Abstract
The hierarchical porosity and active sites of porous carbon materials have significant impacts on the oxygen reduction reaction (ORR) process. The heteroatom-doped porous carbon materials (Z67-900, Z8-900, Z11-900, Z12-900) were synthesized by pyrolysis of ZIFs to reveal the synergistic effect of hierarchical porosity and Co-Nx sites. The structures of prepared materials were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectra, and nitrogen adsorption. The results of electrocatalytic performance show that Z67-900 has the best performance among the four materials prepared. The onset potential (E0) of Z67-900 is close to commercial Pt/C (20%), and the half-wave potential (E1/2) of Z67-900 is 80 mV positive than that of Pt/C in an O2-saturated 0.1 M KOH solution (1600 rpm) with sweep rate of 5 mV·s−1. Moreover, Z67-900 has better methanol resistance. The hierarchical pore structure of Z67-900 facilitates mass transfer, while the Co-Nx sites provide active catalytic centers. This study provides a solid foundation for the rational design of highly efficient ZIF-derived heteroatom-doped catalysts.
1. Introduction
With the excessive consumption of fossil fuels, the resulting energy shortages and environmental pollution are becoming increasingly serious. In order to achieve sustainable development, it is necessary to develop new clean energy equipment and technology. Direct methanol fuel cells (DMFCs) have drawn considerable attention due to its high energy density, simple construction, environment-friendly qualities, and other merits [1,2,3]. However, the ORR at the cathode exhibits slower kinetic behavior, which is detrimental to the improvement of battery efficiency [4]. This situation is also seen in some air-based battery systems. It is notable that platinum-based catalysts Pt/C are effective in decreasing the overpotential for ORRs [5,6]. Nevertheless, platinum-based catalysts are limited in large-scale applications because of scarce reserves, high cost, and poor methanol resistance. Therefore, it is of great practical significance to develop non-platinum catalysts with high catalytic activity and excellent methanol resistance.
In recent years, heteroatom-doped (e.g., B, S, N, or metal) porous carbon materials have been demonstrated as promising non-precious metal ORR catalysts because of their high electrocatalytic activity, economical, and eco-friendly properties [7,8,9]. As far as we know, the ORR catalytic performance of porous carbon materials is closely related to factors such as specific surface area, porous architectures, and heteroatoms as active sites [10,11]. In the continuing effort to improve ORR performance, heteroatom-doped carbon with hierarchically porous architectures (incorporating micro-, meso-, and macropores) have received high attention due to the combination of the advantages of multi-level pores [12,13]. For example, macropores are beneficial for facilitating mass transfer, while mesopores and micropores can increase the specific surface area and offer more active sites. From the perspective of synthetic strategy, zeolitic imidazolate frameworks (ZIFs), which contain rich carbon, nitrogen, and transition metal elements, as well as abundant micropores and controllable regular pore structures have emerged as ideal precursors to prepare heteroatom-doped porous carbon catalysts [14,15]. In general, the pyrolysis of ZIFs under a certain inert atmosphere and temperature can produce transition metals and nitrogen atoms co-doped (M-N-C) porous carbon materials, which is regarded as a simple and effective method. Sometimes, the carbonization of the skeleton of ZIFs and the generation of defects during the pyrolysis process can lead to the formation of mesopores, even macropores in the material, resulting in multi-level pores. The obtained heteroatom-doped hierarchically porous carbon materials can expose more dispersed active sites on the one hand and accelerate the adsorption and transfer of reactants and active species on the other hand [16,17,18]. At present, the above two points have been proven to be crucial factors in ORR catalysis [19]. However, it is difficult to dominate and confirm the interactions between the carbon carriers and metal because of the highly unpredictable formation process of porous carbon materials. Therefore, it is meaningful to design porous carbon catalysts with controllable regular pore structures and transition metal elements. In this work, ZIFs (ZIF-8, ZIF-67, ZIF-11, and ZIF-12) with similar micropores were synthesized and pyrolyzed in a nitrogen atmosphere to produce heteroatom-doped porous carbon materials. Furthermore, the electrocatalytic activity of ZIF-derived heteroatom-doped porous carbon materials for ORR were investigated to reveal the interaction between the carriers and metal. This work provides good foundation for designing efficient heteroatom-doped carbon catalysts.
2. Materials and Methods
2.1. Materials
All reagents are of analytical grade. The Pt/C (20%) and raw reagents such as 2-methylimidazole, 1H-benzimidazole, metal salts, and Nafion solution were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) Other reagents such as NaOH, KOH, methanol, toluene, and ammonia were obtained from Tianjin Damao Chemical Reagent Factory (Tianjin, China).
2.2. The Preparation of ZIFs
Preparation of ZIF-8: Zn (NO3)2·6H2O (4.641 g, 15.6 mmol) was dissolved in 20 mL of water to form a zinc nitrate solution, while NaOH (1.256 g, 31.4 mmol) was dissolved in 16 mL of water to give a sodium hydroxide solution. The NaOH solution was then dropped into the Zn(NO3)2 solution, which immediately produced a Zn(OH)2 white precipitate. NH3·H2O (6mol·L−1) was slowly added to the above precipitates until the system became clear and transparent. The resulting solution was recorded as A. The 2-methylimidazole (2.578 g, 31.4 mmol) was fully dissolved in 40 mL of methanol, which was counted as solution B. The solution A and B were mixed to obtain a white precipitate. After aging for 24 h, the precipitate was filtered and washed with water, followed by methanol. Finally, white solid of ZIF-8 was obtained after drying. Yield: 64% based on Zn(II).
Preparation of ZIF-67: Co(NO3)2·6H2O (1.106 g, 3.8 mmol) and 2-methylimidazole (1.231 g, 15 mmol) were dissolved in 30 mL methanol, respectively. The obtained two solutions were mixed and sonicated for 10 min. After stirring at room temperature for 24 h, purple precipitates of ZIF-67 were centrifuged, washed with methanol and dried at 60 °C. Yield: 47% based on Co(II).
Preparation of ZIF-11: The 1H-benzimidazole (1.181 g, 10 mmol) was dissolved in 60 mL methanol, and then 53 mL toluene, 10 mmol ammonia, and Zn(CH3COO)2·2H2O (1.097 g, 5 mmol) were added in turn. The mixture was stirred for 4 h at room temperature. The obtained suspension was centrifuged and washed with methanol three times. White powder of ZIF-11 was collected after drying at 60 °C. Yield: 54% based on Zn(II).
