Surface Observation and Magnetism of Oil-Extracted Botryococcus braunii Residues before and after Carbonization
Abstract
:1. Introduction
2. Results and Discussion
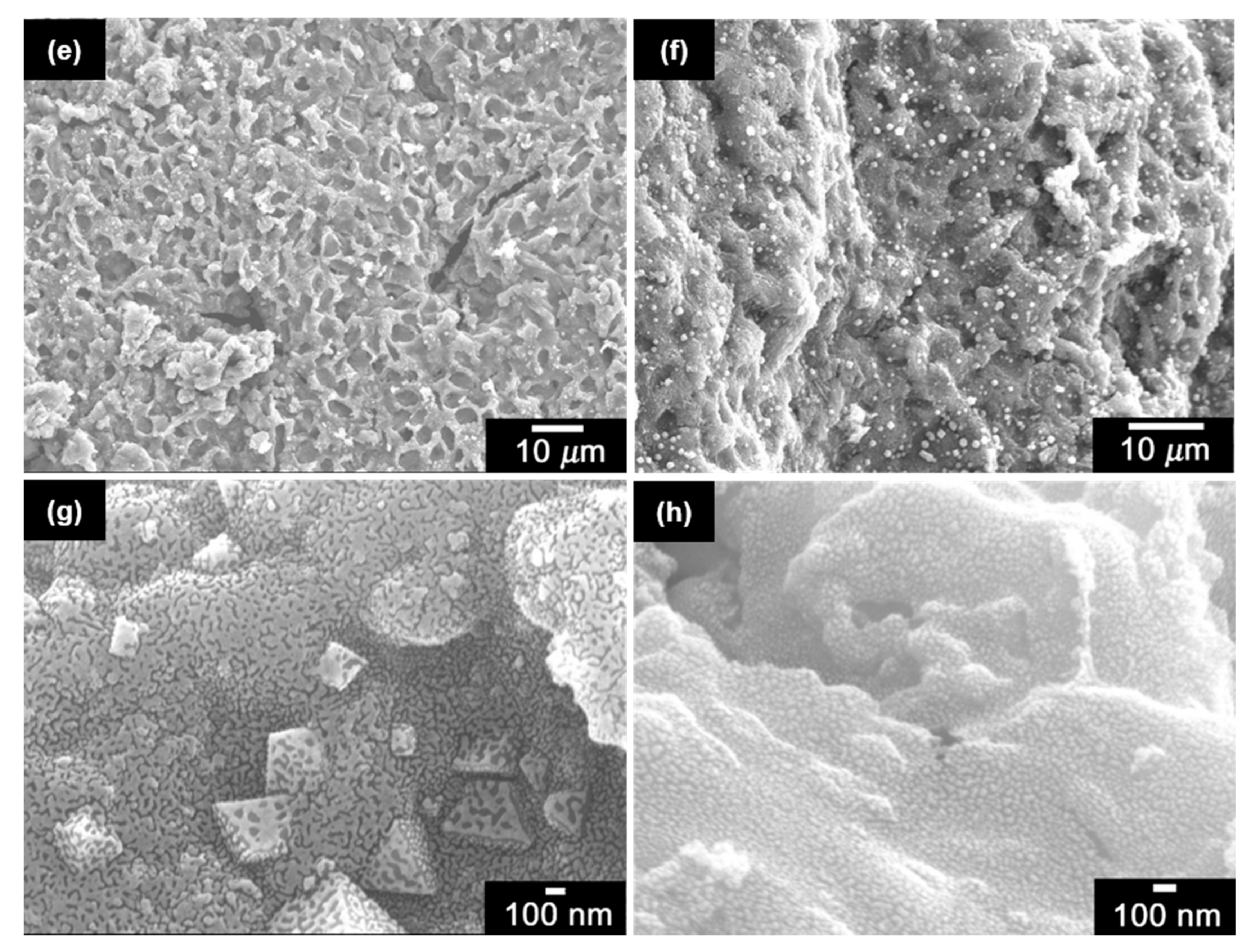
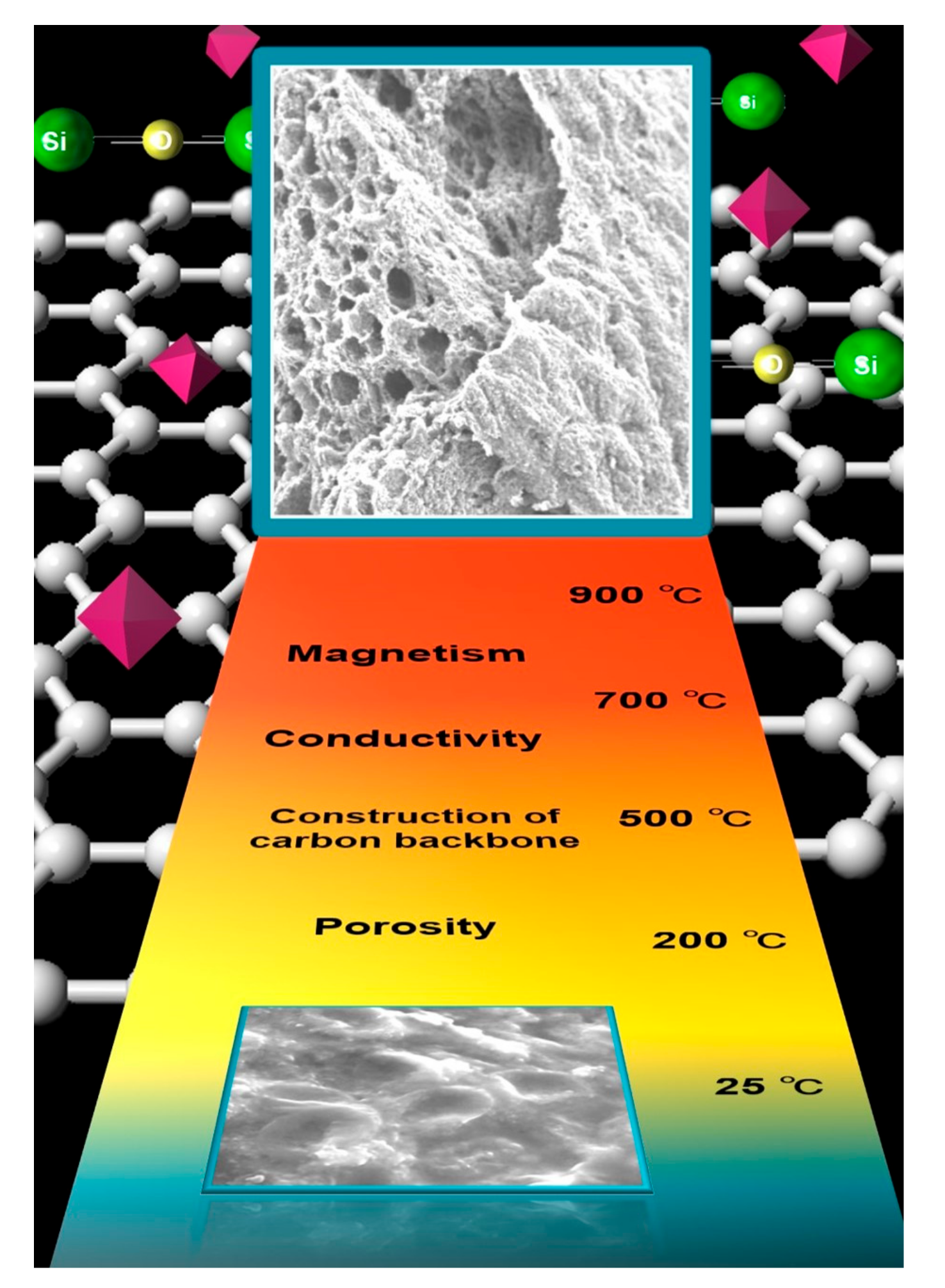
2.1. Scanning Electron Microscopy (SEM)
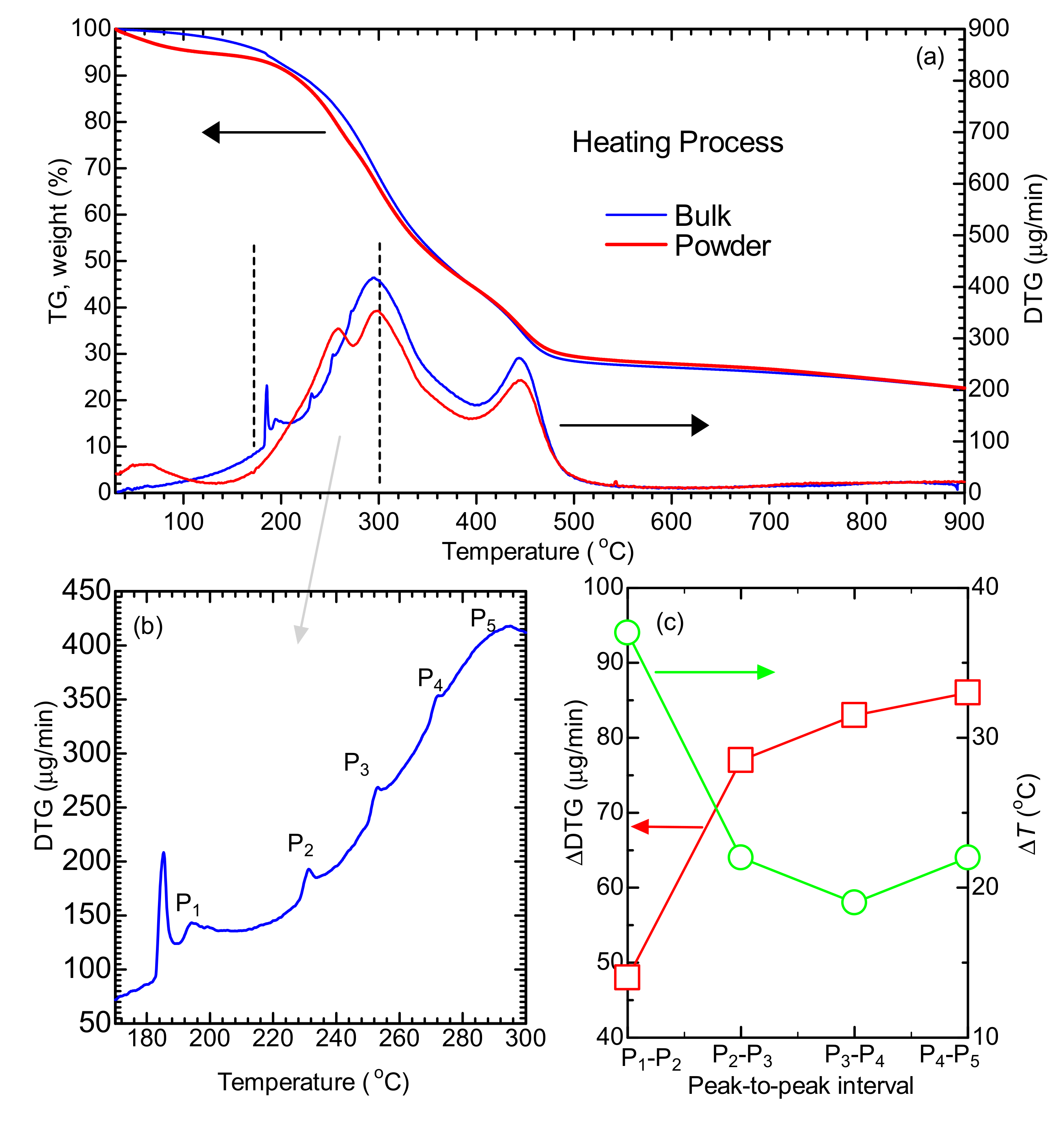
2.2. Thermogravimetric (TG) Analysis and Relationship to Morphology
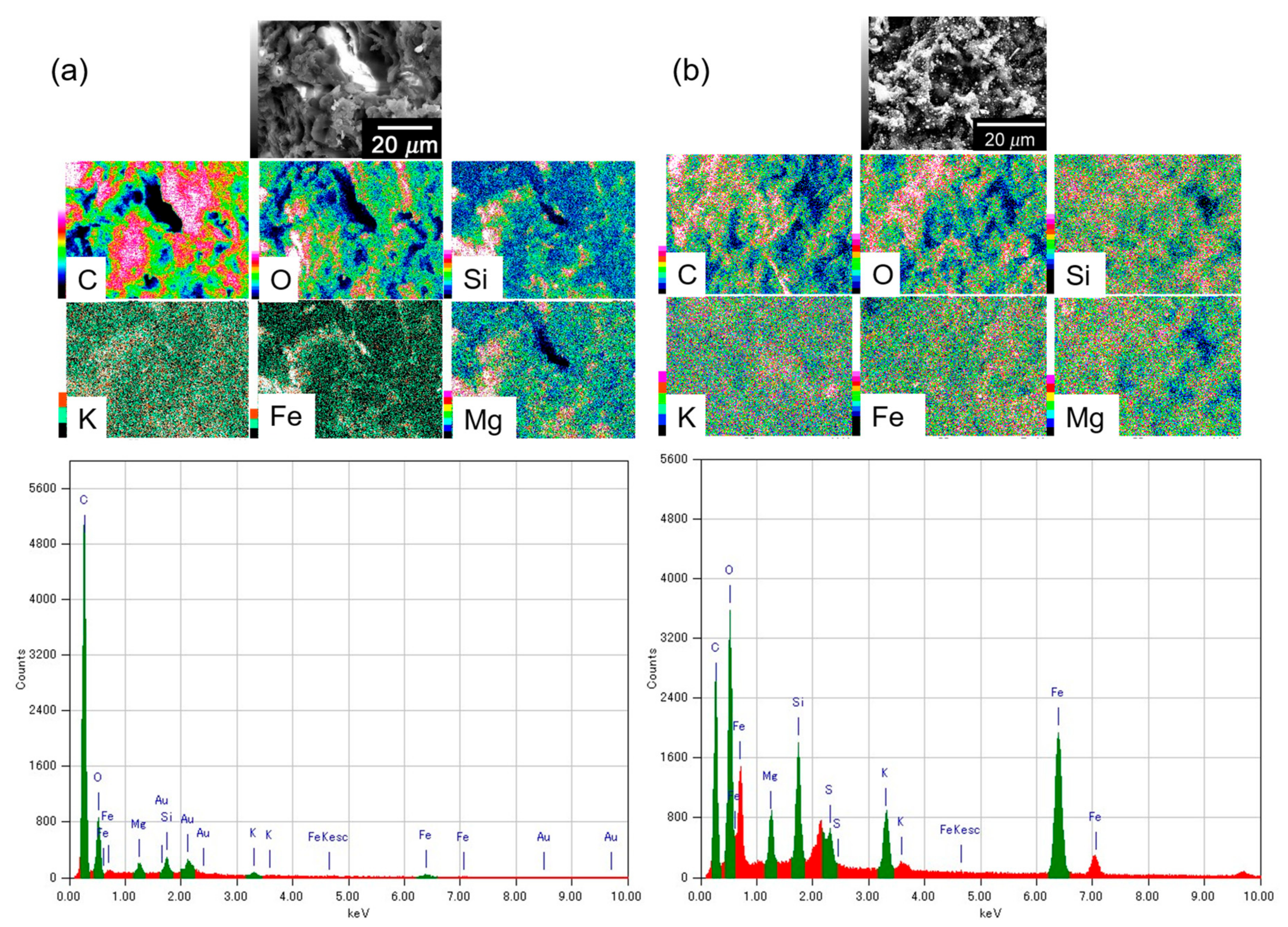
2.3. Energy Dispersive X-ray Spectroscopy (EDS)
2.4. Infrared Absorption (IR)
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Electron Spin Resonance (ESR)
2.7. X-ray Diffraction and 57Fe Mössbauer Spectroscopy
2.8. Superconducting Quantum Interference Device (SQUID) Measurement
2.8.1. SQUID Measurement (Sample before Carbonization)
2.8.2. SQUID Measurement (Carbonized Sample at 900 °C)
2.9. Mechanism of Generation of Magnetic Material
2.10. Prospects for Future Applicaiton
3. Materials and Methods
3.1.Preparation of B. braunii Residue Samples
3.2. Carbonization of B. braunii Residue Samples
3.3. Infrared (IR) Absorbance Measurement
3.4. Thermogravimetric (TG) Analysis
3.5. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
3.6. X-ray Photoelectron Spectroscopy (XPS) Measurement
3.7. Electron Spin Resonance (ESR) Measurement
3.8. X-ray Diffraction (XRD) Spectroscopy
3.9. Techniques and Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lapuerta, M.; Armas, O.; Rodriguez-Fernandez, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Bartle, J.R.; Abadi, A. Toward sustainable production of second generation bioenergy feedstocks. Energy Fuels 2010, 24, 2–9. [Google Scholar] [CrossRef]
