Carbon Dots for Sensing and Killing Microorganisms
Abstract
1. Introduction
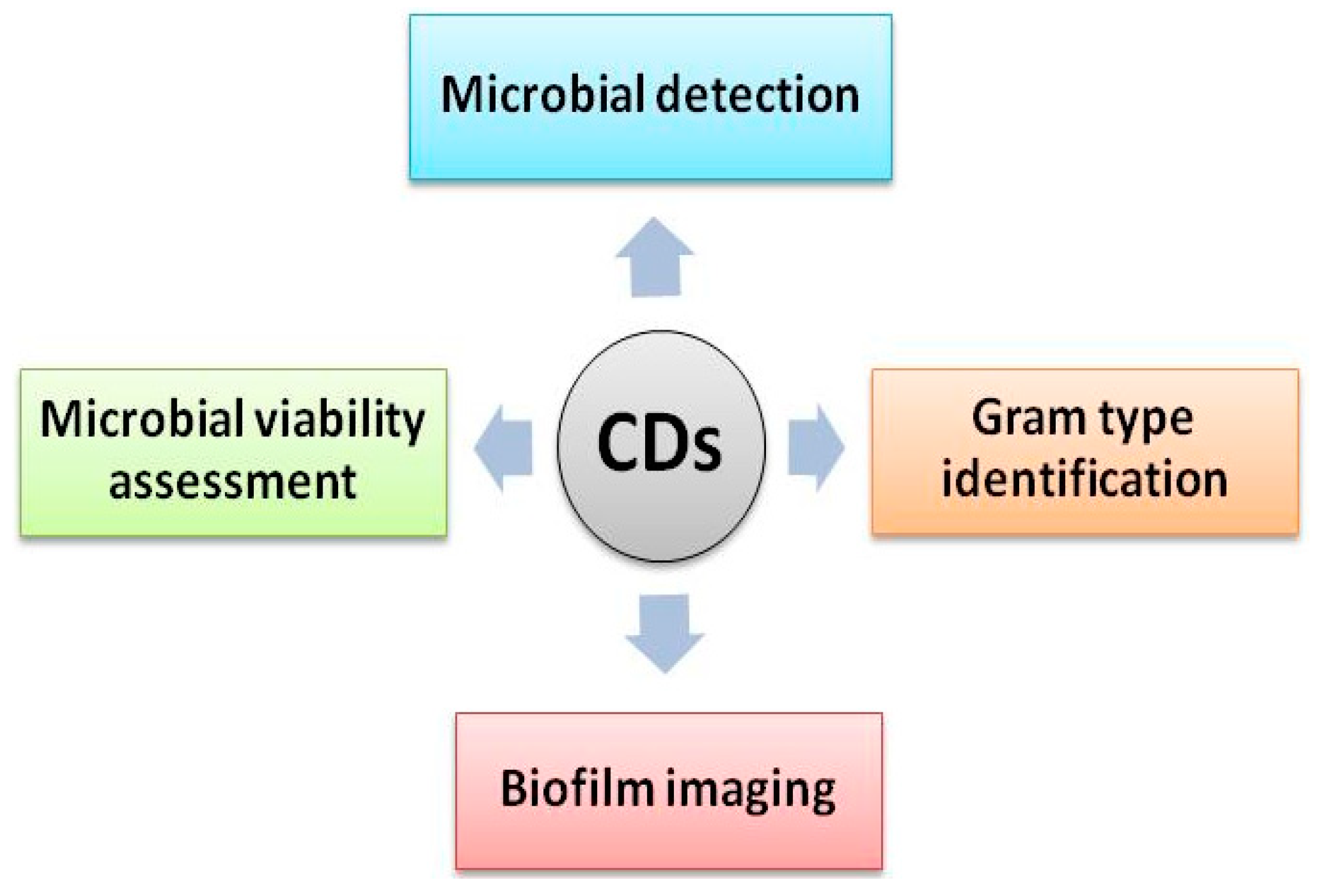
2. CDs for Sensing Microorganisms
2.1. Microbial Detection
2.1.1. CDs Derived from Natural Sources for Microbial Imaging
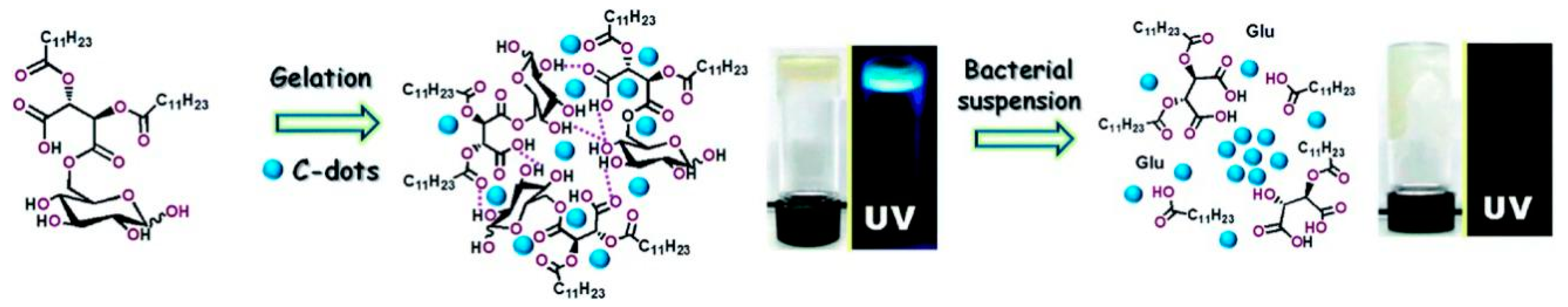
2.1.2. Amphiphilic CDs for Microbial Monitoring
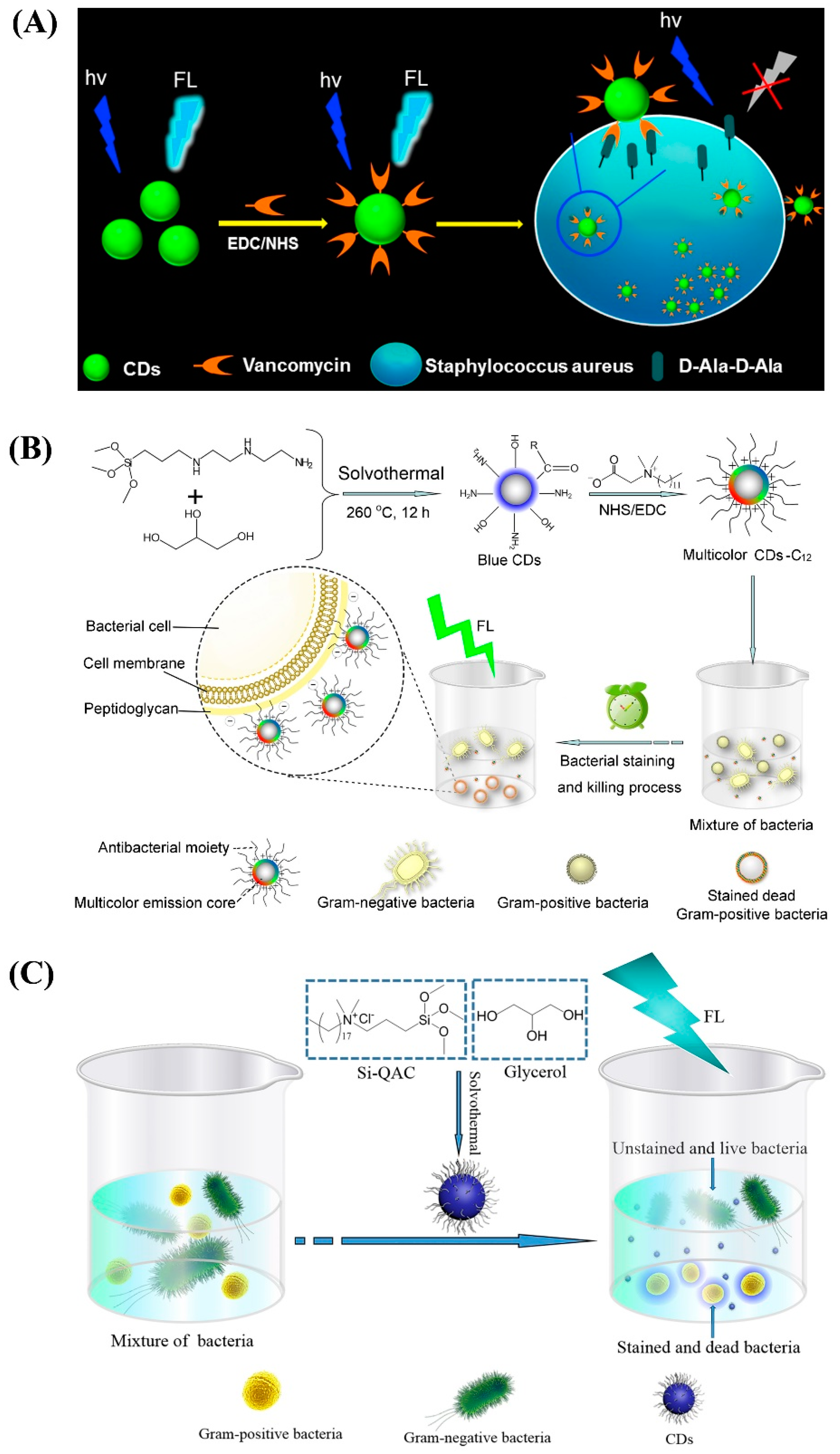
2.1.3. Functionalized CDs for Microbial Labeling
2.2. Gram Type Identification Using CDs
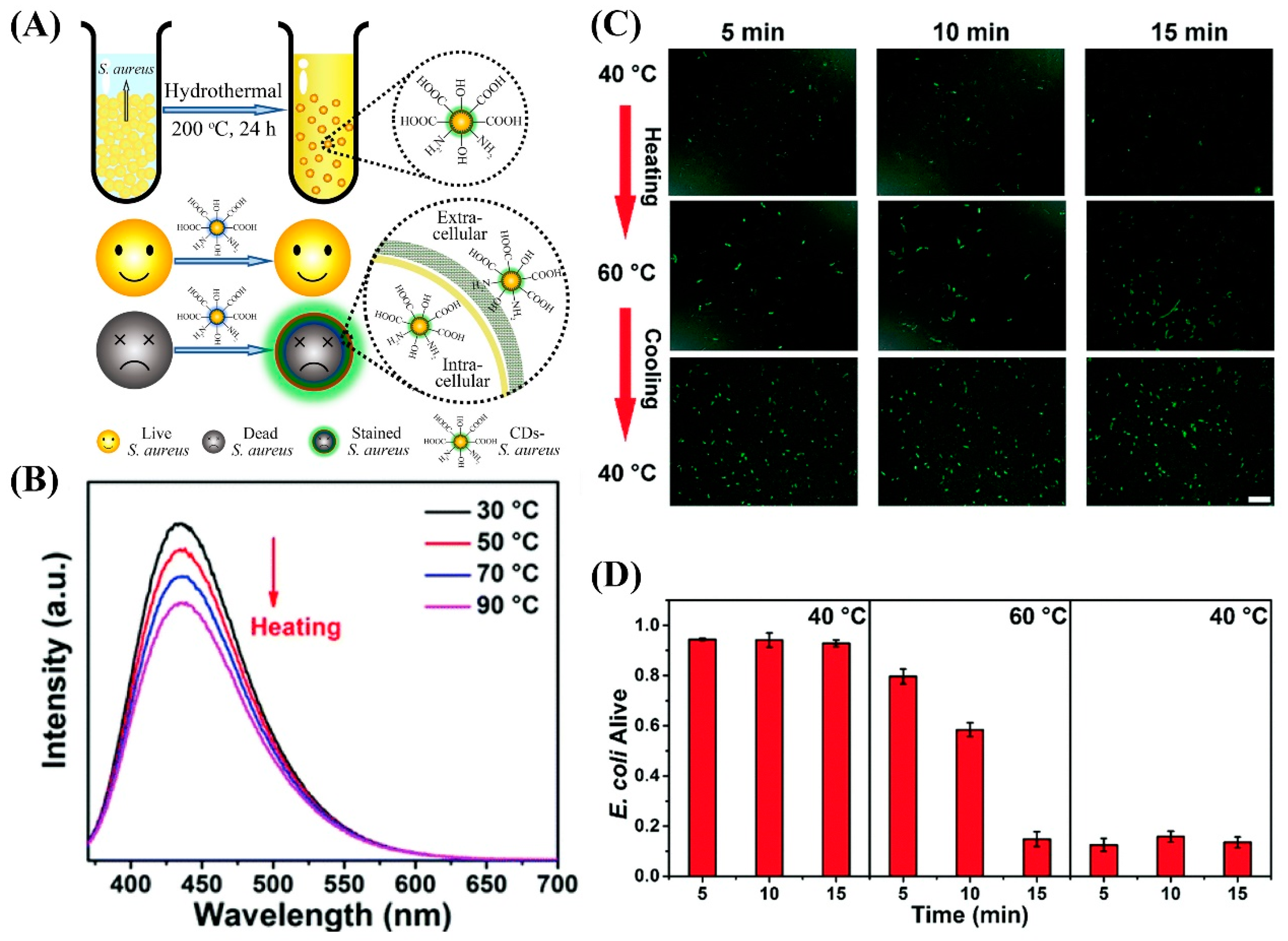
2.3. Microbial Viability Assessment Using CDs
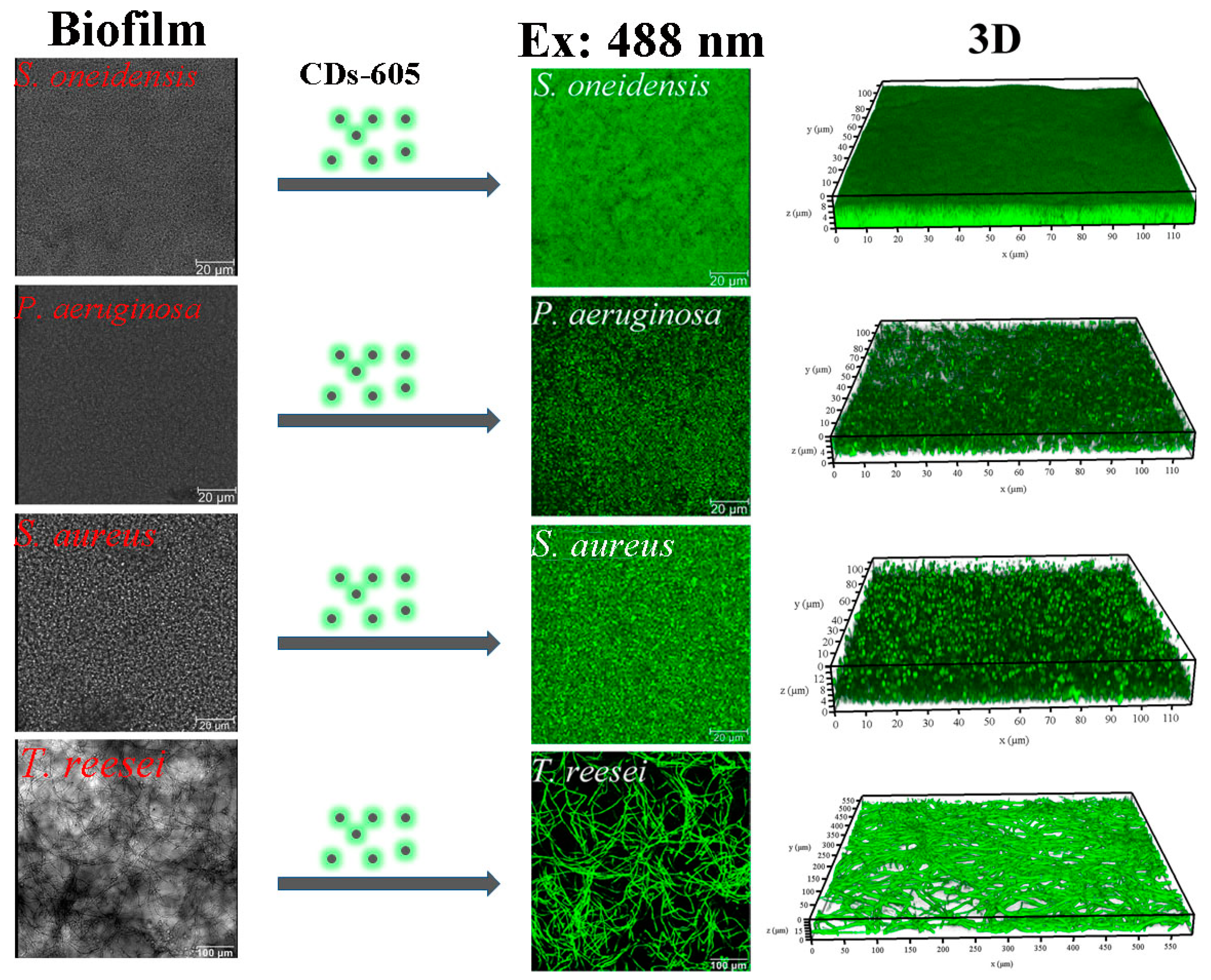
2.4. CDs for Biofilm Imaging
3. CDs for Killing Microorganisms
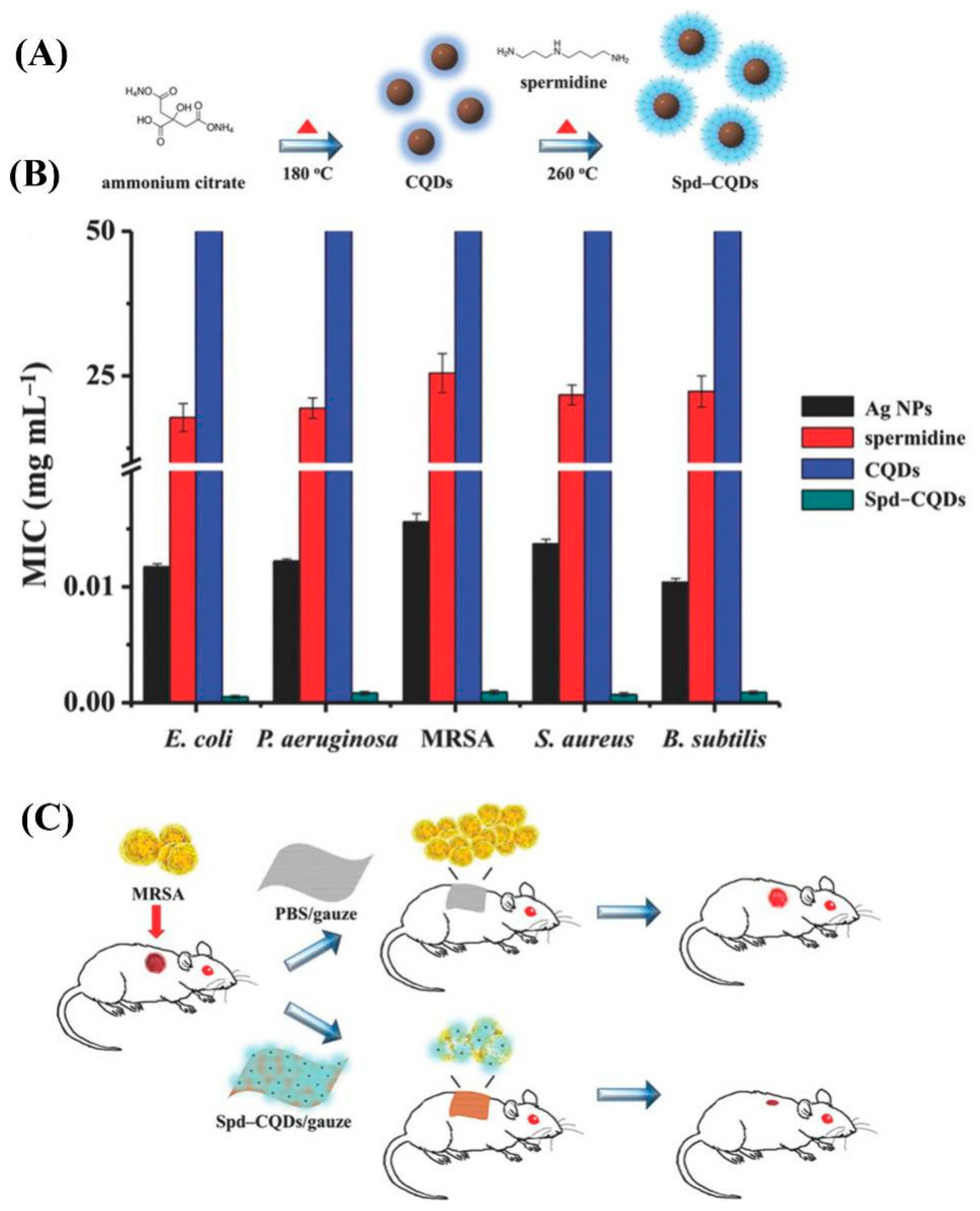
3.1. Positively Charged CDs
3.2. Uniquely Shaped CDs
3.3. Photosensitive CDs
3.3.1. PDT
3.3.2. PTT
3.4. Antibiotic-Modified CDs
3.5. Metal-Based CDs as Antimicrobial Agents
3.6. Other CDs
4. Bacterial Theranostic Systems Based on CDs
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Sun, W.; Wu, F.-G. Fluorescent quantum dots for microbial imaging. Chin. Chem. Lett. 2018, 29, 1475–1485. [Google Scholar] [CrossRef]
- Sun, W.; Wu, F.-G. Two-dimensional materials for antimicrobial applications: Graphene materials and beyond. Chem.-Asian J. 2018, 13, 3378–3410. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Qian, Z. Functional carbon quantum dots: A versatile platform for chemosensing and biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, C.; Chen, Z. Bacteria-derived carbon dots inhibit biofilm formation of Escherichia coli without affecting cell growth. Front. Microbiol. 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.-H.; Jia, H.-R.; Jiang, Y.-W.; Wang, Z.; Wu, F.-G. Ultrasmall and photostable nanotheranostic agents based on carbon quantum dots passivated with polyamine-containing organosilane molecules. Nanoscale 2017, 9, 15441–15452. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-W.; Hua, X.-W.; Li, Y.-H.; Jia, H.-R.; Wu, F.-G. Hyperthemia-promoted cytosolic and nuclear delivery of copper/carbon quantum dot-crosslinked nanosheets: Multimodal imaging-guided photothermal cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 1544–1555. [Google Scholar] [CrossRef]
- Hua, X.-W.; Bao, Y.-W.; Zeng, J.; Wu, F.-G. Ultrasmall all-in-one nanodots formed via carbon dot-mediated and albumin-based synthesis: Multimodal imaging-guided and mild laser-enhanced cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 42077–42087. [Google Scholar] [CrossRef]
- Hua, X.-W.; Bao, Y.-W.; Chen, Z.; Wu, F.-G. Carbon quantum dots with intrinsic mitochondrial targeting ability for mitochondria-based theranostics. Nanoscale 2017, 9, 10948–10960. [Google Scholar] [CrossRef]
