hu, J.; Hu, A.; Li, M.; Mao, D. Oxidation of the copper alloy with pure copper plating. J. Adhes. Sci. Technol. 2012, 26, 2653–2660. [Google Scholar] [CrossRef]
- Liu, C.-W.; Wang, Y.-L.; Tsai, M.-S.; Feng, H.-P.; Chang, S.-C.; Hwang, G.-J. Effect of plating current density and annealing on impurities in electroplated Cu film. J. Vac. Sci. Technol. A 2005, 23, 658–662. [Google Scholar] [CrossRef][Green Version]
- Wu, C.-W.; Harroun, S.G.; Lien, C.-W.; Chang, H.-T.; Unnikrishnan, B.; Lai, I.P.-J.; Chang, J.-Y.; Huang, C.-C. Self-templated formation of aptamer-functionalized copper oxide nanorods with intrinsic peroxidase catalytic activity for protein and tumor cell detection. Sens. Actuators B Chem. 2016, 227, 100–107. [Google Scholar] [CrossRef]
- Kim, K.; Johnson, R.W.; Tanskanen, J.T.; Liu, N.; Kim, M.G.; Pang, C.; Ahn, C.; Bent, S.F.; Bao, Z. Selective metal deposition at graphene line defects by atomic layer deposition. Nat. Comm. 2014, 5, 4781. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Pal, A.K. Incorporation of nanocrystalline silver on carbon nanotubes by electrodeposition technique. AIP Conf. Proc. 2008, 1063, 98–104. [Google Scholar]
