Formation of Improved Metallurgical Properties and Carbon Structure of Coke by Optimizing the Composition of Petrographically Heterogeneous Interbasin Coal Batches
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Detailed Study of American Coal
3.2. Using American Coal in Coking Batches
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suárez-Ruiz, I.; Crelling, J.C. Applied Coal Petrology. The Role of Petrology in Coal Utilization; Elsevier: Amsterdam, The Netherlands, 2018; 388p. [Google Scholar]
- December 2022 Crude Steel Production and 2022 Global Crude Steel Production Totals. World Steel Association. Available online: https://worldsteel.org/media-centre/press-releases/2023/december-2022-crude-steel-production-and-2022-global-totals/ (accessed on 31 January 2023).
- Metallurgical Coke Global Market Report 2023. Available online: https://www.researchandmarkets.com/report/metallurgical-coke (accessed on 2 August 2023).
- Kovalyov, E.T.; Vasiliev, Y.S.; Kuznichenko, V.M.; Krivonis, V.V.; Danilov, A.B.; Solovyov, M.A. Theory and practice of production of high-quality blast furnace coke from rammed charges of reduced spiciness. J. Coal Chem. 2009, 3–4, 24–30. [Google Scholar]
- Gordienko, A.I.; Redin, V.A.; Dolgarev, G.V.; Chalenko, V.I.; Wegera, I.M. Experience in the development and operation of the experimental and industrial plant for thermal preparation of the charge at OJSC “Yasiniv Coke Chemical Plant”. J. Coal Chem. 2008, 5–6, 22–25. [Google Scholar]
- Chernyshov, Y.A.; Ovchinnikova, S.A.; Podlubny, A.V. The use of petrographic characteristics and new complex indicators to evaluate the properties of coal and interbasin charges of Zaporizhkoks JSC. J. Coal Chem. 2009, 1–2, 12–20. [Google Scholar]
- National Standard of Ukraine 3472-2015; Lignite, Stone and Anthracite Coal. Classification. Derzhspozhivstandard of Ukraine: Kyiv, Ukraine, 2015.
- Miroshnichenko, D.; Shmeltser, K.; Kormer, M. Factors affecting the formation the carbon structure of coke and the method of stabilizing its physical and medical properties. C-J. Carbon Res. 2023, 9, 66. [Google Scholar] [CrossRef]
- North, L.; Blackmore, K.; Nesbitt, K.; Mahoney, M.R. Models of coke quality prediction and the relationships to input variables: A review. Fuel 2018, 219, 426–445. [Google Scholar] [CrossRef]
- Gupta, S.; Shen, F.; Lee, W.J.; O’Brien, G. Improving coke strength prediction using automated coal petrography. Fuel 2012, 94, 368–373. [Google Scholar] [CrossRef]
- Dash, P.S.; Guha, M.; Chakraborty, D.; Banerjee, P.K. Prediction of coke CSR from coal blend characteristics using various techniques: A comparative evaluation. Int. J. Coal Prep. Util. 2012, 32, 92–169. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Ulanovskij, M. Reactivity of coke: Methods of measuring and influence factors. Coke Chem. 2004, 5, 21–31. [Google Scholar]
- Gupta, A.; Das, A.K.; Chauhan, G.I.S. A coal-blending model: A tool for better coal blend preparation. Coal Prep. 2007, 27, 28–38. [Google Scholar] [CrossRef]
- Nag, D.; Haldar, S.K.; Choudhary, P.K.; Banerjee, P.K. Prediction of coke CSR from ash chemistry of coal blend. Int. J. Coal Prep. Util. 2009, 29, 243–250. [Google Scholar] [CrossRef]
- Todoschuk, T.W.; Price, J.; Gransden, J. Development of coke strength after reaction (CSR) at Dofasco. Iron Steel Technol. 2004, 1, 73–84. [Google Scholar]
- Wang, Q.; Guo, R.; Zhao, X.-F.; Sun, J.-F.; Zhang, S.; Liu, W.-Z. A new testing and evaluating method of cokes with greatly varied CRI and CSR. Fuel 2016, 182, 879–885. [Google Scholar] [CrossRef]
- Jordan, P. Characterising Coals for Coke Production and Assessing Coke: Predicting Coke Quality Based on Coal Petrography, Rheology and Coke Petrography. Master’s Thesis, Faculty of Engineering and the Built Environment, University of Witwatersrand, Johannesburg, South Africa, 2008; p. 121. [Google Scholar]
- Pusz, S.; Buszko, R. Reflectance parameters of cokes in relation to their reactivity index (CRI) and the strength after reaction (CSR), from coals of the Upper Silesian Coal Basin, Poland. Int. J. Coal Geol. 2012, 90–91, 43–49. [Google Scholar] [CrossRef]
