Comparative Study of Graphite Exfoliation Techniques Using Nafion as a Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- spectral graphite rods of grade EC-22 (length 19.9 cm, diameter 0.6 cm); density 1.65 g/cm3, porosity 25%, electrical resistivity ≤ 20 µOhm [37];
- -
- graphite foil made of thermally expanded graphite from Graflex®(Graflex, Podolsk, Russia), density ≤ 0.05 g/cm3;
2.2. Methods
2.2.1. UV-Vis
2.2.2. DTA
2.2.3. XRD
2.2.4. DLS
2.2.5. XPS
2.2.6. TEM and Electron Diffraction
2.3. Calculations and Data Processing
2.4. Technologies of Few-Layer Graphene
2.4.1. Ultrasound-Assisted Liquid-Phase Exfoliation (High-Power Ultrasound)
- -
- number of graphene layers;
- -
- particle size;
- -
- monodispersity;
- -
- stability of the dispersion;
- -
- overall process efficiency, including product yield and exfoliation duration.
2.4.2. Electrochemical Exfoliation Followed by Liquid-Phase Exfoliation Using Mild US Treatment
3. Results
3.1. UV-Vis Spectroscopy and Sedimentation Curves
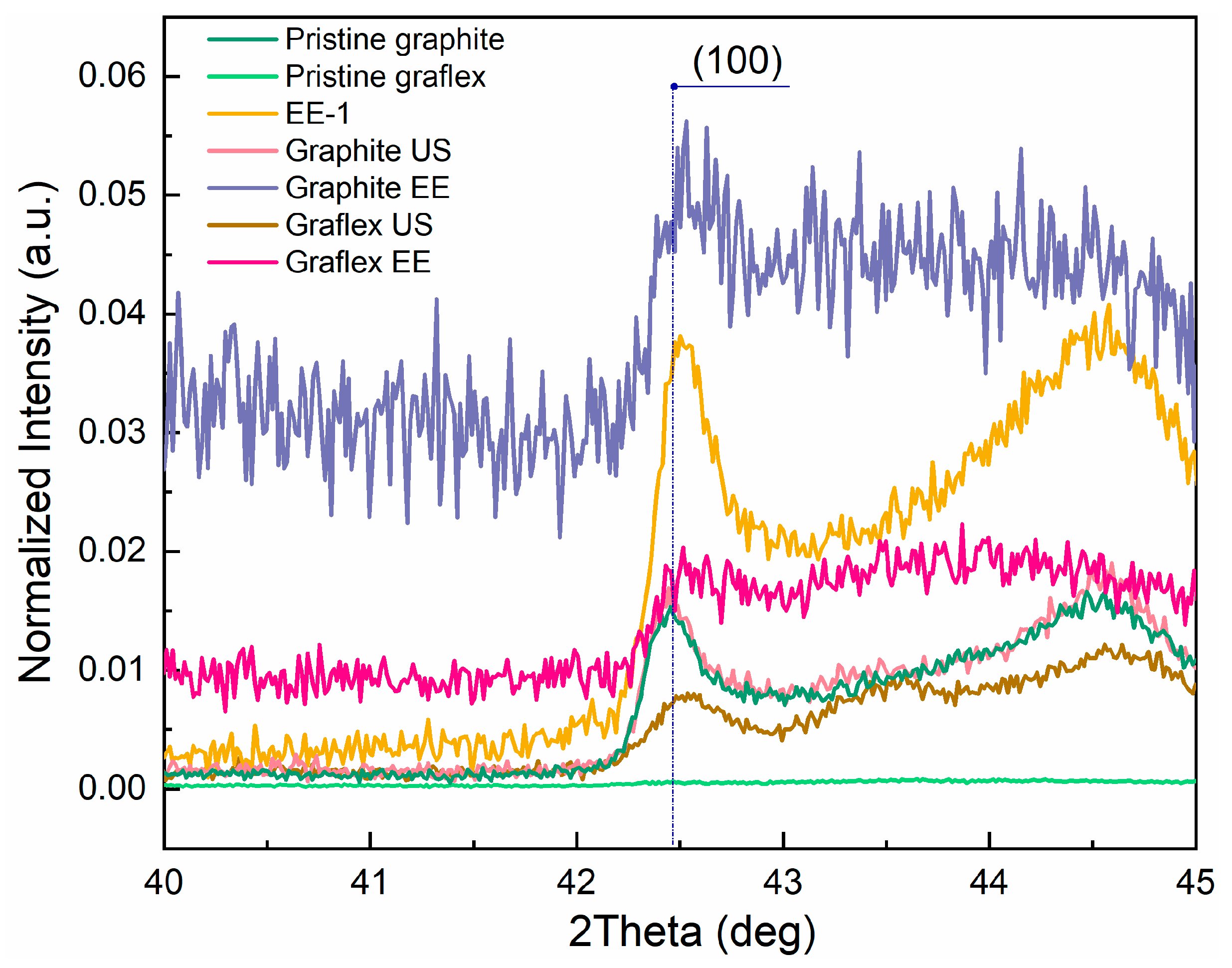
3.2. TEM, ED, and XRD
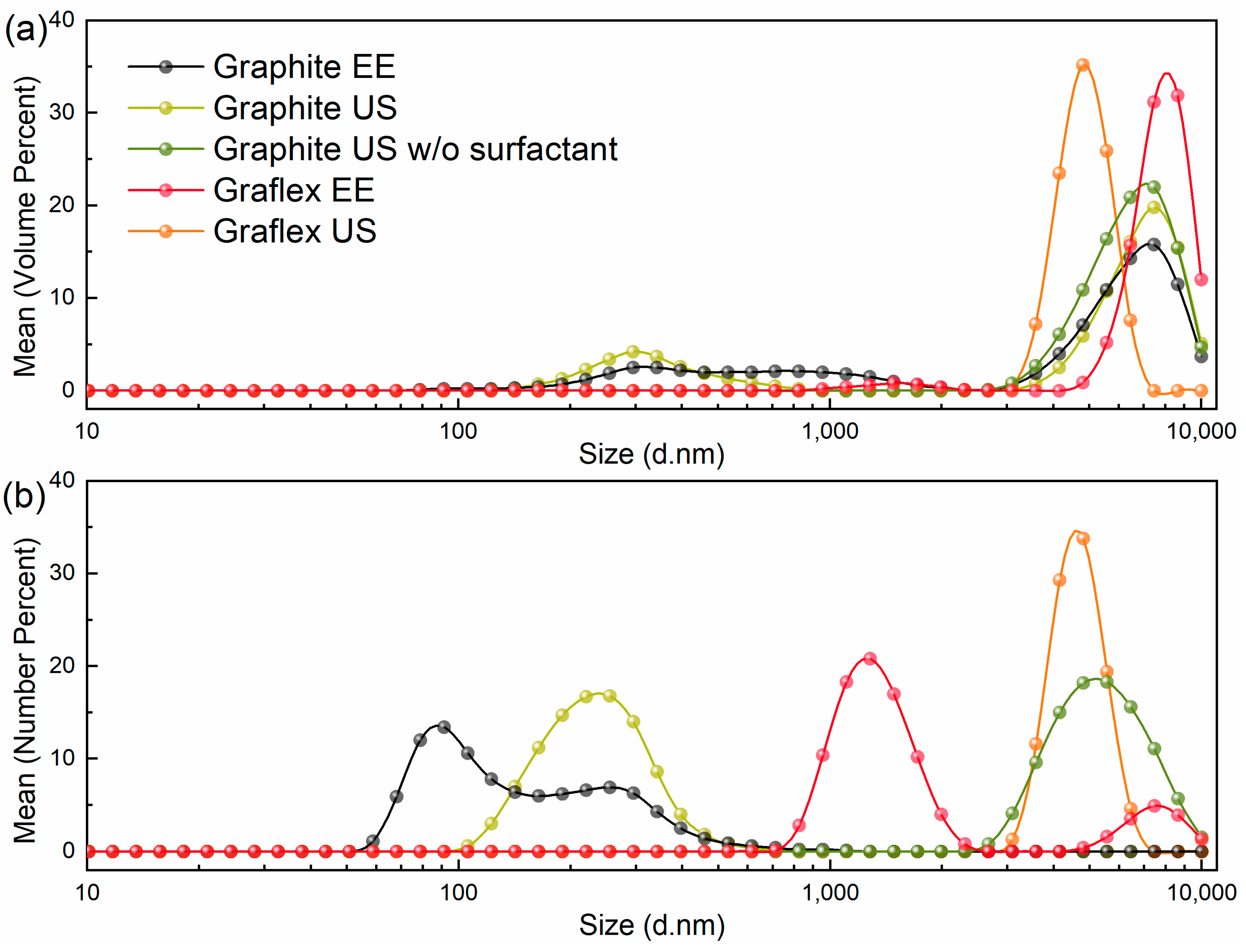
3.3. DLS
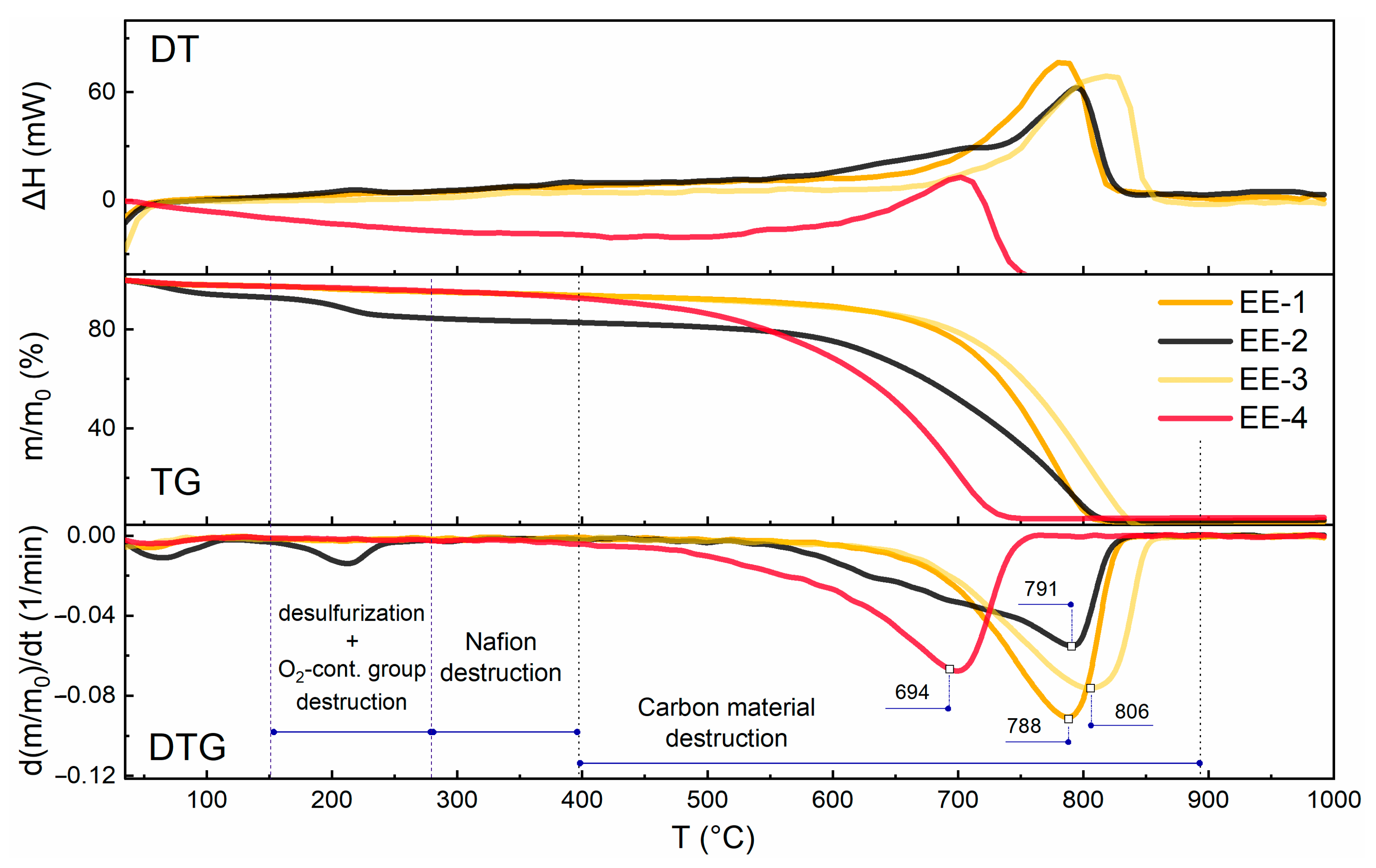
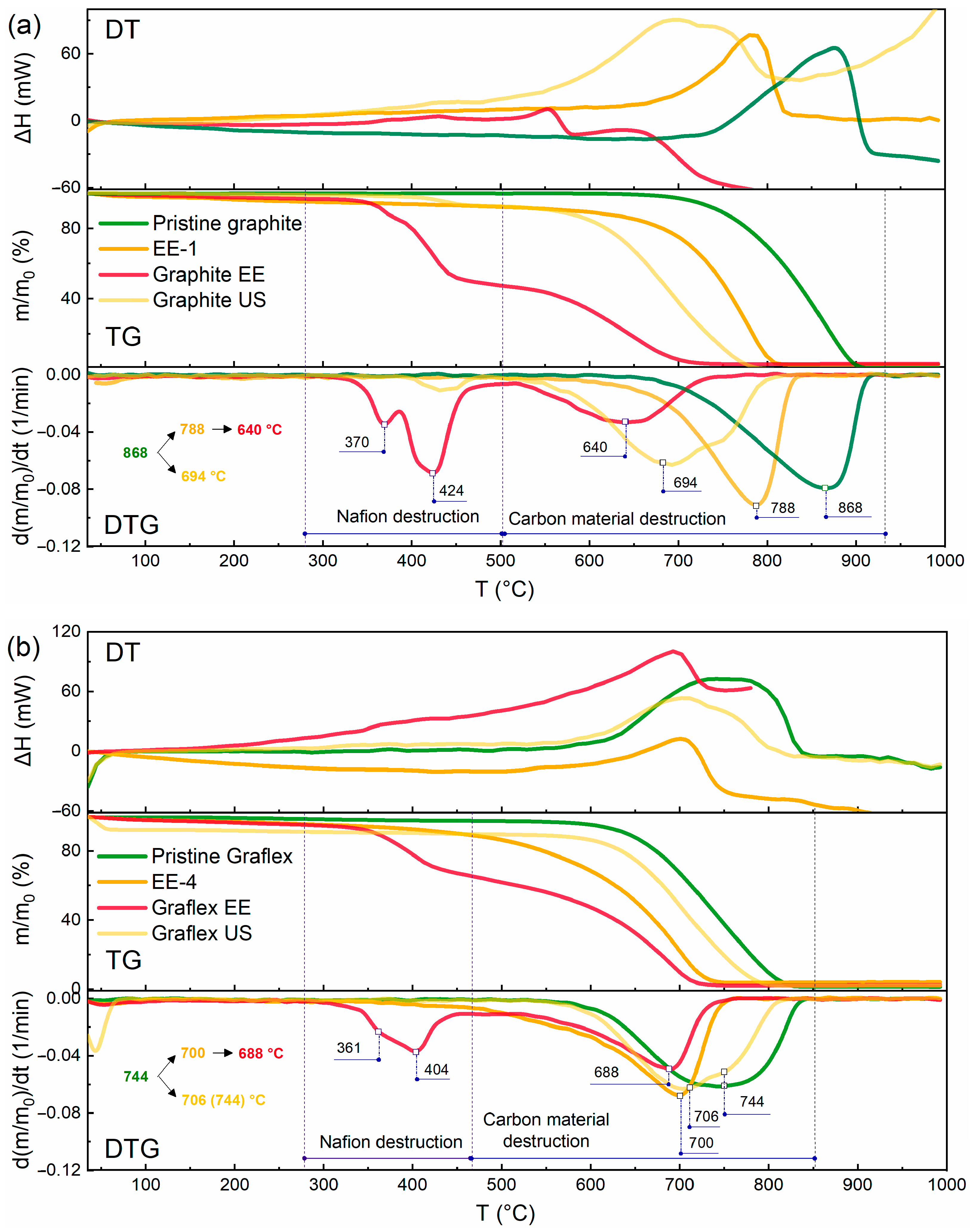
3.4. DTA
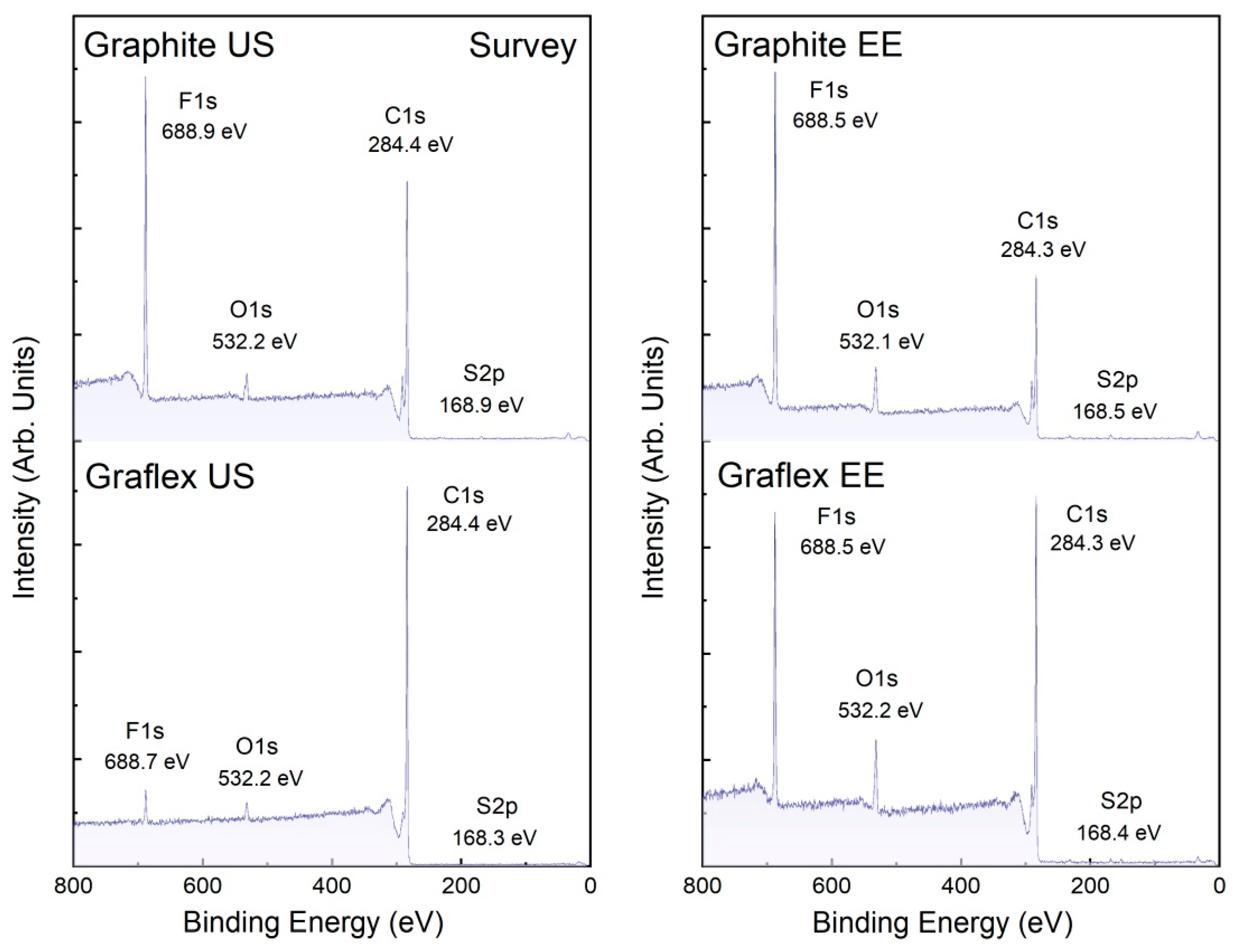
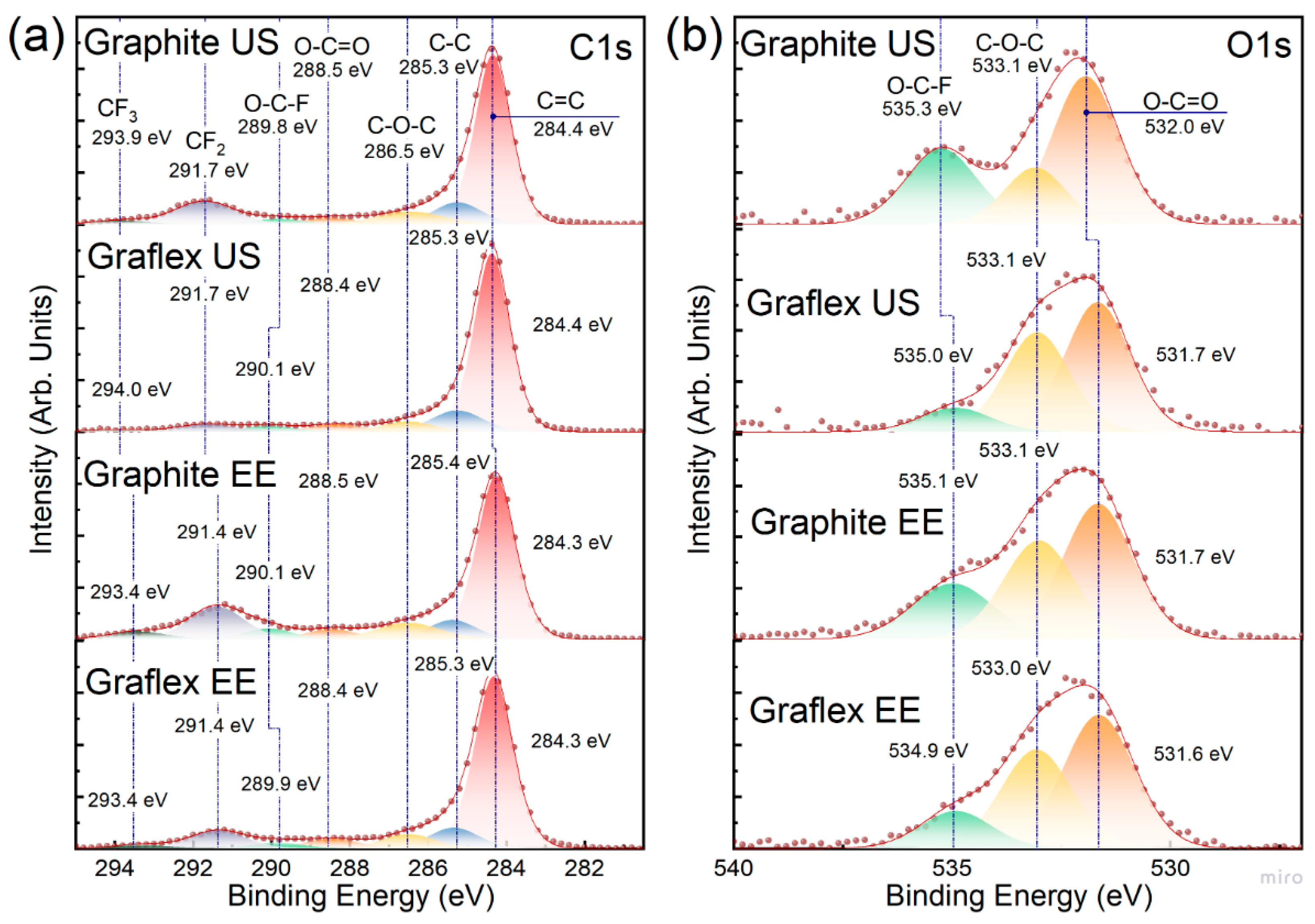
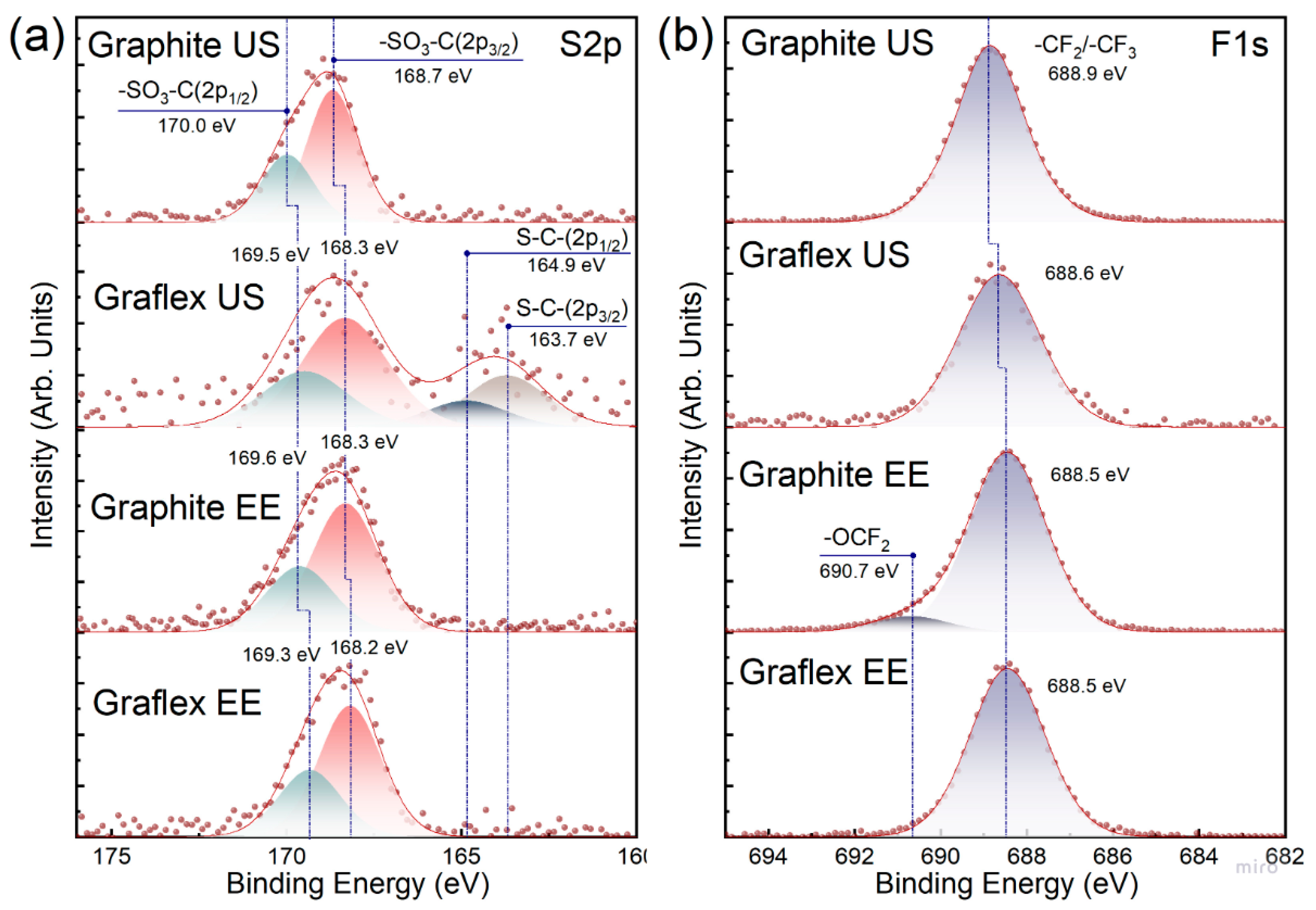
3.5. XPS
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction |
| TGA | thermogravimetric analysis |
| ED | electron diffraction |
| DLS | dynamic light scattering |
| EDX | energy-dispersive X-ray spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| PEM | proton exchange membrane |
| PEM WE | proton exchange membrane water electrolyzer |
| MEAs | membrane electrode assemblies |
| PTFE | polytetrafluoroethylene |
| PEMFC | proton exchange membrane fuel cells |
| rGO | reduced graphene oxide |
| GO | graphene oxide |
| TEM | transmission electron microscopy |
| AFM | atomic force microscopy |
| SEM | scanning electron microscopy |
| US | ultrasound |
| CV | current-voltage |
| TG | thermogravimetric |
| DT | differential thermal |
| CSR | coherent scattering region |
| EE | electrochemical exfoliation |
References
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Baig, N. Two-Dimensional Nanomaterials: A Critical Review of Recent Progress, Properties, Applications, and Future Directions. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107362. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, P.; Zhou, M.; Yu, H. Nafion-Endowed Graphene Super-Anticorrosion Performance. ACS Sustain. Chem. Eng. 2020, 8, 15344–15353. [Google Scholar] [CrossRef]
- Tang, G.; Hou, X.; Wang, Y.; Yan, Z.; Ren, T.; Ma, L.; Huang, X.; Wang, C. Hexagonal Boron Nitride/Polyaniline Nanocomposites for Anticorrosive Waterborne Epoxy Coatings. ACS Appl. Nano Mater. 2022, 5, 361–372. [Google Scholar] [CrossRef]
