Enhanced Removal of Photosensitive Antibiotics in Water Using CO2: A Beneficial Exploration of CO2 Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. CIP Degradation Experiments
2.3. Analysis Methods
3. Results and Discussion
3.1. Influence of Operating Parameter on CIP Degradation by UV/CO2 Process
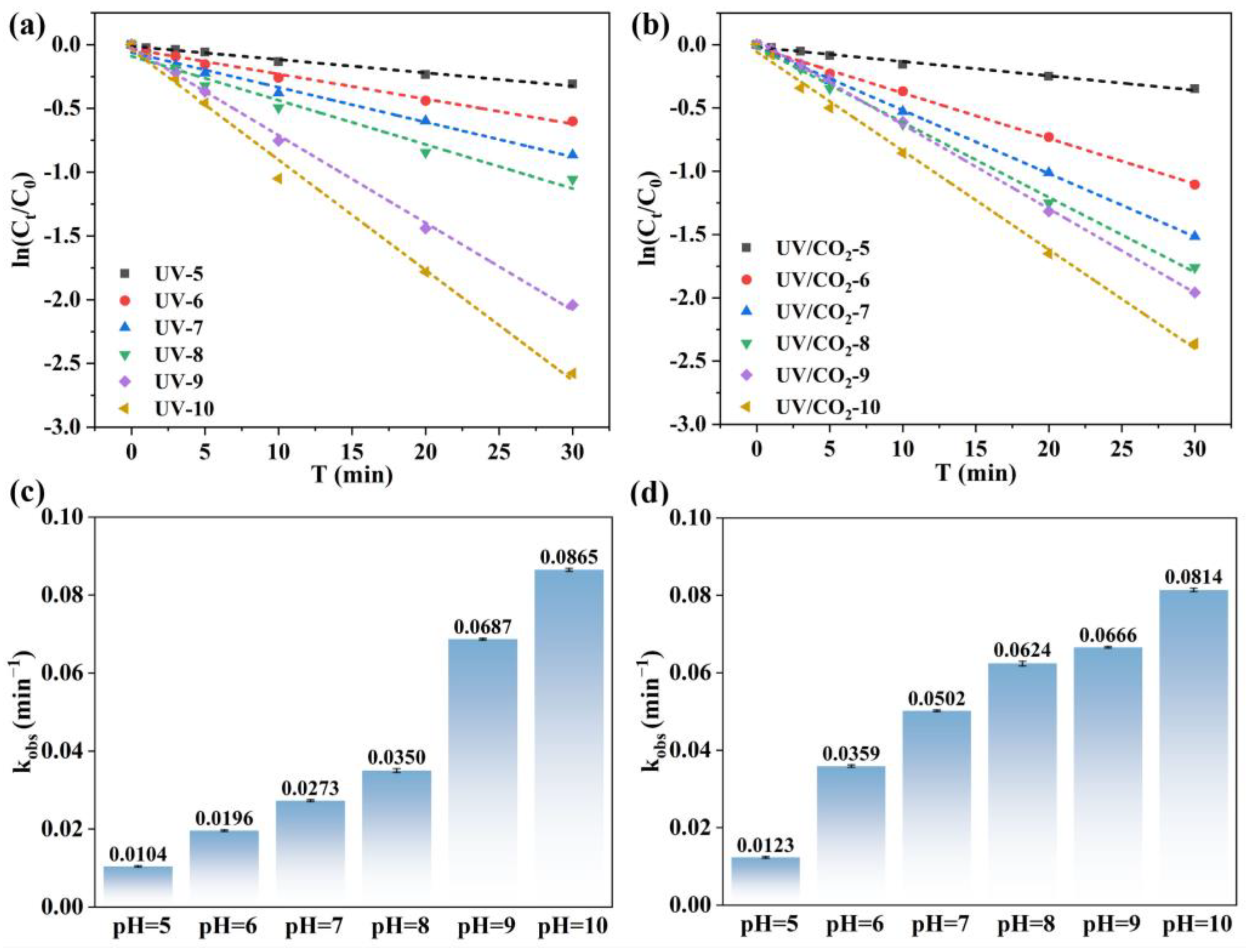
3.1.1. Effect of pH Value
3.1.2. Effect of Initial Concentration of CO2
3.1.3. Effect of Initial Concentration of CIP
3.1.4. Effect of Temperature
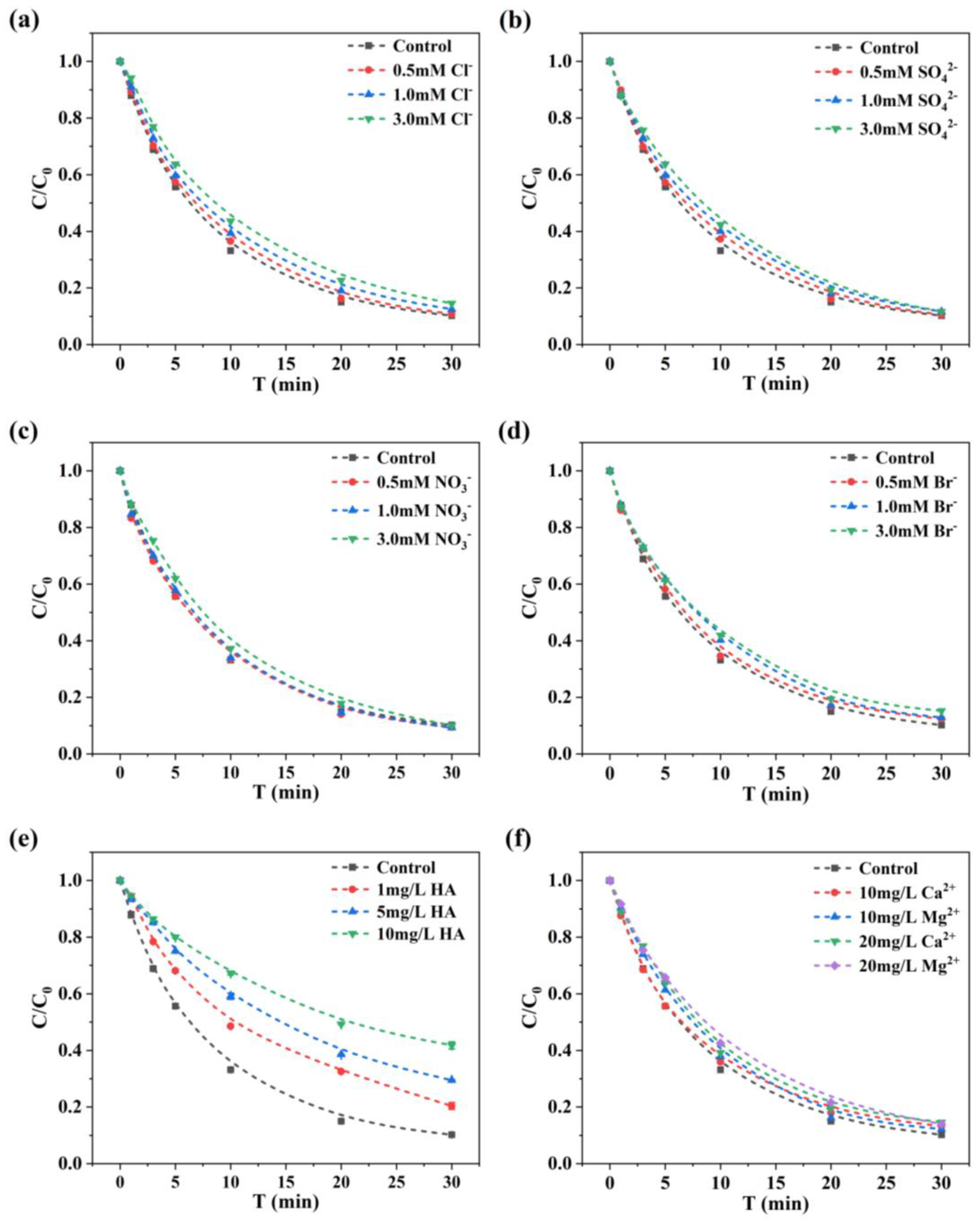
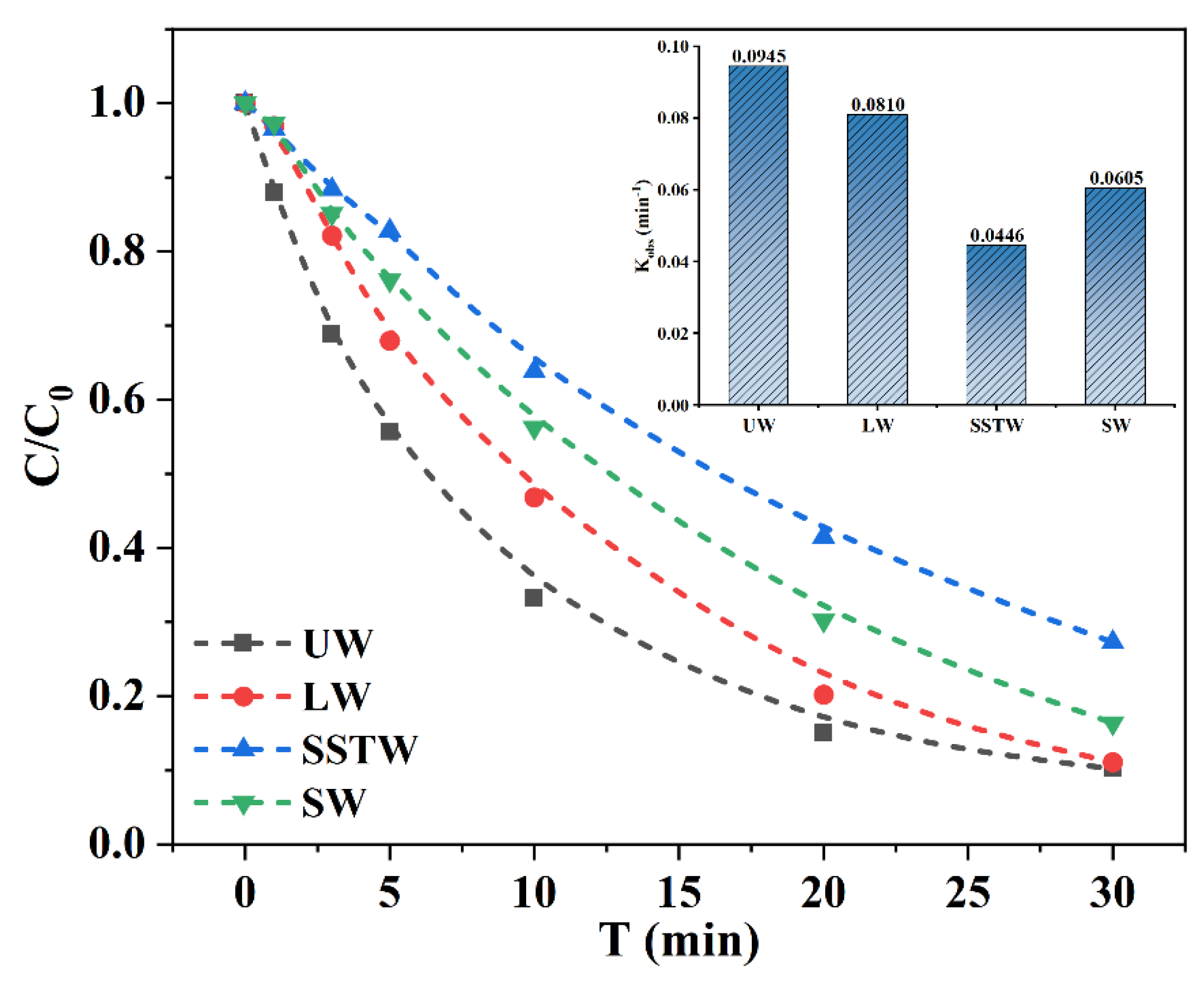
3.2. Influence of Water Matrices
3.3. Degradation Mechanism
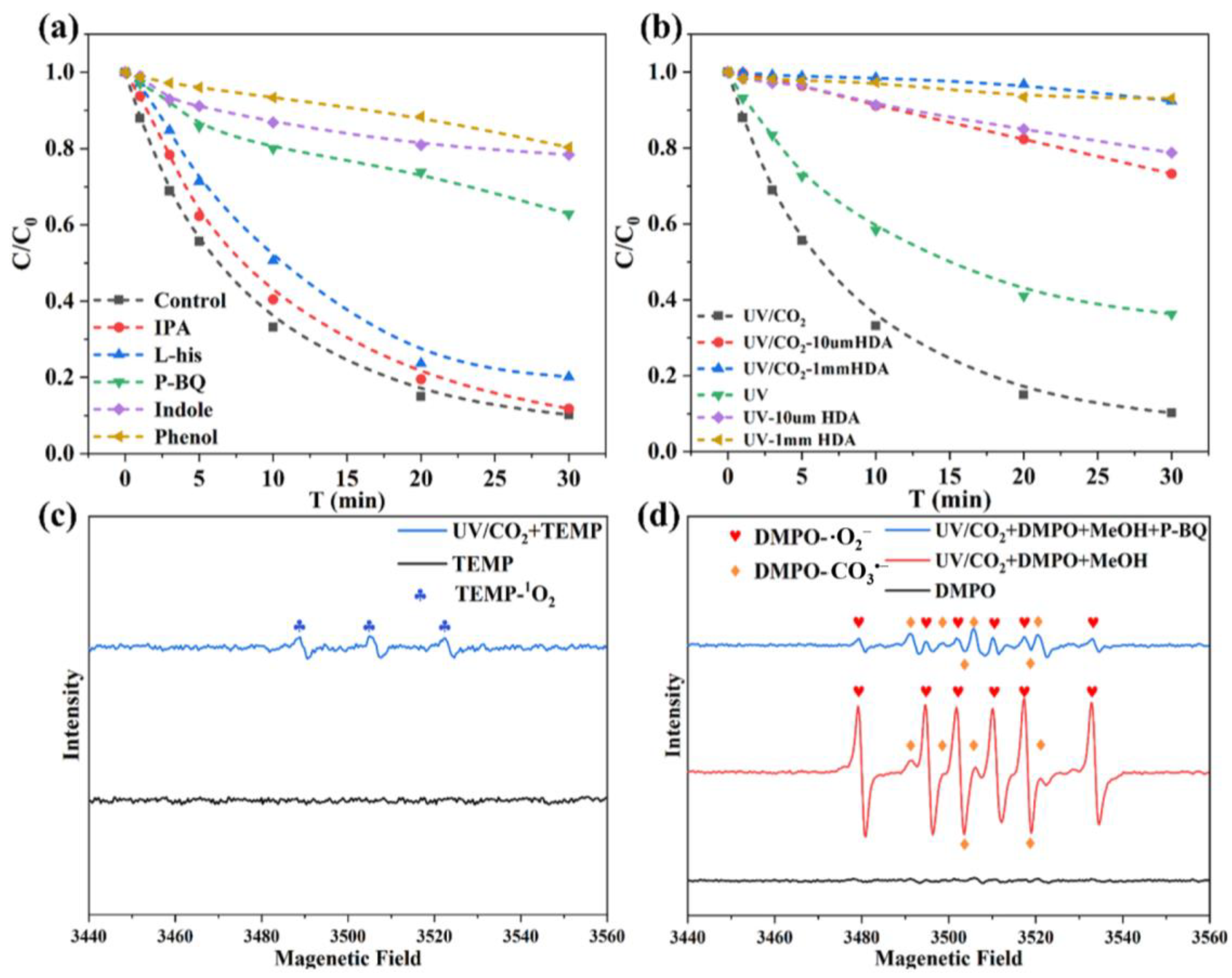
3.3.1. Identification of Reactive Species Contributing to CIP Degradation
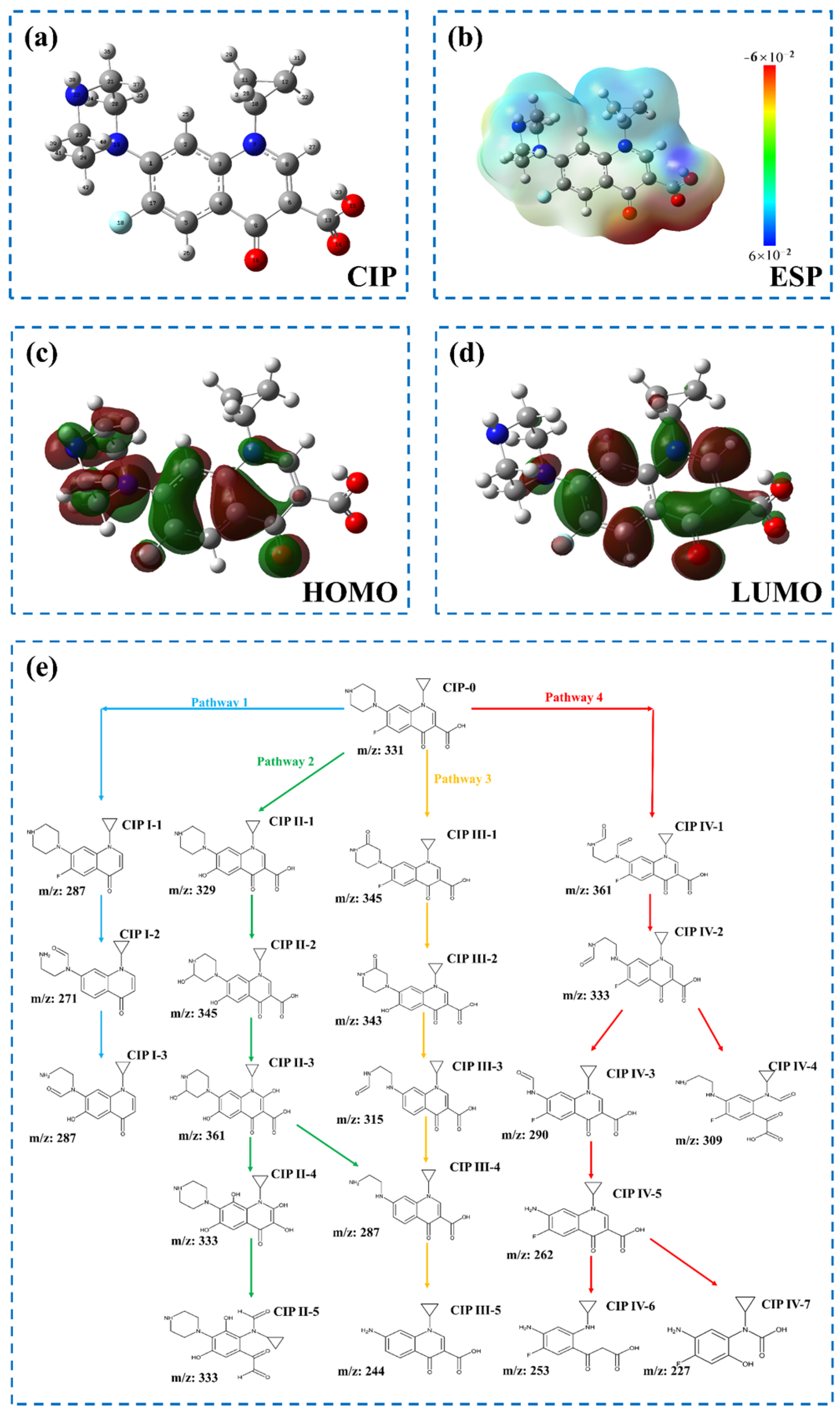
3.3.2. Transformation Products
3.4. Toxicity Assessment of Transformation Products
3.5. Actual Water Testing and Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIP | Ciprofloxacin |
| 3CIP* | Excited Triplet State of CIP |
| AOPs | Advanced Oxidation Processes |
| DMPO | 5,5-dimethyl-1-pyrroline-N-oxide |
| TEMP | 2,6,6-tetramethyl-4-piperidone |
| EPR | Electron Paramagnetic Resonance |
| EEM | Excitation–Emission Matrix |
| NOM | Natural Organic Matter |
| ROS | Reactive Oxygen Species |
| IPA | Isopropanol |
| P-BQ | Benzoquinone |
| L-his | L-histidine |
| PhOH | Phenol |
| 1CIP* | Excited Singlet State of CIP |
| DFT | Density Functional Theory |
| LBO | Laplacian Bond Order |
| GHS | Globally Harmonized System |
| LW | Lake Water |
| SSTW | Secondary Sedimentation Tank Effluent |
| SW | Seawater |
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Loddo, V.; Palmisano, L.; Parrino, F. Photocatalytic ozonation for a sustainable aquaculture: A long-term test in a seawater aquarium. Appl. Catal. B 2019, 253, 69–76. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, M.; Pan, Z.; Li, L.; Liang, J.; Yu, K.; Zhang, Y. Ultraviolet/peroxydisulfate degradation of ofloxacin in seawater: Kinetics, mechanism and toxicity of products. Sci. Total Environ. 2020, 705, 135960. