Bituminous Coal-Derived Carbon Anode: Molten Salt-Assisted Synthesis and Enhanced Performance in Sodium-Ion Battery
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Carbon Materials
2.1.1. Pre-Treatment of Raw Materials
2.1.2. Preparation of Molten Salt
2.1.3. Synthesis of Hard Carbon
2.2. Characterization
2.3. Preparation and Performance
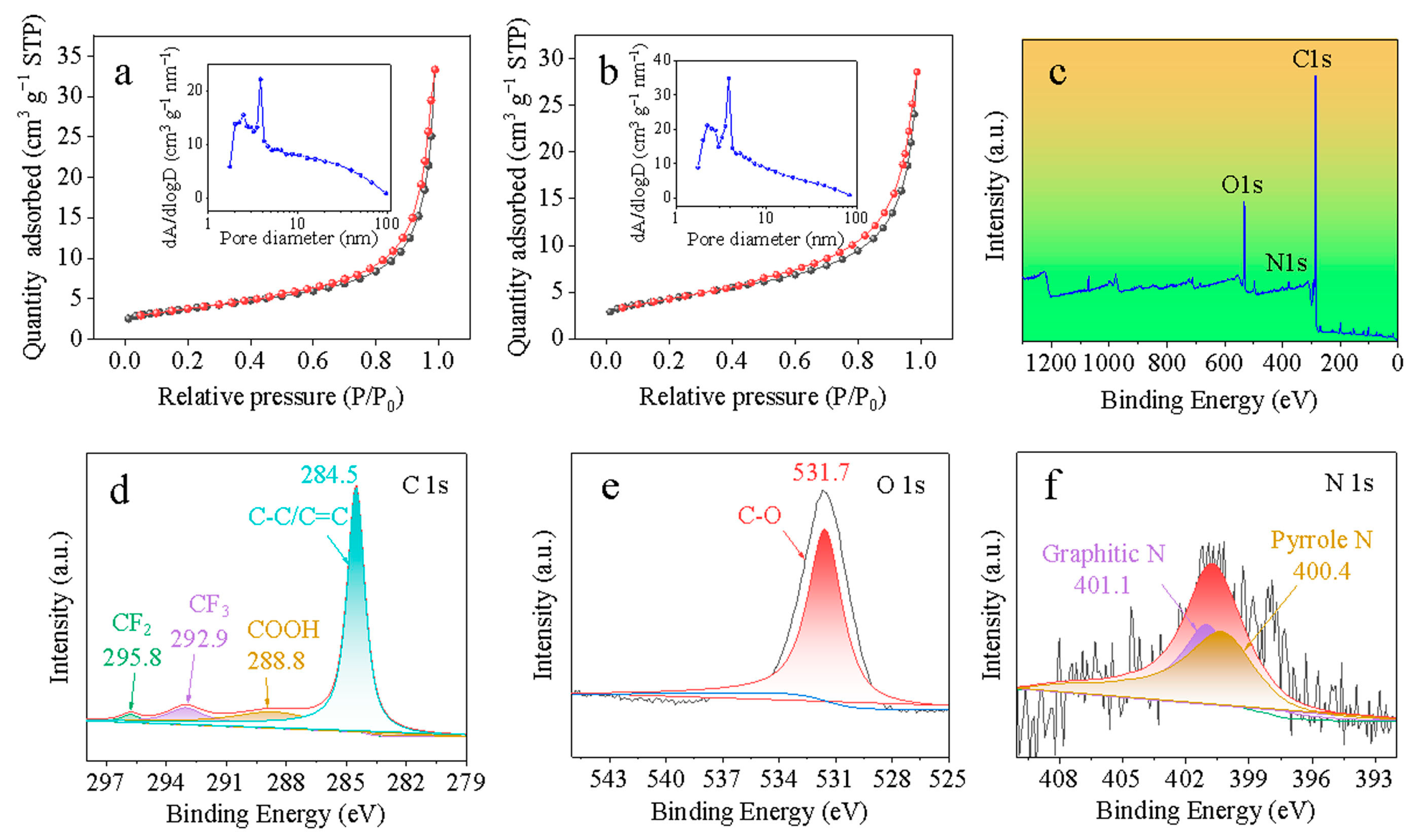
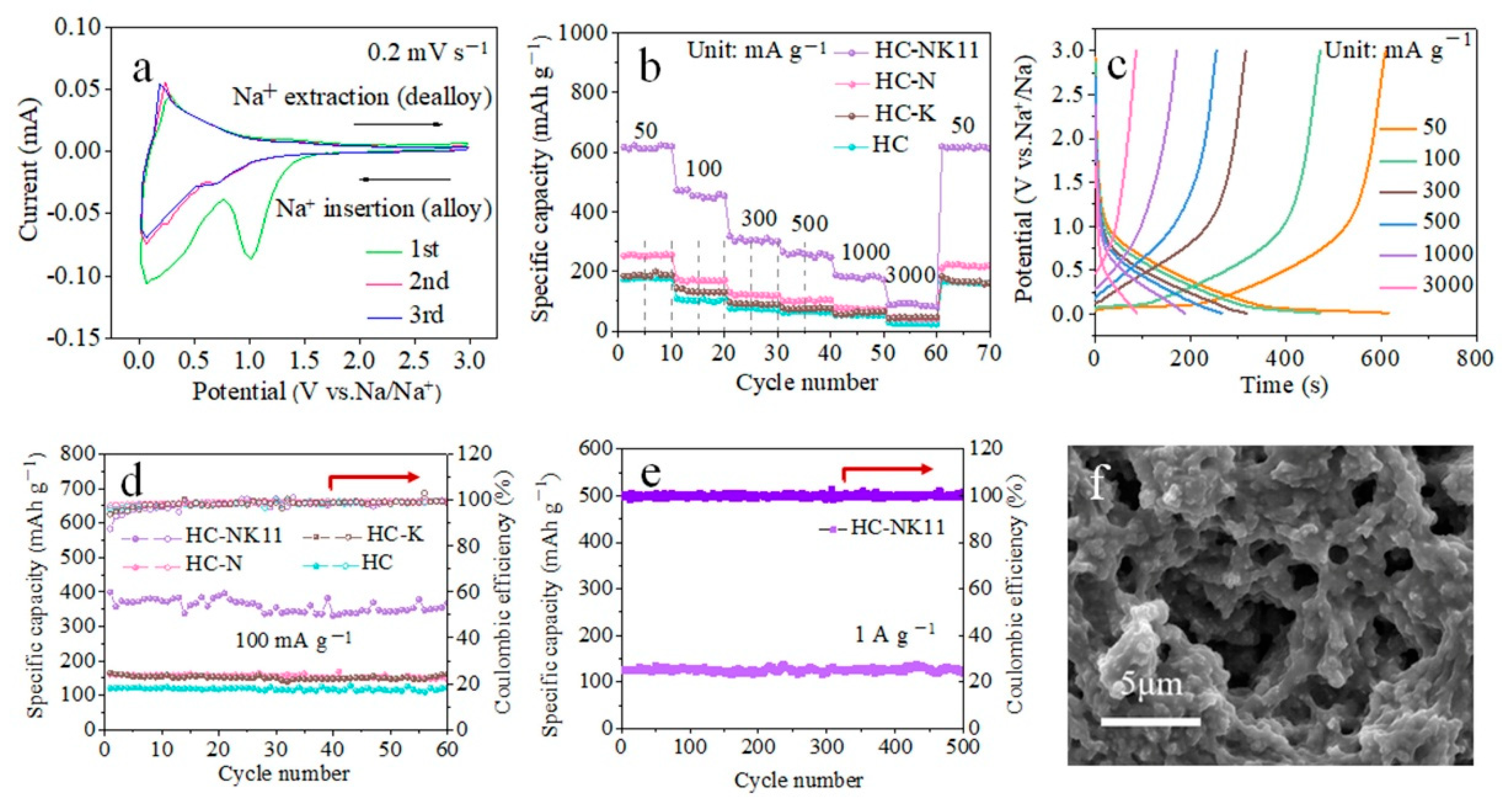
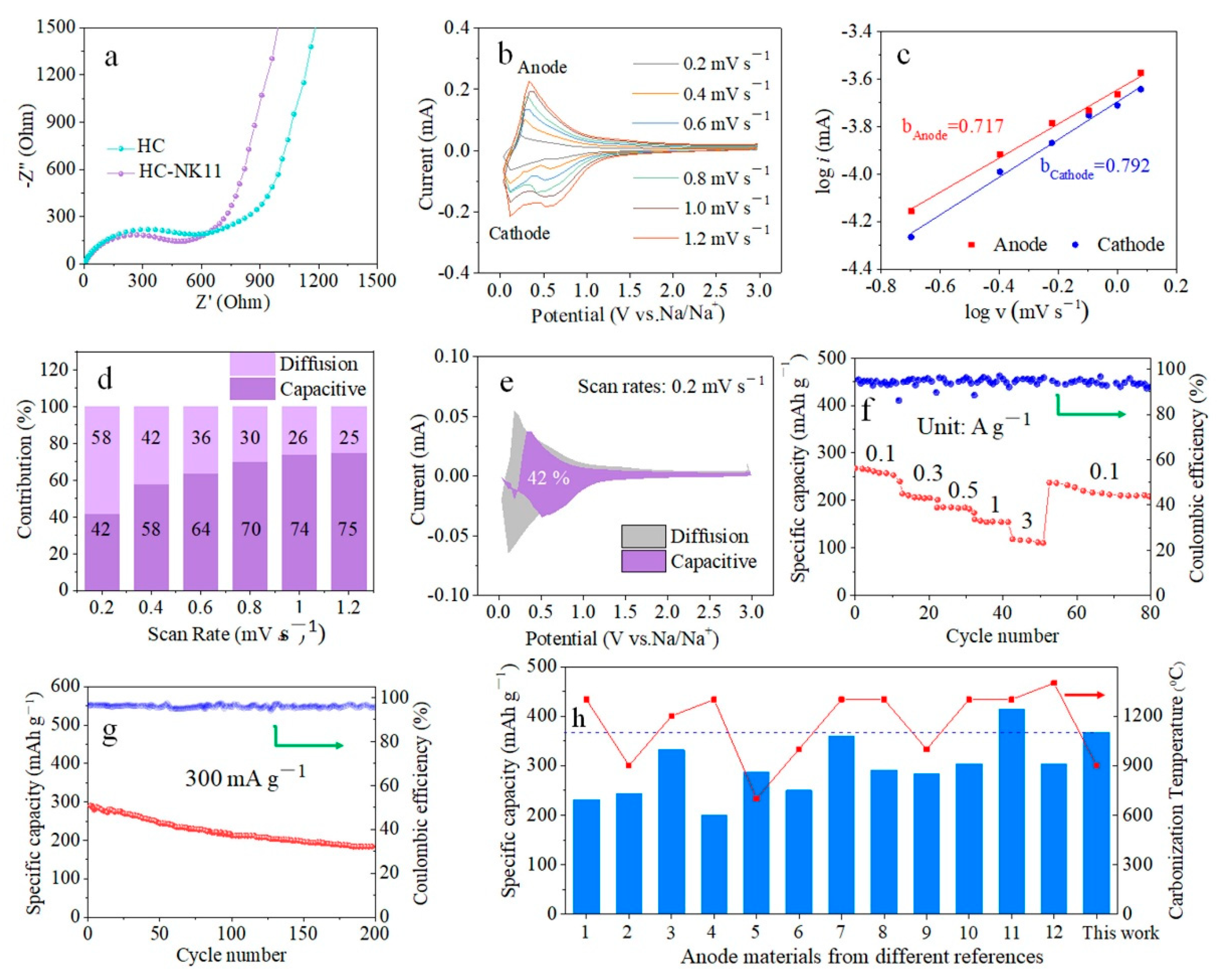
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Yang, Z.; Zhou, X.Z.; Hao, Z.Q.; Chen, J.; Wang, Z.M.; Chen, X.M.; Wu, X.Q.; Li, L.; Li, L.; et al. Recent progress on electrolyte boosting initial coulombic efficiency in lithium-ion batteries. Adv. Funct. Mater. 2024, 34, 2303457. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-ion batteries toward Na-ion chemistries: Challenges and opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Liu, X.M.; Wu, Z.; Xie, L.Q.; Sheng, L.; Liu, J.H.; Wang, L.; Wu, K.; He, X.M. Prelithiation Enhances Cycling life of lithium-ion batteries: A mini review. Energy Environ. Mater. 2023, 6, e12501. [Google Scholar] [CrossRef]
- Wang, X.H.; Dong, J.H.; Ren, J.; Nan, D.; Huang, N.; Li, J.H.; Liu, J. Storage mechanisms, modification strategies, and prospects of hard carbon anode for sodium-ion batteries. J. Electroanal. Chem. 2025, 988, 119152. [Google Scholar] [CrossRef]
- Qin, H.; Wang, S.; Zhou, H.Y.; Pan, S.; Cui, D.; Wang, Q.; Jia, C.X.; Zhang, L.D.; Bai, J.R. From graphite to hard carbon: Multifaceted sodium storage reactions and future perspectives of carbon anode materials for sodium-ion batteries. J. Electroanal. Chem. 2025, 993, 119270. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, N.; Xu, B. Recent advances in heterostructured carbon materials as anodes for sodium ion batteries. Small Struct. 2021, 2, 2100132. [Google Scholar] [CrossRef]