- Glebova, N.V.; Krasnova, A.O.; Nechitailov, A.A. Thermal degradation of nafion in the presence of nanostructured materials: Thermally expanded graphite, carbon black, and platinum. Russ. J. Appl. Chem. 2020, 93, 1034–1041. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal Conductivity and Structure of Non-Covalent Functionalized Graphene/Epoxy Composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. One-Pot Synthesis of Sulfur and Nitrogen Co-Functionalized Graphene Material Using Deep Eutectic Solvents for Supercapacitors. ChemSusChem 2019, 12, 3326–3335. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.-X.; Wu, D.-L.; Xia, W.; Jia, D.-Z. Interaction between Nitrogen and Sulfur in Co-Doped Graphene and Synergetic Effect in Supercapacitor. Sci. Rep. 2015, 5, 9591. [Google Scholar] [CrossRef]
- Pavko, L.; Gatalo, M.; Finšgar, M.; Ruiz-Zepeda, F.; Ehelebe, K.; Kaiser, P.; Geuß, M.; Đukić, T.; Surca, A.K.; Šala, M.; et al. Graphene-Derived Carbon Support Boosts Proton Exchange Membrane Fuel Cell Catalyst Stability. ACS Catal. 2022, 12, 9540–9548. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ishitobi, H.; Nakagawa, N. Different Functional Groups Cross-Linked Graphene Oxide Membranes for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2024, 85, 586–597. [Google Scholar] [CrossRef]
- Samantaray, S.; Mohanty, D.; Satpathy, S.K.; Hung, I.-M. Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules 2024, 29, 2937. [Google Scholar] [CrossRef]
- Pham, N.N.T.; Nguyen, V.K.T.; Guo, H.; Lee, S.G. Influence of Phosphorus-Doped Bilayer Graphene Configuration on the Oxygen Reduction Reaction in Acidic Solution. Carbon 2023, 210, 118012. [Google Scholar] [CrossRef]
- Strativnov, E.; Khovavko, A.; Guachao, N. Obtaining of Globular Graphene Based on Thermally Expanded Graphite. Appl. Nanosci. 2022, 12, 2791–2811. [Google Scholar] [CrossRef]
- Vacacela Gomez, C.; Tene, T.; Guevara, M.; Tubon Usca, G.; Colcha, D.; Brito, H.; Molina, R.; Bellucci, S.; Tavolaro, A. Preparation of Few-Layer Graphene Dispersions from Hydrothermally Expanded Graphite. Appl. Sci. 2019, 9, 2539. [Google Scholar] [CrossRef]
- Li, M.; Yin, B.; Gao, C.; Guo, J.; Zhao, C.; Jia, C.; Guo, X. Graphene: Preparation, Tailoring, and Modification. Exploration 2023, 3, 20210233. [Google Scholar] [CrossRef]
- Cheng, C.; Jia, P.; Xiao, L.; Geng, J. Tandem Chemical Modification/Mechanical Exfoliation of Graphite: Scalable Synthesis of High-Quality, Surface-Functionalized Graphene. Carbon 2019, 145, 668–676. [Google Scholar] [CrossRef]
- Barsukov, M.G.; Ritt, C.L.; Barsukov, I.V.; Syth, E.M.; Elimelech, M. Influence of Graphite Geography on the Yield of Mechanically Exfoliated Few-Layer Graphene. Carbon 2023, 208, 355–364. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Pang, S.; Englert, J.M.; Tsao, H.N.; Hernandez, Y.; Hirsch, A.; Feng, X.; Müllen, K. Extrinsic Corrugation-Assisted Mechanical Exfoliation of Monolayer Graphene. Adv. Mater. 2010, 22, 5374–5377. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.F.; Álvarez-Rubiera, E.; Villar-Rodil, S.; Martínez-Jódar, A.; Tascón, J.M.D.; Suárez-García, F.; Paredes, J.I. Chemically Tuning Graphene via Anodic Exfoliation for Enhanced Performance in Aqueous Zinc-Based Electrochemical Energy Storage Applications. Carbon 2024, 228, 119293. [Google Scholar] [CrossRef]