- Petrou, E.C.; Papppis, C.P. Biofuels: A survey on Pros and Cons. Energy Fuels 2009, 23, 1055–1066. [Google Scholar] [CrossRef]
- Perego, C.; Bosetti, A. Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater. 2011, 144, 28–39. [Google Scholar] [CrossRef]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.B. Commercial cellulosic ethanol: The role of plant-expressed enzymes. In Vitro Cell. Dev. Biol. Plant 2009, 45, 314–329. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomasspyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other application: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Shimamura, R.; Watanabe, S.; Sagakura, Y.; Shiho, M.; Kaya, K.; Watanabe, M.M. Development of Botryococcus seed culture system for future mass culture. Procedia Environ. Sci. 2012, 15, 80–89. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Wang, F.; Guo, C.; Liu, C. Improved biomass and hydrocarbon productivity of Botryococcus braunii by periodic ultrasound stimulation. Bioenergy Res. 2014, 7, 986–992. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Low-cost production of green microalga Botryococcus braunii biomass with high lipid content through mixotrophic and photoautotrophic cultivation. Appl. Biochem. Biotechnol. 2014, 174, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.; Hu, Y.; Stiles, A.R.; Guo, C.; Liu, C. Magnetic flocculant for high efficiency harvesting of microalgal cells. ACS Appl. Mater. Interfaces 2014, 6, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.; Wagner, M.; El-Sheekh, M.; Hanelt, D. Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: Screening studies towards biodiesel production. J. Appl. Phycol. 2013, 25, 931–936. [Google Scholar] [CrossRef]
- Watanabe, H.; Li, D.; Nakagawa, Y.; Tomishige, K.; Kaya, K.; Watanabe, M.M. Characterization of oil-extracted residue biomass of Botryococcus braunii as a biofuel feed stock and its pyrolytic behavior. Appl. Energy 2014, 132, 475–484. [Google Scholar] [CrossRef]
- Watanabe, H.; Li, D.; Nakagawa, Y.; Tomishige, K.; Watanabe, M.M. Catalytic gasification of oil-extracted residue biomass of Botryococcus braunii. Bioresour. Technol. 2015, 191, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Garciano, L.O.; Tran, N.H.; Kannangara, G.S.K.; Milev, A.S.; Wilson, M.A.; McKirdy, D.M.; Hall, P.A. Pyrolysis of a Naturally Dried Botryococcus braunii Residue. Energy Fuels 2012, 26, 3874–3881. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Liu, L.; Sun, X. Self-Assembled Magnetically Modified Flocculant for Separating Microalgae and Preparation Method and Application Thereof. Patent CN104480016 A 20150401, 1 April 2015. [Google Scholar]
- Bell, R.J.; Bird, N.F.; Dean, P. The vibrational spectra of vitreous silica, germania and beryllium fluoride. J. Phys. C Solid State Phys. 1968, 1, 299–303. [Google Scholar] [CrossRef]
- Devine, R.A.B. Ion implantation- and radiation-induced structural modifications in amorphous SiO2. J. Non-Cryst. Solids 1993, 152, 50–58. [Google Scholar] [CrossRef]
