Development of La Doped Ni/CeO2 for CH4/CO2 Reforming
Abstract
:1. Introduction
- -
- Dry impregnation of the lanthanum precursor on the not calcined support of ceria;
- -
- Dry impregnation of the lanthanum precursor on the calcined support of ceria;
- -
- Co-precipitation of lanthanum and cerium precursors.
2. Materials and Methods
2.1. Catalysts Synthesis
- -
- incipient wetness impregnation of La(NO2)3·6H2O aqueous solution on iCe and calcination at 550 °C (iLaCe);
- -
- incipient wetness impregnation of La(NO2)3·6H2O aqueous solution on oCe and calcination at 550 °C (oLaCe);
- -
- Co-precipitation of (NH4)2Ce(NO3)6 and La(NO2)3·6H2O and calcination at 550 °C (cLaCe).
2.2. Characterization Techniques
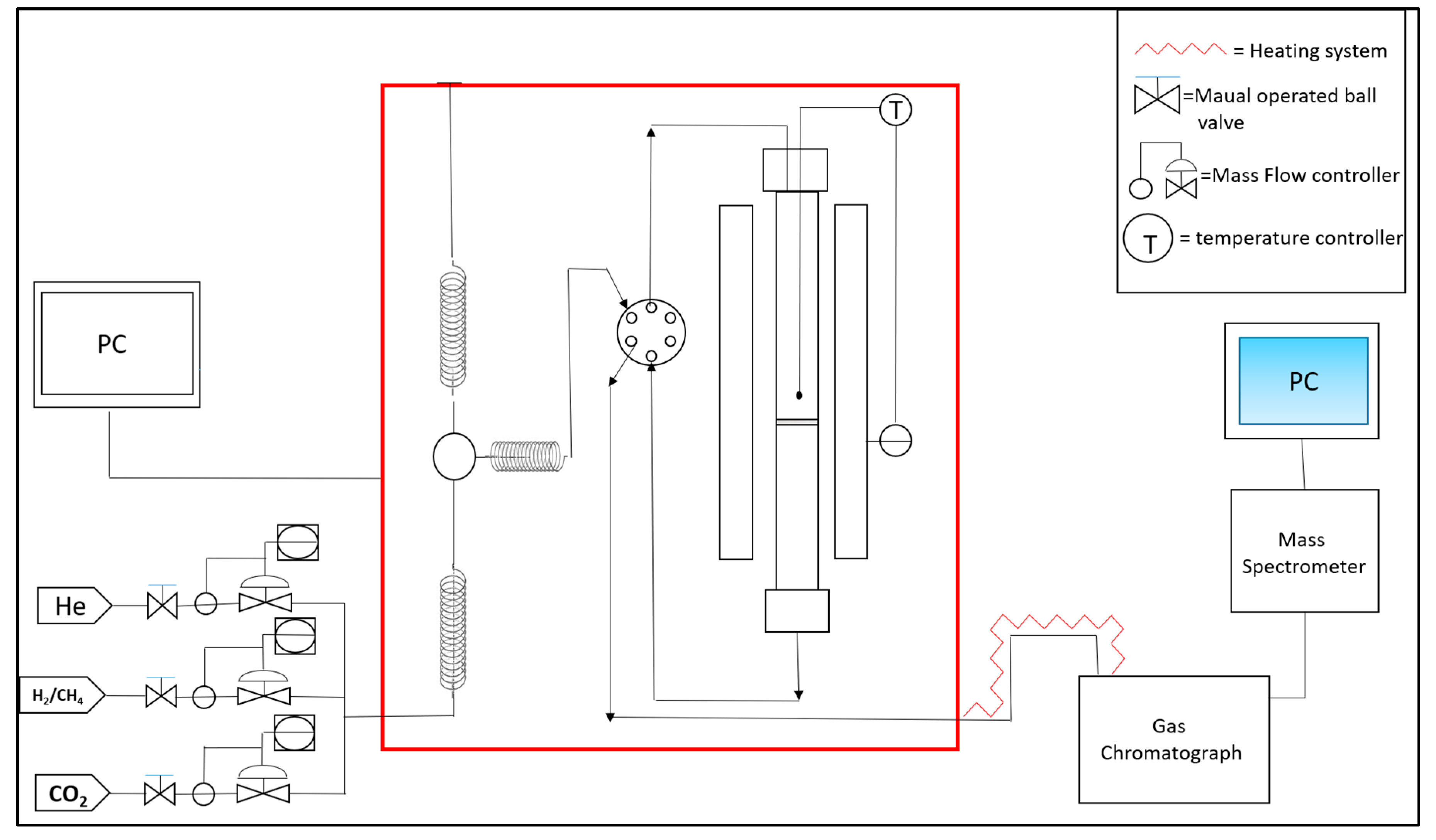
2.3. Catalytic Tests
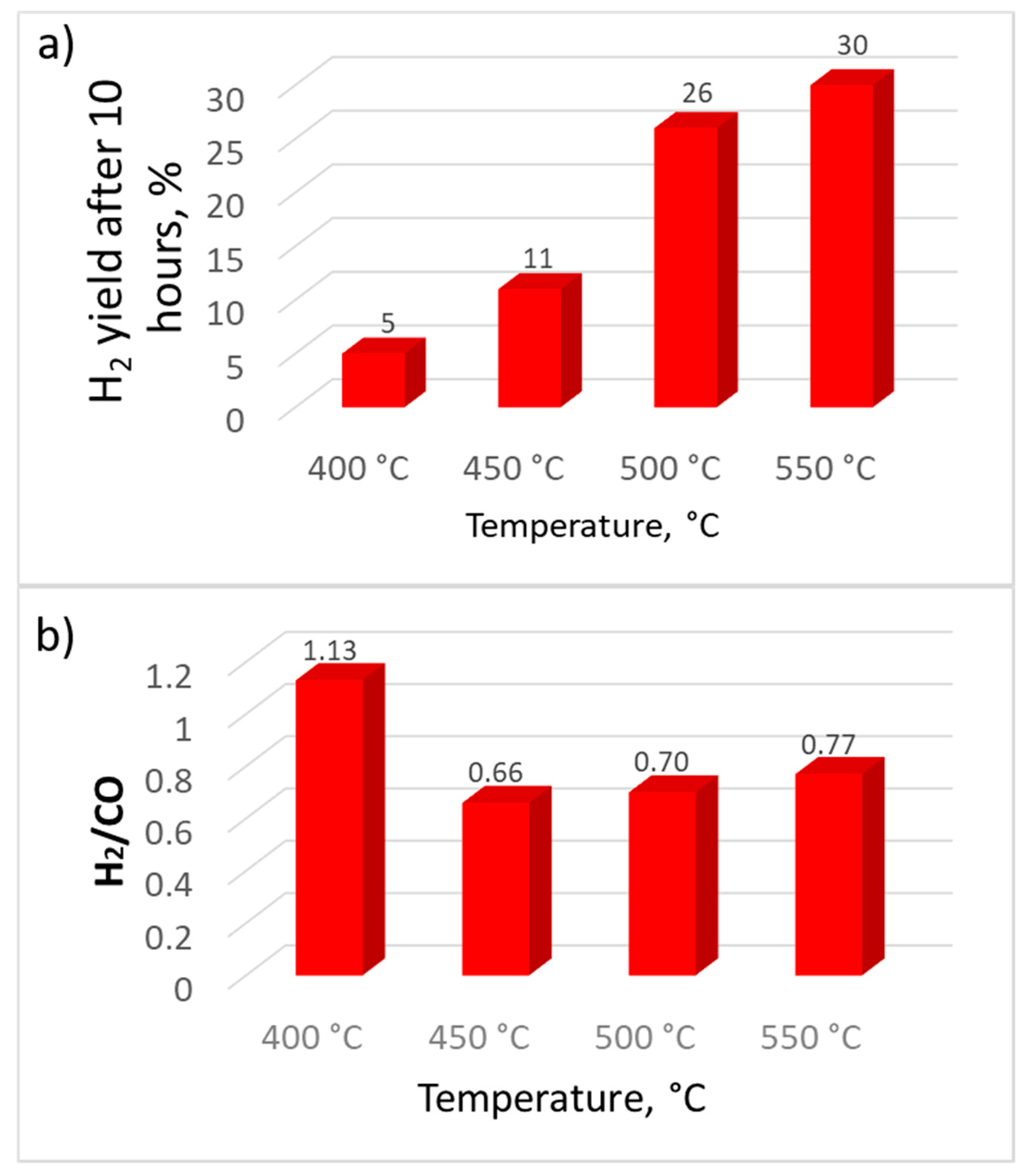