- Gürsu, H.; Gençten, M.; Şahin, Y. One-Step Electrochemical Preparation of Graphene-Coated Pencil Graphite Electrodes by Cyclic Voltammetry and Their Application in Vanadium Redox Batteries. Electrochim. Acta 2017, 243, 239–249. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Mariatti, M.; Chin, S.Y. Fabrication of Graphene by Electrochemical Intercalation Method and Performance of Graphene/PVA Composites as Stretchable Strain Sensor. Arab. J. Sci. Eng. 2020, 45, 7677–7689. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Eslami-Farsani, R.; Torabian, M.; Amousa, N. Recent Advances in One-Pot Functionalization of Graphene Using Electrochemical Exfoliation of Graphite: A Review Study. Synth. Met. 2020, 269, 116549. [Google Scholar] [CrossRef]
- Girish, S.; Tambe, P. Surfactant Assisted Exfoliation of High Purity Graphene in Aqueous Solution as a Nanofluid Using Kitchen Blender: Influence on Dispersion, Thermal Conductivity and Rheological Properties. Adv. Powder Technol. 2022, 33, 103767. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, S.O. Surfactant Mediated Liquid Phase Exfoliation of Graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef]
- Griffin, A.; Nisi, K.; Pepper, J.; Harvey, A.; Szydłowska, B.M.; Coleman, J.N.; Backes, C. Effect of Surfactant Choice and Concentration on the Dimensions and Yield of Liquid-Phase-Exfoliated Nanosheets. Chem. Mater. 2020, 32, 2852–2862. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-Visible Spectroscopy of Graphene Oxides. AIP Adv. 2012, 2, 032146. [Google Scholar] [CrossRef]
- Baggio, A.R.; Santos, M.S.C.; Souza, F.H.V.; Nunes, R.B.; Souza, P.E.N.; Báo, S.N.; Patrocinio, A.O.T.; Bahnemann, D.W.; Silva, L.P.; Sales, M.J.A.; et al. Quenching Effects of Graphene Oxides on the Fluorescence Emission and Reactive Oxygen Species Generation of Chloroaluminum Phthalocyanine. J. Phys. Chem. A 2018, 122, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Lee, D.-J.; Park, S.-S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Graphite Electrodes for Spectral Analysis. Available online: https://www.shj-carbon.com/high-purity-graphite/spectral-analysis-graphite-electrode.html (accessed on 18 August 2025).
- Kumar, V.; Alam, N.; Lee, D.-J.; Giese, U. Role of low surface area few layer graphene in enhancing mechanical properties of poly (1,4-cis-isoprene) rubber nanocomposites. Rubber Chem. Technol. 2020, 93, 172–182. [Google Scholar] [CrossRef]
