Carbon Black Nanoparticles in Non-Instrumental Immunoassays Development for Diagnostic Applications
Abstract
1. Introduction
2. Carbon Black Nanoparticles, General Information
2.1. Characteristics of Carbon Black Nanoparticles
2.2. CB Preparation Methods
3. Detection Reagents Based on Carbon Black Nanoparticles
3.1. Functionalization of Carbon Black Nanoparticles with Recognition Molecules
| No | CBNP’s Sources | Bioconjugation Method | Recognition Molecule | Ref. |
|---|---|---|---|---|
| 1 | Spezial Schwartz 4 | physical adsorption | anti-albumin antibody | [10] |
| 2 | Spezial Schwartz 4 | physical adsorption | anti-alpha-amylase antibody | [43] |
| 3 | Spezial Schwartz 4 | physical adsorption | monoclonal antibody | [44] |
| 4 | N/S | physical adsorption | Mouse anti-rat IgG F(ab′)2 fragment specific antibody | [45] |
| 5 | Spezial Schwartz 4 | physical adsorption | Anti-IgE immunoglobulin G | [46] |
| 6 | N/S | physical adsorption | monoclonal antibodies vs Verotoxin-Producing Escherichia coli | [47] |
| 7 | Carbon nanoparticles, in the form of nanostrings, were purchased from Maiia Diagnostics (Uppsala, Sweden) | physical adsorption | anti-influenza A nucleoprotein Monoclonal antibody | [48] |
| 8 | carbon black anti-EPO suspension, MAIIA Diagnostics | physical adsorption | anti-EPO antibody | [49] |
| 9 | N/S | N/S | anti-SFTSV antibody | [50] |
| 10 | Carbon black N115 | physical adsorption | Mouse anti-human IgG monoclonal antibody | [51] |
| 11 | Carbon black 100 | physical adsorption | Dengue Virus NS1 glycoprotein mouse monoclonal antibody | [12] |
| 12 | Spezial Schwartz 4 | physical adsorption | avidin | [11] |
| 13 | Spezial Schwartz 4 | physical adsorption | antibody | [52] |
| 14 | Spezial Schwartz 4 | physical adsorption | Polyclonal antibody | [4] |
| 15 | N/S | physical adsorption | neutravidin | [53] |
| 16 | Spezial Schwartz 4 | physical adsorption | neutravidin | [54] |
| 17 | Spezial Schwartz 4 | physical adsorption | Polyclonal antibody | [55] |
| 18 | Candle soot | physical adsorption followed by treatment with glutaraldehyde | monoclonal antibody and receptor | [14] |
| 19 | Candle soot | physical adsorption followed by treatment with glutaraldehyde | monoclonal antibody | [15] |
| 20 | Spezial Schwartz 4 | physical adsorption | Polyclonal antibody | [56] |
| 21 | Spezial Schwartz 4 | physical adsorption | Polyclonal antibody | [57] |
| 22 | Spezial Schwartz 4 | physical adsorption | neutravidin | [58] |
| 23 | Special blacks and other grades from Degussa AG | physical adsorption | monoclonal antibody | [59] |
| 24 | Spezial Schwartz 4 | physical adsorption | scCro DNA binding protein | [60] |
| 25 | Maiia Diagnostics | physical adsorption | antibiotin antibody or Oligonucleotide | [61] |
| 26 | Spezial Schwartz 4 | physical adsorption | Polyclonal antibody | [62] |
| 27 | Special Black 100 | covalent cross-linking by a Silane | monoclonal antibody | [35] |
| 28 | Spezial Schwartz 4 | physical adsorption | neutravidin | [63] |
| 29 | Amorphous CNPs | physical adsorption | Polyclonal antibody | [64] |
| 30 | Spezial Schwartz 4 | physical adsorption | p48 protein | [65] |
| 31 | Spezial Schwartz 4 | physical adsorption | neutravidin | [66] |
| 32 | Spezial Schwartz 4 | physical adsorption | Goat anti-Mouse IgG FcY | [67] |
| 33 | Candle soot | covalent cross-linking with glutaraldehyde | monoclonal antibody | [16] |
| 34 | Degussa, Düsseldorf, Germany | physical adsorption | polyclonal antibodies | [68] |
| 35 | N/S | physical adsorption | monoclonal antibody | [69] |
| 36 | Special Black 4 | physical adsorption | monoclonal antibody | [70] |
| 37 | Candle soot | covalent cross-linking with glutaraldehyde and biotin-streptavidin interaction | DNA-aptemer And biotin | [71] |
| 38 | Special Black 100 | physical adsorption | monoclonal antibody | [72] |
| 39 | Spezial Schwartz 4 | physical adsorption | avidin | [73] |