Preparation of ZIF-12: The synthesis of ZIF-12 was similar to that of ZIF-11, except that Zn(CH3COO)2·2H2O (1.097 g, 5 mmol) was replaced by Co(CH3COO)2·4H2O (1.245 g, 5 mmol). The purple solid of ZIF-12 was obtained with a yield of 44% based on Co(II).
2.3. Synthesis of ZIF-Derived Heteroatom-Doped Porous Carbon Materials
The prepared ZIFs were placed in a tube furnace filled with N2 gas and heated at 900 °C for 3 h with N2 flow rate of 100 mL·min−1. Then, ZIF-derived porous carbon materials were obtained and marked as Z8-900, Z11-900, Z12-900, and Z67-900 [14], respectively. The preparation process is simply represented in Scheme 1.
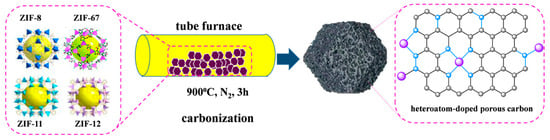
Scheme 1.
The diagrammatic sketch of the synthesis process of ZIF-derived porous carbon materials (grey spheres: carbon atoms, blue spheres: nitrogen atoms, purple spheres: transition metal elements. The arrow indicates the process of transformation from one state to another).
2.4. Characterization
The XRD data were collected on a D2PHASER diffractometer (Cu anode, λ = 0.154 nm, Bruker Corporation, Billerica, MA, USA) with 2θ from 5 to 80°. The IR was determined on a TENSOR II spectrometer (Bruker Corporation, Billerica, MA, USA), covering the spectral range of 4000 to 400 cm−1. The Raman spectra were measured on a LabRAM HR spectrometer (HORIBA, Ltd., Tokyo, Japan) with 532 nm laser excitation. The N2 adsorption–desorption isotherms were performed on a Micromeritics ASAP 2460 instrument (Micromeritics, Norcross, GA, USA) at 77K. JEM-2010 electron microscope (JEOL Ltd., Tokyo, Janpan) was used for TEM images. XPS was recorded by an ESCALAB250Xi spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with an Al (Mono) Kα X-ray source (1486.6 eV).
2.5. Evaluation of the Electrocatalytic Activity
The electrochemical workstation (CHI660E, Shanghai Chenhua Instrument, Shanghai, China) was used for testing in this work. Firstly, a catalytic ink was prepared by dispersing the catalyst in the mixing of isopropanol (5 mg·mL−1) and Nafion solution (2 wt.%) with the assistance of ultrasonic technology for 0.5 h. Secondly, the resulting catalyst ink (15 μL) was loaded on the working electrode. Finally, a three-electrode system was used for a cyclic voltammetry (CV) test. The catalyst ink modified electrode, a platinum wire, and saturated Hg/Hg2Cl2 electrode (SCE) were used as the working electrode, the counter electrode, and reference electrode, respectively. The CV test was conducted in a N2- or O2-saturated 0.1 M KOH electrolyte with the scan rate of 100 mV·s−1. The test on a rotating disk electrode (RDE) was carried out under quasi-stationary conditions (5mV·s−1 sweep rate) at 25 °C. A glassy carbon electrode with a rotating disk (5.61 mm dia) was used as the working electrode. The catalyst ink was immobilized on the surface of the RDE, which was performed on a CHI660E electrochemical workstation equipped with an AF-MSRCE modulator rate rotator (Dr. Hans Hugo Klein GmbH & Co., KG, Eisingen, Germany).
3. Results and Discussion
3.1. Analysis of XRD Curves and Raman Spectra
Graphitization degree is important for the catalytic performance for ORRs. The XRD and Raman spectra were investigated to study the graphitization degree. From Figure 1a, it can be seen that isostructural ZIF-8(Zn) and ZIF-67(Co) display characteristic diffraction peaks at 7.3°, 10.3°, 12.8°, 14.9°, 16.6°, and 18.0°, which correspond to (011), (002), (112), (022), (013), and (222) crystal planes. Similarly, sharp diffraction peaks for isostructural ZIF-11(Zn) and ZIF-12(Co) were observed at 7.3°, 10.3°, 12.8°, 14.9°, 16.6°, and 18.0° (Figure 1b), corresponding to (011), (112), (015), (044), and (006) crystal planes, respectively. This indicated that ZIF materials were successfully synthesized and had high crystallinity. In contrast to crystalline ZIF materials, the XRD curves of porous carbon (Z8-900, Z11-900, Z12-900, and Z67-900, Figure 1c) exhibit weak and broad diffraction peaks at 20–30°, which indicate amorphous carbon in obtained doped carbon materials [20]. The results indicated that ZIFs are completely carbonized at 900 °C in a nitrogen atmosphere. Moreover, for Z8-900 and Z11-900, no peaks belonging to Zn or ZnO were observed on their XRD curves because Zinc’s elemental (boiling point 907 °C) is easily vaporized during the high-temperature pyrolysis process [21]. The XRD curves of Z12-900 and Z67-900 showed three sharp peaks appeared at ~43°, ~52°, ~75°, corresponding to the characteristic diffraction peaks of cobalt (111), (200), (220) [22].
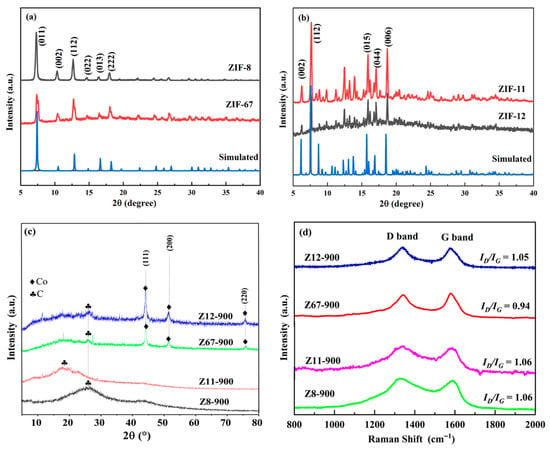
Figure 1.