- Fitch, J.T.; Lucovsky, G.; Kobeda, E.; Irene, E.A. Effects of thermal history on stress-related properties of very thin films of thermally grown silicon dioxide. J. Vac. Sci. Technol. B 1989, 7, 153–162. [Google Scholar] [CrossRef]
- Agarwal, A.; Davis, K.M.; Tomozawa, M. A simple IR spectroscopic method for determining fictive temperature of silica glasses. J. Non-Cryst. Solids 1995, 185, 191–198. [Google Scholar] [CrossRef]
- Manoharan, C.; Venkatachalapathy, R.; Dhanapandian, S.; Deenadayalan, K. FTIR and Mössbauer spectroscopy applied to study of archaeological artefacts from Maligaimedu, Tamil Nadu, India. Indian J. Pure Appl. Phys. 2007, 45, 860–865. [Google Scholar]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wagoner, G. Spin resonance of charge carries in graphite. Phys. Rev. 1960, 118, 647–653. [Google Scholar] [CrossRef]
- Feher, G.; Kip, A.F. Electron spin resonance absorption in metals. I. Experimental. Phys. Rev. 1955, 98, 337–348. [Google Scholar] [CrossRef]
- Dyson, F.J. Electron spin resonance absorption in metals. II. Theory of electron diffusion and the skin effect. Phys. Rev. 1955, 98, 349–359. [Google Scholar] [CrossRef]
- Joshi, J.P.; Bhat, S.V. On the analysis of broad Dysonian electron paramagnetic resonance spectra. J. Magn. Reson. 2004, 168, 284–287. [Google Scholar] [CrossRef] [PubMed]
- ESR of Material-Carbon. JEOP Application Note: ER-110001E. Available online: https://www.jeol.co.jp/en/applications/detail/1293.html (accessed on 27 January 2018).