| 40 | Candle soot | covalent cross-linking with glutaraldehyde | Protein A | [73] |
3.2. Characterization Method of Bioconjugates Based on CBNPs
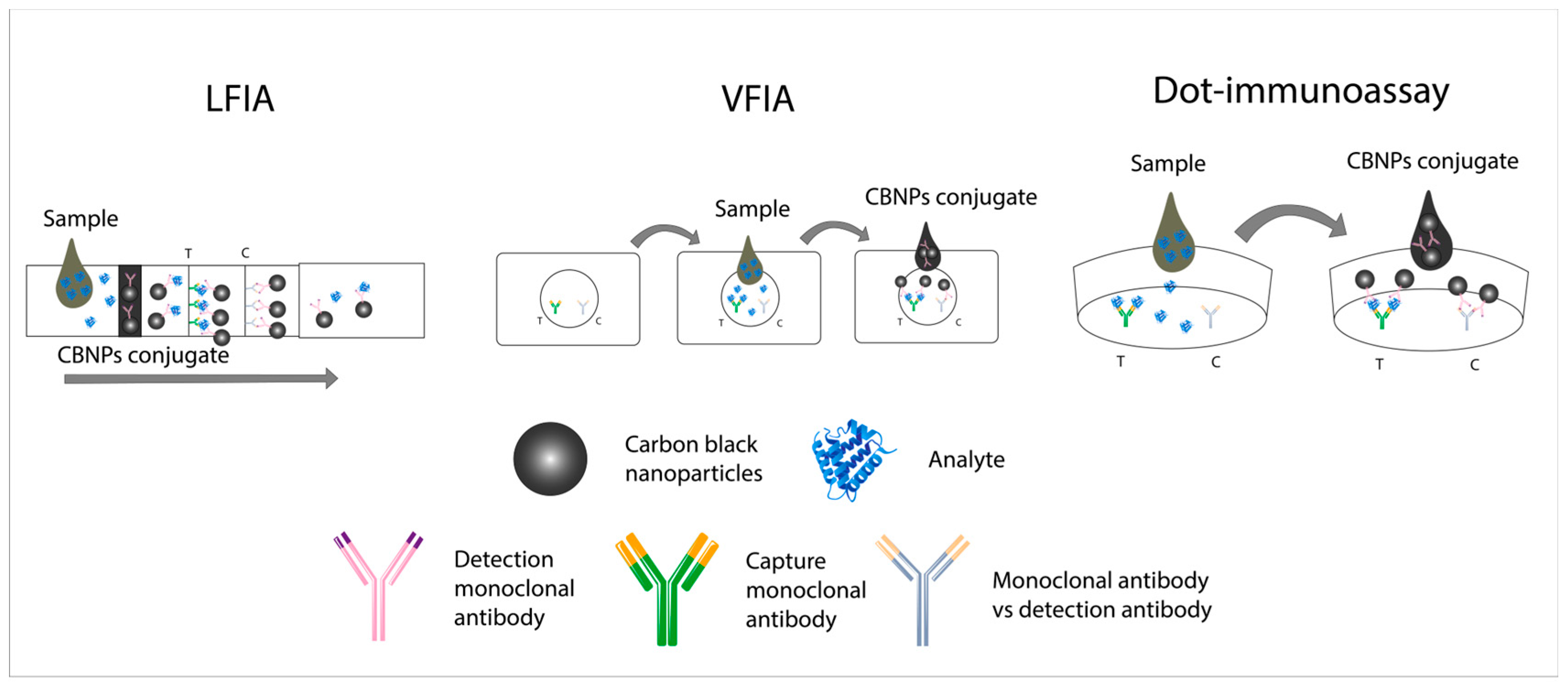
4. Use of Diagnostic Reagents Based on CBNPs in Non-Instrumental Immunoassays for Visual Assessment
4.1. Non-Instrumental Immunoassays Based on CBNPs for Visual Assessment
4.2. CBNPs in Assay Design Compared to Other Labels
5. Conclusions: Future Perspectives and Recommendation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a-C | Amorphous carbon |
| CB | Carbon black |
| CBNPs | Carbon black nanoparticles |
| LFIA | Lateral flow immunoassay |
| VFIA | Vertical flow immunoassay |
| LOD | Limit of detection |
| GA | Glutaraldehyde |
References
- Farka, Z.; Brandmeier, J.C.; Mickert, M.J.; Pastucha, M.; Lacina, K.; Skládal, P.; Soukka, T.; Gorris, H.H. Nanoparticle-Based Bioaffinity Assays: From the Research Laboratory to the Market. Adv. Mater. 2024, 36, 2307653. [Google Scholar] [CrossRef]
- Dede, M.; van Dam, A. Conjugation of Visual Enhancers in Lateral Flow Immunoassay for Rapid Forensic Analysis: A Critical Review. Anal. Bioanal. Chem. 2025, 417, 15–31. [Google Scholar] [CrossRef]
- Robertson, L.J.; Moore, J.S.; Blighe, K.; Ng, K.Y.; Quinn, N.; Jennings, F.; Warnock, G.; Sharpe, P.; Clarke, M.; Maguire, K.; et al. Evaluation of the IgG Antibody Response to SARS CoV-2 Infection and Performance of a Lateral Flow Immunoassay: Cross-Sectional and Longitudinal Analysis over 11 Months. BMJ Open 2021, 11, e048142. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Tong, B.; Zhang, Y.; Sheng, W.; Pan, M.; Wang, S. Development and Comparison of Immunochromatographic Strips with Three Nanomaterial Labels: Colloidal Gold, Nanogold-Polyaniline-Nanogold Microspheres (GPGs) and Colloidal Carbon for Visual Detection of Salbutamol. Biosens. Bioelectron. 2016, 85, 337–342. [Google Scholar] [CrossRef]
- Kuswandi, B.; Ensafi, A.A. Perspective—Paper-Based Biosensors: Trending Topic in Clinical Diagnostics Developments and Commercialization. J. Electrochem. Soc. 2020, 167, 037509. [Google Scholar] [CrossRef]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold Nanoparticles Based Optical Biosensors for Cancer Biomarker Proteins: A Review of the Current Practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Mucha, J.; Busta-Flores, P.; Lazo-Porras, M.; Vetter, B.; Safary, E.; Moran, A.E.; Gupta, R.; Bernabé-Ortiz, A. Facilitators and Barriers of the Implementation of Point-of-Care Devices for Cardiometabolic Diseases: A Scoping Review. BMC Health Serv. Res. 2023, 23, 412. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Álvarez-Diduk, R.; Parolo, C.; Piper, A.; Merkoçi, A. Toward Next Generation Lateral Flow Assays: Integration of Nanomaterials. Chem. Rev. 2022, 122, 14881–14910. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; McNamee, S.E.; Vance, P.; McLaughlin, J. High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors 2022, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, A.; Wichers, J.H.; Berendsen, L.B.J.M.; Timmermans, A.J.M.; Keizer, G.D.; van Doorn, A.W.J.; Bantjes, A.; van Gelder, W.M.J. Colloidal Carbon Particles as a New Label for Rapid Immunochemical Test Methods: Quantitative Computer Image Analysis of Results. J. Biotechnol. 1993, 30, 185–195. [Google Scholar] [CrossRef]
- Porras, J.C.; Bernuz, M.; Marfa, J.; Pallares-Rusiñol, A.; Martí, M.; Pividori, M.I. Comparative Study of Gold and Carbon Nanoparticles in Nucleic Acid Lateral Flow Assay. Nanomaterials 2021, 11, 741. [Google Scholar] [CrossRef]