XRD curves of (a) ZIF-8 and ZIF-67, (b) ZIF-11 and ZIF-12, (c) obtained porous carbon materials. (d) Raman spectra of Z8-900, Z67-900, Z11-900, and Z12-900.
The Raman spectra were used to determine the disordered and graphitic carbon content of the porous carbon materials in this work. As shown in Figure 1d, the D band (1340 cm−1) arises from disordered carbon or defects, while the G band (1580 cm−1) corresponds to sp2-hybridized graphitic carbon [23]. The calculated ID/IG value (intensity ratio) of Z8-900, Z11-900, Z12-900, and Z67-900 are 1.06, 1.06, 1.05, and 0.94, respectively. Z67-900 has a smaller ID/IG ratio and implies a lower amount of disordered carbon or defects. In summary, the ratio is near one, which is far from graphitization, indicating low graphitization degree with abundant defective sites in obtained porous carbon materials. It is consistent with the XRD results.
3.2. Analysis of Infrared Spectroscopy
Infrared spectroscopy was used to identify functional groups and structures of obtained ZIFs (Figure 2). ZIF-8 shows peaks at 2927 cm−1 (C-H stretches), 1583 cm−1 (imidazole ring C=N vibration), 1423, 1307 cm−1 (ring stretching), 1146 cm−1 (C-N stretch), 996(m), 759, 693 cm−1 (out-of-plane bends), and 421 cm−1 (Zn-N stretch). ZIF-67 displays a nearly identical pattern with subtle shifts: 2929, 1581, 1422, 1303, 1142, 992, 757, 694, and 426 cm−1. In contrast, ZIF-11 and ZIF-12 show weakened aliphatic C-H stretches at 3062 cm−1 and 3047 cm−1, with prominent peaks at 1468/1460 cm−1 (benzene ring), 1248/1238 cm−1 (C-N-C asymmetric stretch), and 1005 cm−1 (in-plane ring deformation). The low-frequency regions feature Zn-N and Co-N vibrations at 425 and 427 cm−1, respectively. The similar infrared spectra of ZIF-8 and ZIF-67 (ZIF-11 and ZIF-12) originate from their identical ligand and framework, which is consistent with the XRD results.
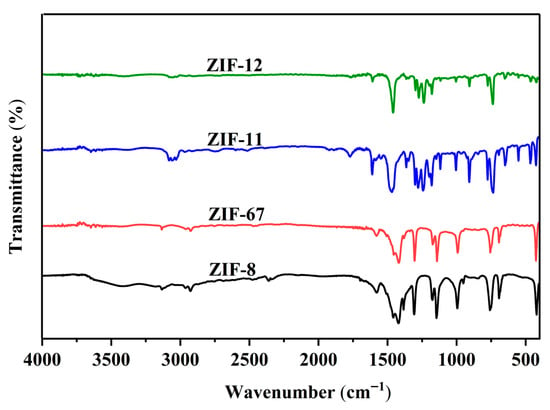
Figure 2.
The infrared spectra of ZIF-8, ZIF-67, ZIF-11, and ZIF-12.
3.3. The Morphology
The morphology of the prepared ZIFs and porous carbon samples were investigated by the high-resolution TEM. It can be seen that ZIF-8, ZIF-67, ZIF-11, and ZIF-12 show uniform rhombic polyhedron crystals with well-defined boundaries (Figure 3a–d). In contrast, the carbonized Z8-900, Z67-900, Z11-900, and Z12-900 retain the original rhombic dodecahedron and size of the ZIFs precursor after carbonization [14,15,17]. However, the morphological structure of porous carbon samples becomes roughly relative to the ZIFs precursor, which is caused by a collapse of the framework. Z8-900, especially, exhibited a flake-like pyrolytic carbon morphology, resulting from the structural collapse of the ZIF-8 and volatilization of Zn species during pyrolysis at 900 °C. The escaping of Zn atoms can result in various amounts of defects.
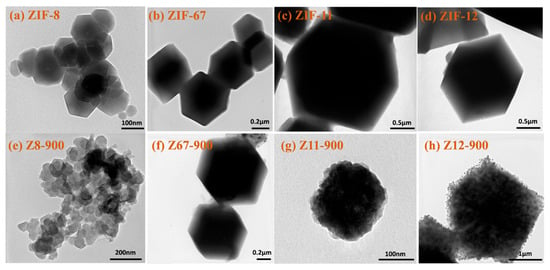
Figure 3.
TEM images of (a) ZIF-8, (b) ZIF-67, (c) ZIF-11, (d) ZIF-12, (e) Z8-900, (f) Z67-900, (g) Z11-900, and (h) Z12-900.
3.4. X-Ray Photoelectron Spectroscopy
XPS was used to characterize the composition of the ZIF-derived heteroatom-doped porous carbon materials (Figure 4). The XPS survey pattern confirmed that the ZIF-67 and Z67-900 were composed of C, N, O, and Co elements (Figure 4a, Table 1), indicating that the basic elements of ZIF-67 have been maintained after pyrolysis. The high-resolution spectrum of C 1s revealed the presence of C=C/C-C (284.8 eV), C=N (285.8 eV) bonds, and C-N (289.0 eV) bonds for ZIF-67 and Z67-900 (Figure 4b). Worthily, besides pyridinic-N (398.5 eV) and pyrrolic-N (400 eV), an additional signal located at 401.1 eV for graphite nitrogen was observed in the N 1s spectrum of Z67-900 compared to ZIF-67 (Figure 4c) [24]. In addition, the content of C in Z67-900 (83.55%) is significantly higher than that in ZIF-67 (58%), but the content of N (4.42%) is lower than that in ZIF-67 (21.51%). These results indicate that the graphitization degree increases. The graphitized carbon–nitrogen structure in doped carbon materials derived from ZIFs can anchor target single atoms. In this way, it was effective to avoid the migration and aggregation of target atoms as active sites. Interestingly, Co0 was formed in Z67-900. As shown in Figure 3d, the Co 2p3/2 of Z67-900 can be separated into three peaks of 778.4 eV, 780.2 eV, and 782.2 eV and are attributed to Co0, Co3+, and Co2+, respectively [25,26]. The above results indicate the presence of Co-N-C structures in Z67-900 [27,28].