- Hua, X.-W.; Bao, Y.-W.; Wu, F.-G. Fluorescent carbon quantum dots with intrinsic nucleolus-targeting capability for nucleolus imaging and enhanced cytosolic and nuclear drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 10664–10677. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Ma, C.; Ge, C.; Yan, M.; Yang, J.; Zhang, Y.; Morais, P.C.; Bi, H. Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and L-lysine for bioimaging. J. Mater. Chem. B 2014, 2, 4631–4639. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Teng, X.; Yan, M.; Bi, H.; Morais, P.C. Mitochondria-targeting nanoplatform with fluorescent carbon dots for long time imaging and magnetic field-enhanced cellular uptake. ACS Appl. Mater. Interfaces 2015, 7, 10201–10212. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Yang, J.; Wu, F.-G. Mitochondria-targetable carbon quantum dots for differentiating cancerous cells from normal cells. Nanoscale 2017, 9, 18368–18378. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Yang, J.; Wu, F.-G. On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 2018, 134, 232–243. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Liu, J.; Wu, J.; Chen, H.; Bi, H. Design and preparation of a ternary composite of graphene oxide/carbon dots/polypyrrole for supercapacitor application: Importance and unique role of carbon dots. Carbon 2017, 115, 134–146. [Google Scholar] [CrossRef]
- Jaleel, J.A.; Pramod, K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control. Release 2018, 269, 302–321. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Kasibabu, B.S.B.; D’souza, S.L.; Jha, S.; Singhal, R.K.; Basu, H.; Kailasa, S.K. One-step synthesis of fluorescent carbon dots for imaging bacterial and fungal cells. Anal. Methods 2015, 7, 2373–2378. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater. Sci. Eng. C 2014, 38, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kasibabu, B.S.B.; D’souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from Carica papaya juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.-W.; Bao, Y.-W.; Wang, H.-Y.; Chen, Z.; Wu, F.-G. Bacteria-derived fluorescent carbon dots for microbial live/dead differentiation. Nanoscale 2017, 9, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Rawat, K.; Prasad, T.; Bohidar, H.B. Antifungal efficacy of Au@carbon dots nanoconjugates against opportunistic fungal pathogen, Candida albicans. Colloid Surf. B-Biointerfaces 2018, 163, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable carbon dots with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Pagneux, Q.; Sane, F.; Wang, Q.; Boukherroub, R.; Hober, D.; Szunerits, S. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces 2016, 8, 9004–9013. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Dong, X.; Moyer, M.M.; Yang, F.; Sun, Y.P.; Yang, L. Carbon dots’ antiviral functions against noroviruses. Sci. Rep. 2017, 7, 519. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Huang, S.; Gu, J.; Ye, J.; Fang, B.; Wan, S.; Wang, C.; Ashraf, U.; Li, Q.; Shao, L.; Song, Y.; et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019, 542, 198–206. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Chandra, S.; Mahto, T.K.; Chowdhuri, A.R.; Das, B.; Sahu, S.K. One step synthesis of functionalized carbon dots for the ultrasensitive detection of Escherichia coli and iron(III). Sens. Actuators B 2017, 245, 835–844. [Google Scholar] [CrossRef]
- Chandra, S.; Chowdhuri, A.R.; Mahto, T.K.; Samui, A.; Sahu, S.K. One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv. 2016, 6, 72471–72478. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nandi, S.; Jelinek, R. Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv. 2017, 7, 588–594. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Gedda, G.; Khan, M.S.; Wu, H.-F. Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal. Chim. Acta 2016, 920, 63–71. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.-C. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N. Rapid detection of bacteria by carbon quantum dots. J. Biomed. Nanotechnol. 2011, 7, 846–848. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Sonker, A.K.; Sonkar, S.K.; Sarkar, S. Pollutant soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E. coli and sensing cholesterol. RSC Adv. 2014, 4, 30100–30107. [Google Scholar] [CrossRef]
- Wang, N.; Fan, H.; Sun, J.; Han, Z.; Dong, J.; Ai, S. Fluorine-doped carbon nitride quantum dots: Ethylene glycol-assisted synthesis, fluorescent properties, and their application for bacterial imaging. Carbon 2016, 109, 141–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Fan, Y.; Guo, X.; Zhou, L.; Lv, Y.; Lin, J. One-step microwave synthesis of N-doped hydroxyl-functionalized carbon dots with ultra-high fluorescence quantum yields. Nanoscale 2016, 8, 15281–15287. [Google Scholar] [CrossRef]
- Nandi, S.; Ritenberg, M.; Jelinek, R. Bacterial detection with amphiphilic carbon dots. Analyst 2015, 140, 4232–4237. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Malishev, R.; Kootery, K.P.; Mirsky, Y.; Kolusheva, S.; Jelinek, R. Membrane analysis with amphiphilic carbon dots. Chem. Commun. 2014, 50, 10299–10302. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Kennedy, S.R.; Soriano, M.L.; Jones, C.D.; Valcárcel, M.; Steed, J.W. Fluorescent carbon dot–molecular salt hydrogels. Chem. Sci. 2015, 6, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl. Nano Mater 2015, 7, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, A.; Tian, Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-I.; Chang, H.-T.; Lin, C.-H.; Shen, Y.-W.; Unnikrishnan, B.; Li, Y.-J.; Huang, C.-C. One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosens. Bioelectron. 2015, 68, 1–6. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar]