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A review of processes for removing antibiotics from breeding wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Lu, Z.; Ling, Y.; Sun, W.; Liu, C.; Mao, T.; Ao, X.; Huang, T. Antibiotics degradation by UV/chlor(am)ine advanced oxidation processes: A comprehensive review. Environ. Pollut. 2022, 308, 119673. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, W.; Wang, X.; Blaney, L.; Peng, G.; Sharma, V.K. Chloride- enhanced degradation of micropollutants in natural water by the iron/biochar/peroxymonosulfate system: Role of iron(iv) and radicals. J. Hazard. Mater. 2025, 492, 137952. [Google Scholar] [CrossRef]
- Liu, X.; Fang, L.; Zhou, Y.; Zhang, T.; Shao, Y. Comparison of UV/PDS and UV/H2O2 processes for the degradation of atenolol in water. J. Environ. Sci. 2013, 25, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Lin, Z.; Chen, P.; Feng, M.; Liu, H.; Xiao, Z.; Lin, Z.; Li, D.; Liu, D.; Zhang, Y.; et al. Enhanced photochemical degradation and transformation of ciprofloxacin in a uv/calcium peroxide system: pH effects, defluorination kinetics, and different components numerical analysis. J. Clean. Prod. 2023, 414, 137706. [Google Scholar] [CrossRef]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; Zhou, Y.; Fang, L.; Shao, Y. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chemosphere 2013, 93, 2717–2724. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X. Degradation of aqueous polycyclic musk tonalide by ultraviolet-activated free chlorine. Processes 2019, 7, 95. [Google Scholar] [CrossRef]
- Lang, Z.; Song, X.; Song, G.; Han, L.; Zhang, Q.; Zhou, M. A flow-through UV/electro-chlorine process for cost-effective and multifunctional purification of marine aquaculture wastewater. J. Environ. Chem. Eng. 2022, 10, 107262. [Google Scholar] [CrossRef]