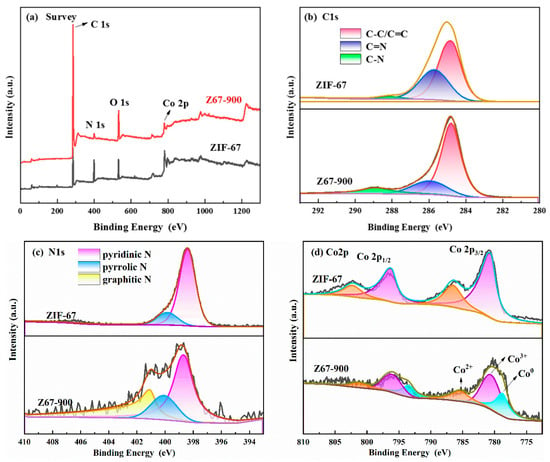
Figure 4.
(a) The XPS survey spectrum. (red line: Z67-900, black line: ZIF-67). (b) C1s high-resolution spectrum (red: C-C/C=C, dark blue: C=N, green: C-N). (c) N1s high-resolution spectrum (pink: pyridinic N, blue: pyrrolic N, yellow: graphitic N). (d) Co2p high-resolution spectrum (light purple: Co3+, orange: Co2+, cyan: Co0).

Table 1.
Element content of ZIF-67 and Z67-900 based on XPS analysis.
3.5. The Specific Surface and Pore Size Analysis
The electrocatalytic activity of three-dimensional Co/N-doped porous carbon materials is closely related to good electrolyte permeability, large specific surface area, and high conductivity. The isotherm and pore size distribution are shown in Figure 5. It can be seen that Z11-900 displays type I isotherm, which indicates it only contains micropores. However, Z8-900, Z12-900, and Z67-900 have a combination of type I and type IV isotherms with H3 hysteresis loops (Figure 5a,c) [29,30] which indicate irregular pore structures. The BET surface area of Z8-900, Z11-900, Z12-900, and Z67-900 are 1084, 248, 680, and 124 m2·g−1, respectively. The BET surface area of Z67-900 is about 13% of ZIF-67, which should be interpreted as the collapse of the 3D framework of ZIF-67 after carbonization [29]. In addition, the DFT model of porosity distribution curves show that there are only micropores (1–1.86 nm) in Z11-900. But for Z67-900, Z12-900, and Z8-900 there are micropores, mesopores, and macropores (Figure 5b,d), which is consistent with the conclusion given by isotherms. As we known, the micropores are beneficial for exposing more active sites, while mesopores and macropores are conducive to accelerate mass transport of ORR-relevant species (O2, H2O, etc.), which can boost catalytic activity [31,32,33].
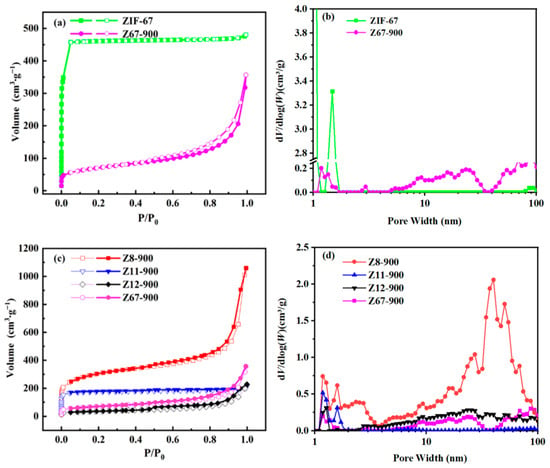
Figure 5.
N2 adsorption–desorption isotherms: (a) ZIF-67 and Z67-900, (c) Z8-900, Z11-900, Z12-900, and Z67-900. Porosity Distribution by N2-DFT model: (b) ZIF-67 and Z67-900. (d) Z8-900, Z11-900, Z12-900, and Z67-900.
3.6. Effects of Co/N-Doped Porous Carbon for ORR
The electrocatalytic activity of ZIF-derived porous carbon for ORRs was first investigated via the CV technique. As shown in Figure 6a–e, a sharp reduction peak was observed in the O2 saturated electrolyte, indicating that oxygen interacts with the N-C/M-N-C structure. The reduction potential of Z12-900 (0.80 V vs. RHE) is the same as Pt/C (0.80 V vs. RHE); however, the peak current density of Z12-900 is (0.33 mV·cm−2), which is much lower than that of commercial Pt/C (0.95 mV·cm−2) (Figure 6f). The reduction potential of Z67-900 is 0.84 V (vs. RHE), which is slightly more positive than Pt/C, and the peak current density is higher than Z12-900. These results indicated that Z67-900 effectively decreased the overpotential of the ORR. Compared to Z67-900 and Z12-900, Z8-900 and Z11-900 have larger specific surface areas, but they do not have metal sites. The higher electrochemical activity of Z67-900 and Z12-900 indicate that the Co-N-C structure is more favorable to ORRs than the C-N structure. In addition, the higher activity of Z67-900 compared to Z12-900 is attributed to the former having a larger specific surface area and abundant mesopores and macropores. Overall, the electrocatalytic activity for ORR is significantly associated with the active Co-Nx sites as well as hierarchical porosity.
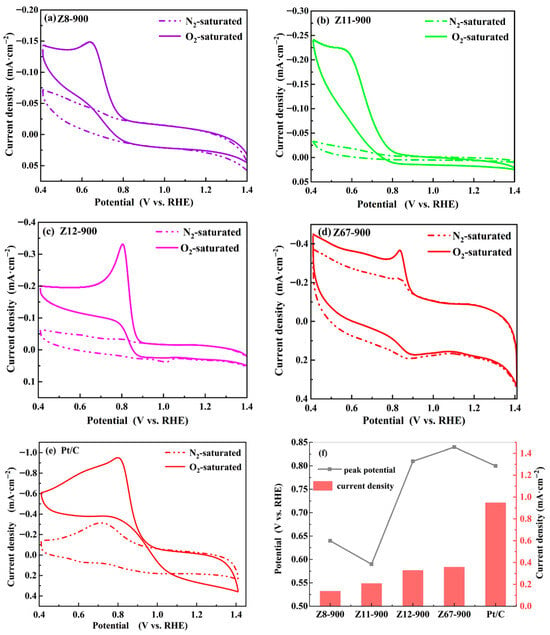
Figure 6.