- Linares, E.M.; Kubota, L.T.; Michaelis, J.; Thalhammer, S. Enhancement of the Detection Limit for Lateral Flow Immunoassays: Evaluation and Comparison of Bioconjugates. J. Immunol. Methods 2012, 375, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Thakur, A.K. The Toxicological Mechanisms of Environmental Soot (Black Carbon) and Carbon Black: Focus on Oxidative Stress and Inflammatory Pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, F.; Sun, Y.; Mi, T.; Wang, L.; Li, Q.; Li, J.; Ma, W.; Liu, W.; Zuo, J.; et al. Development of a Highly Sensitive Lateral Flow Immunoassay Based on Receptor-Antibody-Amorphous Carbon Nanoparticles to Detect 22 β-Lactams in Milk. Sens. Actuators B: Chem. 2020, 321, 128458. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wen, K.; Li, C.; Mujtaba Mari, G.; Jiang, H.; Shi, W.; Shen, J.; Wang, Z. Multiplex Lateral Flow Immunoassays Based on Amorphous Carbon Nanoparticles for Detecting Three Fusarium Mycotoxins in Maize. J. Agric. Food Chem. 2017, 65, 8063–8071. [Google Scholar] [CrossRef]
- Rayev, M.; Shmagel, K. Carbon–Protein Covalent Conjugates in Non-Instrumental Immunodiagnostic Systems. J. Immunol. Methods 2008, 336, 9–15. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental Concentrations of Engineered Nanomaterials: Review of Modeling and Analytical Studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Watson, A.Y.; Valberg, P.A. Carbon Black and Soot: Two Different Substances. AIHAJ Am. Ind. Hyg. Assoc. 2001, 62, 218–228. [Google Scholar] [CrossRef]
- Medalia, A.I.; Rivin, D.; Sanders, D.R. A Comparison of Carbon Black with Soot. Sci. Total Environ. 1983, 31, 1–22. [Google Scholar] [CrossRef]
- Jeong, B.O.; Kwon, S.W.; Kim, T.J.; Lee, E.H.; Jeong, S.H.; Jung, Y. Effect of Carbon Black Materials on the Electrochemical Properties of Sulfur-Based Composite Cathode for Lithium-Sulfur Cells. J. Nanosci. Nanotechnol. 2013, 13, 7870–7874. [Google Scholar] [CrossRef]
- Yan, H.; Pan, G. Toxicity and Bioaccumulation of Copper in Three Green Microalgal Species. Chemosphere 2002, 49, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Dernov, A.; Kowalik, M.; van Duin, A.C.T.; Dumitrică, T. Mapping the Structural–Mechanical Landscape of Amorphous Carbon with ReaxFF Molecular Dynamics. J. Appl. Phys. 2025, 137, 065107. [Google Scholar] [CrossRef]
- Ma, T.-B.; Wang, L.-F.; Hu, Y.-Z.; Li, X.; Wang, H. A Shear Localization Mechanism for Lubricity of Amorphous Carbon Materials. Sci. Rep. 2014, 4, 3662. [Google Scholar] [CrossRef]
- Steele, B.A.; Bastea, S.; Kuo, I.-F.W. Ab Initio Structural Dynamics of Pure and Nitrogen-Containing Amorphous Carbon. Sci. Rep. 2023, 13, 19657. [Google Scholar] [CrossRef]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef]
- Fitzer, E.; Kochling, K.-H.; Boehm, H.P.; Marsh, H. Recommended Terminology for the Description of Carbon as a Solid (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 473–506. [Google Scholar] [CrossRef]
- Mypгa, M.C.; Murga, M.S. The Carbon Universe: Types of carbon particles, mechanisms of their formation and distribution. In Proceedings of Physics of the Cosmos: Proceedings of the 50th International Student Scientific Conference, Yekaterinburg, 2023; Ural University Press: Yekaterinburg, Russia, 2023; pp. 88–97. [Google Scholar]
- What Is Carbon Black? Orion Engineered Carbons. Available online: https://www.thecarycompany.com/media/pdf/specs/orion-what-is-carbon-black.pdf (accessed on 30 August 2025).
- International Carbon Black Association (ICBA). Carbon Black User’s Guide. 2016. Available online: https://static1.squarespace.com/static/5fd161c5b1bc2872873bd5ee/t/67992a438085086153e286f3/1738091075852/icba+carbon+black+user%27s+guide+2024_092024.pdf (accessed on 30 August 2025).
- Specialty Carbon Black for UV Curing Printing Inks. Orion Engineered Carbons. Available online: https://orioncarbons.com/wp-content/uploads/2023/04/scb_for_uv_curing_printing_inks_en.pdf (accessed on 30 August 2025).
- Specialty Carbon Blacks. Orion Engineered Carbons. Available online: https://orioncarbons.com/wp-content/uploads/2023/04/22_06_21_td_0112_farbrusstabelle_emea_web_2.pdf (accessed on 30 August 2025).