- Banerji, S.; Adhya, S.K.; Ghosh, S.K. ESR studies of carbon containing finely dispersed iron. Carbon 1988, 26, 461–463. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, J.; Zong, Y.; Miao, H.; Hu, X.; Bai, J.; Li, X. Hydrophobic graphene nanosheets decorated by monodispersed superparamagnetic Fe3O4 nanocrystals as synergistic electromagnetic wave absorbers. J. Mater. Chem. C 2015, 3, 4452–4463. [Google Scholar] [CrossRef]
- Ino, H.; Moriya, T.; Fujita, E. Mössbauer Effect Study of the Iron-Carbon Martensite and Its Tempering Process. Tetsu-to-Hagane 1968, 1, 34–48. [Google Scholar] [CrossRef]
- Mendonca, F.G.; Andisson, J.D.; Rosmaninho, M.G.; Lago, R.M.; Tristão, J.C. Mössbauer study of carbon coated iron magnetic nanoparticles produced by simultaneous reduction/pyrolysis. Hyperfine Interact. 2011, 202, 123–129. [Google Scholar] [CrossRef]
- Strijkers, G.J.; Kohlhepp, J.T.; Swagten, H.J.M.; de Jonge, W.J.M. Formation of nonmagnetic c-Fe1-xSi in antiferromagnetically coupled epitaxial Fe/Si/Fe. Phys. Rev. B 1999, 60, 9583–9587. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Electronic conduction of magnetite (Fe3O4) and its transition point at low temperatures. Nature 1939, 14, 327–328. [Google Scholar] [CrossRef]
- Bernal-Villamil, I.; Gallego, S. Electronic phase transitions in ultrathin magnetite films. J. Phys. Condens. Matter 2015, 27, 293292. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 6th ed.; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
- Chaddha, G.; Seehra, M.S. Magnetic components and particle size distribution of coal fly ash. J. Phys. D Appl. Phys. 1983, 16, 1767–1776. [Google Scholar] [CrossRef]