- Ye, M.; Li, C.; Liu, X.; Wang, L.; Chen, R. UV-activated permanganate process for micro-organic pollutant degradation: Efficiency, mechanism and influencing factors. Water Sci. Technol. 2021, 83, 1278–1285. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Guo, K.; Wu, Z.; Wang, L.; Hua, Z.; Fang, J. Kinetics and pathways of the degradation of PPCPs by carbonate radicals in advanced oxidation processes. Water Res. 2020, 185, 116231. [Google Scholar] [CrossRef]
- Behar, D.; Czapski, G.; Duchovny, I. Carbonate radical in flash photolysis and pulse radiolysis of aqueous carbonate solutions. J. Phys. Chem. 1970, 74, 2206–2210. [Google Scholar] [CrossRef]
- Cope, V.W.; Chen, S.-N.; Hoffman, M.Z. Intermediates in the photochemistry of of carbonato-amine complexes of cobalt(III). Carbonate(-) radicals and the aquocarbonato complex. J. Am. Chem. Soc. 1973, 95, 3116–3121. [Google Scholar] [CrossRef]
- Moore, J.S.; Phillips, G.O.; Sosnowski, A. Reaction of the carbonate radical anion with substituted phenols. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 31, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, Z.; Zhang, L.; Yoshimura, C.; Ye, Z.; Yu, P.; Qian, Y.; Hatano, Y.; Wang, J.; Niu, J. Photodegradation of propranolol in surface waters: An important role of carbonate radical and enhancing toxicity phenomenon. Chemosphere 2022, 297, 134106. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.; Zhang, G.; Li, M.; Liu, B.; Kang, C. Hydroxyl radical dominated ibuprofen degradation by UV/percarbonate process: Response surface methodology optimization, toxicity, and cost evaluation. Chemosphere 2023, 329, 138681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, P.; Boyer, T.H.; Zhao, L.; Huang, C.-H. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Wu, C.; Linden, K.G. Phototransformation of selected organophosphorus pesticides: Roles of hydroxyl and carbonate radicals. Water Res. 2010, 44, 3585–3594. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Boczkaj, G.; Aubry, O.; Aravind, U.K.; Aravindakumar, C.T. Advanced oxidation processes for degradation of water pollutants—Ambivalent impact of carbonate species: A review. Water 2023, 15, 1615. [Google Scholar] [CrossRef]