- Dresselhaus, M.S.; Pimenta, M.A.; Ecklund, P.C.; Dresselhaus, G. Raman Scattering in Materials Science; Springer: Berlin, Germany, 2000. [Google Scholar]
- Schönfelder, R.; Avilés, F.; Knupfer, M.; Azamar-Barrios, J.A.; González-Chi, P.I.; Rümmeli, M.H. Influence of architecture on the Raman spectra of acid-treated carbon nanostructures. J. Exp. Nanosci. 2014, 9, 931–941. [Google Scholar] [CrossRef]
- Ferkel, H.; Müller, B.; Riehemann, W. Electrodeposition of particle-strengthened nickel films. Mater. Sci. Environ. A 1997, 234, 474–476. [Google Scholar] [CrossRef]
- Low, C.T.J.; Walsh, F.C. Multifunctional Materials for Tribological Applications; Wood, R.J.K., Ed.; Pan Stanford: Singapore, 2015. [Google Scholar]

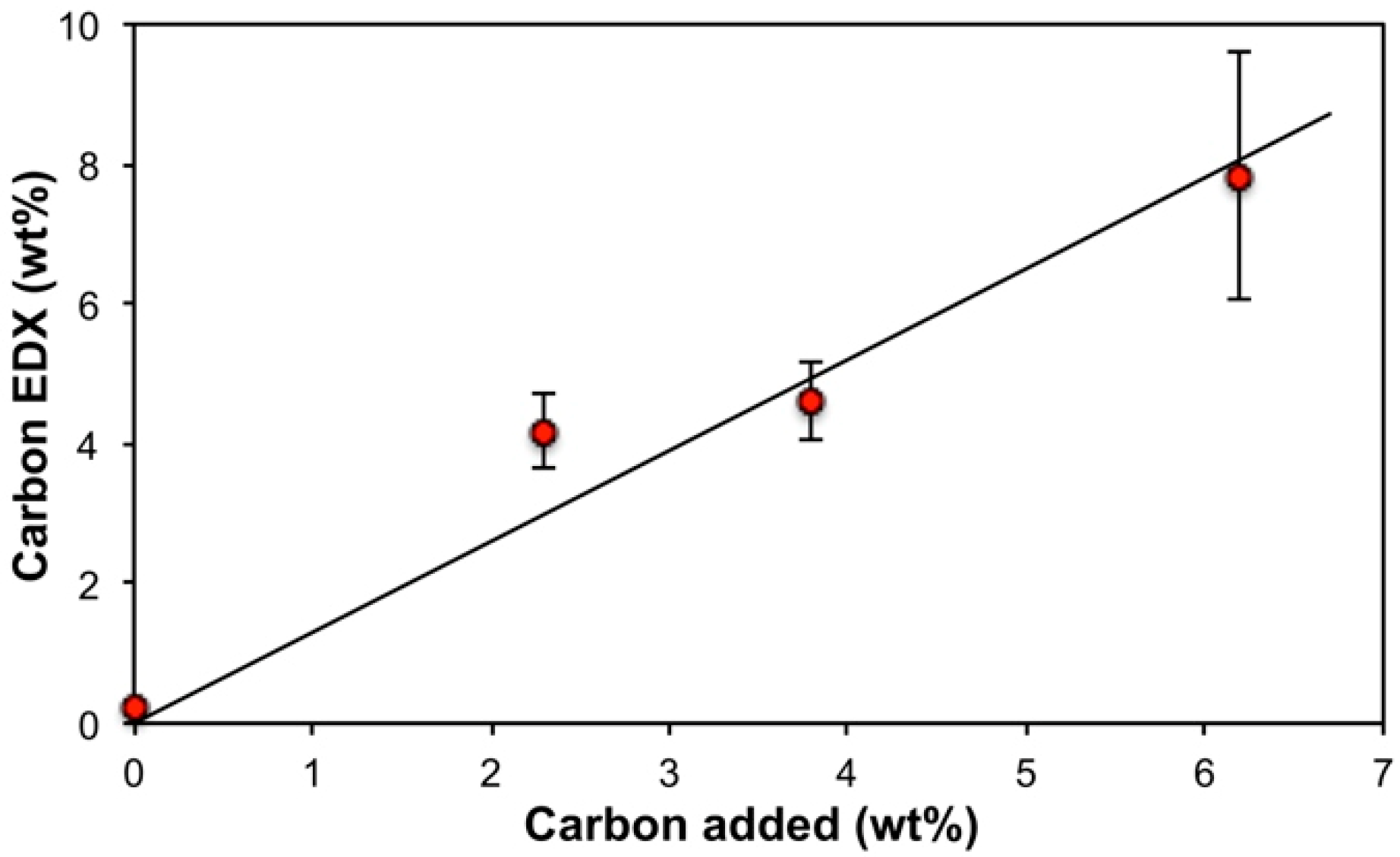

| Reaction Volume (mL) | SWCNT (mg) | Electrodeposition Time (min) | Air-Dried Product (mg) | SWCNT (wt%) |
|---|---|---|---|---|
| 75 | 0 | 90 | 350 | 0.0 |
| 150 | 17 | 210 | 753 | 2.3 |