- Galimberti, M.; Kumar, V.; Coombs, M.; Cipolletti, V.; Agnelli, S.; Pandini, S.; Conzatti, L. Filler networking of a nanographite with a high shape anisotropy and synergism with carbon black in poly(1,4-cis-isoprene)–based nanocomposites. Rubber Chem. Technol. 2014, 87, 197–218. [Google Scholar] [CrossRef]
- Mauro, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Chemically Reduced Graphite Oxide with Improved Shape Anisotropy. J. Phys. Chem. C 2012, 116, 24809–24813. [Google Scholar] [CrossRef]
- Backes, C.; Paton, K.R.; Hanlon, D.; Yuan, S.; Katsnelson, M.I.; Houston, J.; Smith, R.J.; McCloskey, D.; Donegan, J.F.; Coleman, J.N. Spectroscopic Metrics Allow in Situ Measurement of Mean Size and Thickness of Liquid-Exfoliated Few-Layer Graphene Nanosheets. Nanoscale 2016, 8, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lu, Y.; Somers, L.A.; Johnson, A.T.C. High Yield Preparation of Macroscopic Graphene Oxide Membranes. J. Am. Chem. Soc. 2009, 131, 898–899. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Benalcázar Palacios, F.; Morocho Barrionuevo, T.P.; Vacacela Gomez, C.; Bellucci, S. Optical Properties of Graphene Oxide. Front. Chem. 2023, 11, 1214072. [Google Scholar] [CrossRef]
- Xia, Z.; Bellani, V.; Sun, J.; Palermo, V. Electrochemical Exfoliation of Graphite in H2SO4, Li2SO4 and NaClO4 Solutions Monitored in Situ by Raman Microscopy and Spectroscopy. Faraday Discuss. 2021, 227, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Liu, R.; Niu, Y. Synthesis of High-Quality Graphene by Electrochemical Anodic and Cathodic Co-Exfoliation Method. Chem. Eng. J. 2023, 461, 141985. [Google Scholar] [CrossRef]
- Redox Tech. Persulfate. Available online: https://www.redox-tech.com/persulfate (accessed on 18 August 2025).
- Han, J.; Xin, J.; Zheng, X.; Kolditz, O.; Shao, H. Remediation of Trichloroethylene-Contaminated Groundwater by Three Modifier-Coated Microscale Zero-Valent Iron. Environ. Sci. Pollut. Res. 2016, 23, 14442–14450. [Google Scholar] [CrossRef]
- Alexander, C.M.; Goodisman, J. Size Histograms of Gold Nanoparticles Measured by Gravitational Sedimentation. J. Colloid Interface Sci. 2014, 418, 103–112. [Google Scholar] [CrossRef]
- Shi, G.; Michelmore, A.; Jin, J.; Li, L.H.; Chen, Y.; Wang, L.; Yu, H.; Wallace, G.; Gambhir, S.; Zhu, S.; et al. Advancement in Liquid Exfoliation of Graphite through Simultaneously Oxidizing and Ultrasonicating. J. Mater. Chem. A 2014, 2, 20382–20392. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, S.; Kim, Y.-I.; Kim, J.-G.; Min, B.-K.; Lee, K.-I.; Park, Y.; John, P. The Seeded Growth of Graphene. Sci. Rep. 2014, 4, 5682. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Obergfell, D.; Roth, S.; Girit, C.; Zettl, A. On the Roughness of Single- and Bi-Layer Graphene Membranes. Solid State Commun. 2007, 143, 101–109. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Liu, G.; Li, J.; Tian, H.; Li, J.; Ma, Y. Scalable Self-Propagating High-Temperature Synthesis of Graphene for Supercapacitors with Superior Power Density and Cyclic Stability. Adv. Mater. 