The CV curves in O2-saturated and N2-saturated 0.1 M KOH solution with sweep speed of 5 mV·s−1: (a) Z8-900, (b) Z11-900, (c) Z12-900, (d) Z67-900, and (e) Pt/C. (f) Peak potential and current density of porous carbon and Pt/C.
To further investigate the effects of Co/N-doped porous carbon for ORRs, Z67-900 was selected as a representative and thoroughly studied. The LSV test was executed by using a rotating disk electrode (RDE) in an O2-saturated 0.1 mol·L−1 KOH solution with a sweep rate of 5 mV·s−1. The polarization curves were tested at speeds ranging from 400 to 2500 rpm using Z67-900 and Pt/C modified electrodes (Figure 7a,c). The current density increased sharply with the rise of RDE velocity, which represented the rapid diffusion of reactants on the catalyst surface. The Koutecky–Levich (K-L) equation (Figure 7b,d), which shows good linearity, implies a first-order reaction kinetic corresponding to the dissolved oxygen percentage [16,25]. Usually, the onset potential and half-wave potential are the critical indicators to evaluate the ORR performance of catalysts. The LSV curves at a rotation speed of 1600 rpm are shown in Figure 7e. The onset potential E0 of Z67-900 (0.90 V vs. RHE) is the same as commercial Pt/C (0.90 V vs. RHE), and the half-wave potential E1/2 of Z67-900 is 0.82 V (vs. RHE). So Z67-900 has a positive E1/2 of 80 mV over Pt/C (0.74 V, vs. RHE) (Figure 7f). This empirical evidence proved that the obtained porous carbon Z67-900 exhibited excellent ORR activity. The electrocatalytic activity of Z67-900 was enhanced by the synergistic effect of the hierarchical porosity and Co-Nx sites.
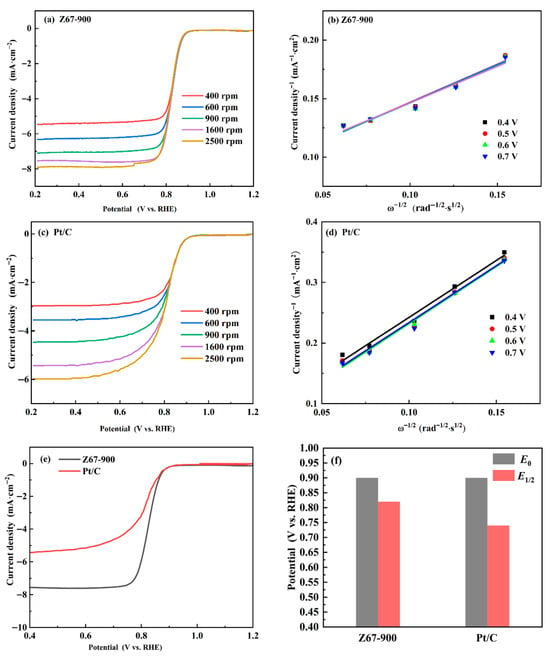
Figure 7.
The LSV curves of (a) Z67-900 and (c) Pt/C in an O2-saturated 0.1 M KOH solution at different rotations of 400–2500 rpm: (b) K-L curves of Z67-900. (d) K-L curves of Pt/C. (e) LSV curves of Z67-900 and Pt/C in an O2-saturated 0.1 M KOH solution (1600 rpm) with a sweep rate of 5 mV·s−1. (f) The comparison of onset potential E0 and half-wave potential E1/2 of Z67-900 and Pt/C.
In addition, it is well known that the four-electron reduction path (H2O as a product) is preferable for ORRs than that of the two-electron process (H2O2 as a product) because peroxides are unfavorable to the stability of the battery [34,35]. The total number (n) of transferred electrons in the ORR is calculated by the K-L equation. The n for Z67-900 was 3.80–3.92 in a potential window of 0.2–1.3 V (vs. RHE), which indicated that ORRs mainly proceeded a four-electron pathway for Z67-900 [36,37]. The yields of peroxide (H2O2) were tested to further study the pathway and mechanism of the ORR by rotating ring-disk electrode (RRDE) in an O2-saturated KOH solution (0.1 M) at 1600 rpm. The disk current ID and ring current IR curves based on Pt/C (20 wt%) are shown in Figure 8a. It was found that Z67-900 produced a lower amount of H2O2 (8.9%) at 0.25 V (vs. RHE) and the yield of H2O2 decreases with the decreasing of positive potential (Figure 8b). This is consistent with the CV and RDE test results.
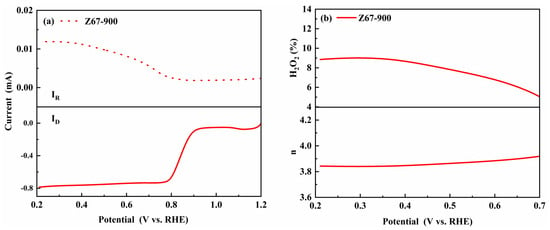
Figure 8.
(a) The disk current ID and ring current IR of Z67-900. (b) The H2O2 yield and n value for Z67-900 in O2-saturated 0.1 M KOH solution (1600 rpm).
In the process of the ORR, the oxidation of methanol may lead to the deactivation of the catalyst; therefore, high performance electrocatalytic materials should have good methanol tolerance. In order to investigate the methanol tolerance, cyclic voltammetry curves of both Pt/C and Z67-900 were tested in an O2-saturated KOH (0.1 M) containing 3 M methanol. The comparison of results is shown in Figure 9a. The oxygen reduction peak of Pt/C at 0.80 V (vs. RHE) decreased after injecting methanol into the electrolyte, and a methanol oxidation peak at 0.86 V (vs. RHE) appeared. The reduction reaction of O2 on the surface of Pt/C was restricted, which revealed that the commercial Pt/C has weak methanol resistance. Moreover, the CV curve of Z67-900 (Figure 9b) does not show new oxidation peaks in a methanol-containing electrolyte, the potential and current density of the oxygen reduction peak just shows a slightly negative shift. These results show that Z67-900 has better methanol resistance performance in an alkaline methanol-containing electrolyte, which makes it possible to be used as a substitute for commercial Pt/C catalysts.
