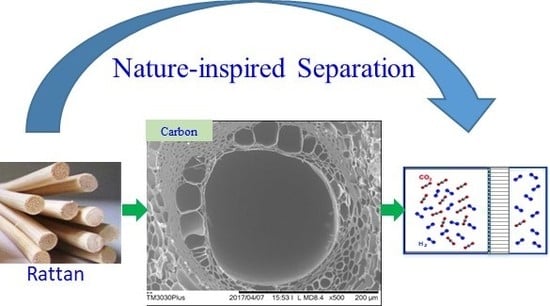
Novel Tubular Carbon Membranes Prepared from Natural Rattans
Abstract
:1. Introduction
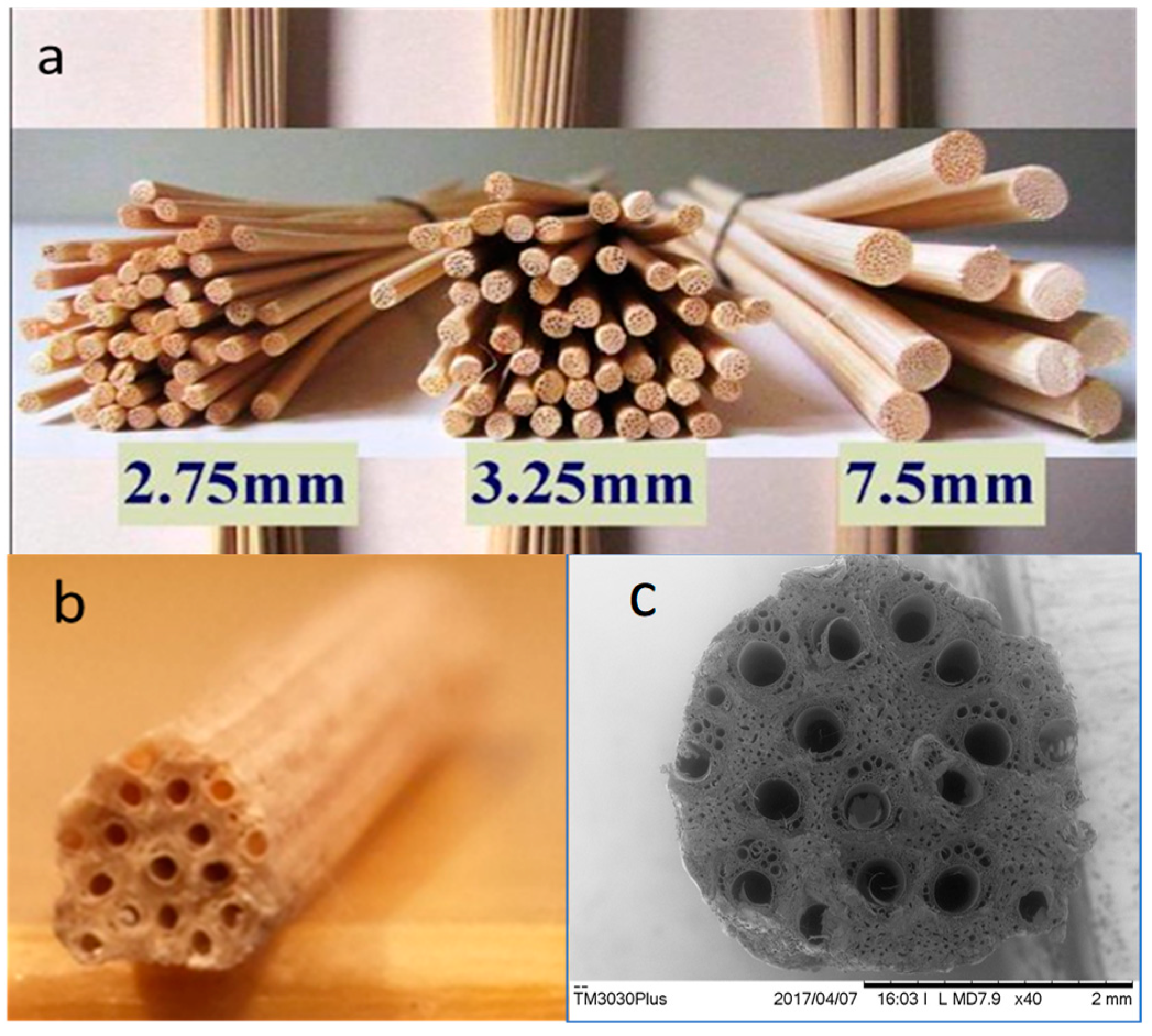
2. Material and Methods
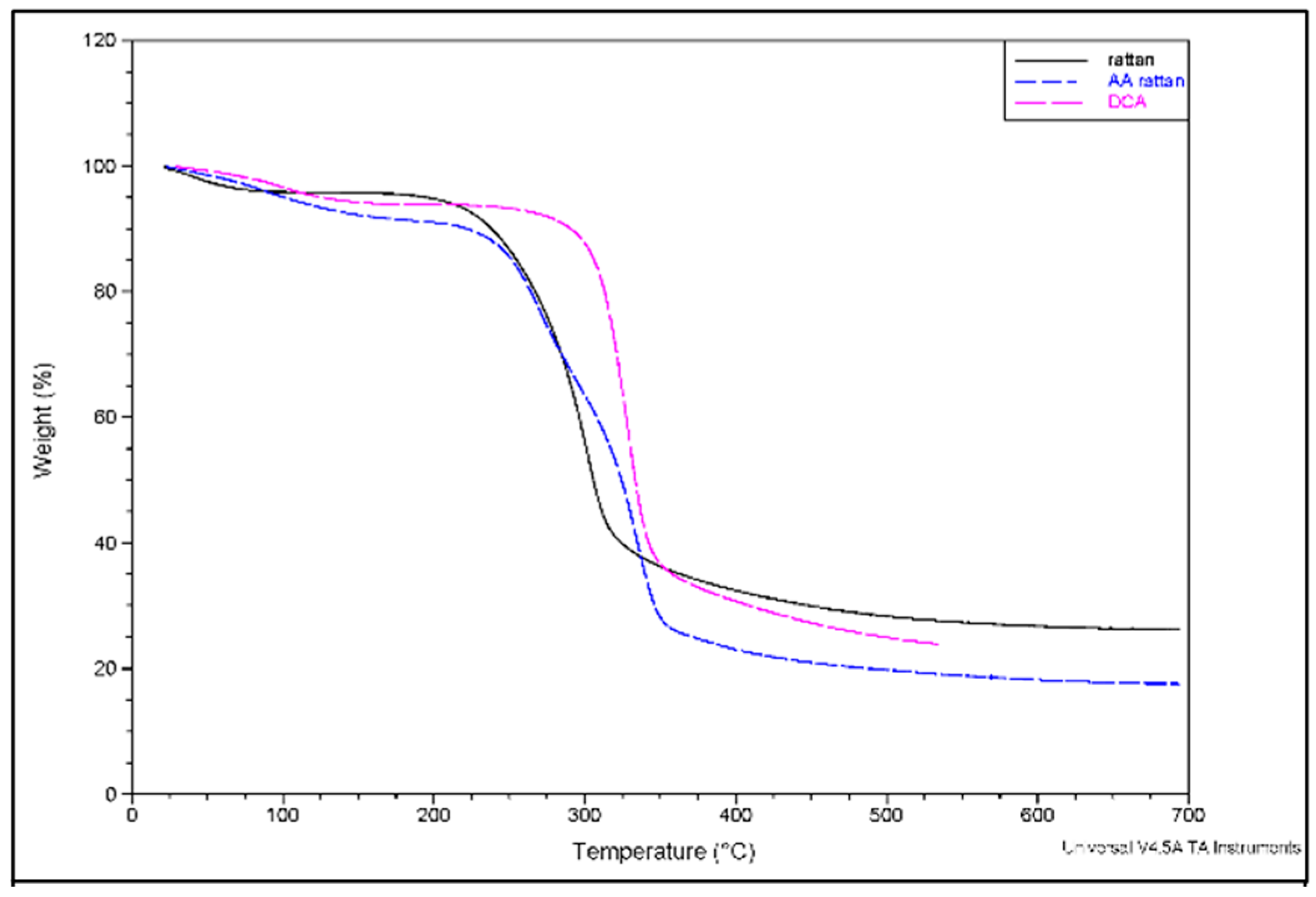
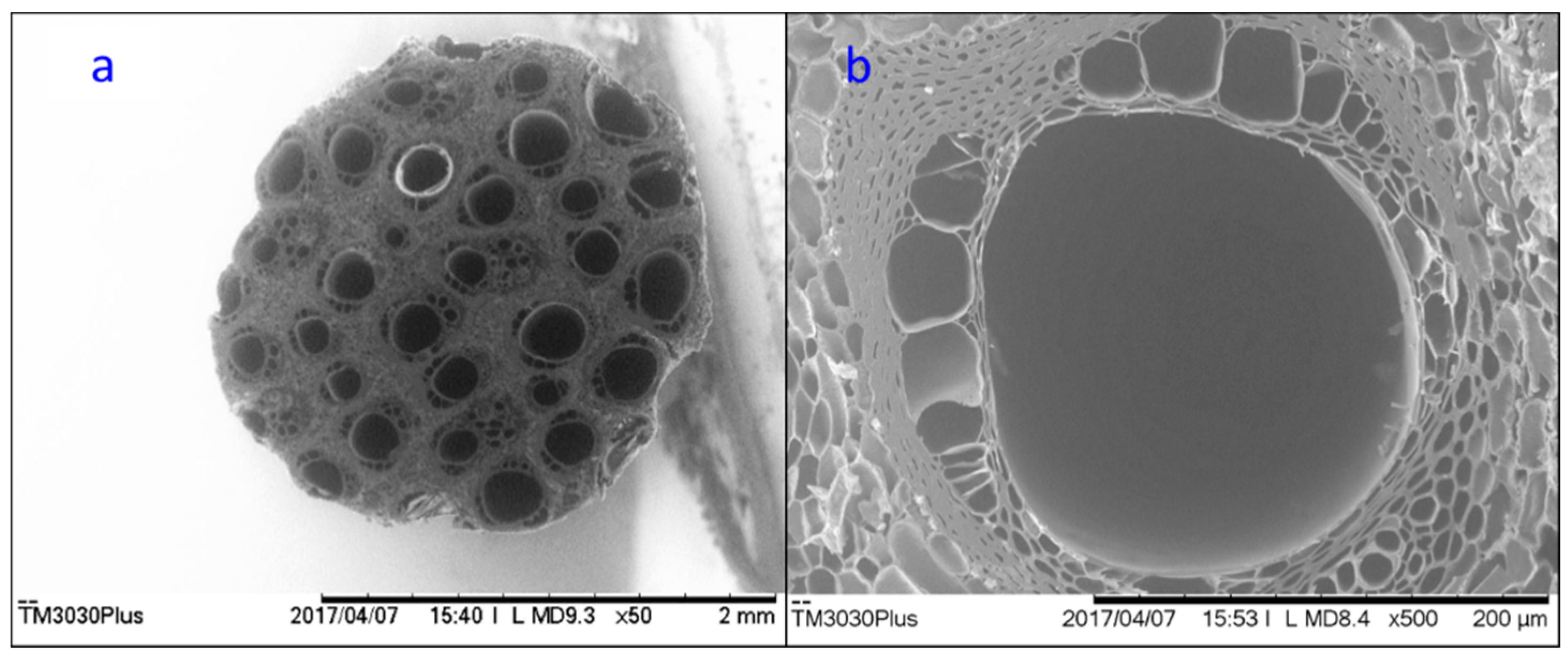
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, X.; Hägg, M.-B. Membranes for environmentally friendly energy processes. Membranes 2012, 2, 706–726. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yu, Q.; Hägg, M.-B.; Hoek, E.M.V.; Tarabara, V.V. CO2 capture. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Briceño, K.; Montané, D.; Garcia-Valls, R.; Iulianelli, A.; Basile, A. Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation. J. Membr. Sci. 2012, 415, 288–297. [Google Scholar] [CrossRef]
- Briceño, K.; Iulianelli, A.; Montané, D.; Garcia-Valls, R.; Basile, A. Carbon molecular sieve membranes supported on non-modified ceramic tubes for hydrogen separation in membrane reactors. Int. J. Hydrogen Energy 2012, 37, 13536–13544. [Google Scholar] [CrossRef]