2017, 29, 1604690. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Kong, B.-S.; Yang, S.B.; Jung, H.-T. Preparation of Graphene Relying on Porphyrin Exfoliation of Graphite. Chem. Commun. 2010, 46, 5091. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The Structure of Suspended Graphene Sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The Proton Conductivity and Mechanical Properties of Nafion®/ ZrP Nanocomposite Membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef]
- Xu, G.; Wei, Z.; Li, S.; Li, J.; Yang, Z.; Grigoriev, S.A. In-Situ Sulfonation of Targeted Silica-Filled Nafion for High-Temperature PEM Fuel Cell Application. Int. J. Hydrogen Energy 2019, 44, 29711–29716. [Google Scholar] [CrossRef]
- Li, K.; Ye, G.; Pan, J.; Zhang, H.; Pan, M. Self-Assembled Nafion®/Metal Oxide Nanoparticles Hybrid Proton Exchange Membranes. J. Membr. Sci. 2010, 347, 26–31. [Google Scholar] [CrossRef]
- Kim, H.J.; Talukdar, K.; Choi, S.-J. Tuning of Nafion® by HKUST-1 as Coordination Network to Enhance Proton Conductivity for Fuel Cell Applications. J. Nanopart. Res. 2016, 18, 47. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Green and Facile Production of High-Quality Graphene from Graphite by the Combination of Hydroxyl Radicals and Electrical Exfoliation in Different Electrolyte Systems. RSC Adv. 2019, 9, 3693–3703. [Google Scholar] [CrossRef]
- Karakoti, M.; Jangra, R.; Pandey, S.; Dhapola, P.S.; Dhali, S.; Mahendia, S.; Singh, P.K.; Sahoo, N.G. Binder-Free Reduced Graphene Oxide as Electrode Material for Efficient Supercapacitor with Aqueous and Polymer Electrolytes. High Perform. Polym. 2020, 32, 175–182. [Google Scholar] [CrossRef]
- Sadek, R.; Sharawi, M.S.; Dubois, C.; Tantawy, H.; Chaouki, J. Superior Quality Chemically Reduced Graphene Oxide for High Performance EMI Shielding Materials. RSC Adv. 2022, 12, 22608–22622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Huang, L.; Sun, L.-C.; Xie, S.-Y.; Xu, G.-L.; Chen, S.-R.; Xu, Y.-F.; Li, J.-T.; Chou, S.-L.; Dou, S.-X.; et al. Facile Synthesis of a Interleaved Expanded Graphite-Embedded Sulphur Nanocomposite as Cathode of Li–S Batteries with Excellent Lithium Storage Performance. J. Mater. Chem. 2012, 22, 4744. [Google Scholar] [CrossRef]
- Vittore, A.; Acocella, M.R.; Guerra, G. Graphite Functionalization by Ball Milling with Sulfur. SN Appl. Sci. 2019, 1, 169. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Shen, L.; Bao, N. Revisiting the Oxidation of Graphite: Reaction Mechanism, Chemical Stability, and Structure Self-Regulation. ACS Omega 2020, 5, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Hwang, H.; Park, J.; Hwang, H.; Shul, Y.-G. Temperature-Dependent Performance of the Polymer Electrolyte Membrane Fuel Cell Using Short-Side-Chain Perfluorosulfonic Acid Ionomer. Int. J. Hydrogen Energy 2014, 39, 11690–11699. [Google Scholar] [CrossRef]