- Zhou, J.H.; Ma, K.N.; Lian, X.Y.; Shi, Q.T.; Wang, J.Q.; Chen, Z.J.; Guo, L.L.; Liu, Y.; Bachmatiuk, A.; Sun, J.Y.; et al. Eliminating graphite exfoliation with an artificial solid electrolyte interphase for stable lithium-ion batteries. Small 2021, 18, 2107460. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Huang, Y.; Chen, C.; Wang, M.Y.; Liu, P.B. Phosphorus-doped porous biomass carbon with ultra-stable performance in sodium storage and lithium storage. Electrochim. Acta 2019, 321, 134698. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Li, Y.; Guo, R.; Pei, H.J.; Luo, Y.; Xie, J.Y. Lithium/sodium storage behavior of an amorphous carbon derived from the used acticarbon for rechargeable batteries. J. Electrochem. Soc. 2019, 166, A1585. [Google Scholar] [CrossRef]
- Jin, Z.; Cui, Z.W.; Long, X.Y.; Millan, M.; Yuan, G.M.; Dong, Z.J.; Cong, Y.; Zhang, J.; Li, Y.J.; Li, X.K. Understanding the correlation between microstructure and electrochemical performance of hybridized pitch cokes for lithium-ion battery through tailoring their evolutional structures from ordered soft carbon to disordered hard carbon. J. Alloys Compd. 2021, 887, 161357. [Google Scholar] [CrossRef]
- Sun, N.; Qiu, J.S.; Xu, B. Understanding of sodium storage mechanism in hard carbons: Ongoing development under debate. Adv. Energy Mater. 2022, 12, 2200715. [Google Scholar] [CrossRef]
- Zhu, J.H.; Roscow, J.; Chandrasekaran, S.; Deng, L.B.; Zhang, P.X.; He, T.S.; Wang, K.; Huang, L.C. Biomass-derived carbons for sodium ion batteries and sodium ion capacitors. ChemSusChem 2020, 13, 1275–1295. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Liu, B.H.; Ren, Q.J.; Fan, L.L.; Shi, Z.Q.; Zhang, Q.Y. A solvothermal pre-oxidation strategy converting pitch from soft carbon to hard carbon for enhanced sodium storage. Chin. Chem. Lett. 2023, 34, 107526. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, D.; Mo, Y.; Tang, R.; Gao, P.; Han, M.M.; Chen, S.; Wakabayashi, K.; Yoshii, T.; Nishihara, H.; et al. Tuning surface reactivity towards high-performance hard carbon in Li/Na/K-ion batteries. J. Energy Chem. 2025, 103, 27–36. [Google Scholar] [CrossRef]