- Lie, J.A.; He, X.; Kumakiri, I.; Kita, H.; Hägg, M.-B. 14 carbon based membranes. In H2 Production and Separation/Purification; Basile, A., Dalena, F., Tong, J., Veziroglu, N., Eds.; Senior Commissioning Editor-Books: London, UK, 2017; pp. 405–431. [Google Scholar]
- Okamoto, K.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation through carbonized membranes derived from an asymmetric polyimide hollow fiber membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Carbon membranes for gas separation processes: Recent progress and future perspective. J. Membr. Sci. Res. 2015, 1, 2–15. [Google Scholar]
- Rungta, M.; Xu, L.; Koros, W.J. Carbon molecular sieve dense film membranes derived from matrimid® for ethylene/ethane separation. Carbon 2012, 50, 1488–1502. [Google Scholar] [CrossRef]
- Bhuwania, N.; Labreche, Y.; Achoundong, C.S.K.; Baltazar, J.; Burgess, S.K.; Karwa, S.; Xu, L.; Henderson, C.L.; Williams, P.J.; Koros, W.J. Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 2014, 76, 417–434. [Google Scholar] [CrossRef]
- He, X.; Hagg, M.-B. Optimization of carbonization process for preparation of high performance hollow fiber carbon membranes. Ind. Eng. Chem. Res. 2011, 50, 8065–8072. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.-B. Hollow fiber carbon membranes: Investigations for CO2 capture. J. Membr. Sci. 2011, 378, 1–9. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.-B. Structural, kinetic and performance characterization of hollow fiber carbon membranes. J. Membr. Sci. 2012, 390, 23–31. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.-B. Hollow fiber carbon membranes: From material to application. Chem. Eng. J. 2013, 215, 440–448. [Google Scholar] [CrossRef]
- He, X.; Lie, J.A.; Sheridan, E.; Hagg, M.-B. Preparation and characterization of hollow fiber carbon membranes from cellulose acetate precursors. Ind. Eng. Chem. Res. 2011, 50, 2080–2087. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Ruffini, A.; Will, J.; Greil, P.; Müller, F.; Martínez, F.J.; Torres, R.C.; Varela, F.F.M.; Ramírez, R.J. Implants for Load Bearing Bone Substitutions Having Hierarchical Organized Architecture Deriving from Transformation of Vegetal Structures. U.S. Patent No. 9,326,948, 8 November 2018. [Google Scholar]
- Li, H.; Saeed, A.; Jahan, M.S.; Ni, Y.; van Heiningen, A. Hemicellulose removal from hardwood chips in the pre-hydrolysis step of the kraft-based dissolving pulp production process. J. Wood Chem. Technol. 2010, 30, 48–60. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X. Novel Tubular Carbon Membranes Prepared from Natural Rattans. C 2019, 5, 9. https://doi.org/10.3390/c5010009
He X. Novel Tubular Carbon Membranes Prepared from Natural Rattans. C. 2019; 5(1):9. https://doi.org/10.3390/c5010009
Chicago/Turabian StyleHe, Xuezhong. 2019. "Novel Tubular Carbon Membranes Prepared from Natural Rattans" C 5, no. 1: 9. https://doi.org/10.3390/c5010009
APA StyleHe, X. (2019). Novel Tubular Carbon Membranes Prepared from Natural Rattans. C, 5(1), 9. https://doi.org/10.3390/c5010009