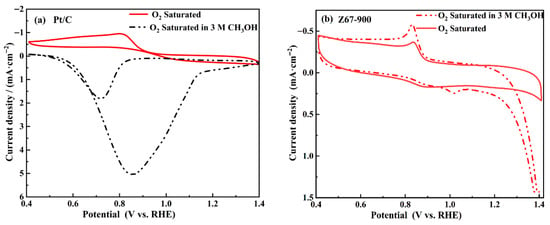
Figure 9.
The CV curves in an O2-saturated 0.1 M KOH solution and 3 M CH3OH-0.1 M KOH mixed solution with sweep rate of 5 mV·s−1: (a) Pt/C and (b) Z67-900.
A mechanism was proposed for an ORR catalyzed by a three-dimensional Co/N-doped ZIF-derived porous carbon based on the experimental results and the literature (Figure 10) [38]. The electron transfer number n for Z67-900 was 3.80–3.92. It was found that Z67-900 produced a lower amount of H2O2 (8.9%) at 0.25 V (vs. RHE) and the yield of H2O2 decreases with the decreasing of positive potential. The result was indicative of a four-electron pathway of water (H2O) production for Z67-900. First, the O2 molecule is bonded to the Co center (d-filling of 3d74s2) in prepared catalysts by bridge adsorption. Then, O2ads-Co-Z67-900 is reduced to *OOH, which is further converted into adsorbed Oads-Co-Z67-900, and OHads-Co-Z67-900 by a four-electron pathway of water (H2O) production. The Co-N-doped catalyst Z67-900 derived from ZIF-67 precursors preserved a distinctive coordination environment (Co-Nx sites), which promotes the electron transfer rate of the first step and thus accelerated the ORR [39,40]. Moreover, the hierarchical pores of ZIF-derived carbon were conducive to the exposure of active sites and mass transfer, so that it could more conveniently form a collaborative effect. Benefiting from the synergistic effect, the ZIF-derived porous carbon lowered the activation energy of the *OOH dissociation step in the ORR process through adjusting the charge density around the Co sites, as supported by the previous literature [38].
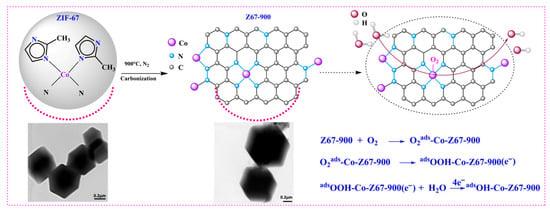
Figure 10.
The mechanism for ORRs catalyzed by Z67-900 (dark grey spheres: carbon atoms, blue spheres: nitrogen atoms, purple spheres: cobalt element, light grey spheres: hydrogen atoms, red spheres: oxygen atoms).
Notably, established studies (Table 2) consistently demonstrate the synergistic effects between hierarchical porosity and Co-Nx sites in ZIF-derived heteroatom-doped carbon materials. This synergy manifests through two key approaches: (i) the creation of hierarchical pore architectures via either additive-mediated methods (e.g., NaCl templating [41], organic molecule incorporation [42,43], or ZIF-8/ZIF-67 co-precipitation [41,44,45,46] during pyrolysis. (ii) The enhancement of Co-Nx site density through coordination (e.g., vb2-Co2+ complexation). These structural modifications collectively contribute to the ORRs catalytic performance, with the interdependence of hierarchical porosity and Co-Nx sites being particularly crucial [47,48,49,50,51].

Table 2.
The established studies in ZIF-derived heteroatom-doped carbon materials.
4. Conclusions
In this work, a series of structurally associated Co/N-doped porous carbon materials (Z67-900, Z8-900, Z11-900, Z12-900) were synthesized by pyrolysis of ZIF precursors. The electrocatalytic ORR performance was systematically studied to investigate the synergistic interplay between hierarchical porosity and Co-Nx active sites. The results of electrocatalytic performance show that Z67-900 has the best performance among the four materials prepared. The onset potential (E0) of Z67-900 is close to commercial Pt/C (20%), and the half-wave potential (E1/2) of Z67-900 is 80 mV more positive than that of Pt/C in an O2-saturated 0.1 M KOH solution. The higher electrochemical activity of Z67-900 indicates that the Co-N-C structure is more favorable to ORRs than the C-N structure of Z8-900 and Z11-900. In addition, the higher activity of Z67-900 compared to Z12-900 is attributed to the former having a larger specific surface area and abundant mesopores and macropores. The synergistic cooperation between the hierarchical pore architecture and Co-Nx sites was found to intensely influence the one-step four-electron transfer pathway. These findings provide a good foundation for designing and optimizing heteroatom-doped carbon catalysts.
Author Contributions
Y.Y.: Writing—original draft, Formal analysis. A.-M.T.: Data curation: Visualization. Q.-X.R.: Investigation, Validation. G.Z.: Writing—review & editing, Conceptualization, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data can be accessed upon reasonable request from the corresponding author at zhanggai@xatu.edu.cn.