- Likhomanov, V.S.; Primachenko, O.N.; Ivanchev, S.S. Thermodynamic Properties of Water in Perfluorinated Membranes of Nafion and Aquivion Types, Prepared by Emulsion Polymerization. Russ. J. Appl. Chem. 2014, 87, 1314–1318. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; Hong, W.H.; Choi, Y.S.; Lee, H. Influence of Morphology on the Transport Properties of Perfluorosulfonate Ionomers/Polypyrrole Composite Membrane. Macromolecules 2005, 38, 2289–2295. [Google Scholar] [CrossRef]
- Düngen, P.; Schlögl, R.; Heumann, S. Non-Linear Thermogravimetric Mass Spectrometry of Carbon Materials Providing Direct Speciation Separation of Oxygen Functional Groups. Carbon 2018, 130, 614–622. [Google Scholar] [CrossRef]
- Friedel Ortega, K.; Arrigo, R.; Frank, B.; Schlögl, R.; Trunschke, A. Acid–Base Properties of N-Doped Carbon Nanotubes: A Combined Temperature-Programmed Desorption, X-Ray Photoelectron Spectroscopy, and 2-Propanol Reaction Investigation. Chem. Mater. 2016, 28, 6826–6839. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the Identification of Sulfides and Polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
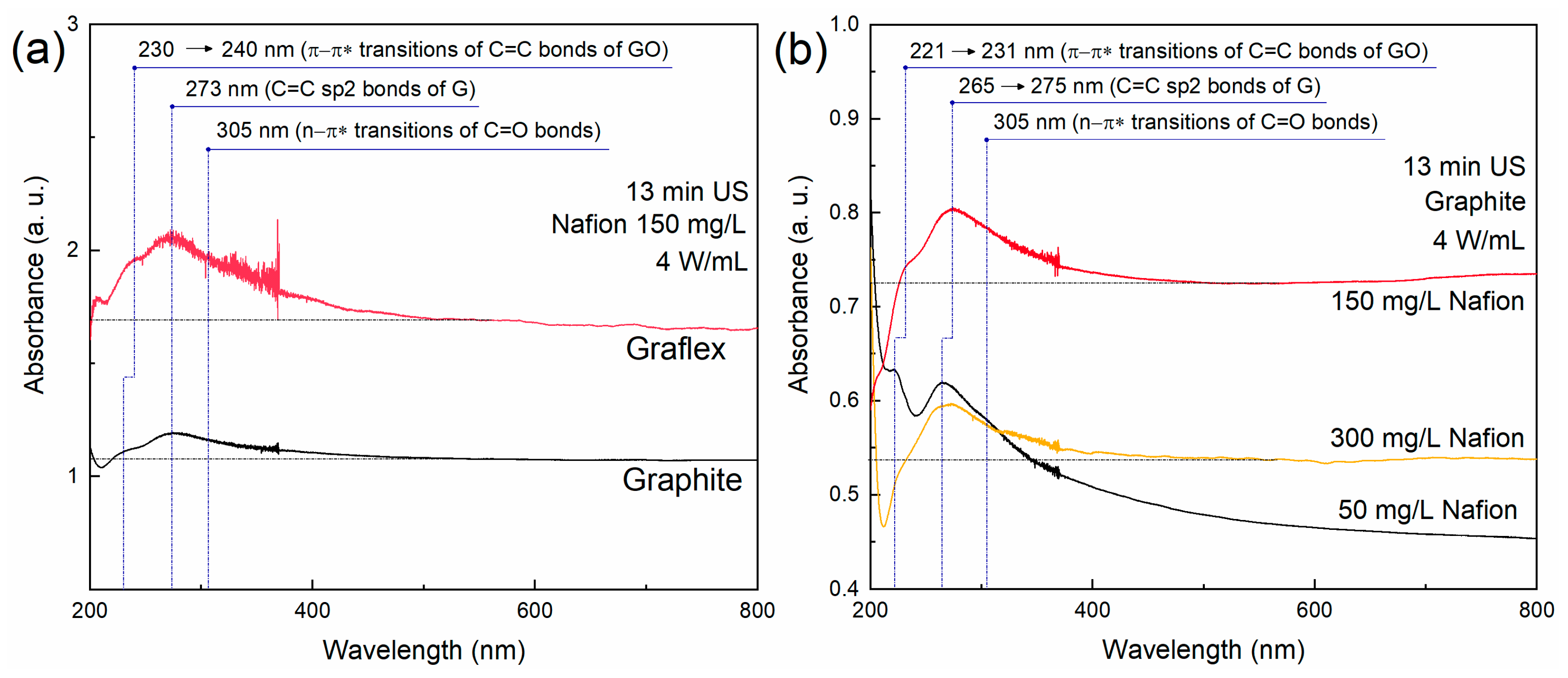
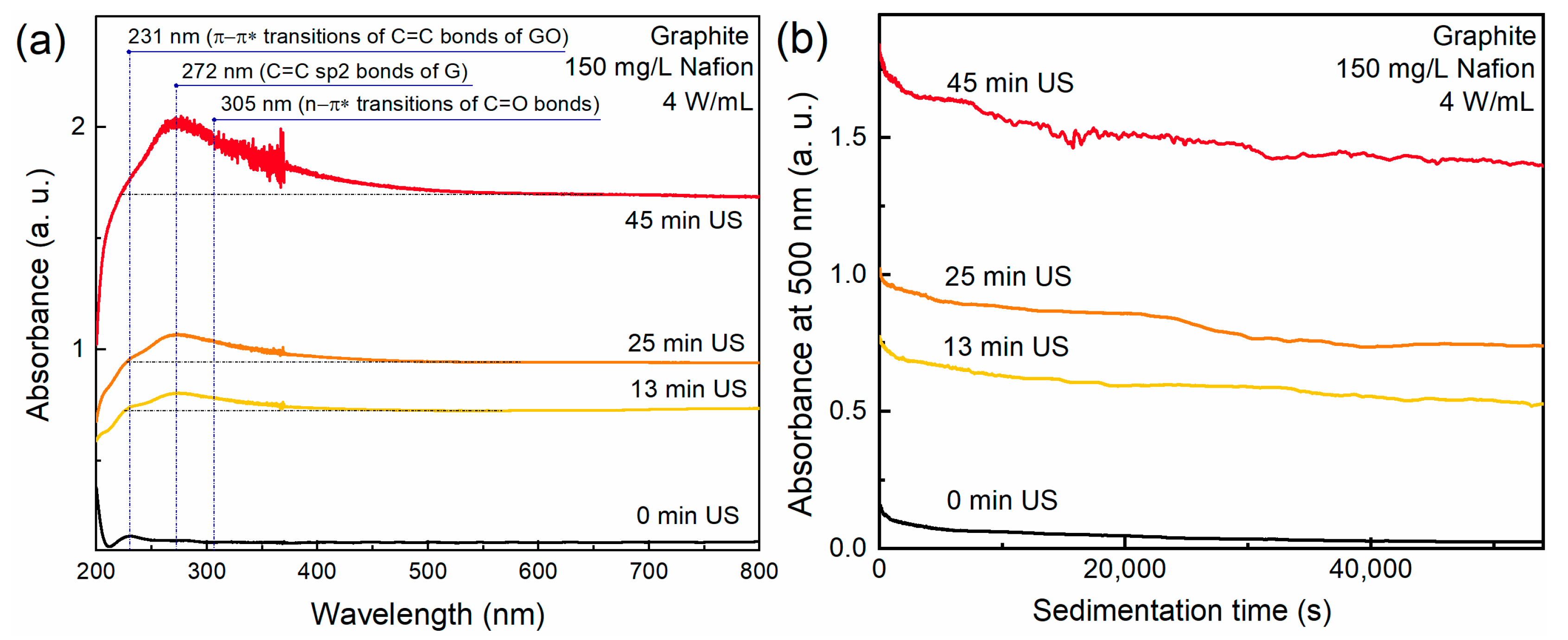
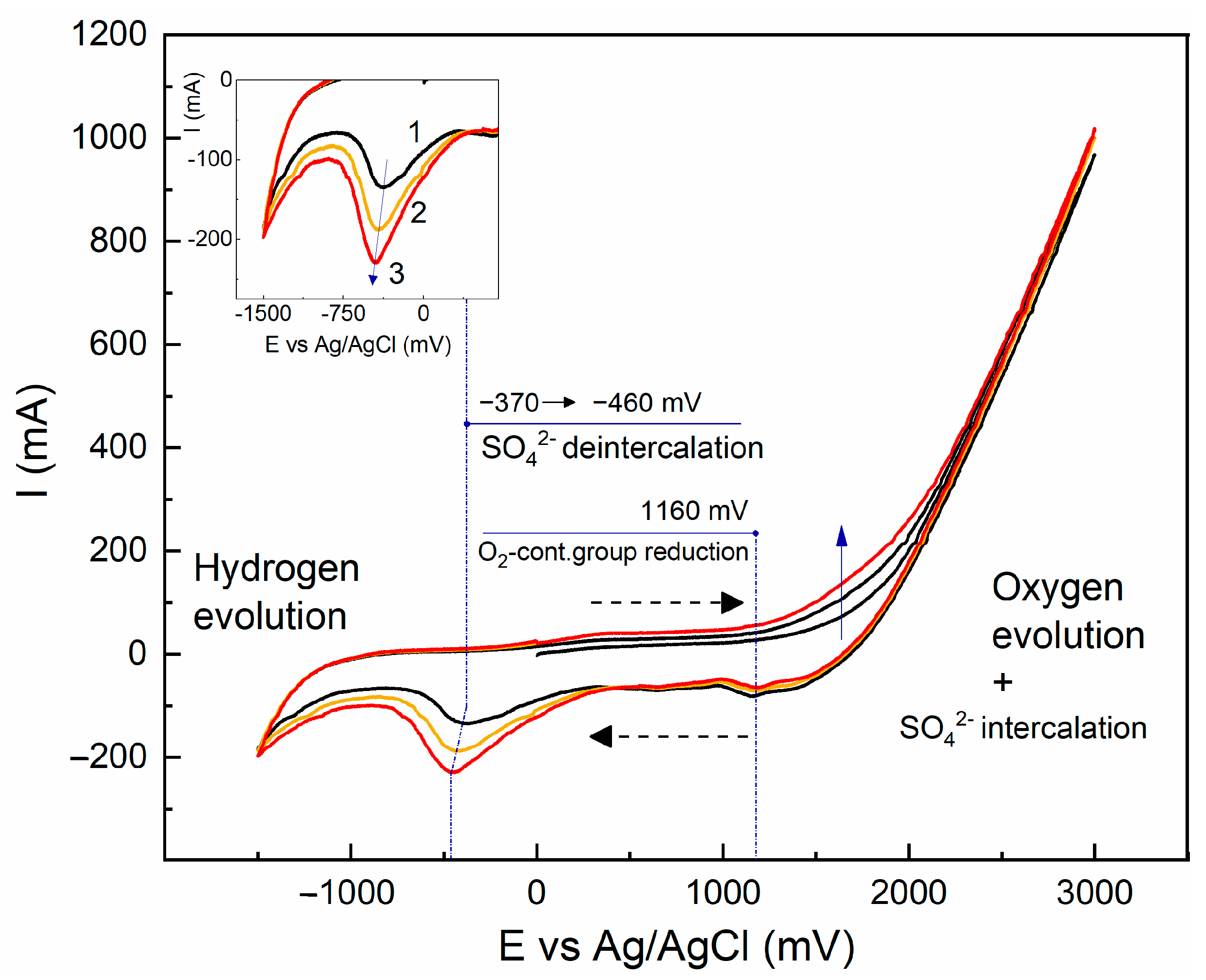

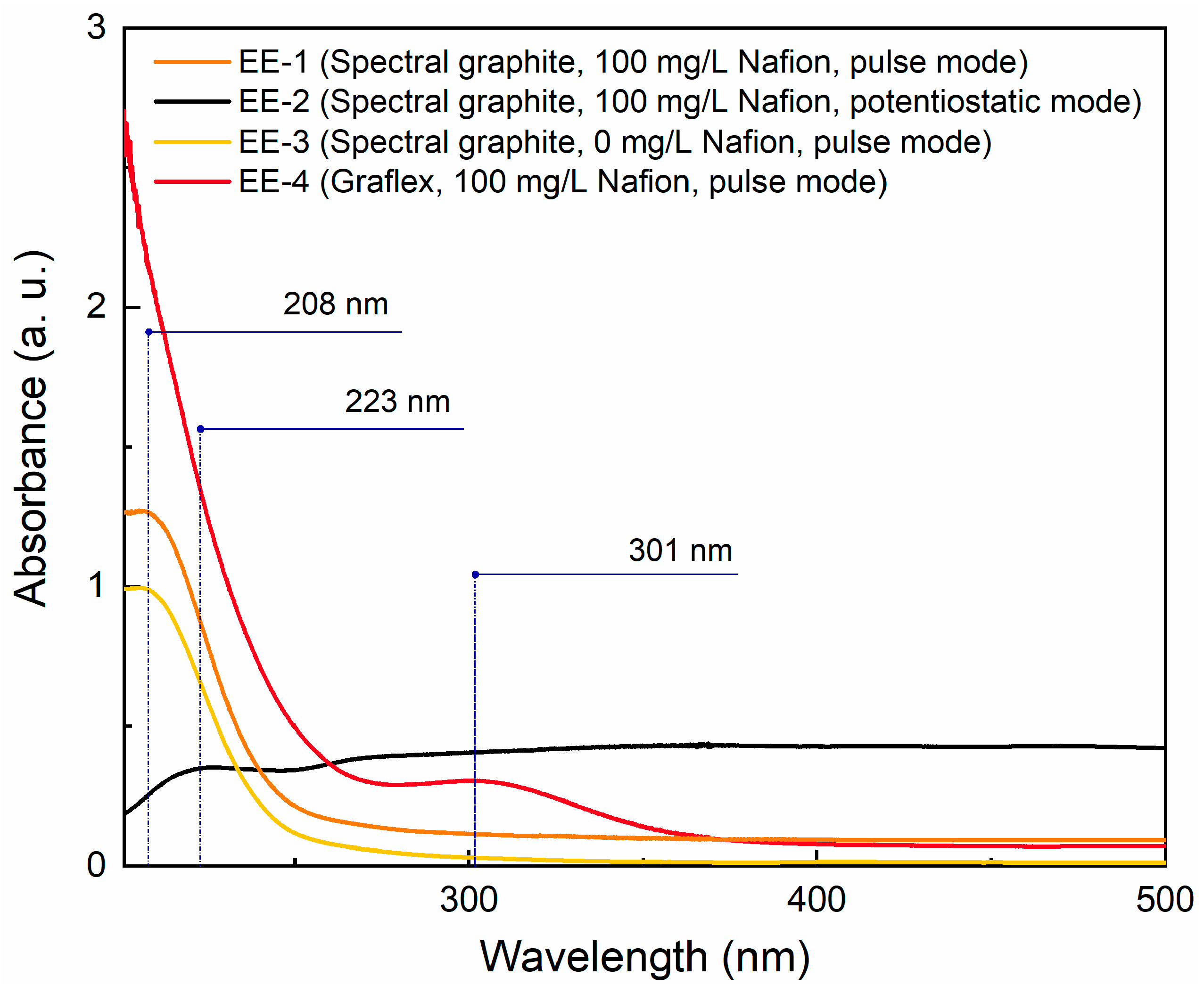
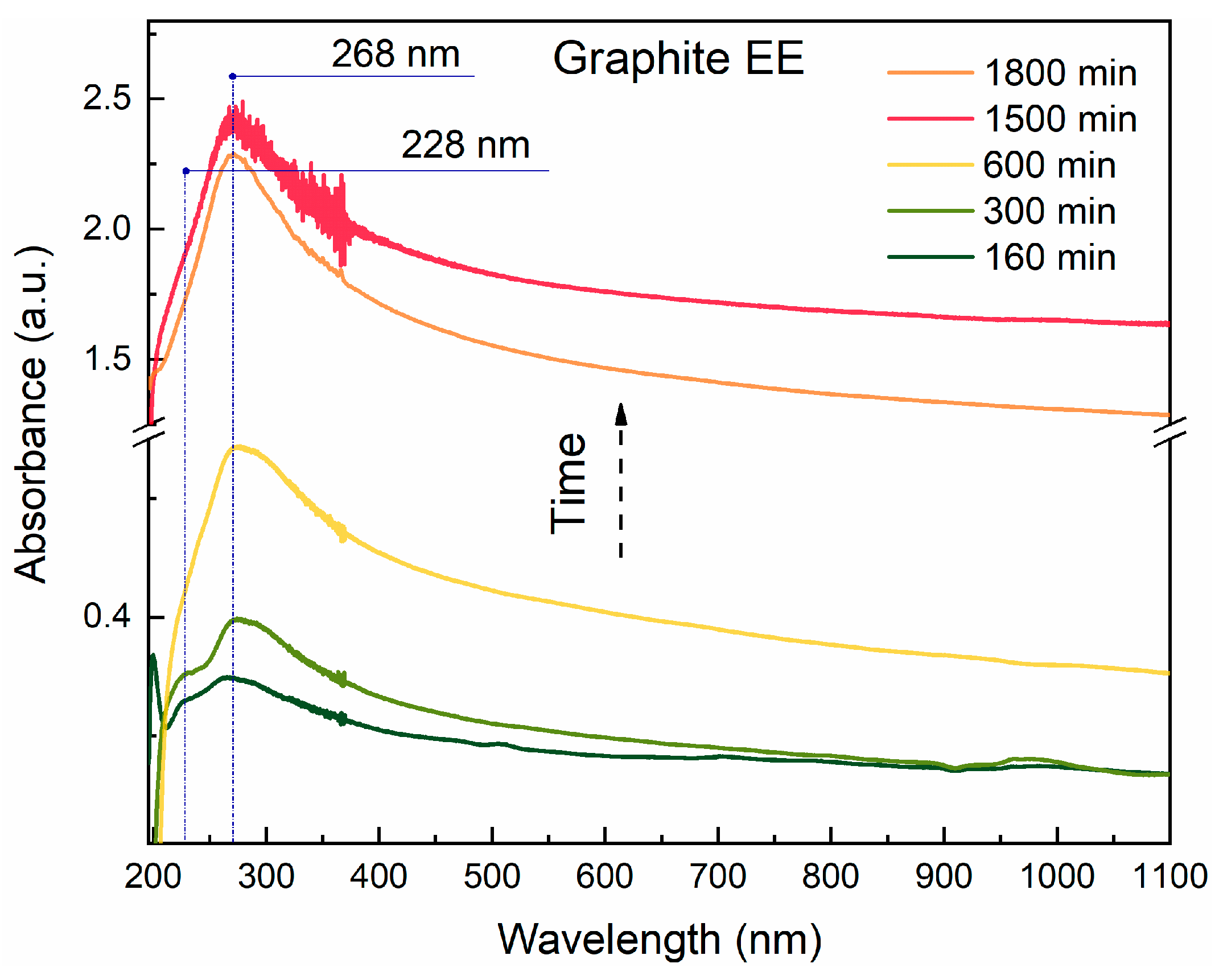
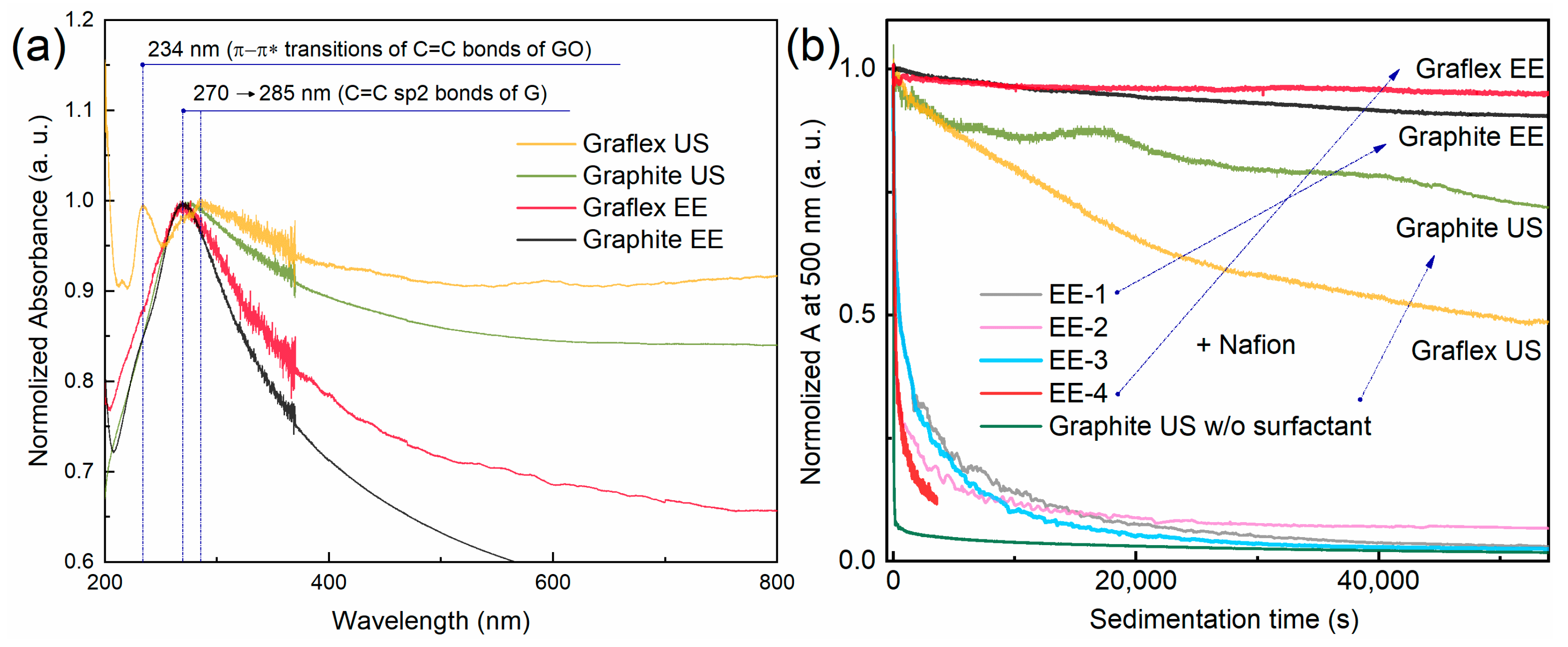
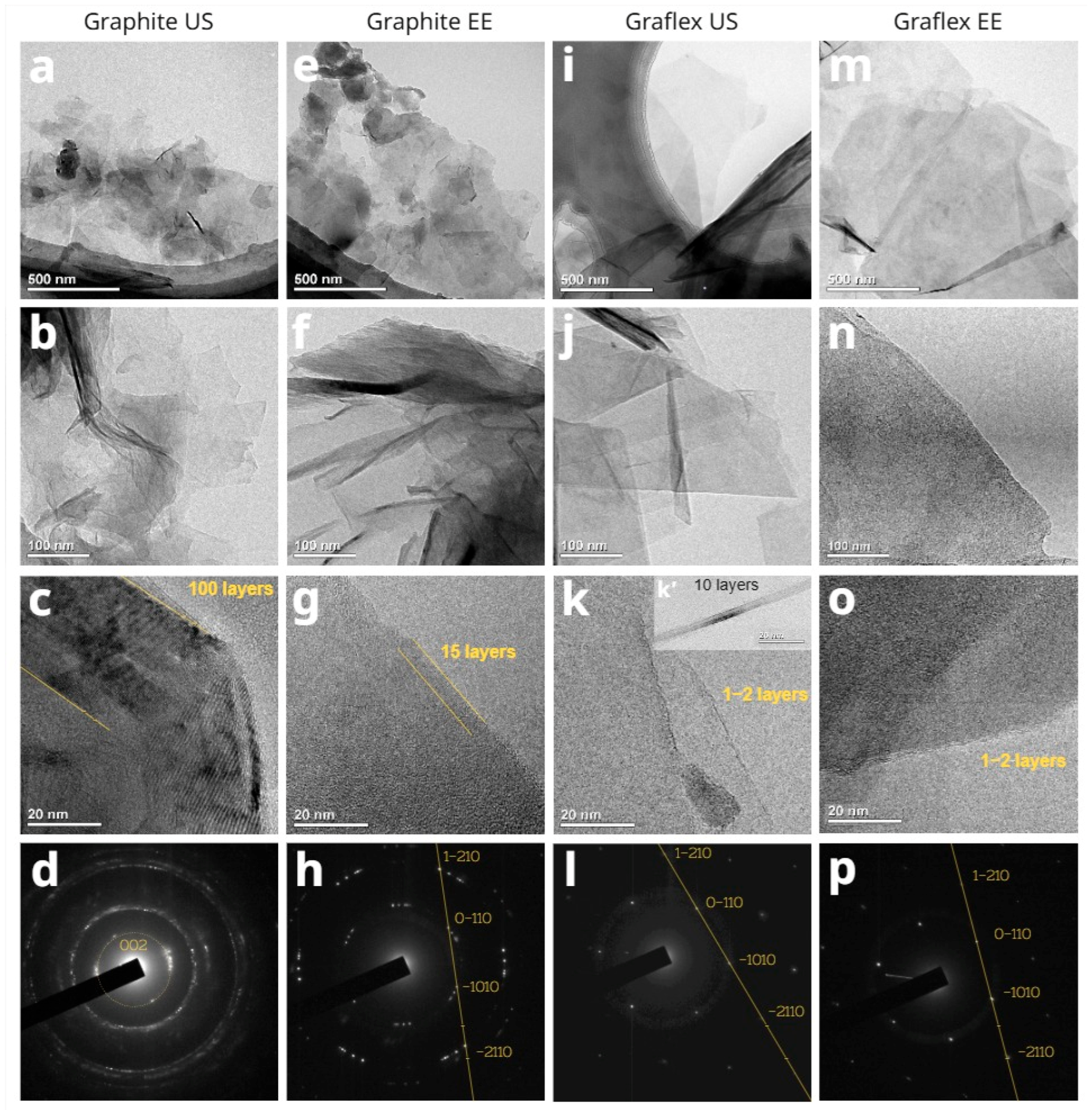
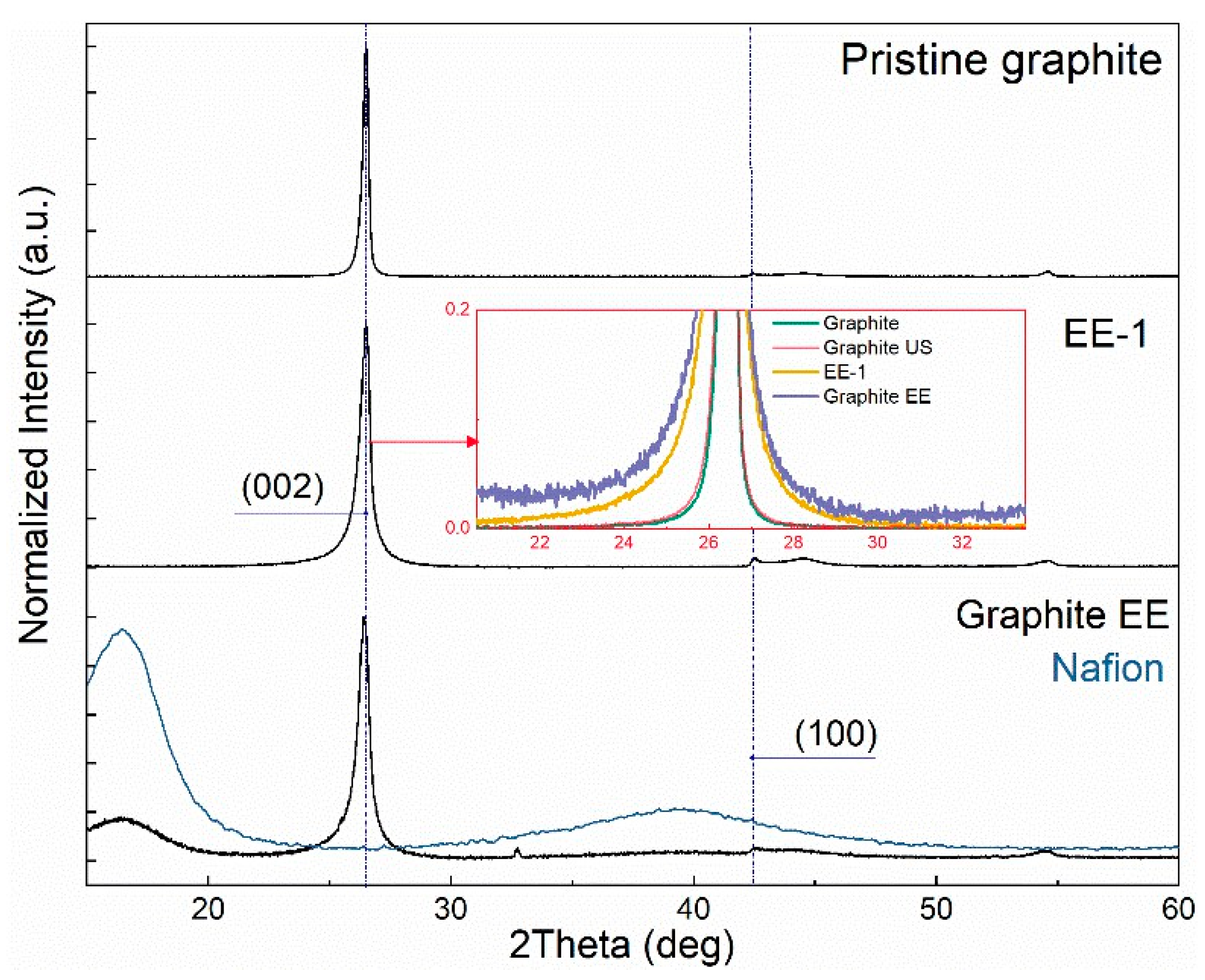

| Sample | Precursor | С (Surfactant), mg/L | Sonication Time, min |
|---|---|---|---|
| Graphite US 0 mg/L | Spectral graphite | 0 | 13 |
| Graphite US 50 mg/L | Spectral graphite | 50 | 13 |
| Graphite US 150 mg/L | Spectral graphite | 150 | 13 |
| Graphite US 300 mg/L | Spectral graphite | 300 | 13 |
| Graphite US 0 min | Spectral graphite | 150 | 0 |
| Graphite US 13 min | Spectral graphite | 150 | 13 |
| Graphite US 25 min | Spectral graphite | 150 | 25 |
| Graphite US 45 min | Spectral graphite | 150 | 45 |
| Graflex US 13 min | Graflex | 150 | 13 |
| Sample | A550 | Amax (270) | N |
|---|---|---|---|
| Graphite US 0 mg/L | 1.034 | 1.116 | >11 |
| Graphite US 150 mg/L | 0.622 | 0.710 | >11 |
| Graphite US 300 mg/L | 0.538 | 0.597 | >11 |
| Graphite US 13 min | 0.724 | 0.804 | >11 |
| Graphite US 25 min | 0.943 | 1.068 | >11 |
| Graphite US 45 min | 1.706 | 2.023 | 10 |
| Graflex US 13 min | 1.690 | 2.055 | 10 |
| Graphite US 13 min (replicate) | 1.077 | 1.192 | >11 |
| Sample | Sonication Time, min | τ1/2, s | A54000/A0 |
|---|---|---|---|
| Graphite US 0 min | 0 | 1390 | 0.13 |
| Graphite US 13 min | 13 | 6900 | 0.68 |
| Graphite US 25 min | 25 | 10,700 | 0.73 |
| Graphite US 45 min | 45 | 7700 | 0.76 |
| Sample | Composition | С (Surfactant), mg/L | Product Yield | |
|---|---|---|---|---|
| Carbon Material | Surfactant | |||
| Graphite US | Spectral graphite | Nafion | 100 | 0.83 |
| Graflex US | Graflex | Nafion | 100 | (1.28) associated with partial exfoliation of the auxiliary electrode |