- Abdul-Razzaq, W.; Gautam, M. Discovery of magnetite in the exhausted material from a diesel engine. Appl. Phys. Lett. 2001, 78, 2018–2019. [Google Scholar] [CrossRef]
- Amorim, C.C.; Leão, M.M.D.; Dutra, P.R.; Tristão, J.C.; Magalhães, F.; Lago, R.M. Use of tar pitch as a binding and reductant of BFD waste to produce reactive materials for environmental applications. Chemosphere 2014, 109, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kadouri, A.; Derenne, S.; Largeau, E.; Casadevall, E.; Berkaloff, C. Resistant biopolymer in the outer walls of Botryococcus braunii, B race. Phytochemistry 1988, 27, 551–557. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, H.; Shang, N.; Gao, S.; Wang, C. Magnetic graphene nanocomposite as an efficient catalyst for hydrogenation of nitroarenes. Chin. Chem. Lett. 2013, 24, 539–541. [Google Scholar] [CrossRef]
- He, G.; Liu, W.; Sun, X.; Chen, Q.; Wang, X.; Chen, H. Fe3O4@graphene oxide composite: A magnetically separable and efficient catalyst for the reduction of nitroarenes. Mater. Res. Bull. 2013, 48, 1885–1890. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional iron-oxide-nanoflake/graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 14446–14455. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Liang, M.; Han, D.; Wang, Z. Multifunctional sandwich-like mesoporous silica-Fe3O4-graphene oxide nanocomposites for removal of methylene blue from water. RSC Adv. 2015, 5, 39964–39972. [Google Scholar] [CrossRef]