- Wilkin, R.T.; DiGiulio, D.C. Geochemical impacts to groundwater from geologic carbon sequestration: Controls on pH and inorganic carbon concentrations from reaction path and kinetic modeling. Environ. Sci. Technol. 2010, 44, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilization: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Lyu, H.; Kong, J.; Ma, J.; Wang, Z.; Feng, S. Review on carbon dioxide fixation coupled with nutrients removal from wastewater by microalgae. J. Clean. Prod. 2021, 292, 125975. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X. Fast Degradation of monochloroacetic acid by BiOI-enhanced UV/S(IV) process: Efficiency and mechanism. Catalysts 2019, 9, 460. [Google Scholar] [CrossRef]
- Schollenberger, C.J. Thymolphthalein as indicator for titrimetric estimation of carbon dioxide. Ind. Eng. Chem. 1928, 20, 1101. [Google Scholar] [CrossRef]
- Geng, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yuan, C.; Du, J.; Wang, C. Energy-saving photo-degradation of three fluoroquinolone antibiotics under VUV/UV irradiation: Kinetics, mechanism, and antibacterial activity reduction. Chem. Eng. J. 2020, 383, 123145. [Google Scholar] [CrossRef]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Yu, Z.; Cheng, H. Influence of chemical speciation on photochemical transformation of three fluoroquinolones (FQs) in water: Kinetics, mechanism, and toxicity of photolysis products. Water Res. 2019, 148, 19–29. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Jiang, X.; Wen, J.; Li, J.; Zhong, Y.; Chi, L.; Wang, Y. Percarbonate persistence under different water chemistry conditions. Chem. Eng. J. 2020, 389, 123422. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- He, X.; Pelaez, M.; Westrick, J.A.; O’Shea, K.E.; Hiskia, A.; Triantis, T.; Kaloudis, T.; Stefan, M.I.; de la Cruz, A.A.; Dionysiou, D.D. Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res. 2012, 46, 1501–1510. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Guo, J.; Xiong, W.; Leung, M.K.H. Advances and prospects in electrocatalytic processes for wastewater treatment. Processes 2024, 12, 1615. [Google Scholar] [CrossRef]