| Graphite US w/o surfactant | Spectral graphite | - | 0 | 0.82 |
| Sample | Composition | Mode | Product Yield | Charge | Specific Charge, C/g | |
|---|---|---|---|---|---|---|
| Carbon Material | Nafion, mg/L | |||||
| EE-1 | Spectral graphite | 100 | pulse +3 V, −1.5 V, 30 s | 0.78 | +3 V: 9010 C −1.5 V: 3310 C | 24,641 |
| EE-2 | Spectral graphite | 100 | potentiostatic +3 V | 0.97 | +3 V: 8493 C | 11,634 |
| EE-3 | Spectral graphite | 0 | pulse +3 V, −1.5 V, 30 s | 0.53 | +3 V: 13,285 C −1.5 V: 4953 C | 45,644 |
| EE-4 | Graflex | 100 | pulse +3 V, −1.5 V, 30 s | 0.35 | +3 V: 27,887 C −1.5 V: 13,465 C | 143,340 |
| Sample | Composition, %wt | С (Nafion), mg/L | N (UV-Vis) | |
|---|---|---|---|---|
| Carbon Material | Surfactant (Nafion) | |||
| Graphite EE | 80 (EE-1) | 20 | 2000 | 7 |
| Graflex EE | 80 (EE-4) | 9 | ||
| Sample | A550 | Amax (270) | N |
|---|---|---|---|
| Graphite US | 0.910 | 1.066 | 10 |
| Graflex US | 1.337 | 1.477 | >11 |
| Graphite US w/o surfactant | 0.459 | 0.483 | >11 |
| Graphite EE | 1.505 | 2.289 | 8 |
| Graphite EE (replicate) | 0.821 | 1.348 | 7 |
| Graflex EE | 1.341 | 1.894 | 9 |
| Sample | C Carbon, g/L | τ1/2, s | A54000/A0 |
|---|---|---|---|
| EE-1 | 15 | 534 | 0.0302 |
| EE-2 | 15 | 72 | 0.0675 |
| EE-3 | 15 | 504 | 0.0253 |
| EE-4 | 15 | 170 | - |
| Graphite EE | 0.24 | >54,000 | 0.905 |
| Graphite US | 3.8 | >54,000 | 0.720 |
| Graflex US | 0.38 | 46,200 | 0.488 |
| Graphite US w/o surfactant | 15 | 19 | 0.0131 |
| Graflex EE | 0.24 | >54,000 | 0.949 |
| Sample | Position (hkl), 2θ | Average CSR Size (hkl), Å | FWHM (hkl), 2θ | d (002), Å | Number of Layers | G | AS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (002) | (100) | (002) | (100) | (002) | (100) | N = Lc/d(002) | A = La/Lc | |||
| Graphite pristine | 26.504 | 42.393 | 308.480 | 270.100 | 0.276 | 0.329 | 3.360 | 91.8 | 0.927 | 0.876 |
| EE-1 | 26.474 | 42.439 | 169.700 | 264.190 | 0.487 | 0.342 | 3.364 | 50.4 | 0.883 | 1.557 |
| EE-1 (replicate) | 26.487 | 42.462 | 168.300 | 249.000 | 0.510 | 0.358 | 3.363 | 50.1 | 0.900 | 1.480 |
| Graphite US | 26.463 | 42.405 | 378.913 | 259.140 | 0.225 | 0.343 | 3.365 | 112.6 | 0.867 | 0.684 |
| Graphite EE | 26.448 | - | 149.711 | 17.733 | 0.570 | 5.040 | 3.367 | 44.5 | 0.845 | 0.118 |
| Graflex pristine | 26.500 | - | 220.833 | - | 0.386 | - | 3.361 | 65.7 | 0.921 | - |
| Graflex US | 26.471 | 42.486 | 169.922 | 266.130 | 0.502 | 0.333 | 3.364 | 50.5 | 0.879 | 1.566 |
| Graflex EE | 26.468 | - | 103.344 | - | 0.825 | - | 3.365 | 30.7 | 0.874 | - |
| Sample | Max, nm | Particle Fraction, % | Size Range, nm | FWHM, nm | ζ-Potential, mV |
|---|---|---|---|---|---|
| Graphite EE (1st peak) | 91 | 13 | 59–1106 | 198 | −35 |
| Graphite EE (2nd peak) | |||||
| Graphite US | 255 | 17 | 106–712 | 195 | −28.1 |
| Graphite US w/o surfactant | 5560 | 18 | 2669–10,000 | 4180 | −5.9 |