- Wang, Q.D.; Zhao, C.L.; Lu, Y.X.; Li, Y.M.; Zheng, Y.H.; Qi, Y.R.; Rong, X.H.; Jiang, L.W.; Qi, X.G.; Shao, Y.J.; et al. Advanced nanostructured anode materials for sodium ion batteries. Small 2017, 13, 1701835. [Google Scholar] [CrossRef]
- Chen, D.Q.; Zhang, W.; Luo, K.Y.; Song, Y.; Zhong, Y.J.; Liu, Y.X.; Wang, G.K.; Zhong, B.H.; Wu, Z.G.; Guo, X.D. Hard carbon for sodium storage: Mechanism and optimization strategies toward commercialization. Energy Environ. Sci. 2021, 14, 2244–2262. [Google Scholar] [CrossRef]
- Tian, Q.Q.; Li, X.M.; Xie, L.J.; Su, F.Y.; Yi, Z.L.; Dong, L.; Chen, C.M. Insights into the carbonization mechanism of bituminous coal-derived carbon materials for lithium-ion and sodium ion batteries. New Carbon Mater. 2023, 38, 939–953. [Google Scholar] [CrossRef]
- Niu, J.Z.; Cheng, J.Y.; Yi, Z.L.; Wang, C.J.; Li, X.M.; Chen, J.P.; Xie, L.J.; Chang, Y.G.; Li, X.; Su, F.Y.; et al. Promoting cross-linking reactivity of coal molecules via swelling strategy to realize high performance coal-derived hard carbon anode. J. Electroanal. Chem. 2025, 984, 119060. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, D.X.; Xu, C.R.; Peng, B.; Li, J.C.; Yang, J.; Xu, S.; Sun, X.Y.; Zhou, H.S.; Guo, S.H. A “grafting technique” to tailor the interfacial behavior of hard carbon anodes for stable sodium-ion batteries. Energy Environ. Sci. 2025, 18, 1911. [Google Scholar] [CrossRef]
- Shi, M.; Song, C.L.; Tai, Z.G.; Zou, K.Y.; Duan, Y.; Dai, X.; Sun, J.J.; Chen, Y.Z.; Liu, Y.N. Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 2021, 292, 120250. [Google Scholar] [CrossRef]
- Xing, B.L.; Meng, W.B.; Liang, H.; Kang, W.W.; Zeng, H.H.; Zhang, C.X.; Egun, I.L.; Li, P.; Cao, Y.J.; Chen, Z.F. Flexible coal-derived carbon fibers via electrospinning for self-standing lithium-ion battery anodes. Int. J. Min. Sci. Technol. 2024, 34, 1753–1763. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Tang, X.F.; Kong, Z.H.; You, Z.L.; Zhang, Y.X.; Duan, Y.F.; Zhang, Y.T. Pre-oxidation modification of bituminous coal-based hard carbon for high-quality sodium ion storage. Solid State Ion. 2024, 416, 116668. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, K.Y.; Su, M.Y.; Liu, H.H.; Gu, Z.Y.; Dai, D.M.; Li, B.; Wang, J.W.; He, X.Y.; Wu, X.L. Coal-derived flaky hard carbon with fast Na transport kinetic as advanced anode material for sodium-ion batteries. Carbon 2024, 229, 119526. [Google Scholar] [CrossRef]
- Pang, Z.Y.; Li, G.S.; Xiong, X.L.; Ji, L.; Xu, Q.; Zou, X.L.; Lu, X.G. Molten salt synthesis of porous carbon and its application in supercapacitors: A review. J. Energy Chem. 2021, 61, 622–640. [Google Scholar] [CrossRef]
- Lin, P.L.; Pelton, A.D.; Bale, C.W. Computation of ternary molten salt phase diagrams. J. Am. Ceram. Soc. 1979, 62, 414–422. [Google Scholar] [CrossRef]
- Yang, L.; Hu, M.X.; Zhang, H.W.; Yang, W.; Lv, R.T. Pore structure regulation of hard carbon: Towards fast and high-capacity sodium ion storage. J. Colloid Interface Sci. 2020, 566, 257–264. [Google Scholar] [CrossRef]
- Kim, J.; Yu, D.; Kim, D.; Kim, J.; Yang, J. Development of bio-graphite from waste coffee grounds via catalytic graphitization for sustainable Lithium ion batteries anodes. FlatChem 2025, 51, 100867. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Shu, Q.Q.; Liang, C.L.; Bai, Y.B.; Wang, X.M. F, O co-doped porous hard carbon from conjugated microporous polymer for efficient lithium storage. J. Electroanal. Chem. 2024, 964, 118355. [Google Scholar] [CrossRef]
- Guo, P.; Song, H.H.; Chen, X.H. Hollow graphene oxide spheres self-assembled by W/O emulsion. J. Mater. Chem. 2010, 20, 4867–4874. [Google Scholar] [CrossRef]
- Yan, F.; Yang, Q.; Li, M.Q.; Chen, G.; Zhang, W.; Chen, Y.W. Facile synthesis of hollow stalagmite-like N, S-doped C and its capacity attenuation mechanism as anodes in K-ion batteries. Carbon 2022, 200, 56–62. [Google Scholar] [CrossRef]