- Zhong, D.; Zhuo, Y.; Feng, Y.; Yang, X. Employing carbon dots modified with vancomycin for assaying Gram-positive bacteria like Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 546–553. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.-H.; Gao, G.; Chen, X.; Jia, H.-R.; Li, Y.-H.; Chen, Z.; Wu, F.-G. Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.-H.; Chen, X.; Wu, F.-G. One-step synthesized carbon dots with bacterial contact-enhanced fluorescence emission property: Fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon 2019, 146, 827–839. [Google Scholar] [CrossRef]
- Zhao, E.; Hong, Y.; Chen, S.; Leung, C.W.T.; Chan, C.Y.K.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Highly fluorescent and photostable probe for long–term bacterial viability assay based on aggregation–induced emission. Adv. Healthc. Mater. 2014, 3, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Keer, J.T.; Birch, L. Molecular methods for the assessment of bacterial viability. J. Microbiol. Methods 2003, 53, 175–183. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, Y.; Zhao, X.; Chen, X.; Wang, P.; Zheng, Z. Discrimination of viable and dead microbial materials with Fourier transform infrared spectroscopy in 3–5 micrometers. Opt. Express 2018, 26, 15842–15850. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Barbero, R.J.; Gray, D.S.; Belcher, A.M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010, 5, 280–285. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, D.; Ivleva, N.P.; Mircescu, N.E.; Schubert, S.; Niessner, R.; Wieser, A.; Haisch, C. Label-free in situ discrimination of live and dead bacteria by surface-enhanced Raman scattering. Anal. Chem. 2015, 87, 6553–6561. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Lu, F.; Wang, H.; Zhang, M.; Yang, J.; Huang, J.; Huang, H.; Liu, Y.; Kang, Z. Fluorescent carbon dots with highly negative charges as a sensitive probe for real-time monitoring of bacterial viability. J. Mater. Chem. B 2017, 5, 6008–6015. [Google Scholar] [CrossRef]
- Lu, F.; Song, Y.; Huang, H.; Liu, Y.; Fu, Y.; Huang, J.; Li, H.; Qu, H.; Kang, Z. Fluorescent carbon dots with tunable negative charges for bio-imaging in bacterial viability assessment. Carbon 2017, 120, 95–102. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Chen, Z. Exopolysaccharide-derived carbon dots for microbial viability assessment. Front. Microbiol. 2018, 9, 2697. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef]
- Natalio, F.; André, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Denkhaus, E.; Meisen, S.; Telgheder, U.; Wingender, J. Chemical and physical methods for characterisation of biofilms. Microchim. Acta 2007, 158, 1–27. [Google Scholar] [CrossRef]
- Wolf, G.; Crespo, J.G.; Reis, M.A.M. Optical and spectroscopic methods for biofilm examination and monitoring. Rev. Environ. Sci. Biotechnol. 2002, 1, 227–251. [Google Scholar] [CrossRef]