- Fan, Y.; Fowler, G.D.; Zhao, M. The Past, Present and Future of Carbon Black as a Rubber Reinforcing Filler—A Review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Buxbaum, G.; Pfaff, G. Industrial Inorganic Pigments: Third Edition. In Industrial Inorganic Pigments, 3rd ed.; Wiley GmbH & Co. KgaA: Weinheim, Germany, 2005; pp. 1–300. [Google Scholar] [CrossRef]
- Pfaff, G. Carbon Black Pigments. Phys. Sci. Rev. 2022, 7, 109–125. [Google Scholar] [CrossRef]
- Mattsson, L.; Jungmann, C.; Lieberzeit, P.A.; Preininger, C. Modified Carbon Black as Label in a Colorimetric On-Chip Immunoassay for Histamine. Sens. Actuators B: Chem. 2017, 246, 1092–1099. [Google Scholar] [CrossRef]
- Bakken, J.A.; Jensen, R.; Monsen, B.; Raaness, O.; Wærnes, A.N. Thermal Plasma Process Development in Norway. Pure Appl. Chem. 1998, 70, 1223–1228. [Google Scholar] [CrossRef]
- Fulcheri, L.; Probst, N.; Flamant, G.; Fabry, F.; Grivei, E.; Bourrat, X. Plasma Processing: A Step towards the Production of New Grades of Carbon Black. Carbon. 2002, 40, 169–176. [Google Scholar] [CrossRef]
- Fabry, F.; Flamant, G.; Fulcheri, L. Carbon Black Processing by Thermal Plasma. Analysis of the Particle Formation Mechanism. Chem. Eng. Sci. 2001, 56, 2123–2132. [Google Scholar] [CrossRef]
- da Costa Labanca, A.R. Carbon Black and Hydrogen Production Process Analysis. Int. J. Hydrog. Energy 2020, 45, 25698–25707. [Google Scholar] [CrossRef]
- Chen, T.-T.; Chuang, K.-J.; Chiang, L.-L.; Chen, C.-C.; Yeh, C.-T.; Wang, L.-S.; Gregory, C.; Jones, T.; BéruBé, K.; Lee, C.-N.; et al. Characterization of the Interactions between Protein and Carbon Black. J. Hazard. Mater. 2014, 264, 127–135. [Google Scholar] [CrossRef]
- van Doorn, A.W.J.; Wichers, J.H.; van Gelder, W.M.J. Method for Determining the Presence or Amount of Analyte Using a Stable Colloidal Carbon Sol. Int. Patent 5,529,901, 5 October 1994. [Google Scholar]
- Doorn, A.W.J.V.; Wichers, J.H.; Gelder, W.M.J.V. Stable Aqueous Carbon Sol Composition for Determining Analyte. Int. Patent 5,641,689, 12 May 1994. [Google Scholar]
- Koets, M.; Sander, I.; Bogdanovic, J.; Doekes, G.; Amerongen, A. van A Rapid Lateral Flow Immunoassay for the Detection of Fungal Alpha-Amylase at the Workplace. J. Environ. Monit. 2006, 8, 942–946. [Google Scholar] [CrossRef]
- van Dam, G.J.; Wichers, J.H.; Ferreira, T.M.F.; Ghati, D.; van Amerongen, A.; Deelder, A.M. Diagnosis of Schistosomiasis by Reagent Strip Test for Detection of Circulating Cathodic Antigen. J. Clin. Microbiol. 2004, 42, 5458–5461. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Crabbe, P.; Salden, M.; Wichers, J.; Van Peteghem, C.; Kohen, F.; Pieraccini, G.; Moneti, G. Preliminary Evaluation of a Lateral Flow Immunoassay Device for Screening Urine Samples for the Presence of Sulphamethazine. J. Immunol. Methods 2003, 278, 117–126. [Google Scholar] [CrossRef]
- Lönnberg, M. Membrane-Assisted Isoform ImmunoAssay: Separation and Determination of Protein Isoforms. J. Immunol. Methods 2000, 246, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Capps, K.L.; McLaughlin, E.M.; Murray, A.W.A.; Aldus, C.F.; Wyatt, G.M.; Peck, M.W.; Van Amerongen, A.; Ariëns, R.M.C.; Wichers, J.H.; Baylis, C.L.; et al. Validation of Three Rapid Screening Methods for Detection of Verotoxin-Producing Escherichia Coli in Foods: Interlaboratory Study. J. AOAC Int. 2004, 87, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wiriyachaiporn, N.; Sirikett, H.; Maneeprakorn, W.; Dharakul, T. Carbon Nanotag Based Visual Detection of Influenza A Virus by a Lateral Flow Immunoassay. Microchim. Acta 2017, 184, 1827–1835. [Google Scholar] [CrossRef]
- Lönnberg, M.; Drevin, M.; Carlsson, J. Ultra-Sensitive Immunochromatographic Assay for Quantitative Determination of Erythropoietin. J. Immunol. Methods 2008, 339, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ji, F.; Ding, S.-N. Carbon Black as a Colorimetric Label for an Immunochromatographic Test Strip for Severe Fever with Thrombocytopenia Syndrome Virus Detection. Analyst 2023, 148, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Kropaneva, M.; Khramtsov, P.; Bochkova, M.; Lazarev, S.; Kiselkov, D.; Rayev, M. Vertical Flow Immunoassay Based on Carbon Black Nanoparticles for the Detection of IgG against SARS-CoV-2 Spike Protein in Human Serum: Proof-of-Concept. Biosensors 2023, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.S.; Salentijn, G.I.; Nielen, M.W.F. A Critical Comparison between Flow-through and Lateral Flow Immunoassay Formats for Visual and Smartphone-Based Multiplex Allergen Detection. Biosensors 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, L.H.; Moers, A.; Norde, W.; van Amerongen, A. Rapid Mastitis Detection Assay on Porous Nitrocellulose Membrane Slides. Anal. Bioanal. Chem. 2013, 405, 7469–7476. [Google Scholar] [CrossRef]
- Noguera, P.; Posthuma-Trumpie, G.A.; van Tuil, M.; van der Wal, F.J.; de Boer, A.; Moers, A.P.H.A.; van Amerongen, A. Carbon Nanoparticles in Lateral Flow Methods to Detect Genes Encoding Virulence Factors of Shiga Toxin-Producing Escherichia Coli. Anal. Bioanal. Chem. 2011, 399, 831–838. [Google Scholar] [CrossRef]