| Graflex EE (1st peak) | 1281 | 21 | 712–2305 | 755 | - |
| Graflex EE (2nd peak) | 7456 | 5 | 4801–10,000 | 3050 | |
| Graflex US | 4801 | 34 | 3091–6439 | 1959 | −27.2 |
| Sample | Atomic % (XPS Survey Spectrum) | Ratios | ||||
|---|---|---|---|---|---|---|
| C1s | F1s | O1s | S2p |
C1s/O1s
(Carbon Material) | F1s/S2p | |
| Graphite US | 71.40 | 24.19 | 3.90 | 0.51 | 37.48 | 47.43 |
| Graflex US | 94.86 | 2.68 | 2.32 | 0.15 | 45.31 | 17.87 |
| Graphite EE | 61.17 | 31.00 | 7.16 | 0.67 | 11.22 | 46.27 |
| Graflex EE | 75.27 | 17.03 | 7.27 | 0.43 | 11.97 | 39.60 |
| Nafion | 28.14 | 64.25 | 6.05 | 1.55 | - | 41.45 |
| Sample | C=C | C–C | –CF2 | –CF3 | O–C–F | C–O–C | O–C=O | sp2/sp3 | C/O |
|---|---|---|---|---|---|---|---|---|---|
| Graphite US | 59.9 | 10.3 | 13.4 | 2.0 | 2.7 | 8.4 | 3.4 | 5.8 | 13.38 |
| Graflex US | 69.0 | 11.7 | 4.6 | 1.3 | 3.3 | 5.8 | 4.4 | 5.9 | 35.56 |
| Graphite EE | 54.5 | 7.9 | 15.5 | 4.0 | 4.6 | 9.1 | 4.5 | 6.9 | 5.50 |
| Graflex EE | 63.5 | 9.8 | 9.5 | 2.0 | 2.9 | 7.3 | 5.0 | 6.5 | 7.82 |
| Nafion | 0 | 12.8 | 67.7 | 14.3 | 0 | 5.2 | 0 | - | - |
| Sample | Graphite US | Graflex US | Graphite EE | Graflex EE |
|---|---|---|---|---|
| Number of graphene layers (XRD data) | ~100 | ~50 | ~45 | ~30 |
| Number of graphene layers (TEM data) | 100 | 1–2 | 15 | 1–2 |
| Lateral size (DLS and TEM data), nm | 100–300 | >1000 | 100–300 | >1000 |
| C/O (C1s XPS spectra) | 13.38 | 35.56 | 5.50 | 7.82 |
| Product yield, % | 98 | 98 | 76 | 33 |
| Time of synthesis, min | 45 | 45 | 500 + 1800 | 528 + 1800 |
| Energy input, kWh | 0.75 | 0.75 | 0.012 + 3.9 | 0.034 + 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnova, A.O.; Glebova, N.V.; Nechitailov, A.A.; Kastsova, A.G.; Pelageikina, A.O.; Kirilenko, D.A.; Shvidchenko, A.V.; Shestakov, M.S.; Koroleva, A.V.; Khrapova, E.K. Comparative Study of Graphite Exfoliation Techniques Using Nafion as a Surfactant. C 2025, 11, 76. https://doi.org/10.3390/c11040076
Krasnova AO, Glebova NV, Nechitailov AA, Kastsova AG, Pelageikina AO, Kirilenko DA, Shvidchenko AV, Shestakov MS, Koroleva AV, Khrapova EK. Comparative Study of Graphite Exfoliation Techniques Using Nafion as a Surfactant. C. 2025; 11(4):76. https://doi.org/10.3390/c11040076
Chicago/Turabian StyleKrasnova, Anna O., Nadezhda V. Glebova, Andrey A. Nechitailov, Angelina G. Kastsova, Anna O. Pelageikina, Demid A. Kirilenko, Alexander V. Shvidchenko, Mikhail S. Shestakov, Aleksandra V. Koroleva, and Ekaterina K. Khrapova. 2025. "Comparative Study of Graphite Exfoliation Techniques Using Nafion as a Surfactant" C 11, no. 4: 76. https://doi.org/10.3390/c11040076
APA StyleKrasnova, A. O., Glebova, N. V., Nechitailov, A. A., Kastsova, A. G., Pelageikina, A. O., Kirilenko, D. A., Shvidchenko, A. V., Shestakov, M. S., Koroleva, A. V., & Khrapova, E. K. (2025). Comparative Study of Graphite Exfoliation Techniques Using Nafion as a Surfactant. C, 11(4), 76. https://doi.org/10.3390/c11040076