Acknowledgments
The authors extend their gratitude to Gao-Hong Li for his support in XPS and TEM testing, as well as for the financial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ismail, A.; Kee, Y.W. Investigation on voltage loss mechanism for direct methanol fuel cell. Energy Rep. 2023, 10, 535–543. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Falcão, D.S.; Oliveira, V.B.; Rangel, C.; Pinto, A. Review on micro-direct methanol fuel cells. Renew. Sustain. Energy Rev. 2014, 34, 58–70. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Antolini, E. The oxygen reduction on Pt-Ni and Pt-Ni-M catalysts for low-temperature acidic fuel cells: A review. Int. J. Energy Res. 2018, 42, 3747–3769. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.L.; Guo, S. Towards high-efficiency nano electrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Hira, S.A.; Prabakar, K.; Ha, C.-S.; Joo, S.H.; Nam, K.M.; Park, K.H. Heteroatom-doped nanomaterials/core–shell nanostructure based electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2022, 10, 987–1021. [Google Scholar] [CrossRef]
- Arif, M.; Mahsud, A.; Muhmood, T.; Deepak, F.L. Design, synthesis, and electronic structure modulation of ORR electrocatalysts. J. Environ. Chem. Eng. 2024, 12, 113417. [Google Scholar] [CrossRef]
- Xue, B.; Xu, J.; Xiao, R. Synthesis of Hierarchically Porous Carbon with Tailored Porosity and Electrical Conductivity Derived from Hard−Soft Carbon Precursors for Enhanced Capacitive Performance. ACS Sustain. Chem. Eng. 2021, 9, 15925–15934. [Google Scholar] [CrossRef]
- Wu, M.; Qiao, J.; Liu, Y.; Zhang, J.; Zhou, X.; Li, K. A large-scale synthesis of heteroatom (N and S) co-doped hierarchically porous carbon (HPC) derived from polyquaternium for superior oxygen reduction reactivity. Green Chem. 2016, 18, 2699–2709. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; He, Y.; Liu, Y.; Xu, C. Highly performed nitrogen-doped porous carbon electrocatalyst for oxygen reduction reaction prepared by a simple and slight regulation in hydrolyzing process of ZIF-8. J. Solid State Chem. 2021, 302, 122415. [Google Scholar] [CrossRef]
- Chu, Y.; Jiang, Q.L.; Chang, L.Y.; Jin, Y.H.; Wang, R.Z. Cobalt nanoparticles embedded in nitrogen-doped porous carbon derived the electrodeposited ZnCo-ZIF for high-performance ORR electrocatalysts. J. Electroanal. Chem. 2023, 928, 117041. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.; Zhang, Y.; Wu, D.; Bai, Y.; Liu, S.; Zhao, H. An attempt to confirm the contribution to ORR activity of different N-species in M-N-C (M = Fe, Co, Ni) catalysts with XPS analysis. Chem. Commun. 2023, 59, 4535–4538. [Google Scholar] [CrossRef]
- Yong, C.; Xu, Y.; Yu, H.; Wu, P.; Wang, J.; Shen, L.L.; Zhang, G.R.; Mei, D. Toward quantification of active site density and size-dependent ORR activity of ZIF-derived carbons in alkaline electrolyte. J. Catal. 2023, 428, 115148. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; Liu, B.; Chen, Z.; Wu, Q.; Zhao, X.; He, D.; Mu, S. 3D-ZIF scaffold derived carbon encapsulated iron nitride as a synergistic catalyst for ORR and zinc-air battery cathodes. Carbon 2021, 171, 368–375. [Google Scholar] [CrossRef]
- Lin, M.L.; Huang, C.C.; Lo, M.Y.; Mou, C.Y. Well-Ordered Mesoporous Carbon Thin Film with Perpendicular Channels: Application to Direct Methanol Fuel Cell. J. Phys. Chem. C 2008, 112, 867–873. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Guo, X.S.; Tang, Y.; Ye, J.S.; Liu, H.Y.; Xiao, X.Y. Metalloporphyrin doped macroporous ZIF-8 metal-organic framework derived M-Nx carbon material for oxygen reduction reactions. J. Alloys Compd. 2023, 947, 169441. [Google Scholar] [CrossRef]
- Bugday, N.; Altin, S.; Bulut, F.; Altin, E.; Yasar, S. Boron-doped porous carbon material derived from ZIF-11: Investigation of cotton fabric supercapacitor and Li-ion battery performances. Int. J. Energy Res. 2022, 46, 7732–7748. [Google Scholar] [CrossRef]
- He, H.; Lei, Y.; Liu, S.; Thummavichai, K.; Zhu, Y.; Wang, N. Tunable active-sites of Co-nanoparticles encapsulated in carbon nanofiber as high performance bifunctional OER/ORR electrocatalyst. J. Colloid Interface Sci. 2023, 630, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liu, M.Y.; Li, Y.; Yuan, L.; Zhang, P.; Cai, Z.; Chen, H.; Zou, J.L. ZIF-8@ZIF-67 derived ZnCo2O4@nitrogenedoped carbon/carbon nanotubes wrapped by a carbon layer: A stable oxygen reduction catalyst with a competitive strength in acid media. Mater. Today Energy 2021, 19, 100574. [Google Scholar] [CrossRef]
- Wang, T.; He, Y.; Liu, Y.; Guo, F.; Li, X.; Chen, H.; Li, H.; Lin, Z. A ZIF-triggered rapid polymerization of dopamine renders Co/N-codoped cage-in-cage porous carbon for highly efficient oxygen reduction and evolution. Nano Energy 2021, 79, 105487. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Miao, X.; Yang, W.; Wang, C.; Pan, Q. ZIF-L-Co@carbon fiber paper composite derived Co/Co3O4@C electrocatalyst for ORR in alkali/acidic media and overall seawater splitting. Int. J. Hydrogen Energy 2020, 45, 33028–33036. [Google Scholar] [CrossRef]
- Mokhtarnejad, M.; Ribeiro, E.L.; Almasi, S.; Khomami, B. Role of laser ablation synthesis parameters in ORR electrocatalytic performance of MOF-derived hybrid nanocomposites. RSC Adv. 2025, 15, 25707–25716. [Google Scholar] [CrossRef]
- Cui, J.; Cao, X.; Wang, X.; Liu, J.; Yuan, N.; Ding, J. Self-template synthesized ZIF-derived polyhedron-connected porous Co–N–C as an oxygen reduction catalyst for Zn–air batteries. N. J. Chem. 2025, 49, 12243–12251. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Sheng, M.; Gan, C.; Hu, H.; Sun, B.; Wang, X.; Jiang, H. ZIF-67 template-assisted porous carbon based RuCo synergistic effect for efficient NH3BH3 hydrolysis & 4-nitrophenol reduction: Effect of morphology and pore structure adjustment. Int. J. Hydrogen Energy 2023, 48, 36795–36809. [Google Scholar]