- Kuntzleman, T.S.; Sturgis, A. Effect of temperature in experiments involving carbonated beverages. J. Chem. Educ. 2020, 97, 4033–4038. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Hirschmann, G.; Eggers, J.; Sommer, R. A standardized method to measure the longitudinal UV emittance of low-pressure-lamps in dependence of water temperature. Water Supply 2022, 22, 900–916. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Gong, S.; Ding, C.; Liu, J.; Fu, K.; Pan, Y.; Shi, J.; Deng, H. Degradation of naproxen by UV- irradiation in the presence of nitrate: Efficiency, mechanism, products, and toxicity change. Chem. Eng. J. 2022, 430, 133016. [Google Scholar] [CrossRef]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; An, Y.; Yang, L.; Sun, Q.; Ma, J.; Zheng, H. Chitin-biocalcium as a novel superior composite for ciprofloxacin removal: Synergism of adsorption and flocculation. J. Hazard. Mater. 2022, 423, 126917. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Carlos, L.; Mártire, D.O.; Gonzalez, M.C.; Gomis, J.; Bernabeu, A.; Amat, A.M.; Arques, A. Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res. 2012, 46, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y.; Quan, X.; Chen, S.; Han, J.; Ou, X.; Zhao, J. Photochemical effect of humic acid components separated using molecular imprinting method applying porphyrin-like substances as templates in aqueous solution. Environ. Sci. Technol. 2010, 44, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Li, H.; Wang, W. Ciprofloxacin photosensitized oxidation of 2′-deoxyguanosine-5′-monophosphate in neutral aqueous solution. Photochem. Photobiol. 2012, 88, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-Z.; Moore, E.G.; Croué, J.-P. Excited triplet state interactions of fluoroquinolone norfloxacin with natural organic matter: A laser spectroscopy study. Environ. Sci. Technol. 2018, 52, 10426–10432. [Google Scholar] [CrossRef]
- Moor, K.J.; Schmitt, M.; Erickson, P.R.; McNeill, K. Sorbic acid as a triplet probe: Triplet energy and reactivity with triplet-state dissolved organic matter via 1O2 phosphorescence. Environ. Sci. Technol. 2019, 53, 8078–8086. [Google Scholar] [CrossRef]