- Yonezawa, N.; Matsuura, H.; Shiho, M.; Kaya, K.; Watanabe, M.M. Effects of soybean curd wastewater on the growth and hydrocarbon production of Botryococcus braunii strain BOT-22. Bioresour. Technol. 2012, 109, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, A.; Matsuura, H.; Sano, T.; Kaya, K.; Watanabe, M.M. Biosynthesis of isoprene units in the C34 botryococcene molecule produced by Botryococcus braunii strain Bot-22. Procedia Environ. Sci. 2012, 15, 56–65. [Google Scholar] [CrossRef]
- Polysilicato-iron mainly contains [SiO2]n·Fe2O3, according to the data sheet (in Japanese) provided by Nankai Chemical.


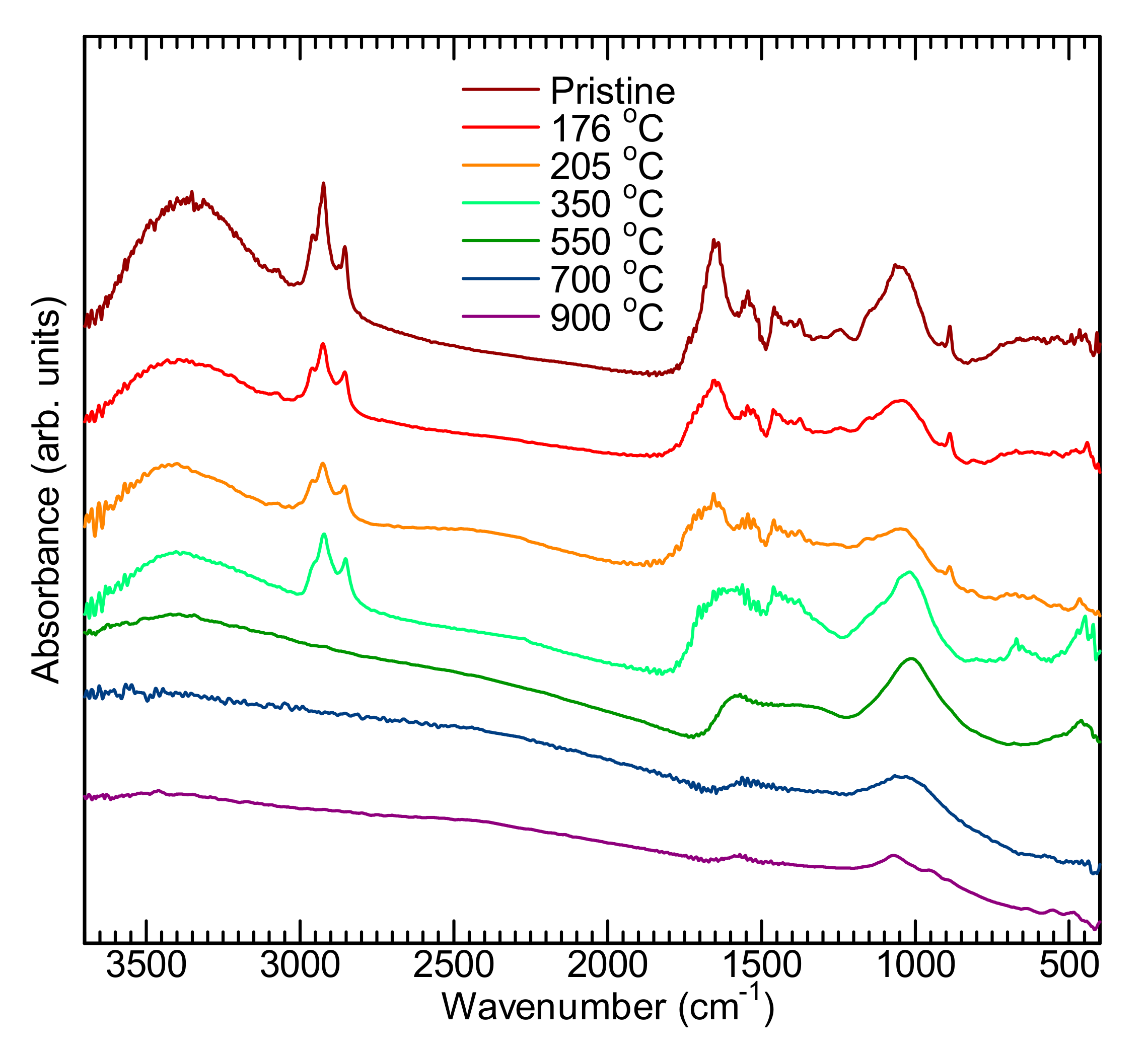
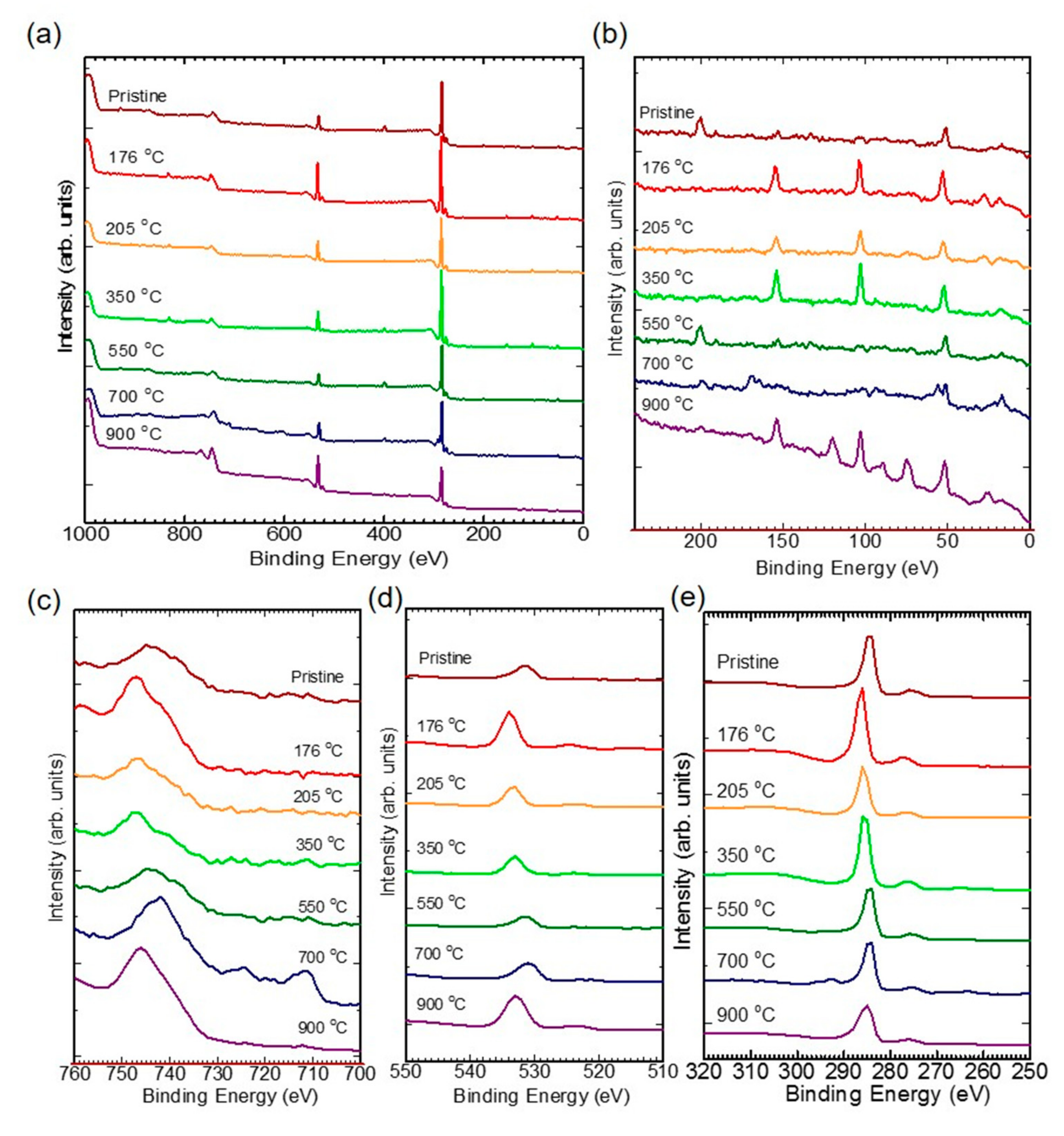
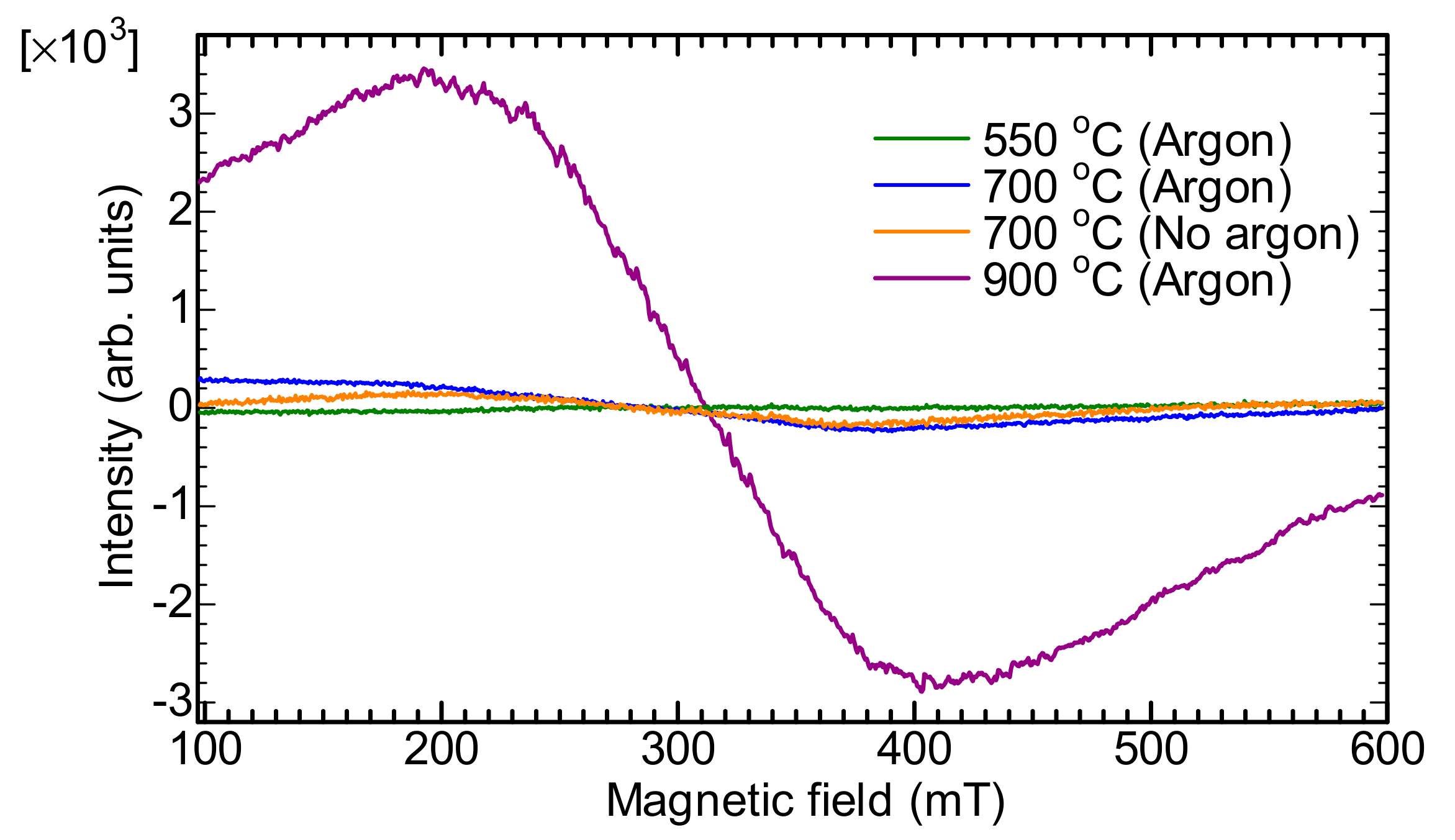
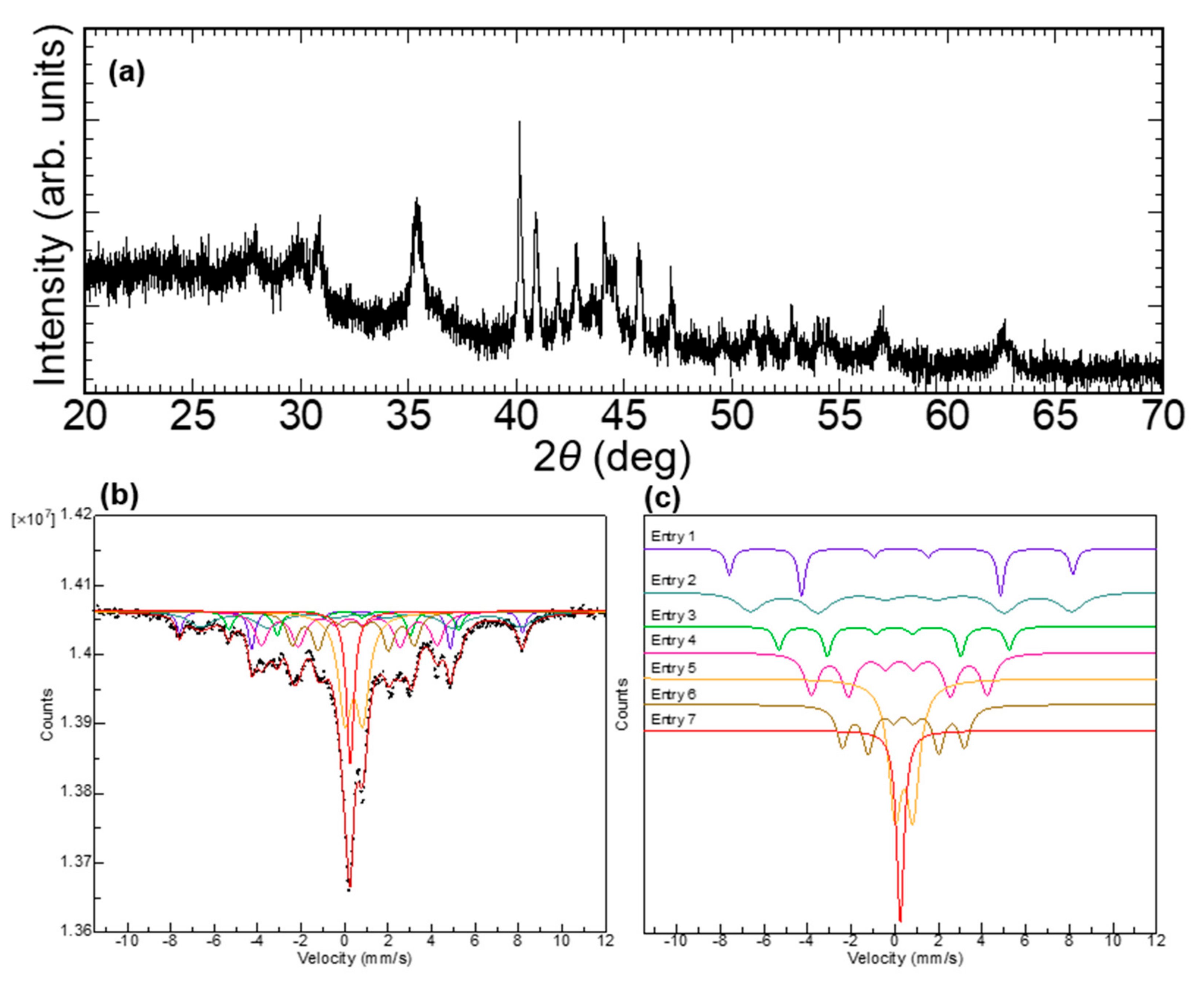

| Temperature | Wavenumber (cm-1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ν OH | =CH2 νasCH2 | –CH3 νasCH | –CH2– νasCH2 | –CH2– νsCH2 | C=C str. | –CH2– δ CH | –CH3 δ CH | =C–O | Si–O–Si stretching | C–H def. | Si–O–Si bending | Si–O–Si rocking | ||
| Pristine | 3366 | 3072 | 2956 | 2923 | 2854 | 1653 | 1543 | 1457 | 1373 | 1246 | 1053 | 887 | - | - |
| 176 °C | 3392 | 3072 | 2960 | 2926 | 2853 | 1653 | 1543 | 1458 | 1375 | 1246 | 1048 | 888 | - | - |
| 205 °C | 3404 | 3072 | 2960 | 2924 | 2853 | 1653 | 1543 | 1455 | 1375 | - | 1045 | 887 | - | - |
| 350 °C | 3402 | - | 2951 | 2922 | 2852 | 1595 | 1542 | 1452 | 1375 | - | 1020 | - | 665 | 447 |
| 550 °C | - | - | - | - | - | 1587 | - | - | - | - | 1011 | - | 669 | 465 |
| 700 °C | - | - | - | - | - | 1540 | - | - | - | - | 1032 | - | × | - |
| 900 °C | - | - | - | - | 1546 | - | - | - | 1072 | - | 669 | - | ||