- Wang, K.F.; Sun, F.; Wang, H.; Wu, D.Y.; Chao, Y.X.; Gao, G.H.; Zhao, G.B. Altering Thermal Transformation Pathway to Create Closed Pores in Coal-Derived Hard Carbon and Boosting of Na+ Plateau Storage for High-Performance Sodium-Ion Battery and Sodium-Ion Capacitor. Adv. Funct. Mater. 2022, 32, 2203725. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Ni, J.J.; Li, T.L.; Li, Y.Z.; Yin, Q.; Xiao, B.; Meng, Q.K.; Sui, Y.W.; Qi, J.Q. Coal-derived boron and phosphorus co-doped activated carbon with expanded interlayer space for high performance sodium ion capacitor anode. J. Colloid Interface Sci. 2025, 677, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, N.; Wang, Y.X.; Soomro, R.A.; Xu, B. Microcrystalline Engineering of Anthracite-Based Carbon Via Salt-Assisted Ball Milling for Enhanced Sodium Storage Performance. Small 2025, 21, 2406497. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.P.; Zhang, D.; Lei, Y.; Zhou, Y.J.; Yang, D.R.; Dong, P.; Xu, B.W.; Yang, B.; Liang, F. Regulating Closed Pore Structure of Coal-Based Hard Carbon Anode by Preoxidation for High-Rate Performance Sodium-Ion Batteries. Langmuir 2025, 41, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, N.; Chen, H.; Soomro, R.A.; Xun, B. Molten salt assisted fabrication of coal-based carbon anode materials for efficient Na ion storage. Inorg. Chem. Front. 2023, 10, 5117–5126. [Google Scholar] [CrossRef]
- Qi, S.X.; Yang, T.; Song, Y.; Zhao, N.; Liu, J.Q.; Tian, X.D.; Wu, J.R.; Li, H.; Liu, Z.J. Impact of pitch fraction oxidation on the structure and sodium storage properties of derived carbon materials. New Carbon Mater. 2025, 40, 409–421. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, W.; Song, X.D.; Yu, C.; Qiu, J.S. Hard carbons prepared by a salt-assisted hydrothermal method as anodes for the sodium-ion battery. New Carbon Mater. 2025, 40, 346–354. [Google Scholar] [CrossRef]