- Sun, L.; Qian, J.; Blough, N.V.; Mopper, K. Insights into the photoproduction sites of hydroxyl radicals by dissolved organic matter in natural waters. Environ. Sci. Technol. Lett. 2015, 2, 352–356. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Y.; Lian, L.; Li, R.; Ma, J.; Zhou, H.; Song, W. Photochemical formation of carbonate radical and its reaction with dissolved organic matters. Water Res. 2019, 161, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rosario-Ortiz, F.L.; Lei, Y.; Pan, Y.; Lei, X.; Westerhoff, P. Multiple roles of dissolved organic matter in advanced oxidation processes. Environ. Sci. Technol. 2022, 56, 11111–11131. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Kitaoka, H. ESR detection of free radical and active oxygen species generated during photolysis of fluoroquinolones. Chem. Pharm. Bull. 1998, 46, 1021–1026. [Google Scholar] [CrossRef]
- Fontmorin, J.M.; Burgos Castillo, R.C.; Tang, W.Z.; Sillanpää, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef]
- He, F.; Zhao, W.; Liang, L.; Gu, B. Photochemical oxidation of dissolved elemental mercury by carbonate radicals in water. Environ. Sci. Technol. Lett. 2014, 1, 499–503. [Google Scholar] [CrossRef]
- Villamena, F.A.; Locigno, E.J.; Rockenbauer, A.; Hadad, C.M.; Zweier, J.L. Theoretical and experimental studies of the spin trapping of inorganic radicals by 5,5-dimethyl-1-pyrrolineN-oxide (DMPO). 2. Carbonate radical anion. J. Phys. Chem. A 2007, 111, 384–391. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Yan, Z.; Zhao, J.; Liu, Y.; Chen, Z.; Fan, M.; Du, W. Systematical comparison of antibiotic degradation in the activated peroxymonocarbonate and Fenton systems: Kinetics, matrix influence, mechanisms and intermediate toxicities. Chem. Eng. J. 2023, 473, 145438. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Chen, Z.-X.; Zhang, J.; Gong, L.; Wang, Y.-X.; Zhao, H.-Q.; Mu, Y. Carbonate-activated hydrogen peroxide oxidation process for azo dye decolorization: Process, kinetics, and mechanisms. Chemosphere 2018, 192, 372–378. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, J.; Huang, Q.; Xiong, C.; Ji, H.; Ren, Q.; Jin, Z.; Chen, S.; Guo, W.; Chen, J. Efficient degradation of ciprofloxacin in wastewater by CuFe2O4/CuS photocatalyst activated peroxynomosulfate. Environ. Res. 2024, 241, 117639. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhou, Z.; Li, W.; Li, L.; Tang, R.; Xiong, S.; Gong, D.; Huang, Y.; Bai, L.; Deng, Y. Revealing the synergistic effect between radical and non-radical species of sulfur-doped carbon nitride for ciprofloxacin removal: Based on density functional theory study. Sci. Total Environ. 2024, 915, 170191. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, L.; Tan, X.; Fang, Y.; Du, C.; Bao, X.; Zeng, Y.; Ma, W.; Yan, Z. Endogenous iron-enriched biochar loaded nickel-foam cathode in electro-Fenton for ciprofloxacin degradation: Performance, mechanism and DFT calculation. Chem. Eng. J. 2024, 498, 155446. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Ma, D.; Wang, Y.; Gao, B.; Yue, Q.; Xu, X. The Application of UV/O3 process on ciprofloxacin wastewater containing high salinity: Performance and its degradation mechanism. Chemosphere 2021, 276, 130220. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Garrido-Zoido, J.M.; García, J. Efficient removal of antibiotic ciprofloxacin by catalytic wet air oxidation using sewage sludge-based catalysts: Degradation mechanism by DFT studies. J. Environ. Chem. Eng. 2023, 11, 109344. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jebalbarezi, B.; Dehghanzadeh, R.; Maryamabadi, A.; Aslani, H. Sulfamethoxazole oxidation in secondary treated effluent using Fe(VI)/PMS and Fe(VI)/H2O2 processes: Experimental parameters, transformation products, reaction pathways and toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 107446. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liu, Y.; Wang, J. Visible light-enhanced interface interaction for pms activation towards the removal of emerging organic pollutants: Performance, mechanism and toxicity. Sep. Purif. Technol. 