| 100 | 17 | 90 | 450 | 3.8 |
| 60 | 17 | 90 | 274 | 6.2 |
| SWCNT (wt%) | C (at%) | O (at%) | Cu (at%) | Fe (at%) |
|---|---|---|---|---|
| 0.0 | 1.1 ± 0.4 | 8.4 ± 0.6 | 90.0 ± 1.7 | 0.7 ± 0.7 |
| 2.3 | 18.3 ± 2.3 | 1.3 ± 0.3 | 77.6 ± 4.0 | 1.5 ± 0.5 |
| 3.8 | 20.0 ± 2.4 | 2.0 ± 0.2 | 76.5 ± 2.5 | 1.3 ± 0.5 |
| 6.2 | 30.6 ± 7.0 | 1.5 ± 0.3 | 65.7 ± 7.2 | 2.2 ± 0.1 |
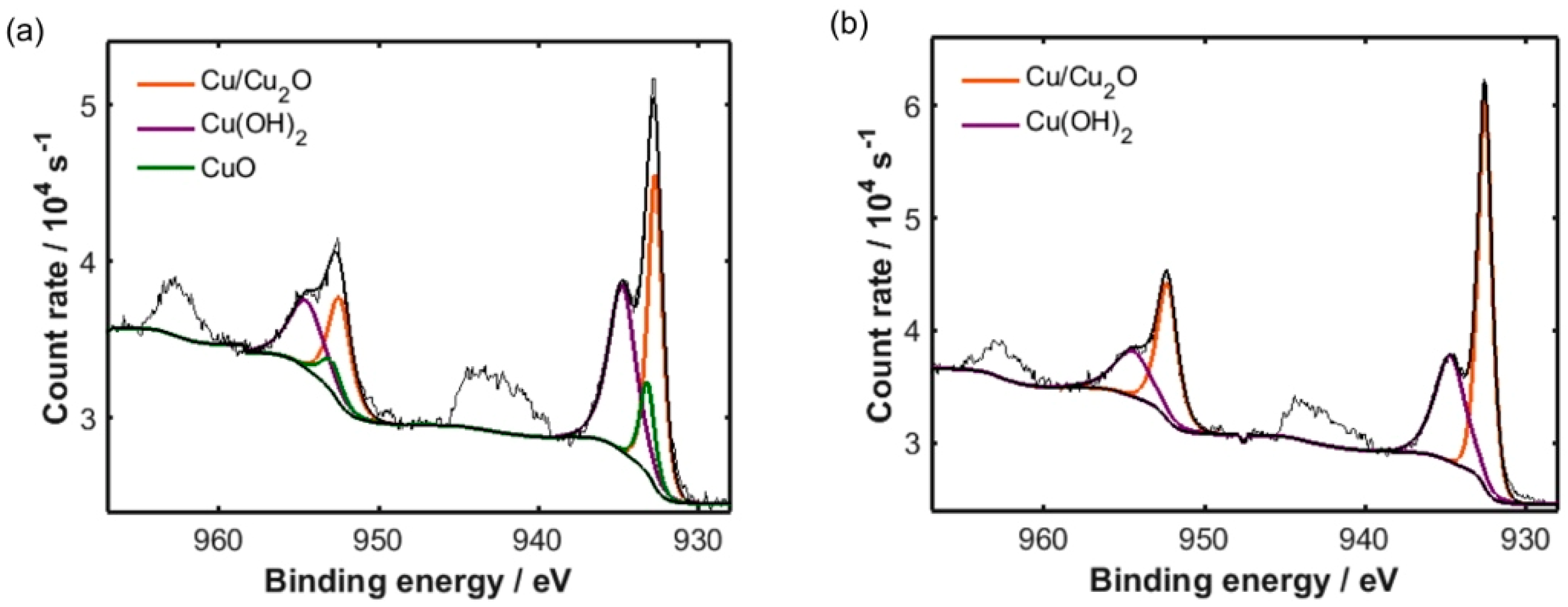
| SWCNT (wt%) | Cu and Cu2O (at%) | CuO (at%) | Cu(OH)2 (at%) |
|---|---|---|---|
| 0.0 | 43.9 | 12.9 | 43.2 |
| 3.8 | 62.7 | − | 37.3 |
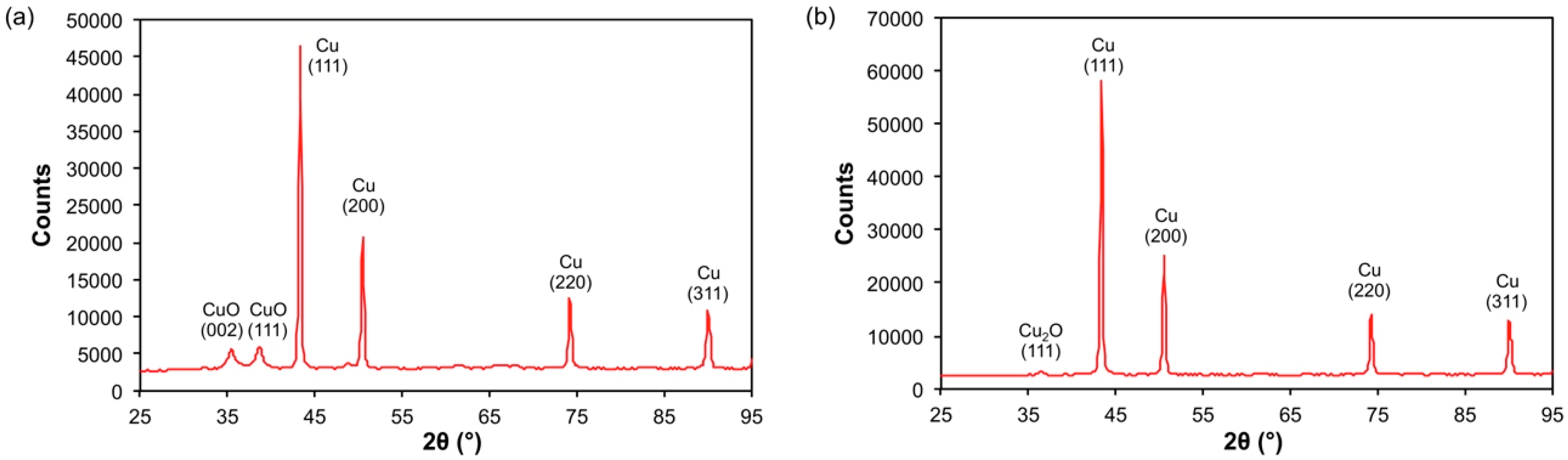
| Composition | Crystal Grain Size | |||||
|---|---|---|---|---|---|---|
| SWCNT (wt%) | Cu (wt%) | Cu2O (wt%) | CuO (wt%) | Cu (Å) | Cu2O (Å) | CuO (Å) |
| 0.0 | 70.0 (4) | − | 30.0(4) | 244 (15) | − | 64 (11) |
| 2.3 | 98.7 (2) | 1.3 (2) | − | 201 (3) | 117 | − |
| 3.8 | 97.5 (2) | 2.5 (2) | − | 254 (17) | 306 | − |
| 6.2 | 99.2 (2) | 0.8 (2) | − | 202 (10) | 145 | − |
| SWCNT (wt%) | IG:ID 1 |
|---|---|
| 0.0 | N/A |
| 2.3 | 11.43 (0.75) |
| 3.8 | 12.43 (1.46) |
| 6.2 | 10.18 (1.93) |