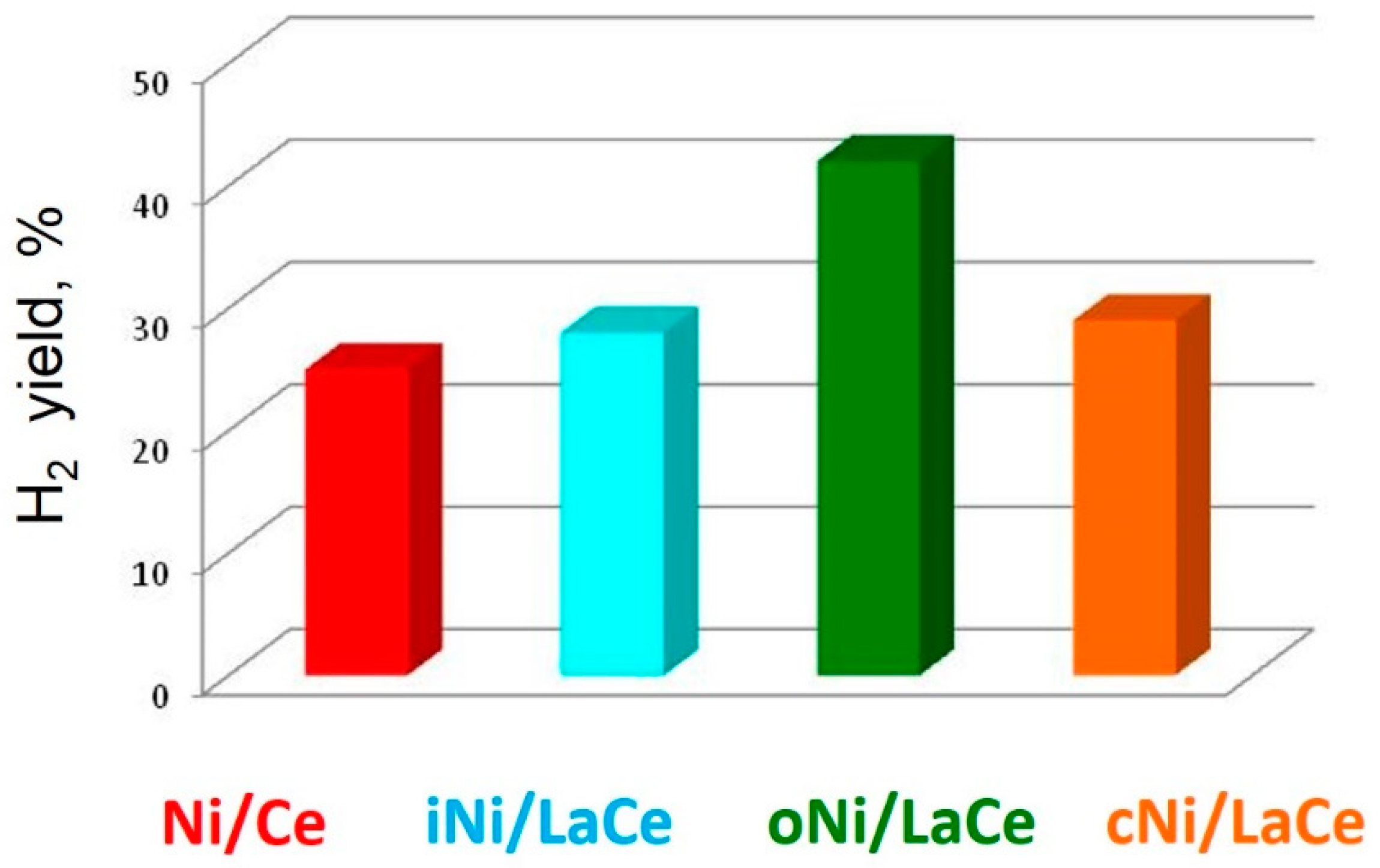
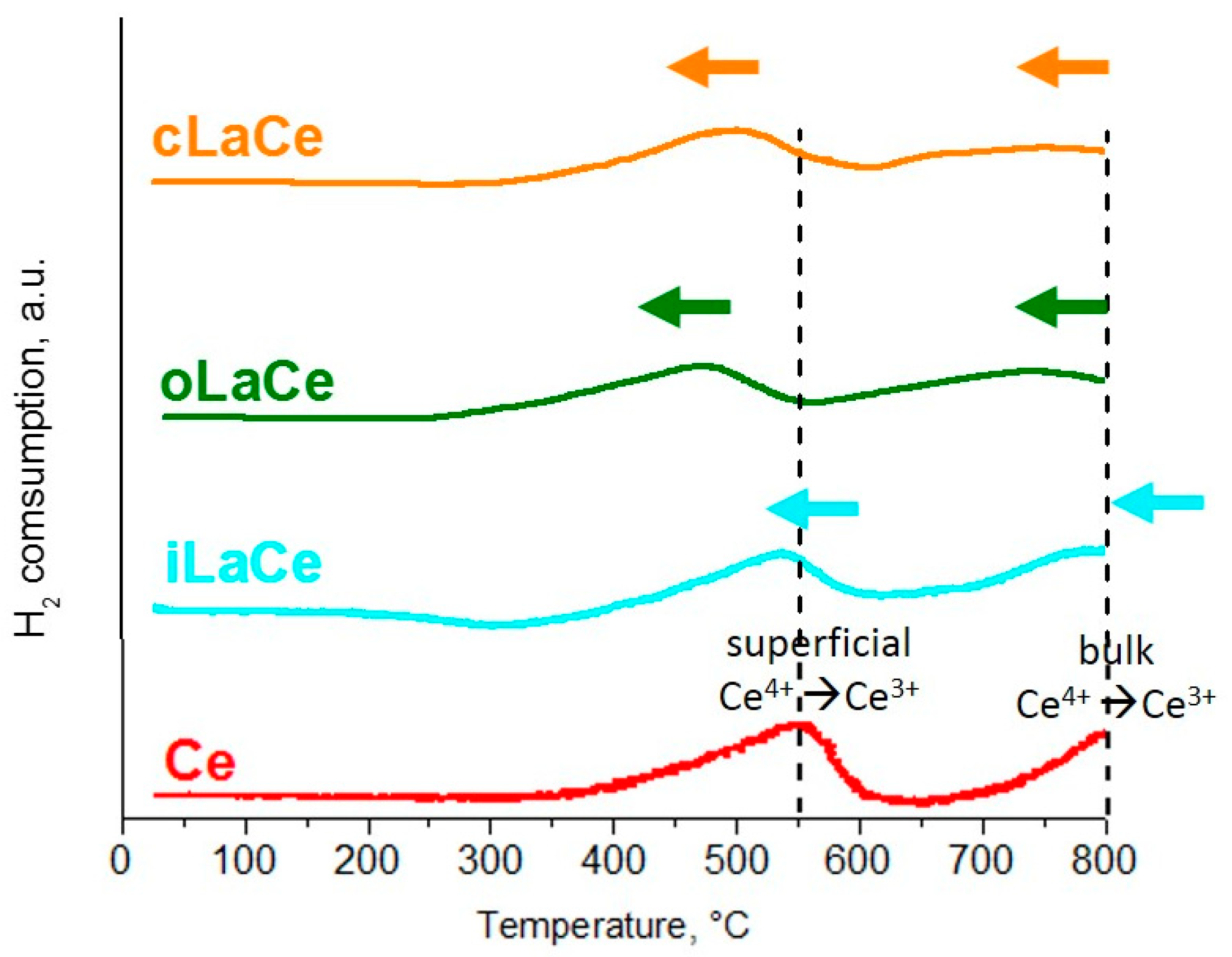
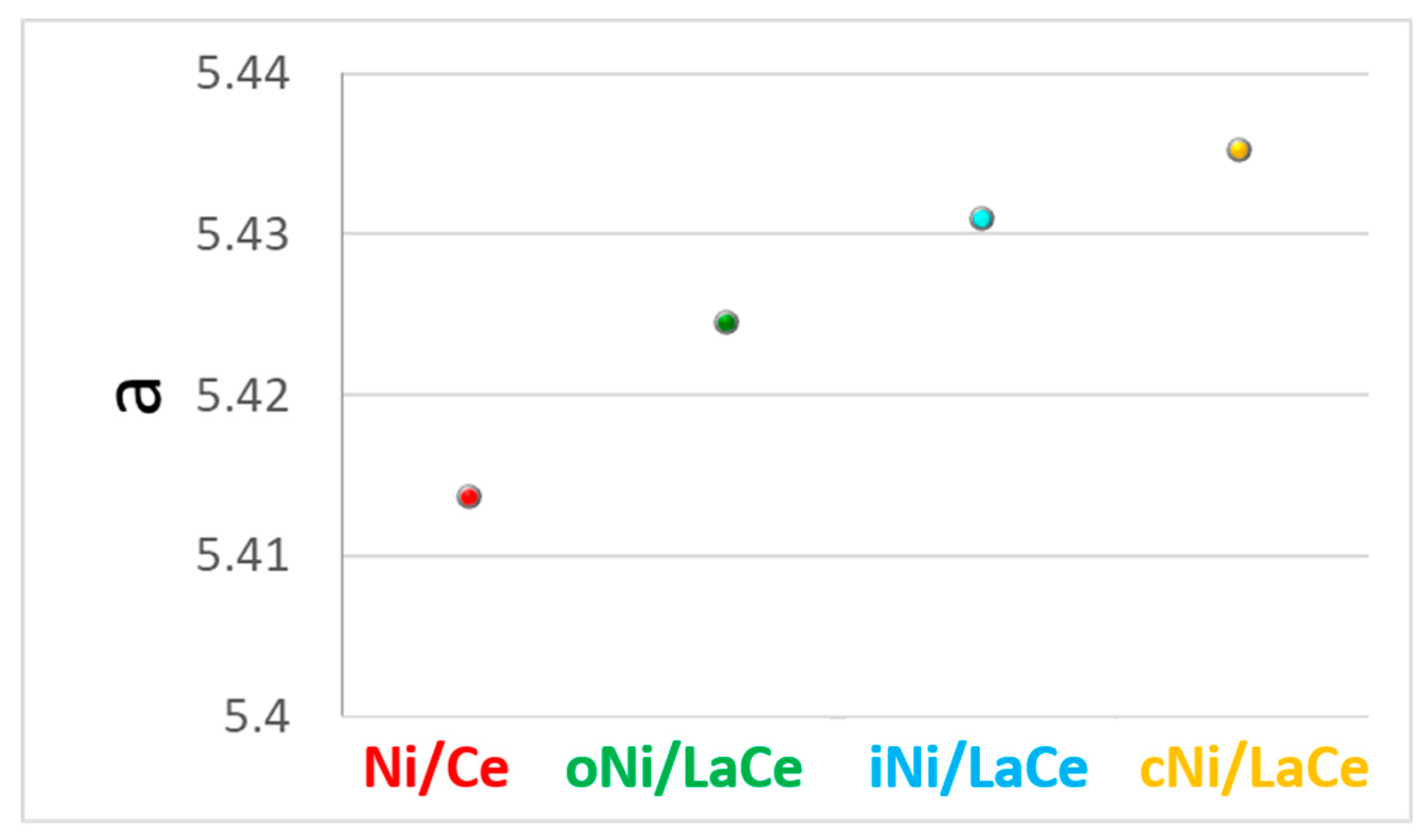
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal control knob governing Earth’s temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuckett, R.P. The Role of Atmospheric Gases in Global Warming Observed. In Impacts on Planet Earth Elsevier, 1st ed.; T.M. Letcher: Amsterdam, The Netherlands, 2009; pp. 3–19. ISBN 978-0-444-53301-2. [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; Available online: http://www.ipcc.ch/ (accessed on 27 September 2018).
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement; Report No. FCCC/CP/2015/L.9/Rev.1; UNFCCC: New York City, NY, USA, 2015; Available online: http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 27 September 2018).
- Rogelj, J.; den elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Toriumi, M.; Sakemi, T.; Masui, N.; Yano, S.; Fujita, H.; Furukawa, H. Conceptual Design of CO2 Transportation System for CCS. Energy Procedia 2013, 37, 2989–2996. [Google Scholar] [CrossRef]
- Onyebuchi, V.E.; Kolios, A.; Hanak, D.P.; Biliyok, C.; Manovic, V. A systematic review of key challenges of CO2 transport via pipelines. Renew. Sustain. Energy Rev. 2018, 81, 2563–2583. [Google Scholar] [CrossRef]
- Bruhn, T.; Naims, H.; Olfe-Kräutlein, B. Separating the debate on CO2 utilisation from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Energy Technology Perspectives 2017; IEA: Paris, France, 2017; Available online: http://www.iea.org/etp/ (accessed on 27 September 2018).