- Neu, T.R.; Swerhone, G.D.W.; Lawrence, J.R. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 2001, 147, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Mustin, C.; Balan, L.; Roques-Carmes, T.; Fontaine-Aupart, M.-P.; Schneider, R. Surface-engineered quantum dots for the labeling of hydrophobic microdomains in bacterial biofilms. Biomaterials 2011, 32, 5459–5470. [Google Scholar] [CrossRef]
- Chalmers, N.I.; Palmer, R.J.; Du-Thumm, L.; Sullivan, R.; Shi, W.; Kolenbrander, P.E. Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Appl. Environ. Microbiol. 2007, 73, 630–636. [Google Scholar] [CrossRef]
- Ritenberg, M.; Nandi, S.; Kolusheva, S.; Dandela, R.; Meijler, M.M.; Jelinek, R. Imaging Pseudomonas aeruginosa biofilm extracellular polymer scaffolds with amphiphilic carbon dots. ACS Chem. Biol. 2016, 11, 1265–1270. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Dong, L.; Fu, D.; Chen, Z. Imaging biofilm-encased microorganisms using carbon dots derived from L. plantarum. Nanoscale 2017, 9, 9056–9064. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.J.; Yan, Z.; Ren, J.; Qu, X. Programmed bacteria death induced by carbon dots with different surface charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Harroun, S.G.; Su, Y.-C.; Huang, C.-F.; Unnikrishnan, B.; Lin, H.-J.; Lin, C.-H.; Huang, C.-C. Synthesis of self-assembled spermidine-carbon quantum dots effective against multidrug-resistant bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Fang, X.; Jiang, S.; Chee, P.L.; Lee, T.-C.; Loh, X.J. Multi-functional fluorescent carbon dots with antibacterial and gene delivery properties. RSC Adv. 2015, 5, 46817–46822. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Hui, L.; Huang, J.; Chen, G.; Zhu, Y.; Yang, L. Antibacterial property of graphene quantum dots (both source material and bacterial shape matter). ACS Appl. Mater. Interfaces 2016, 8, 20–25. [Google Scholar] [CrossRef]
- Li, C.; Lin, F.; Sun, W.; Wu, F.-G.; Yang, H.; Lv, R.; Zhu, Y.-X.; Jia, H.-R.; Wang, C.; Gao, G.; et al. Self-assembled rose bengal-exopolysaccharide nanoparticles for improved photodynamic inactivation of bacteria by enhancing singlet oxygen generation directly in the solution. ACS Appl. Mater. Interfaces 2018, 10, 16715–16722. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Improving the phototherapeutic efficiencies of molecular and nanoscale materials by targeting mitochondria. Molecules 2018, 23, 3016. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef] [PubMed]
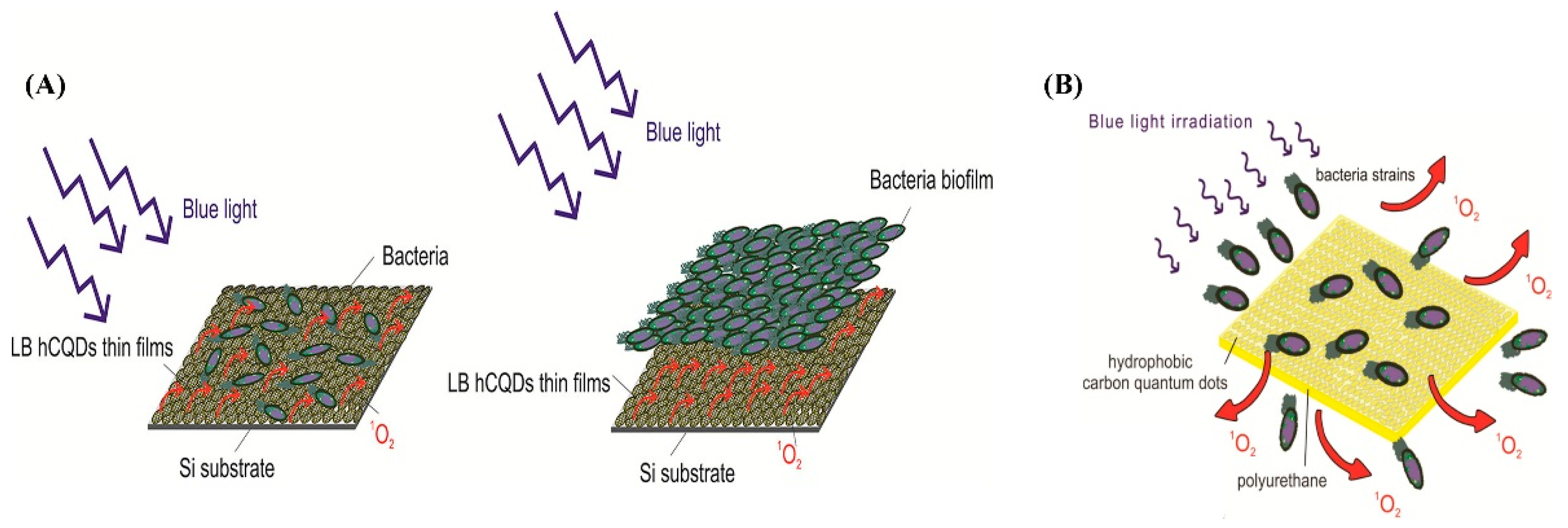