- Suárez-Pantaleón, C.; Wichers, J.; Abad-Somovilla, A.; van Amerongen, A.; Abad-Fuentes, A. Development of an Immunochromatographic Assay Based on Carbon Nanoparticles for the Determination of the Phytoregulator Forchlorfenuron. Biosens. Bioelectron. 2013, 42, 170–176. [Google Scholar] [CrossRef]
- Blažková, M.; Mičková-Holubová, B.; Rauch, P.; Fukal, L. Immunochromatographic Colloidal Carbon-Based Assay for Detection of Methiocarb in Surface Water. Biosens. Bioelectron. 2009, 25, 753–758. [Google Scholar] [CrossRef]
- Blažková, M.; Rauch, P.; Fukal, L. Strip-Based Immunoassay for Rapid Detection of Thiabendazole. Biosens. Bioelectron. 2010, 25, 2122–2128. [Google Scholar] [CrossRef]
- Blažková, M.; Javůrková, B.; Fukal, L.; Rauch, P. Immunochromatographic Strip Test for Detection of Genus Cronobacter. Biosens. Bioelectron. 2011, 26, 2828–2834. [Google Scholar] [CrossRef]
- Lönnberg, M.; Carlsson, J. Quantitative Detection in the Attomole Range for Immunochromatographic Tests by Means of a Flatbed Scanner. Anal. Biochem. 2001, 293, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.B.; Wichers, J.H.; Skouridou, V.; van Amerongen, A.; Masip, L. Nucleic Acid Lateral Flow Assays Using a Conjugate of a DNA Binding Protein and Carbon Nanoparticles. Microchim. Acta 2019, 186, 426. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, D.P.; Boutsika, L.M.; Kouremenou, P.G.; Christopoulos, T.K.; Ioannou, P.C. Carbon Nano-Strings as Reporters in Lateral Flow Devices for DNA Sensing by Hybridization. Anal. Bioanal. Chem. 2011, 400, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Holubová-Mičková, B.; Blažková, M.; Fukal, L.; Rauch, P. Development of Colloidal Carbon-Based Immunochromatographic Strip for Rapid Detection of Carbaryl in Fruit Juices. Eur. Food Res. Technol. 2010, 231, 467–473. [Google Scholar] [CrossRef]
- van Amerongen, A.; Besselink, G.A.J.; Blazkova, M.; Posthuma-Trumpie, G.A.; Koets, M.; Beelen-Thomissen, B. Carbon Nanoparticles as Detection Label for Diagnostic Antibody Microarrays. In Trends in Immunolabelled and Related Techniques; IntechOpen: Rijeka, Croatia, 2012; ISBN 978-953-51-0570-1. [Google Scholar]
- Sharma, R.; Verma, A.; Shinde, N.; Mann, B.; Gandhi, K.; Wichers, J.H.; van Amerongen, A. Adulteration of Cow’s Milk with Buffalo’s Milk Detected by an on-Site Carbon Nanoparticles-Based Lateral Flow Immunoassay. Food Chem. 2021, 351, 129311. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, Y.; Sun, Y.; Chen, C. Development and Application of a Colloidal Carbon Test Strip for the Detection of Antibodies against Mycoplasma Bovis. World J. Microbiol. Biotechnol. 2020, 36, 157. [Google Scholar] [CrossRef]
- Moers, A.P.H.A.; Hallett, R.L.; Burrow, R.; Schallig, H.D.F.H.; Sutherland, C.J.; van Amerongen, A. Detection of Single-Nucleotide Polymorphisms in Plasmodium Falciparum by PCR Primer Extension and Lateral Flow Immunoassay. Antimicrob. Agents Chemother. 2014, 59, 365–371. [Google Scholar] [CrossRef]
- Willemsen, L.; Wichers, J.; Xu, M.; Van Hoof, R.; Van Dooremalen, C.; Van Amerongen, A.; Peters, J. Biosensing Chlorpyrifos in Environmental Water Samples by a Newly Developed Carbon Nanoparticle-Based Indirect Lateral Flow Assay. Biosensors 2022, 12, 735. [Google Scholar] [CrossRef]
- Nauta, M.J.; van der Wal, F.J.; Putirulan, F.F.; Post, J.; van de Kassteele, J.; Bolder, N.M. Evaluation of the “Testing and Scheduling” Strategy for Control of Campylobacter in Broiler Meat in The Netherlands. Int. J. Food Microbiol. 2009, 134, 216–222. [Google Scholar] [CrossRef]
- van Amerongen, A.; van Loon, D.; Berendsen, L.B.J.M.; Wichers, J.H. Quantitative Computer Image Analysis of a Human Chorionic Gonadotropin Colloidal Carbon Dipstick Assay. Clin. Chim. Acta 1994, 229, 67–75. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; Serrano-Pertierra, E.; García, A.C.; López-Martín, S.; Yañez-Mo, M.; Cernuda-Morollón, E.; Blanco-López, M.C. Point-of-Care Detection of Extracellular Vesicles: Sensitivity Optimization and Multiple-Target Detection. Biosens. Bioelectron. 2017, 87, 38–45. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kropaneva, M.; Kalashnikova, T.; Bochkova, M.; Timganova, V.; Zamorina, S.; Rayev, M. Highly Stable Conjugates of Carbon Nanoparticles with DNA Aptamers. Langmuir 2018, 34, 10321–10332. [Google Scholar] [CrossRef]
- Gogalic, S.; Sauer, U.; Doppler, S.; Preininger, C. Investigating Colorimetric Protein Array Assay Schemes for Detection of Recurrence of Bladder Cancer. Biosensors 2018, 8, 10. [Google Scholar] [CrossRef]
- Mussin, J.; Giusiano, G.; Porras, J.C.; Corredor Sanguña, L.H.; Pividori, M.I. Carbon Nanoparticle–Based Lateral Flow Assay for the Detection of Specific Double-Tagged DNA Amplicons of Paracoccidioides spp. Microchim. Acta 2024, 191, 287. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Wichers, J.H.; Koets, M.; Berendsen, L.B.J.M.; van Amerongen, A. Amorphous Carbon Nanoparticles: A Versatile Label for Rapid Diagnostic (Immuno)Assays. Anal. Bioanal. Chem. 2012, 402, 593–600. [Google Scholar] [CrossRef]