2025, 354, 128741. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Ren, X.; Qin, Y.; Zhang, Y.; Xie, J.; Diao, X.; Altaf, M.M. Regional distribution differences of antibiotics in tropical marine aquaculture area: Insights into antibiotic management and risk assessment. Sci. Total Environ. 2024, 954, 176391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; Wang, L.; Shao, Y.; Fang, L. Hydrated electron-based degradation of atenolol in aqueous solution. Chem. Eng. J. 2015, 260, 740–748. [Google Scholar] [CrossRef]
- Strojny, M.; Gładysz, P.; Nowak, W. Preliminary comprehensive assessment of CO2 utilization versus CO2 storage in Poland. Sustain. Energy Technol. Assess. 2024, 66, 103817. [Google Scholar] [CrossRef]
- Mahdi-Ahmed, M.; Chiron, S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J. Hazard. Mater. 2014, 265, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lester, Y.; Avisar, D.; Gozlan, I.; Mamane, H. Removal of pharmaceuticals using combination of UV/H2O2/O3 advanced oxidation process. Water Sci. Technol. 2011, 64, 2230–2238. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Wu, J.; Weng, Q.; Bi, T.; Liu, X. Enhanced Removal of Photosensitive Antibiotics in Water Using CO2: A Beneficial Exploration of CO2 Resource Utilization. C 2025, 11, 75. https://doi.org/10.3390/c11040075
Ye M, Wu J, Weng Q, Bi T, Liu X. Enhanced Removal of Photosensitive Antibiotics in Water Using CO2: A Beneficial Exploration of CO2 Resource Utilization. C. 2025; 11(4):75. https://doi.org/10.3390/c11040075
Chicago/Turabian StyleYe, Miaomiao, Jingqiu Wu, Qiuyuan Weng, Tengchao Bi, and Xiaowei Liu. 2025. "Enhanced Removal of Photosensitive Antibiotics in Water Using CO2: A Beneficial Exploration of CO2 Resource Utilization" C 11, no. 4: 75. https://doi.org/10.3390/c11040075
APA StyleYe, M., Wu, J., Weng, Q., Bi, T., & Liu, X. (2025). Enhanced Removal of Photosensitive Antibiotics in Water Using CO2: A Beneficial Exploration of CO2 Resource Utilization. C, 11(4), 75. https://doi.org/10.3390/c11040075