- Stanković, N.K.; Bodik, M.; Šiffalovič, P.; Kotlar, M.; Mičušik, M.; Špitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots Langmuir–Blodgett thin films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Sens. Actuator B-Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Dong, X.; Awak, M.A.; Wang, P.; Sun, Y.-P.; Yang, L. Carbon dot incorporated multi-walled carbon nanotube coated filters for bacterial removal and inactivation. RSC Adv. 2018, 8, 8292–8301. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, M.; Marković, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajdák, J.; Capáková, Z.; et al. Carbon quantum dots modified polyurethane nanocomposite as effective photocatalytic and antibacterial agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, C.-Y.; Chen, H.-H.; Hsu, C.-L.L.; Wang, J.-Y.; Kao, H.-F.; Chou, L.C.-S.; Chen, Y.-C.; Chen, S.-J.; Chang, W.-T.; et al. Two-photon photoexcited photodynamic therapy and contrast agent with antimicrobial graphene quantum dots. ACS Appl. Mater. Interfaces 2016, 8, 30467–30474. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chen, H.-H.; Chen, S.-Y.; Chang, C.-Y.; Chen, P.-C.; Hou, Y.-I.; Shao, Y.-T.; Kao, H.-F.; Hsu, C.-L.L.; Chen, Y.-C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial amino-functionalized nitrogen-doped graphene quantum dots for eliminating multidrug-resistant species in dual-modality photodynamic therapy and bioimaging under two-photon excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagnosis Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef]
- Dong, X.; Bond, A.E.; Pan, N.; Coleman, M.; Tang, Y.; Sun, Y.-P.; Yang, L. Synergistic photoactivated antimicrobial effects of carbon dots combined with dye photosensitizers. Int. J. Nanomed. 2018, 13, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-L.; Chen, M.-H.; Tung, F.-I.; Liu, T.-Y. A nanovehicle developed for treating deep-seated bacteria using low-dose X-ray. Acta Biomater. 2017, 47, 159–169. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Gupta, K.; Mandal, M.; Karak, N. Biocide immobilized OMMT-carbon dot reduced Cu2O nanohybrid/hyperbranched epoxy nanocomposites: Mechanical, thermal, antimicrobial and optical properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 56, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Kuang, W.; Shi, L.; Ye, X.; Yang, Y.; Xie, X.; Shi, Q.; Tan, S. Carbon quantum dot-decorated TiO2 for fast and sustainable antibacterial properties under visible-light. J. Alloys Compd. 2019, 777, 234–243. [Google Scholar] [CrossRef]
- Liu, J.; Rojas-Andrade, M.D.; Chata, G.; Peng, Y.; Roseman, G.; Lu, J.-E.; Millhauser, G.L.; Saltikov, C.; Chen, S. Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale 2018, 10, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Wang, X.; Mu, L.; Yuan, M.; Liu, B.; Shi, H. Carbon dots-decorated Na2W4O13 composite with WO3 for highly efficient photocatalytic antibacterial activity. J. Hazard. Mater. 2018, 359, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Rezaie-Yazdi, M.; Tondro, G.H.; Akbari, N. Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant Staphylococcus aureus. J. Photochem. Photobiol. B-Biol. 2017, 166, 323–332. [Google Scholar] [CrossRef]