| Entry No. | δ (mm/s) | Δ (mm/s) | H (T) | Yield (%) |
|---|---|---|---|---|
| Entry 1 (Fe3O4 A site) | 0.285 | 2.50 | 48.9 | 7.94 |
| Entry 2 (Fe3O4 B site) | 0.740 | 2.44 | 45.8 | 16.12 |
| Entry 3 (α-Fe) | −0.0397 | 1.68 | 32.8 | 7.13 |
| Entry 4 (Fe3C) | 0.204 | 1.30 | 25.1 | 18.52 |
| Entry 5 (FeO) | 0.418 | 0.815 | – | 23.77 |
| Entry 6 | 0.392 | 0.89 | 17.4 | 16.11 |
| Entry 7 | 0.251 | – | – | 10.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Demura, M.; Watanabe, M.M.; Ohara, K.; Kashiwagi, T.; Kadowaki, K.; Kita, E.; Dong, J.; Goto, H. Surface Observation and Magnetism of Oil-Extracted Botryococcus braunii Residues before and after Carbonization. C 2018, 4, 10. https://doi.org/10.3390/c4010010
Wang A, Demura M, Watanabe MM, Ohara K, Kashiwagi T, Kadowaki K, Kita E, Dong J, Goto H. Surface Observation and Magnetism of Oil-Extracted Botryococcus braunii Residues before and after Carbonization. C. 2018; 4(1):10. https://doi.org/10.3390/c4010010
Chicago/Turabian StyleWang, Aohan, Mikihide Demura, Makoto M. Watanabe, Kotaro Ohara, Takanari Kashiwagi, Kazuo Kadowaki, Eiji Kita, Jiuchao Dong, and Hiromasa Goto. 2018. "Surface Observation and Magnetism of Oil-Extracted Botryococcus braunii Residues before and after Carbonization" C 4, no. 1: 10. https://doi.org/10.3390/c4010010
APA StyleWang, A., Demura, M., Watanabe, M. M., Ohara, K., Kashiwagi, T., Kadowaki, K., Kita, E., Dong, J., & Goto, H. (2018). Surface Observation and Magnetism of Oil-Extracted Botryococcus braunii Residues before and after Carbonization. C, 4(1), 10. https://doi.org/10.3390/c4010010