- Ha, Y.; Ko, S.; Kim, I.; Huang, Y.; Mohanty, K.; Huh, C.; Maynard, J.A. Recent Advances Incorporating Superparamagnetic Nanoparticles into Immunoassays. ACS Appl. Nano Mater. 2018, 1, 512–521. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive Immunosensor for Cancer Biomarker Based on Dual Signal Amplification Strategy of Graphene Sheets and Multienzyme Functionalized Carbon Nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Khani, M.; Shokri, B. Plasma-Assisted Synthesis of Carbon Black with Enhanced Surface Area and Crystallinity from Pyrolysis Fuel Oil: Optimizing Process Parameters. Chem. Eng. Res. Des. 2025, 219, 222–234. [Google Scholar] [CrossRef]
- Sanjuan-Navarro, L.; Moliner-Martínez, Y.; Campíns-Falcó, P. Characterization and Quantitation of Carbon Black Nanomaterials in Polymeric and Biological Aqueous Dispersants by Asymmetrical Flow Field Flow Fractionation. ACS Omega 2021, 6, 31822–31830. [Google Scholar] [CrossRef] [PubMed]
- Boughbina-Portolés, A.; Sanjuan-Navarro, L.; Hakobyan, L.; Gómez-Ferrer, M.; Moliner-Martínez, Y.; Sepúlveda, P.; Campíns-Falcó, P. Reliable Assessment of Carbon Black Nanomaterial of a Variety of Cell Culture Media for in Vitro Toxicity Assays by Asymmetrical Flow Field-Flow Fractionation. Anal. Bioanal. Chem. 2023, 415, 2121–2132. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Carbon Black Particle Exhibits Size Dependent Toxicity in Human Monocytes. Int. J. Inflam. 2014, 2014, 827019. [Google Scholar] [CrossRef]
- Gray, C.A.; Muranko, H. Studies of Robustness of Industrial Aciniform Aggregates and Agglomerates--Carbon Black and Amorphous Silicas: A Review Amplified by New Data. J. Occup. Environ. Med. 2006, 48, 1279–1290. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.; Lee, M.-Y. Biocompatible Dispersion Methods for Carbon Black. Toxicol. Res. 2012, 28, 209–216. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Z.; Zheng, J.; Luo, Z.; Zhu, G.; Xiao, C.; Meng, Z.; Li, Y.; Luo, K. Nitrogen-Doped Graphite Encapsulated Fe/Fe3C Nanoparticles and Carbon Black for Enhanced Performance towards Oxygen Reduction. J. Mater. Sci. Technol. 2019, 35, 2543–2551. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Xu, S. Preparation and Characterization of Water-Dispersible Carbon Black Grafted with Polyacrylic Acid by High-Energy Electron Beam Irradiation. J. Mater. Sci. 2018, 53, 6106–6115. [Google Scholar] [CrossRef]
- Sanjuan-Navarro, L.; Boughbina-Portolés, A.; Moliner-Martínez, Y.; von der Kammer, F.; Campíns-Falcó, P. Isolation of Carbon Black from Soils by Dispersion for Analysis: Quantitation and Characterization by Field Flow Fractionation Techniques. ACS Omega 2023, 8, 34795–34804. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, D.; Li, M.; Du, C.; Fu, S. Surface Modification of Carbon Black by Thiol-Ene Click Reaction for Improving Dispersibility in Aqueous Phase. J. Dispers. Sci. Technol. 2019, 40, 152–160. [Google Scholar] [CrossRef]
- Kiani, A.; Naddeo, M.; Santulli, F.; Volpe, V.; Mazzeo, M.; Acocella, M.R. Mechanochemical Functionalization of Oxidized Carbon Black with PLA. Molecules 2025, 30, 94. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. Carbon Black Analysis Using FT-IR with Germanium and Diamond ATR; Thermo Fisher Scientific Inc.: Munich, Germany, 2013. [Google Scholar]
- Zhang, R.; Jia, C.; Zhao, L.; Pan, J.; Niu, Q.; Liu, R. Characterization of the Interaction between Carbon Black and Three Important Antioxidant Proteins Using Multi Spectroscopy and Modeling Simulations. Chemosphere 2019, 222, 823–830. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Wichers, J.H.; van Amerongen, A.; Reis, N.M. Towards One-Step Quantitation of Prostate-Specific Antigen (PSA) in Microfluidic Devices: Feasibility of Optical Detection with Nanoparticle Labels. BioNanoSci. 2017, 7, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Rayev, M.B.; Ambrosov, I.V.; Briko, N.I. A Novel Method for the Serodiagnosis of Group A Streptococcal Antibodies. In Streptococci and the Host; Horaud, T., Bouvet, A., Leclercq, R., De Montclos, H., Sicard, M., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 1997; Volume 418, pp. 327–329. ISBN 978-1-4899-1827-7. [Google Scholar]
- Hou, F.; Sun, S.; Abdullah, S.W.; Tang, Y.; Li, X.; Guo, H. The Application of Nanoparticles in Point-of-Care Testing (POCT) Immunoassays. Anal. Methods 2023, 15, 2154–2180. [Google Scholar] [CrossRef]
- Nakane, P.K.; Kawaoi, A. Peroxidase-Labeled Antibody. A New Method of Conjugation. J. Histochem. Cytochem. 1974, 22, 1084–1091. [Google Scholar] [CrossRef]
- Degussa Carbon Black (N220). Focus Technology Co., Ltd. Made-in-China. Available online: https://dgm-carbonblack.en.made-in-china.com/product/qopnfskdokrh/china-degussa-carbon-black-n220-.html (accessed on 30 August 2025).