- Bulanov, E.A.; Krutenkov, V.G.; Shilyakov, A.V. Predicting the postreactive strength (CSR) and reactivity (CRI) of dry-slaked metallurgical coke on the basis of Audibert-Arnu dilatometry. Coke Chem. 2009, 52, 404–407. [Google Scholar] [CrossRef]
- Dash, P.S.; Guha, M.; Chakraborty, D.; Krishnan, S.; Banerjee, P. Application of various techniques for predicting coke CSR from coal blend properties through laboratory scale experiments. Tata Search 2005, 1, 89–100. [Google Scholar]
- Kumar, A.; Saxena, V.; Varma, A.; Tiwari, H.; Kumar, A. Effect of dilatation of coal blend on push force and coke quality in stamp charge coke making process. Int. J. Eng. Sci. Res. Technol. 2015, 4, 638–644. [Google Scholar]
- Wang, W.; Fan, G.; Cheng, T. Study on prediction model for coke property under hot state. Fuel Chem. Process. 2009, 1, 002. (In Chinese) [Google Scholar]
- Xie, H.-S.; Liu, Y.-X.; Lu, Q.; Meng, J.-B. Coke quality prediction models. J. Northeast. Univ. Nat. Sci. 2007, 28, 373. (In Chinese) [Google Scholar]
- Jee, K.E. The Effect of Coal Properties on Carbonization Behaviour and Strength of Coke Blends. Master’s Thesis, School of Materials & Science Engineering, Science Engineering, Faculty of Science, The University of New South Wales, New South Wales, Australia, 2012; p. 151. [Google Scholar]
- De Córdova, M.; Madias, J.; Barreiro, J. Review of modeling of coal blends for prediction of coke. In Proceedings of the Contriuciao Tecnica ao 46 Seminario de Reducao de Minerio de Ferro e Materias-Primas, Rio de Janeiro, Brasil, 26–30 September 2016. [Google Scholar]
- Tiwari, H.P.; Banerjee, P.K.; Saxena, V.K. A novel technique for assessing the coking potential of coals/coal blends for non-recovery coke making process. Fuel 2013, 107, 615–622. [Google Scholar] [CrossRef]
- State Standard of Ukraine 4096-2002; Brown Coal, Hard Coal, Anthracite, Combustible Shale and Coal Briquettes. Methods of Sample Selection and Preparation for Laboratory Tests. Technical Committee of Ukraine on Standardization TK-92: Kyiv, Ukraine, 2002.
- ISO 1171-97; Solid Mineral Fuels. Methods for Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 589-81; Hard Coal—Determination of Total Moisture. International Organization for Standardization: Geneva, Switzerland, 1981.
- ISO 562:2024; Hard Coal and Coke—Determination of Volatile Matter. International Organization for Standardization: Geneva, Switzerland, 2024; Technical Committee ISO/TC 27/SC 5 ICS: 73.040 75.160.10.
- ISO 334:2020; Coal and Coke. Determination of Total Sulfur. Eschka Method. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 7404-3-84; Methods for the Petrographic Analysis of Bituminous Coal and Anthracite—Part 3: Method of Determining Maceral Group Composition. International Organization for Standardization: Geneva, Switzerland, 1984.
- ISO 7404-5-85; Methods for the Petrographic Analysis of Coal—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. International Organization for Standardization: Geneva, Switzerland, 1985.
- State Standard of Ukraine 7722:2015; Hard Coal. Method of Determining Plastometric Characteristics. State Enterprise “Ukrainian Scientific Research and Training Center for Problems of Standardization, Certification and Quality”: Kyiv, Ukraine, 2015.
- State Standard of Ukraine 7723:2015; Coal. Determination of the Sintering Index by the Rog Method. State Enterprise “Ukrainian Scientific Research and Training Center for Problems of Standardization, Certification and Quality”: Kyiv, Ukraine, 2015.
- ISO-FDIS 103 29; Coal—Determination of Plastic Properties—Constant-Torque Gieseler Plastometer Method. International Organization for Standardization: Geneva, Switzerland, 2017; Technical Committee ISO/TC 27/SC 5 ICS:73.040.
- ISO 349:2020; Hard Coal—Audibert-Arnu Dilatometer Test. International Organization for Standardization: Geneva, Switzerland, 2020; Technical Committee ISO/TC 27/SC 5 ICS:73.040.
- ISO 501:2025; Hard Coal. Method for Determining the Free Swelling Index. International Organization for Standardization: Geneva, Switzerland, 2025; Technical Committee ISO/TC 27/SC 5ICS: 73.040 75.160.10.
- ISO 502:2025; Hard Coal—Determination of Caking Power—Gray-King Coke Test. International Organization for Standardization: Geneva, Switzerland, 2025; Technical Committee ISO/TC 27/SC 5 ICS:73.040.