- Thakur, M.; Pandey, S.; Mewada, A.; Patil, V.; Khade, M.; Goshi, E.; Sharon, M. Antibiotic conjugated fluorescent carbon dots as a theranostic agent for controlled drug release, bioimaging, and enhanced antimicrobial activity. J. Drug Deliv. 2014, 2014, 282193. [Google Scholar] [CrossRef]
- Hou, P.; Yang, T.; Liu, H.; Li, Y.F.; Huang, C.Z. An active structure preservation method for developing functional graphitic carbon dots as an effective antibacterial agent and a sensitive pH and Al(III) nanosensor. Nanoscale 2017, 9, 17334–17341. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloid Surf. B-Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Mayank; Pandiyan, T.; Kaur, N.; Singh, N. The photochemical degradation of bacterial cell wall using penicillin-based carbon dots: Weapons against multi-drug resistant (MDR) strains. ChemistrySelect 2017, 2, 9277–9283. [Google Scholar] [CrossRef]
- Kumar, V.B.; Dolitzky, A.; Michaeli, S.; Gedanken, A. Antiparasitic ointment based on a biocompatibile carbon dot nanocomposite. ACS Appl. Nano Mater. 2018, 1, 1784–1791. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.; Tang, Q.; Zhang, K.; Yu, W.; Sun, H.; Yang, B. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Habiba, K.; Bracho-Rincon, D.P.; Gonzalez-Feliciano, J.A.; Villalobos-Santos, J.C.; Makarov, V.I.; Ortiz, D.; Avalos, J.A.; Gonzalez, C.I.; Weiner, B.R.; Morell, G. Synergistic antibacterial activity of PEGylated silver–graphene quantum dots nanocomposites. Appl. Mater. Today 2015, 1, 80–87. [Google Scholar] [CrossRef]
- Han, S.; Zhang, H.; Xie, Y.; Liu, L.; Shan, C.; Li, X.; Liu, W.; Tang, Y. Application of cow milk-derived carbon dots/AgNPs composite as the antibacterial agent. Appl. Surf. Sci. 2015, 328, 368–373. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Bandosz, T.J. Carbon quantum dot surface-chemistry-dependent Ag release governs the high antibacterial activity of Ag-metal–organic framework composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, Q.; Geng, L.; Fang, Q.; Gong, J.R. Chiral nanoparticle as a new efficient antimicrobial nanoagent. Adv. Healthc. Mater. 2017, 6, 1601011. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Pramanik, A.; Jones, S.; Pedraza, F.; Vangara, A.; Sweet, C.; Williams, M.S.; Ruppa-Kasani, V.; Risher, S.E.; Sardar, D.; Ray, P.C. Fluorescent, magnetic multifunctional carbon dots for selective separation, identification, and eradication of drug-resistant superbugs. ACS Omega 2017, 2, 554–562. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
| Precursor | Preparation | Microorganism | Ref. |
|---|---|---|---|
| Cationic CDs | |||
| Curcumin | Hydrothermal treatment | Porcine epidemic diarrhea virus | [29] |