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Yang, L.; Lu, F.; Zhou, X.; Wang, X.; Duan, X.; Sun, B. Progress in the studies on the greenhouse gas emissions from reservoirs. Acta Ecol. Sin. 2014, 34, 204–212. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Enger, B.C.; Lodeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; La Parola, V.; Venezia, A.M.; Collard, X.; Aprile, C.; Liotta, L.F. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MOx (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B Environ. 2014, 156–157, 350–361. [Google Scholar] [CrossRef]
- Bobrova, L.N.; Bobin, A.S.; Mezentseva, N.V.; Sadykov, V.A.; Thybaut, J.W.; Marin, G.B. Kinetic assessment of dry reforming of methane on Pt + Ni containing composite of fluorite-like structure. Appl. Catal. B Environ. 2016, 182, 513–524. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, K.; Liu, Z.; Xiao, H.; Deng, W.; Zhou, X. Carbon dioxide reforming of methane over promoted NixMg1−xO (1 1 1) platelet catalyst derived from solvothermal synthesis. Appl. Catal. B Environ. 2014, 148–149, 177–190. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Y.A.; Lu, Y.; Tong, G.; Zhu, K.; Zhou, X. The promoting role of Ag in Ni-CeO2 catalyzed CH4-CO2 dry reforming reaction. Appl. Catal. B Environ. 2015, 165, 43–56. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sopriprun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668. [Google Scholar] [CrossRef]
- Alenazey, F.S. Utilizing carbon dioxide as a regenerative agent in methane dry reforming to improve hydrogen production and catalyst activity and longevity. Int. J. Hydrog. Energy 2014, 39, 18632–18641. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. The State of Zirconia Supported Platinum Catalysts for CO2/CH4 Reforming. J. Catal. 1997, 171, 279–286. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. On the contribution of X-ray absorption spectroscopy to explore structure and activity relations of Pt/ZrO2 catalysts for CO2/CH4 reforming. Top. Catal. 2000, 10, 295–305. [Google Scholar] [CrossRef]
- Nagaoka, K.; Seshan, K.; Lercher, J.A.; Aika, K. Activation mechanism of methane-derived coke (CHx) by CO2 during dry reforming of methane-comparison for Pt/Al2O3 and Pt/ZrO2. Catal. Lett. 2000, 70, 109–116. [Google Scholar] [CrossRef]
- Nagaoka, K.; Seshan, K.; Lercher, J.A.; Aika, K. Mechanism of carbon deposit/removal in methane dry reforming on supported metal catalysts. Stud. Surf. Sci. Catal. 2001, 136, 129–136. [Google Scholar]