- Mohamud, M.A.; Yurtcan, A.B. Platinum decorated on ZIF-8 based nitrogen-doped hierarchical porous carbon composites for PEM fuel cells. J. Phys. Chem. Solids 2022, 171, 111030. [Google Scholar] [CrossRef]
- Zeng, H.J.; Wang, W.; Li, J.; Luo, J.; Chen, S.L. In situ generated dual template method for Fe/N/S Co-doped hierarchically porous honeycomb carbon for high-performance oxygen reduction. ACS Appl. Mater. Interfaces 2018, 10, 8721–8729. [Google Scholar] [CrossRef]
- Shu, J.H.; Niu, Q.J.; Wang, N.N.; Nie, J.; Ma, G.P. Alginate derived Co/N doped hierarchical porous carbon microspheres for efficient oxygen reduction reaction. Appl. Surf. Sci. 2019, 485, 520–528. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, Y.; Wang, Q.; Hu, G.; Mamat, X.; Zhang, S.; Wang, S. Hierarchically Ordered Porous Carbon with Atomically Dispersed FeN4 for Ultraefficient Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2020, 59, 2688. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, Q.; Zhu, J.; Zhong, J.; Tang, Z.; Luo, Y.; Liang, Y. Controllable construction of core-shell polymer@zeolitic imidazolate frameworks fiber derived heteroatom-doped carbon nanofiber network for efficient oxygen electrocatalysis. Small 2018, 14, 1704207. [Google Scholar] [CrossRef]
- Razzaq, A.A.; Yuan, X.; Chen, Y.; Hu, J.; Mu, Q.; Ma, Y.; Zhao, X.; Miao, L.; Ahn, J.-H.; Peng, Y. Anchoring MOF-derived CoS2 on sulfurized polyacrylonitrile nanofibers for high areal capacity lithium-sulfur batteries. J. Mater. Chem. A 2020, 8, 1298–1306. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Zhao, Y.; Song, Y.; Tang, S.; Wang, Q.; Li, W. Trimetallic ZIFs-derived porous carbon as bifunctional electrocatalyst for rechargeable Zn-air battery. J. Power Sources 2022, 542, 231723. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, D.; Zhu, X.; Wang, S.; Zou, Y.; Lan, L.; Chen, R.; Yang, Y.; Liao, Q. ZIF-67-derived Co nanoparticles embedded in N-doped porous carbon composite interconnected by MWCNTs as highly efficient ORR electrocatalysts for a flexible direct formate fuel cell. Chem. Eng. J. 2022, 432, 134192. [Google Scholar] [CrossRef]
- Ma, S.; Han, W.; Han, W.; Dong, F.; Tang, Z. Recent advances and future perspectives in MOF-derived single atom catalysts and their application: A review. J. Mater. Chem. A 2023, 11, 3315–3363. [Google Scholar] [CrossRef]
- Wu, F.; Pan, C.; He, C.T.; Han, Y.; Ma, W.; Wei, H.; Ji, W.; Chen, W.; Mao, J.; Yu, P.; et al. Single-Atom Co-N4 Electrocatalyst Enabling Four-Electron Oxygen Reduction with Enhanced Hydrogen Peroxide Tolerance for Selective Sensing. J. Am. Chem. Soc. 2020, 142, 16861–16867. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.C.; Ding, S.; Lyu, Z.; Feng, S.; Tian, H.; Huyan, C.; Xu, M.; Li, T.; Du, D. 2D Single-atom catalyst with optimized iron sites produced by thermal melting of metal-organic frameworks for oxygen reduction reaction. Small Methods 2020, 4, 1900827. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Wang, P.; Li, K.; Li, S.; Zhang, Z.; He, X.; Duan, Y. Co/N-codoped carbon nanotube hollow polyhedron hybrid derived from salt-encapsulated core-s hell ZIF-8@ZIF-67 for efficient oxygen reduction reaction. J. Alloys Compd. 2022, 904, 164083. [Google Scholar] [CrossRef]
- Ju, Y.; Huang, W.; Gao, Z.; Liu, M.; Huang, N. Research on ORR and OER performance of Co based catalyst from ZIF-67. Appl. Mater. Today 2025, 42, 102576. [Google Scholar] [CrossRef]
- Xili, D.; Zhou, Q.; Zhang, L. Well-defined Co-N-C catalyst based on ZIF-67 in mixed solvents with low amount of ligands for efficient oxygen reduction reaction. J. Alloys Compd. 2022, 911, 165072. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, C.; Cai, M.; Cai, Y.; Ju, H.; Hu, S.; Zhang, M. Porous Co-N-C ORR catalysts of high performance synthesized with ZIF-67 templates. Mater. Res. Bull. 2019, 114, 161–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, N.; Lin, G.; Wang, R.; Yang, M.; Li, K. A carbon catalyst doped with Co and N derived from the metal-organic framework hybrid (ZIF-8@ZIF-67) for efficient oxygen reduction reaction. New Carbon Mater. 2023, 38, 200–209. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; Wang, X.; Cheng, M.; Sun, S.; Hu, S.; Li, C.; Wang, Z. Core-Shell ZIF-8@ZIF-67-Derived Cobalt Nanoparticle-Embedded Nanocage Electrocatalyst with Excellent Oxygen Reduction Performance for Zn–Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 59482–59493. [Google Scholar] [CrossRef]
- Cai, R.; Jiang, J.; Diao, P.; Wei, Z.; Yao, C.; Zhou, B.; Zhang, H.; Liu, W.; Ma, Z. Highly dispersed ZIF-67-derived co-NC confined in carbon pores enables efficient oxygen reduction in alkaline media. J. Electroanal. Chem. 2025, 989, 119212. [Google Scholar] [CrossRef]
- Li, J.; Lai, G.; Li, L.; Zhang, W. Enhanced bifunctional electrocatalysis of Co@NC@NCNT derived from ZIF-67 for advanced rechargeable Zn-air batteries. Colloids Surf. Physicochem. Eng. Asp. 2025, 726, 137772. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Z.; Mei, H.; Song, B.; Gou, Q.; Hu, X.; Qi, D.; Gao, R.; Sun, X. Abundant active-site engineering enables porous Co-N-C electrocatalysts towards superior oxygen reduction reaction activity. Nanoscale 2025, 17, 15720. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.; Xu, Q.; Zhang, F.; Sun, Q.; Xie, H. Noble-metal-free Co-N-C catalyst derived from cellulose-based poly(ionic liquid)s for highly efficient oxygen reduction reaction. Int. J. Biol. Macromol. 2023, 242, 125110. [Google Scholar] [CrossRef]
- Li, L.; Han, G.; Wen, Y.; Liu, Y.; Xiao, R.; Zhang, W.; Kong, F.; Du, L.; Ma, Y.; Zuo, P. Solvent effect to modulate nitrogen dopant in Co-N-C catalysts for oxygen reduction reaction acceleration. Fuel 2023, 345, 128199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).