| Glucose, spermine, and NaCl | Microwave synthesis | E. coli and B. subtilis | [71] |
| Spermidine | Dry heating treatment | Non-multidrug-resistant E. coli, S. aureus, P. aeruginosa, and Salmonella enterica serovar Enteritidis bacteria, and the multidrug-resistant bacterium, MRSA | [72] |
| Ammonium citrate and spermidine | Pyrolysis of solid ammonium citrate to make CDs that are linked with spermidine by heating treatment | Non-multidrug-resistant bacteria like E. coli, S. aureus, B. subtilis, and P. aeruginosa, and MRSA | [73] |
| Uniquely Shaped CDs | |||
| C60 cage | Modified Hummers’ method | S. aureus | [78] |
| Photosensitive CDs | |||
| Graphene | Electrochemical method | MRSA and E. coli | [82] |
| Poloxamer Pluronic F-68 | Bottom-up condensation | E. coli, S. aureus, Bacillus cereus, and P. aeruginosa | [83] |
| Graphene oxide sheet | Ultrasonic shearing reaction method | E. coli and MRSA | [87] |
| Benzene and methylene blue | CDs were synthesized by focusing nanosecond laser pulses into benzene and were then combined with methylene blue | E. coli and M. luteus | [90] |
| Citric acid, zinc stearate, and diethylene glycol | Hydrothermal treatment | E. coli | [95] |
| Ascorbic acid and copper acetate hydrate | Hydrothermal treatment | S. aureus and MRSA | [97] |
| Antibiotic-Modified CDs | |||
| Gum arabic and ciprofloxacin | CDs were prepared from gum arabic by microwave synthesis and conjugated with ciprofloxacin covalently | B. subtilis, S. aureus, E. coli and P. aeruginosa | [98] |
| Metronidazole | Hydrothermal treatment | Porphyromonas gingivalis | [103] |
| Metal-Based CDs | |||
| Critic acid, PEG, and HAuCl4 | CDs were prepared via the microwave treatment of critic acid and PEG, which could reduce HAuCl4 to obtain Au@CDs nanoconjugates | C. albicans | [24] |
| Benzene, silver powder, and PEG | Pulsed laser synthesis | S. aureus and P. aeruginosa | [104] |
| Other CDs | |||
| Ascorbic acid | Electrochemical method | S. aureus, B. subtilis, Bacillus sp. WL-6, E. coli, ampicillin-resistant E. coli, R. solani, and P. grisea | [25] |
| Citric acid and D-Glu | Hydrothermal treatment | E. coli and S. aureus | [107] |
| Graphite | Hummers’ approach | E. coli and S. aureus | [108] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon Dots for Sensing and Killing Microorganisms. C 2019, 5, 33. https://doi.org/10.3390/c5020033
Lin F, Bao Y-W, Wu F-G. Carbon Dots for Sensing and Killing Microorganisms. C. 2019; 5(2):33. https://doi.org/10.3390/c5020033
Chicago/Turabian StyleLin, Fengming, Yan-Wen Bao, and Fu-Gen Wu. 2019. "Carbon Dots for Sensing and Killing Microorganisms" C 5, no. 2: 33. https://doi.org/10.3390/c5020033
APA StyleLin, F., Bao, Y.-W., & Wu, F.-G. (2019). Carbon Dots for Sensing and Killing Microorganisms. C, 5(2), 33. https://doi.org/10.3390/c5020033