- Karemore, A.L.; Vaidya, P.D.; Sinha, R.; Chugh, P. On the dry and mixed reforming of methane over Ni/Al2O3—Influence of reaction variables on syngas production. Int. J. Hydrog. Energy 2016, 41, 22963–22975. [Google Scholar] [CrossRef]
- Črnivec, I.G.O.; Djinović, P.; Erjavec, B.; Pintar, A. Effect of synthesis parameters on morphology and activity of bimetallic catalysts in CO2-CH4 reforming. Chem. Eng. J. 2012, 207–208, 299–307. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Carbon dioxide reforming of methane over ordered mesoporous NiO–MgO–Al2O3 composite oxides. Appl. Catal. B 2011, 108–109, 177–190. [Google Scholar] [CrossRef]
- Min, J.E.; Lee, Y.J.; Park, H.G.; Zhang, C.; Jun, K.W. Carbon dioxide reforming of methane on Ni–MgO–Al2O3 catalysts prepared by sol–gel method: Effects of Mg/Al ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; BaBaluo, A.A.; Jafari, M.F.; Estifaee, P. Synthesis and physicochemical characterizations of Ni/Al2O3–ZrO2 nanocatalyst prepared via impregnation method and treated with non-thermal plasma for CO2 reforming of CH4. J. Ind. Eng. Chem. 2013, 19, 1566–1576. [Google Scholar] [CrossRef]
- Jang, W.J.; Kim, H.M.; Shim, J.O.; Yoo, S.Y.; Jeon, K.W.; Na, H.S.; Lee, Y.L.; Jeong, D.W.; Bae, J.W.; Nah, I.W.; et al. Key properties of Ni–MgO–CeO2, Ni–MgO–ZrO2, and Ni–MgO–Ce(1 − x)Zr(x)O2 catalysts for the reforming of methane with carbon dioxide. Green Chem. 2018, 20, 1621–1633. [Google Scholar] [CrossRef]
- Roh, H.S.; Jun, K.W.; Dong, W.S.; Chang, J.S.; Park, S.E.; Joe, Y.I. Highly active and stable Ni/Ce–ZrO2 catalyst for H2 production from methane. J. Mol. Catal. A Chem. 2002, 181, 137–142. [Google Scholar] [CrossRef]
- Valentini, A.; Carreno, N.L.V.; Probst, L.F.D.; Lisboa-Filho, P.N.; Schreiner, W.H.; Leite, E.R.; Lingo, E. Role of vanadium in Ni:Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. A Gen. 2003, 255, 211–220. [Google Scholar] [CrossRef]
- Li, H.; Wang, J. Study on CO2 reforming of methane to syngas over Al2O3–ZrO2 supported Ni catalysts prepared via a direct sol–gel process. Chem. Eng. Sci. 2004, 59, 4861–4867. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Roman-Martinez, M.C.; Illan-Gomez, M.J. Effect of potassium content in the activity of K-promoted Ni/Al2O3 catalysts for the dry reforming of methan. Appl. Catal. A Gen. 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Luna, A.E.C.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst. Appl. Catal. A Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Yamazaki, O.; Tomishige, K.; Fujimoto, K. Development of highly stable nickel catalyst for methane-steam reaction under low steam to carbon ratio. Appl. Catal. A Gen. 1996, 136, 49–56. [Google Scholar] [CrossRef]