- Gold Au Nanoparticles. SkySpring Nanomaterials, Inc. Available online: https://www.ssnano.com/inc/sdetail/gold_au_nanoparticles__nanopowder_99_9___20nm/22407 (accessed on 30 August 2025).
- Yellow Latex Particles-COOH. Abvigen Inc. Available online: https://www.abvigen.com/content.asp?58136 (accessed on 30 August 2025).
- Maiia Diagnostics. Lateral Flow Test Using Carbon Black Nano-Strings. Available online: https://maiiadiagnostics.com/gammal/research/lateral_flow_test.pdf (accessed on 30 August 2025).
- Ali, F.; Neha, K.; Parveen, S. Current Regulatory Landscape of Nanomaterials and Nanomedicines: A Global Perspective. J. Drug Deliv. Sci. Technol. 2023, 80, 104118. [Google Scholar] [CrossRef]
- Di Ianni, E.; Jacobsen, N.R.; Vogel, U.B.; Møller, P. Systematic Review on Primary and Secondary Genotoxicity of Carbon Black Nanoparticles in Mammalian Cells and Animals. Mutat. Res. /Rev. Mutat. Res. 2022, 790, 108441. [Google Scholar] [CrossRef]
- Rodolpho, J.M.d.A.; Godoy, K.F.d.; Brassolatti, P.; Fragelli, B.D.d.L.; Camillo, L.; Castro, C.A.d.; Assis, M.; Speglich, C.; Longo, E.; Anibal, F.d.F. Carbon Black CB-EDA Nanoparticles in Macrophages: Changes in the Oxidative Stress Pathway and in Apoptosis Signaling. Biomedicines 2023, 11, 1643. [Google Scholar] [CrossRef]
- Koike, E.; Kobayashi, T. Chemical and Biological Oxidative Effects of Carbon Black Nanoparticles. Chemosphere 2006, 65, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Lindner, K.; Ströbele, M.; Schlick, S.; Webering, S.; Jenckel, A.; Kopf, J.; Danov, O.; Sewald, K.; Buj, C.; Creutzenberg, O.; et al. Biological Effects of Carbon Black Nanoparticles Are Changed by Surface Coating with Polycyclic Aromatic Hydrocarbons. Part. Fibre Toxicol. 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed]

| Functionalization Method | Advantages | Disadvantages |
|---|---|---|
| Physical adsorption |
|
|
| Covalent attachment by glutaraldehyde | The functional activity of the conjugates has been demonstrated to be maintained for 10 years or more |
|
| Covalent attachment by a Silane | The diversity of silanes allows the choice of the type of chemical bond for surface functionalization of CBNPs according to analytical purposes. |
|
| No | Assay Format | Scope | Target | Reproducibility of the Diagnostic Reagent Synthesis Method | Stability During Long-Term Storage of the Diagnostic Reagent Synthesis Method | Ref. |
|---|---|---|---|---|---|---|
| 1 | Dot-immuno assay | Food quality control | Soybean Kunitz-type trypsin inhibitor, human serum albumin mouse immunoglobulin isotyping | N/S in manuscript | N/S in manuscript | [10] |
| 2 | LFIA | Food quality control | Fungal alpha-amylase | N/S in manuscript | N/S in manuscript | [43] |
| 3 | LFIA | Medicine | Schistosoma circulating cathodic antigen | N/S in manuscript | Remain stable and do not lose functional stability over the course of a year. Numerical values unspecified. | [44] |
| 4 | LFIA | Medicine | Sulfamethazine | N/S in manuscript | Retains properties for 6 months in a test strip in a vacuum-sealed bag at room temperature | [45] |
| 5 | LFIA | Medicine | IgE | N/S in manuscript | N/S in manuscript | [46] |
| 6 | LFIA | Food quality control | Verotoxin-Producing Escherichia coli | N/S in manuscript | N/S in manuscript | [47] |
| 7 | LFIA | Medicine | N/S in manuscript | N/S in manuscript | [48] | |
| 8 | LFIA | Medicine | Erythropoetin | N/S in manuscript | N/S in manuscript | [49] |
| 9 | LFIA | Medicine | Thrombocytopenia syndrome virus | N/S in manuscript | N/S in manuscript | [50] |
| 10 | VFIA | Medicine | IgG against Spike-protein | Reproducibility confirmed on three batches | Loss of functional activity was noted after one month of storage as a suspension. Numerical values unspecified. | [51] |
| 11 | Dot-immunoassay | Medicine | Dengue virus NS1 glycoprotein | N/S in manuscript | N/S in manuscript | [12] |
| 12 | LFIA | Geno- typing | E. coli | N/S in manuscript | The signal level in the control line is shown to be maintained for 35 days as part of the test strip. | [11] |
| 13 | LFIA VFIA | Food quality control | Hazelnut protein and peanut protein | N/S in manuscript | N/S in manuscript | [52] |
| 14 | LFIA | Food quality control | Salbutamol | N/S in manuscript | It was noted that the diagnostic reagent retains functional activity in the composition for 4.5 months. Numerical values unspecified. | [4] |
| 15 | Microarray immunoassay with colorimetric detection | Veterinary/ Agriculture | DNA for six different mastitis pathogens | N/S in manuscript | N/S in manuscript | [53] |
| 16 | LFIA | Veterinary/ Agriculture | Shiga toxin-producing Escherichia coli virulence factors | N/S in manuscript | N/S in manuscript | [54] |
| 17 | LFIA | Food quality control | Forchlorfenuron | N/S in manuscript | N/S in manuscript | [55] |
| 18 | Multiplex LFIA | Food quality control | 22 β-lactams | N/S in manuscript | N/S in manuscript | [14] |
| 19 | Multiplex LFIA | Food quality control | Deoxynivalenol, T-2 toxin, zearalenone | N/S in manuscript | The diagnostic reagent in the test strip was stable and retained functional activity at room temperature for at least 6 months. | [15] |