- Lu, H.Y.; Sun, S.F.; Xiao, L.F.; Qian, J.F.; Ai, X.P.; Yang, H.X.; Lu, A.H.; Cao, Y.L. High-Capacity Hard Carbon Pyrolyzed from Subbituminous Coal as Anode for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 729–735. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, Y.K.; Liu, J.M.; Xiao, S.K.; Zhang, Y.; Gao, Y.; Yang, C.S.; Liu, L. Mild pretreatment synthesis of coal-based phosphorus-doped hard carbon with extended plateau capacity as anodes for sodium-ion batteries. J. Alloys Compd. 2023, 946, 169384. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.T.; Li, K.K.; Hu, Y.P.; Zhu, Y.Y.; Wang, Y.C.; Liu, Y. Design of cross-linked hard carbon with high initial coulombic efficiency for sodium-ion batteries anode. Chem. Phys. Lett. 2024, 856, 141651. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Y.X.; Li, Q.; Zhang, B.Y.; Chen, F.F.; Leng, C.Y.; Jia, D.Z.; Guo, N.N.; Wang, L.X. Oxygen-driven closing pore formation in coal-based hard carbon for low-voltage rapid sodium storage. Chem. Eng. J. 2024, 493, 152389. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.T.; Dai, X.Q.; Yang, Q.; Tao, J.Y.; Xu, J.K.; Cao, Y.H.; Wu, X.C.; Shan, Z.Q. Scalable silicon@sulfur-doped carbon composites via a low-cost facile method for high-performance lithium-ion battery anodes. J. Alloys Compd. 2023, 946, 169330. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Wang, J.; Li, P.; Wang, Y.; Zhao, Y.; Chen, S. Bituminous Coal-Derived Carbon Anode: Molten Salt-Assisted Synthesis and Enhanced Performance in Sodium-Ion Battery. C 2025, 11, 82. https://doi.org/10.3390/c11040082
Du Y, Wang J, Li P, Wang Y, Zhao Y, Chen S. Bituminous Coal-Derived Carbon Anode: Molten Salt-Assisted Synthesis and Enhanced Performance in Sodium-Ion Battery. C. 2025; 11(4):82. https://doi.org/10.3390/c11040082
Chicago/Turabian StyleDu, Yuxuan, Jian Wang, Peihua Li, Yalong Wang, Yibo Zhao, and Shuwei Chen. 2025. "Bituminous Coal-Derived Carbon Anode: Molten Salt-Assisted Synthesis and Enhanced Performance in Sodium-Ion Battery" C 11, no. 4: 82. https://doi.org/10.3390/c11040082
APA StyleDu, Y., Wang, J., Li, P., Wang, Y., Zhao, Y., & Chen, S. (2025). Bituminous Coal-Derived Carbon Anode: Molten Salt-Assisted Synthesis and Enhanced Performance in Sodium-Ion Battery. C, 11(4), 82. https://doi.org/10.3390/c11040082