- Tomishige, K.; Kanazawa, S.; Sato, M.; Ikushima, K.; Kunimori, K. Catalyst Design of Pt-Modified Ni/Al2O3 Catalyst with Flat Temperature Profile in Methane Reforming with CO2 and O2. Catal. Lett. 2002, 84, 69–74. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Li, B.; Kunimori, K.; Suzuki, K.; Fujimoto, K.I.; Tomishige, K. Performance of NiO–MgO solid solution-supported Pt catalysts in oxidative steam reforming of methane. Appl. Catal. A Gen. 2005, 292, 272–280. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jang, W.J.; Shim, J.O.; Roh, H.S.; Son, I.H.; Lee, S.J. The effect of preparation method on the catalytic performance over superior MgO-promoted Ni–Ce0.8Zr0.2O2 catalyst for CO2 reforming of CH4. Int. J. Hydrog. Energy 2013, 38, 13649–13654. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas production with dry reforming of methane over Ni/ZSM-5 catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. One-Pot Synthesis of Ordered Mesoporous NiO–CaO–Al2O3 Composite Oxides for Catalyzing CO2 Reforming of CH4. ACS Catal. 2012, 2, 1331–1342. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni–X/Al2O3–ZrO2 (X = Mg, Ca, Sr, and Ba) xerogel catalysts: Effect of alkaline earth metal addition. J. Mol. Catal. A 2016, 415, 151–159. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO–Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Xu, B. Durable Ni/MgO catalysts for CO2 reforming of methane: Activity and metal–support interaction. J. Mol. Catal. A Chem. 2009, 299, 44–52. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Rotaru, C.G.; Postole, G.; Florea, M.; Matei-Rutkovska, F.; Parvulescu, V.I.; Gelin, P. Dry reforming of methane on ceria prepared by modified precipitation route. Appl. Catal. A Gen. 2015, 494, 29–40. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Catalyst assisted by non-thermal plasma in dry reforming of methane at low temperature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Signoretto, M.; Menegazzo, F.; Di Michele, A.; Fioriniello, E. Effects of Support and Synthetic Procedure for Sol-Immobilized Au Nanoparticles. Catalysts 2016, 6, 87. [Google Scholar] [CrossRef]
- Tang, M.; Liu, K.; Roddick, M.D.; Fan, M. Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: Synergistic La-Ce effect. J. Catal. 2018, 368, 38–52. [Google Scholar] [CrossRef]