| 20 | LFIA | Environmental safety | Methiocarb | N/S in manuscript | The stability test indicated the strips could be used at least 2 months without change in performance | [56] |
| 21 | LFIA | Food quality control | Thiabendazole | N/S in manuscript | The stability test indicated the strips could be used at least 2 months without change in performance | [57] |
| 22 | LFIA | Genotyping/Medicine | Genus Cronobacter | N/S in manuscript | The stability test indicated the strips could be used at least 6 months without change in performance | [58] |
| 23 | LFIA | Medicine | IgE | N/S in manuscript | N/S in manuscript | [59] |
| 24 | LFIA | Genotyping | DNA (PCR-product) | N/S in manuscript | N/S in manuscript | [60] |
| 25 | LFIA | Genotyping | DNA | N/S in manuscript | N/S in manuscript | [61] |
| 26 | LFIA | Food quality control | Carbaryl | N/S in manuscript | The stability test indicated the strips could be used at least 2 months without change in performance | [62] |
| 27 | Dot-immunoassay in biochip-format | Medicine | Histamine | N/S in manuscript | N/S in manuscript | [35] |
| 28 | Antibody Microarray assay | Food quality control | Food pathogens | N/S in manuscript | N/S in manuscript | [63] |
| 29 | LFIA | Food quality control | Adulteration of cow’s milk with buffalo’s milk | N/S in manuscript | It was noted that the diagnostic reagent retains functional activity for at least a year. Numerical values unspecified. | [64] |
| 30 | LFIA | Veterinary/ Agriculture | Mycoplasma bovi | N/S in manuscript | N/S in manuscript | [65] |
| 31 | LFIA | Genotyping | DNA (PCR-product) | N/S in manuscript | N/S in manuscript | [66] |
| 32 | LFIA | Medicine | Prostate specific antigen | N/S in manuscript | N/S in manuscript | [91] |
| 33 | LFIA | Environmental safety | Chlorpyrifos | N/S in manuscript | N/S in manuscript | [67] |
| 34 | LFIA VFIA Dot-imunoassay | Medicine | Human chorionic gonadotropin, Immunoglobullin, HIV protein | N/S in manuscript | The stability test indicated the diagnostic reagent could be used at least 10 years without change in performance | [16] |
| 35 | LFIA | Medicine | Human chorionic gonadotropin | N/S in manuscript | N/S in manuscript | [69] |
| 36 | LFIA | Medicine | Extracellular vesicles | N/S in manuscript | N/S in manuscript | [70] |
| 37 | Dot-imunoassay | Medicine | IgE | Reproducibility confirmed on three batches | The stability test indicated the diagnostic reagent could be used at least 30 days without change in performance | [71] |
| 38 | Dot-imunoassay in a microarray format | Medicine | Cancer biomarkers | N/S in manuscript | N/S in manuscript | [72] |
| 39 | LFIA | Medicine | DNA (amplicon) | N/S in manuscript | N/S in manuscript | [73] |
| 40 | Dot-imunoassay | Medicine | Group A streptococcal antibodies | N/S in manuscript | N/S in manuscript | [92] |
| Influence | Type of Nanoparticles for Comparison | Numerical Indicators of Effect | Ref. |
|---|---|---|---|
| Carbon black had a low detection limit of 0.01 μg/mL in comparison to 0.1 μg/mL, 1 μg/mL and 1 mg/mL for silver-coated gold nanoparticles, gold nanoparticles and polystyrene beads | [12] | |
| LOD for visual detection of as low as 2.2 × 10−2 pg μL−1 for CBNPs and 8.4 × 10−2 pg μL−1 for AuNPs | [11] | |
| Analytical performance improvement |
| CBNPs provided a higher sensitivity with 2-fold lower cut-off values than that of colloidal gold labels | [14] |
| The visual limit of detection for ACNP-LFAs in buffer was 8-fold better than GNPs and 2-fold better than QDs. | [15] | |
| The detection limit was for b-galactosidase 0.3 amol and for horseradish peroxidase 5 amol enzyme/microplate well. While the detection limit for carbon black was decreased to 0.02 amol /mm2 membrane | [59] | |
| Analytical performance decreased |
| For the nanogold-polyaniline-nanogold microspheres and colloidal carbon, the limit of detection was 10 µg kg−1 in meat samples vs. 5.0 µg kg−1 for the colloidal gold | [4] |
| LOD for CBNPs (9.25 × 106 EVs/μL) significantly lower in comparison with MNPs (1.05 × 106 EVs/μL). At the same time, CBNPs are inferior in sensitivity for gold NPs (LOD = 4.55 × 106 EVs/μL) | [70] | |
| No effect |
| The limits of detection were between 6.9 and 10.4 ng of PCR product for all three labels | [60] |
| The LOD for CBNPs for were comparable with LOD for colloidal gold and fluorescent dye | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitina, M.; Devyatov, S.; Rayev, M. Carbon Black Nanoparticles in Non-Instrumental Immunoassays Development for Diagnostic Applications. C 2025, 11, 79. https://doi.org/10.3390/c11040079
Nikitina M, Devyatov S, Rayev M. Carbon Black Nanoparticles in Non-Instrumental Immunoassays Development for Diagnostic Applications. C. 2025; 11(4):79. https://doi.org/10.3390/c11040079
Chicago/Turabian StyleNikitina, Maria, Stepan Devyatov, and Mikhail Rayev. 2025. "Carbon Black Nanoparticles in Non-Instrumental Immunoassays Development for Diagnostic Applications" C 11, no. 4: 79. https://doi.org/10.3390/c11040079
APA StyleNikitina, M., Devyatov, S., & Rayev, M. (2025). Carbon Black Nanoparticles in Non-Instrumental Immunoassays Development for Diagnostic Applications. C, 11(4), 79. https://doi.org/10.3390/c11040079