| SWCNT (wt%) | C (at%) | O (at%) | Cu (at%) | Fe (at%) |
|---|---|---|---|---|
| Hard coating | 12.3 ± 7.4 | 7.7 ± 4.0 | 76 ± 12 | 3.7 ± 0.5 |
| Porous deposit | 10.1 ± 2.5 | 20.6 ± 7.9 | 65.7 ± 4.4 | 3.4 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raja, P.M.V.; Esquenazi, G.L.; Gowenlock, C.E.; Jones, D.R.; Li, J.; Brinson, B.; Barron, A.R. Electrodeposition of Cu–SWCNT Composites. C 2019, 5, 38. https://doi.org/10.3390/c5030038
Raja PMV, Esquenazi GL, Gowenlock CE, Jones DR, Li J, Brinson B, Barron AR. Electrodeposition of Cu–SWCNT Composites. C. 2019; 5(3):38. https://doi.org/10.3390/c5030038
Chicago/Turabian StyleRaja, Pavan M. V., Gibran L. Esquenazi, Cathren E. Gowenlock, Daniel R. Jones, Jianhua Li, Bruce Brinson, and Andrew R. Barron. 2019. "Electrodeposition of Cu–SWCNT Composites" C 5, no. 3: 38. https://doi.org/10.3390/c5030038
APA StyleRaja, P. M. V., Esquenazi, G. L., Gowenlock, C. E., Jones, D. R., Li, J., Brinson, B., & Barron, A. R. (2019). Electrodeposition of Cu–SWCNT Composites. C, 5(3), 38. https://doi.org/10.3390/c5030038