- Yang, R.; Xing, C.; Lv, C.; Shi, L.; Tsubaki, N. Promoting effect of La2O3 and CeO2 on Ni/γ-Al2O3 catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2010, 385, 92–100. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas Production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Hydrogen production by methane tri-reforming process over Ni–ceria catalysts: Effect of La-doping. Appl. Catal. B 2011, 104, 64–73. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Menegazzo, F.; Ghedini, E.; Innocenti, G.; Di Michele, A.; Cruciani, G.; Cavani, F.; Signoretto, M. Increase of Ceria Redox Ability by Lanthanum Addition on Ni Based Catalysts for Hydrogen Production. ACS Sustain. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Cu- and Ag-Modified Cerium Oxide Catalysts for Methane Oxidation. J. Catal. 1998, 179, 203–221. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: Cambridge, MA, USA, 1982; p. 111. [Google Scholar]
- Dong, W.; Roh, H.; Jun, K.; Park, S.; Oh, Y. Methane reforming over Ni/Ce-ZrO2 catalysts: Effect of nickel content. Appl. Catal. A 2002, 226, 63–72. [Google Scholar] [CrossRef]
- Gould, T.D.; Izar, A.; Weimer, A.; WFalconer, J.L.; Medlin, J.W. Stabilizing Ni Catalysts by Molecular Layer Deposition for Harsh, Dry Reforming Conditions. ACS Catal. 2014, 4, 2714–2717. [Google Scholar] [CrossRef]
- Xie, X.; Otremba, T.; Littlewood, P.; Schomäcker, R.; Thomas, A. One-Pot Synthesis of Supported, Nanocrystalline Nickel Manganese Oxide for Dry Reforming of Methane. ACS Catal. 2013, 3, 224–229. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Liu, Y.; Chen, Y.; Yang, S. Influence of preparation method on performance of Ni-CeO2 catalysts for reverse water-gas shift reaction. J. Rare Earths 2013, 31, 559–564. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Steam reforming of ethanol for production of hydrogen over Ni/CeO2–ZrO2 catalyst: Effect of support and metal loading. Int. J. Hydrog. Energy 2007, 32, 969–980. [Google Scholar] [CrossRef]
- Gonzalez-DelaCruz, V.M.; Holgado, J.P.; Perenìguez, R.; Caballero, A. Morphology changes induced by strong metal–support interaction on a Ni–ceria catalytic system. J. Catal. 2008, 257, 307–314. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Larrondo, S.; Rodríguez-Castellón, E.; Núñez, P. TPR, XRD and XPS characterisation of ceria-based materials synthesized by freeze-drying precursor method. Ceram. Int. 2014, 40, 6807–6814. [Google Scholar] [CrossRef]
- Mandapaka, R.; Madras, G. Aluminium and rhodium co-doped ceria for water gas shift reaction and CO oxidation. Mol. Catal. 2018, 451, 4–12. [Google Scholar] [CrossRef]
- Glass, D.E.; Galvan, V.; Prakash, G.K.S. The Effect of Annealing Temperature on Nickel on Reduced Graphene Oxide Catalysts on Urea. Electrooxidation. Electrochim. Acta 2017, 253, 489–497. [Google Scholar] [CrossRef]
- Keating, P.R.L.; Scanlon, D.O.; Watson, G.W. The nature of oxygen states on the surfaces of CeO2 and La-doped CeO2. Chem. Phys. Lett. 2014, 608, 239–243. [Google Scholar] [CrossRef]
- Shen, W.; Momoi, H.; Komatsubara, K.; Saito, T.; Yoshida, A.; Naito, S. Marked role of mesopores for the prevention of sintering and carbon deposition in dry reforming of methane over ordered mesoporous Ni–Mg–Al oxides. Catal. Today 2011, 171, 150–155. [Google Scholar] [CrossRef]
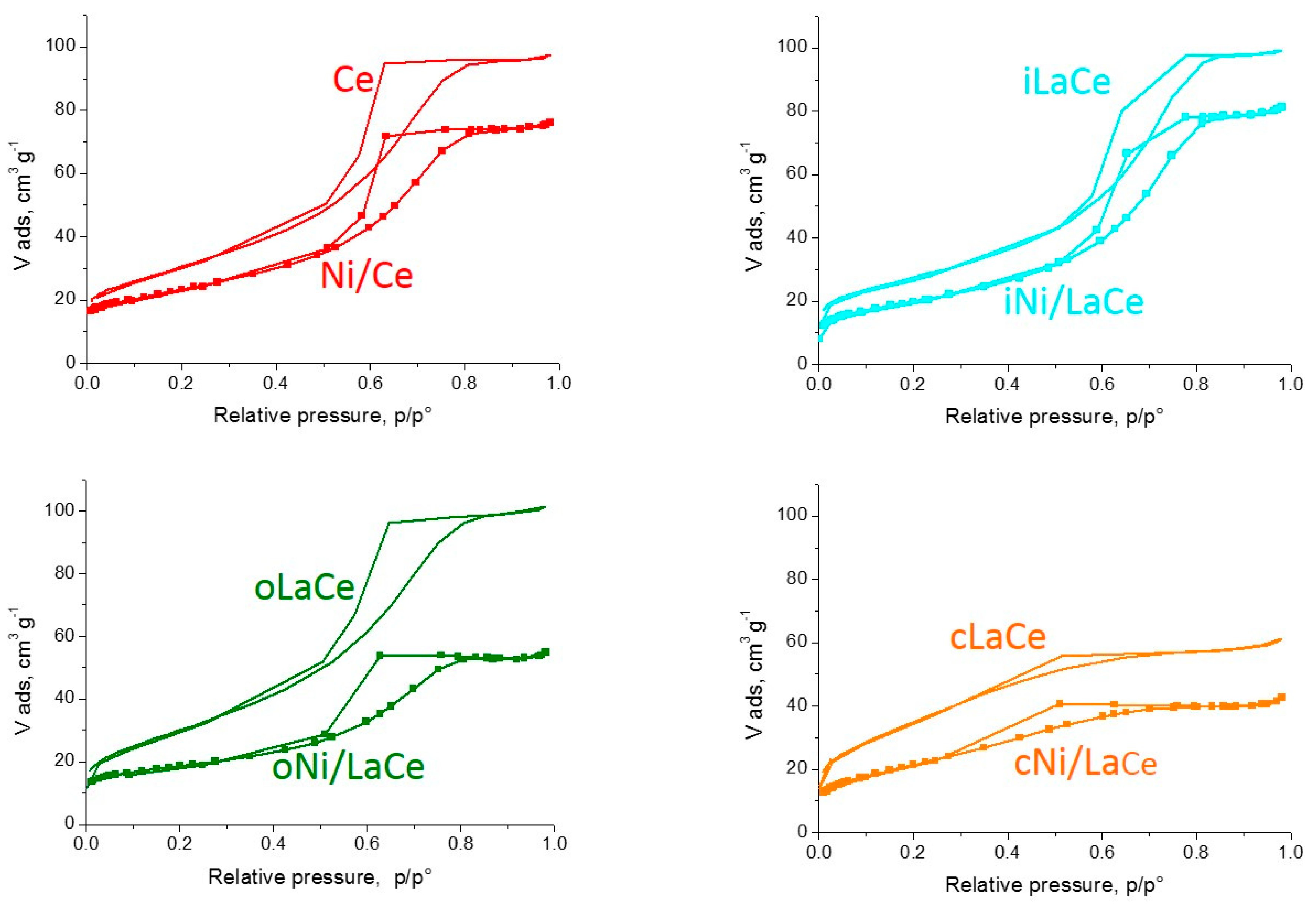
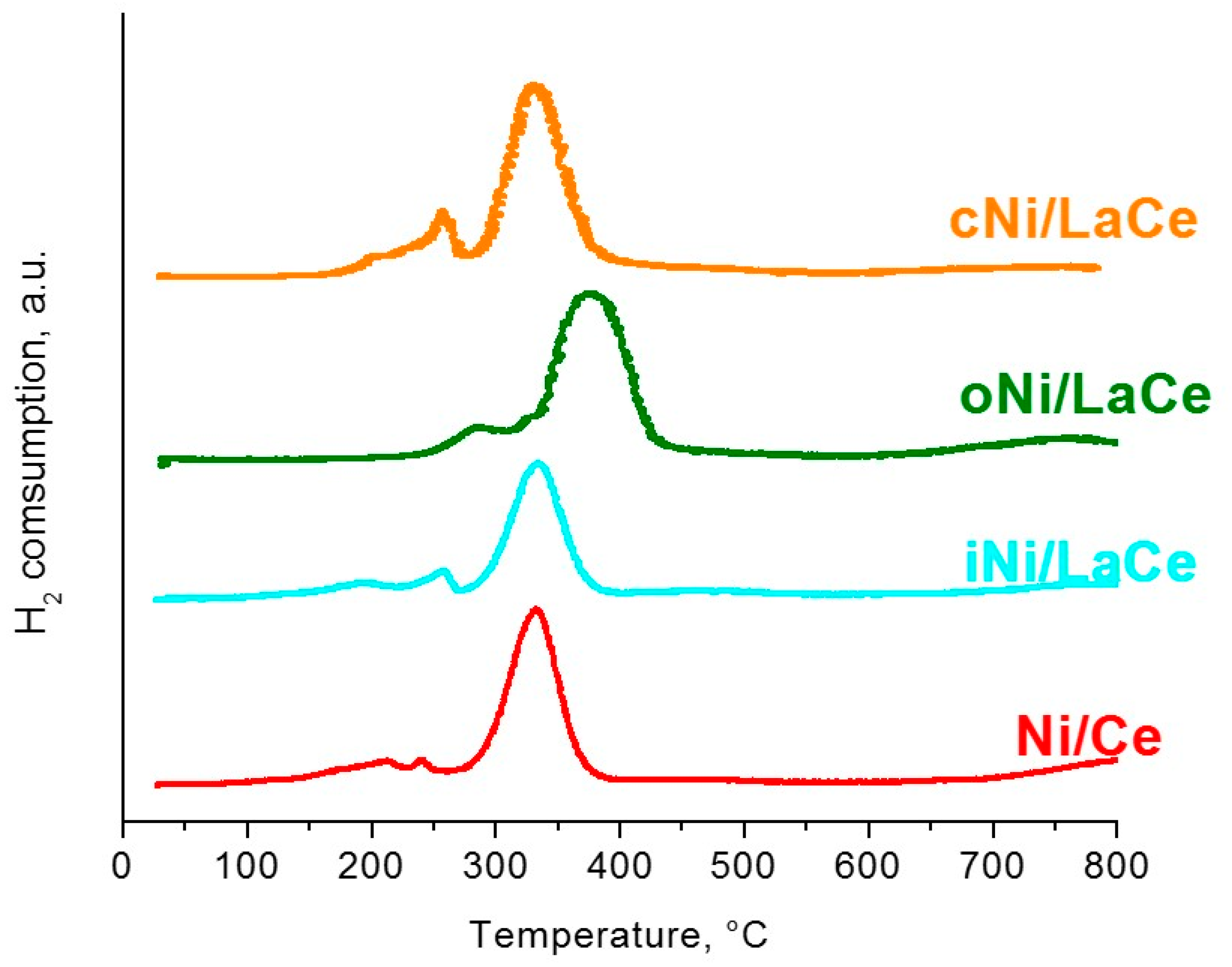
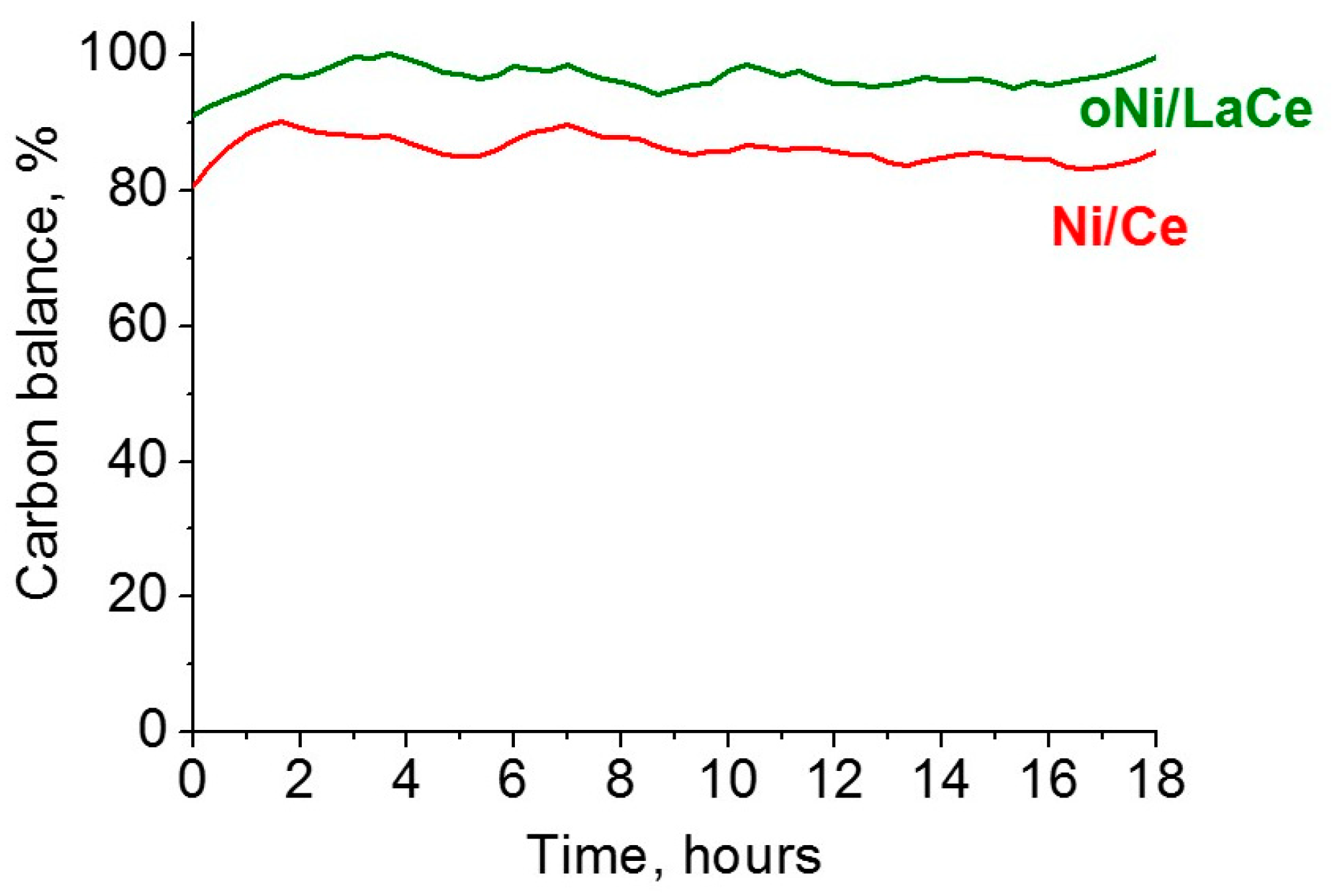
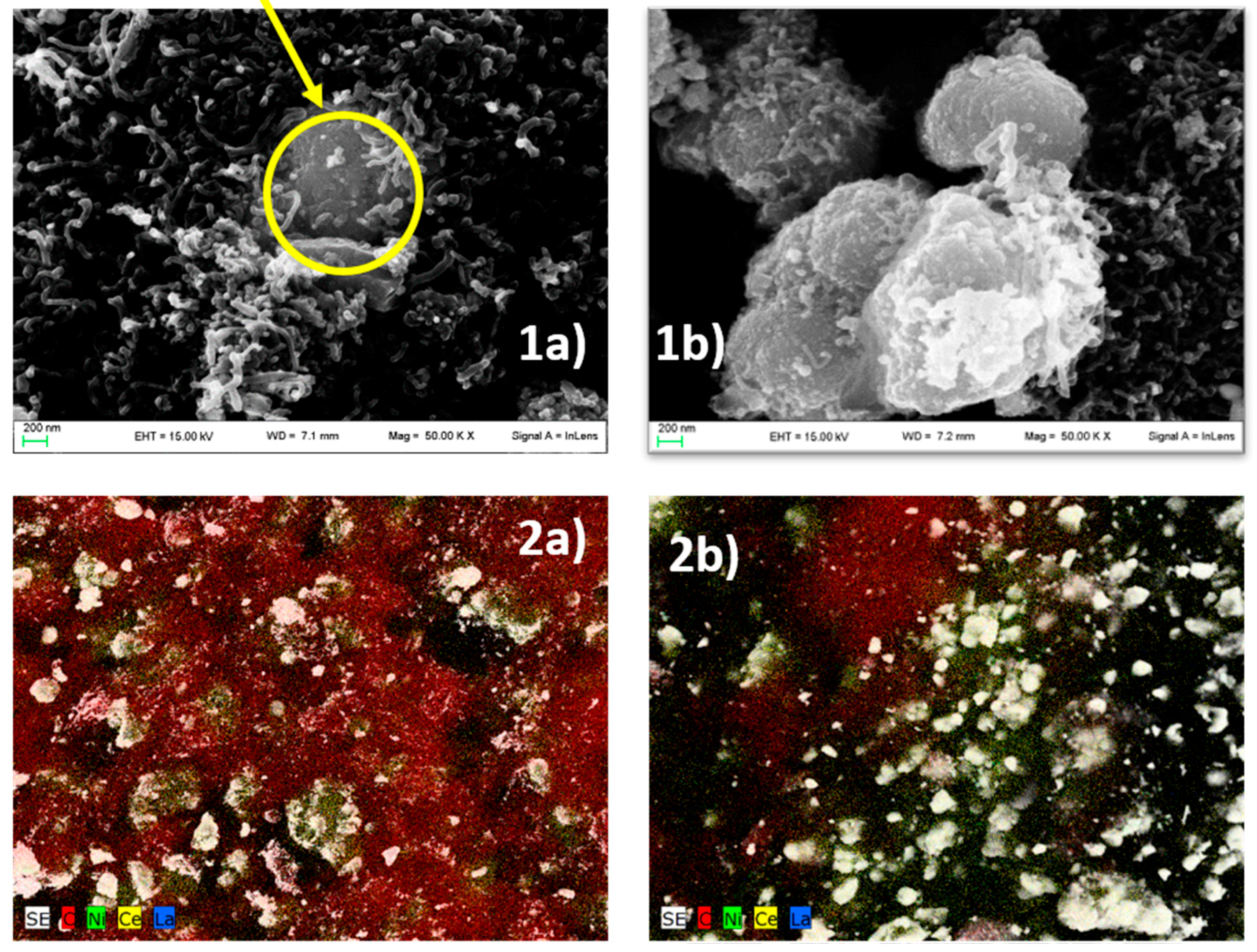
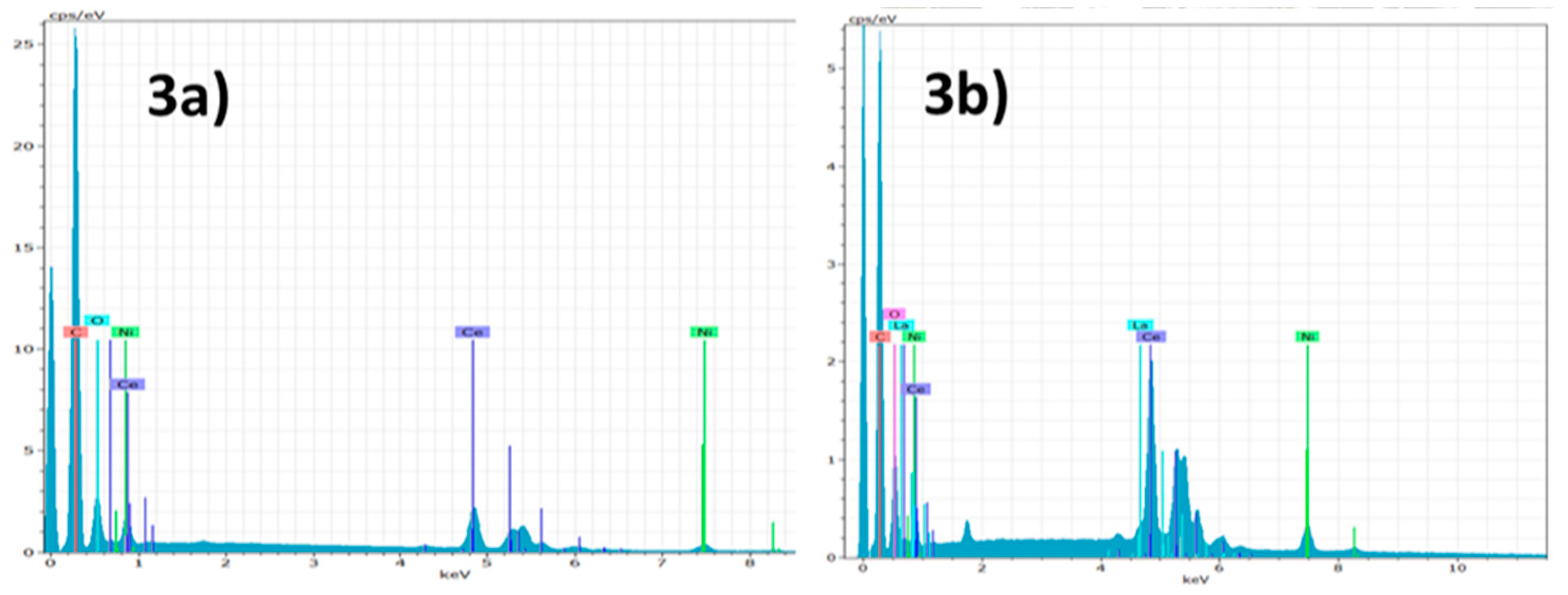
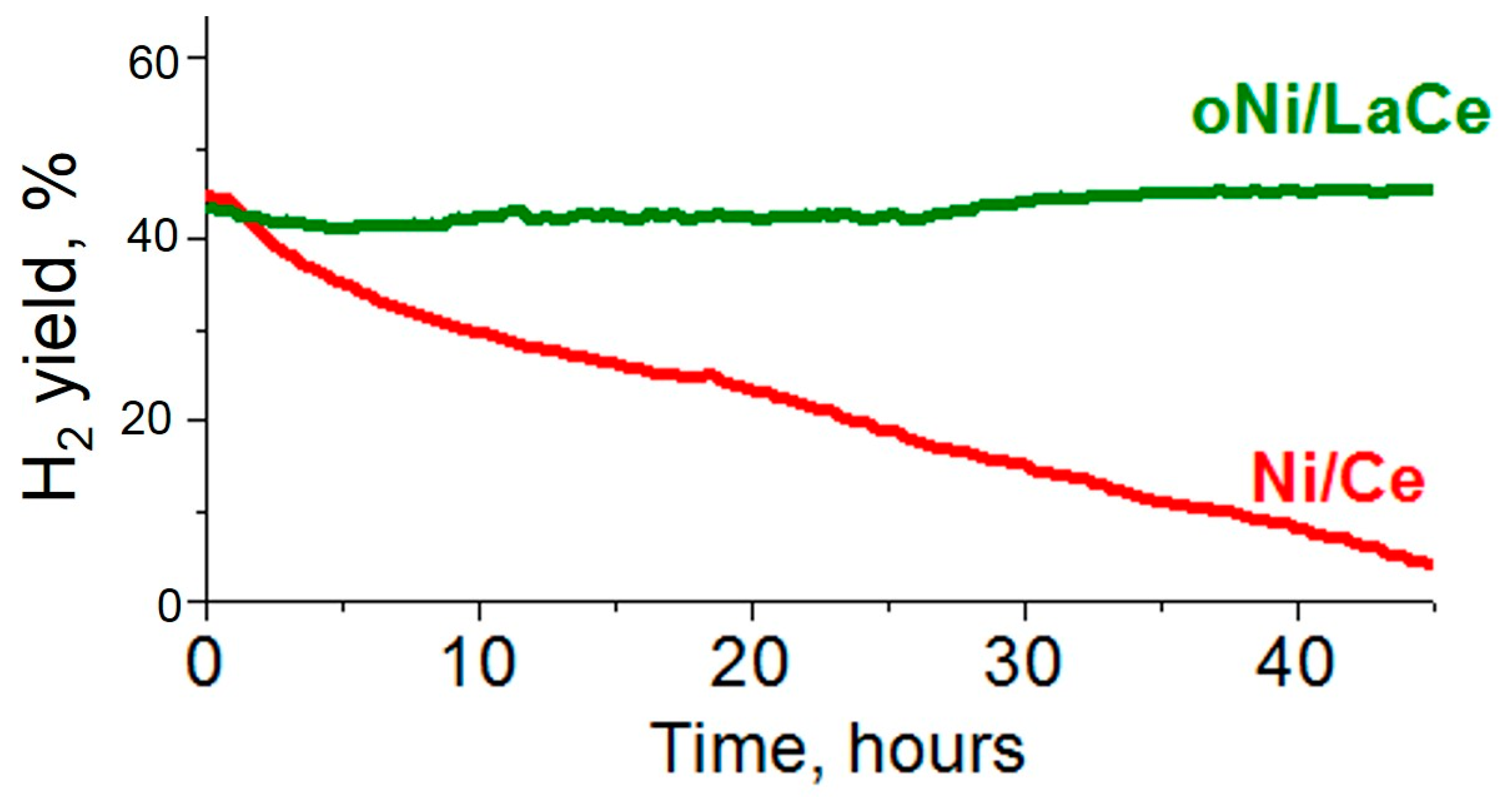
| Methodology | Support | Sample |
|---|---|---|
| precipitation of Ce | Ce | Ni/Ce |
| impregnation of La on iCe | iLaCe | iNi/LaCe |
| impregnation of La on oCe | oLaCe | oNi/LaCe |
| co-precipitation of La and Ce | cLaCe | cNi/LaCe |
| Sample | wt% Ni | m2/g | nm |
|---|---|---|---|
| Ce | 107 (±1) | 6.0 | |
| Ni/Ce | 7.8 (±0.5) | 82 (±1) | 6.1 |
| iLaCe | 97 (±1) | 6.2 | |
| iNi/LaCe | 7.9 (±0.5) | 71 (±1) | 6.5 |
| oLaCe | 110 (±1) | 5.8 | |
| oNi/LaCe | 7.8 (±0.5) | 66 (±1) | 4.7 |
| cLaCe | 128 (±1) | 5.1 | |
| cNi/LaCe | 7.7 (±0.5) | 77 (±1) | 4.7 |
| Sample | Ni Size, nm |
|---|---|
| Ni/Ce | 18 (±1) |
| iNi/LaCe | 16 (±1) |
| oNi/LaCe | 11 (±1) |
| cNi/LaCe | 15 (±1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegazzo, F.; Pizzolitto, C.; Ghedini, E.; Di Michele, A.; Cruciani, G.; Signoretto, M. Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. C 2018, 4, 60. https://doi.org/10.3390/c4040060
Menegazzo F, Pizzolitto C, Ghedini E, Di Michele A, Cruciani G, Signoretto M. Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. C. 2018; 4(4):60. https://doi.org/10.3390/c4040060
Chicago/Turabian StyleMenegazzo, Federica, Cristina Pizzolitto, Elena Ghedini, Alessandro Di Michele, Giuseppe Cruciani, and Michela Signoretto. 2018. "Development of La Doped Ni/CeO2 for CH4/CO2 Reforming" C 4, no. 4: 60. https://doi.org/10.3390/c4040060
APA StyleMenegazzo, F., Pizzolitto, C., Ghedini, E., Di Michele, A., Cruciani, G., & Signoretto, M. (2018). Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. C, 4(4), 60. https://doi.org/10.3390/c4040060