- ISO 556-80; Coke (Greater than 20 mm in Size)-Determination of Mechanical Strength. International Organization for Standardization: Geneva, Switzerland, 1980.
- ISO 18894:2006; Coke-Determination of Coke Reactivity Index (CRI) and Coke Strength After Reaction (CSR). International Organization for Standardization: Geneva, Switzerland, 2006.
- State Standard of Ukraine 8831:2019; Coke. Method for Determining the Resistivity of Coal Coke Powder. UkrNDNC SE: Kyiv, Ukraine, 2019.
- Lyalyuk, V.P.; Kassim, D.A.; Shmeltser, E.O.; Lyakhova, I.A. Improving the technology of preparing coal for the production of blast-Furnace coke under the conditions of multi-basin raw material base. Message 2. Optimizing the degree of crushing by means of petrographic characteristics of the batch. Pet. Coal 2019, 61, 94–99. [Google Scholar]
- Miroshnichenko, D.; Shmeltser, K.; Kormer, M.; Sahalai, D.; Pyshyev, S.; Kukhar, O.; Korchak, B.; Chervinskyy, T. Influence of Raw Materials and Technological Factors on the Sorption Properties of Blast-Fuel Coke. ChemEngineering 2024, 8, 30. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Shmeltser, K.; Kormer, M.; Soloviov, Y.; Pyshyev, S.; Korchak, B.; Shved, M.; Prysiazhnyi, Y. Electrical Resistance as an Aggregate Characteristic of Coke Properties for Electrochemical and Coke Production. Electrochem 2024, 5, 258–273. [Google Scholar] [CrossRef]
- Lyalyuk, V.P.; Sokolova, V.P.; Lyakhova, I.A.; Kassim, D.A.; Shmeltser, E.O.; Miroshnichenko, D.V. The using of coal blends with an increased content of coals of the middle stage of metamorphism for the production of the blastfurnace coke. Message 2. Assessment of coke quality. Pet. Coal 2019, 61, 52–57. [Google Scholar]
- Saranchuk, V.I.; Oshovsky, V.V.; Lavrenko, A.T.; Koshkarev, Y.M. Method for determining the value of electrical resistance of coal depending on temperature. Sci. J. DonNTU Chem. Chem. Technol. 2013, 134, 138–143. [Google Scholar]
- Dang, Y.; Liu, Y.; Xiang, P.; Tan, Z.; Tian, Z.; Greiner, M.; Heumann, S.; Ding, Y.; Qiao, Z.A. Carbon Surface Chemistry: Benchmark for the Analysis of Oxygen Functionalities on Carbon Materials. Adv. Mater. 2025, 37, 2418239. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qiao, Z.A. Carbon Surface Chemistry: New Insight into the Old Story. Adv. Mater. 2022, 34, 2206025. [Google Scholar] [CrossRef] [PubMed]
- Gagarin, S.G. Thermodynamic parameters of coal macerals. Coke Chem. 2008, 51, 163–170. [Google Scholar] [CrossRef]

| Company /Research Institute/ Researchers | Variable Characteristics of Coal | |||
|---|---|---|---|---|
| Petrographic Characteristics | Rheological Properties of Coal in the Plastic State | Characteristics of the Inorganic Part | Others | |
| British Steel | Vitrinite reflectance | Coke ash | ||
| Nippon Steel | Inertinite content, %, vitrinite reflectance | Maximum fluidity | Ash basicity index | |
| BCRA | Inertinite content, % | Maximum fluidity | Content of basic oxides in coal | Carbon and oxygen content in coal, coke, and porosity |
| Kobe Steel | Vitrinite reflectance | Maximum fluidity | Ash basicity | |
| BHP | Content of inert components | Maximum fluidity | Coal ash basicity | Yield of volatile matter |
| Inland Steel | The temperature of transition of coal into a plastic state | Ash basicity index | Sulfur content in coal | |
| ISCOR | Vitrinite reflectance, content of fusite components | Maximum fluidity | Content of basic oxides in coal ash | |
| Tata Steel | Vitrinite reflectance, the ratio between cohesive and inert macerals, reflectogram of the vitrinite component | Maximum fluidity, free swelling index | Ash, SiO2/Al2O3 ratio, alkali index | Composite coking potential (CCP), type of coke residue according to the Gray–King method |
| Materials | Indicator | Methods | |
|---|---|---|---|
| Characteristics of coal raw materials (coals, batches) | Ad | ISO 1171-97 “Solid mineral fuels. Methods for the determination of ash” | [28] |
| Wa | ISO 589-81 “Hard coal—Determination of total moisture” | [29] | |
| Vd, Vdaf | ISO 562:2024 “Hard coal and coke—Determination of volatile matter “ | [30] | |
| Sdt | ISO 334:2020; Coal and Coke. Determination of Total Sulfur. Eschka Method | [31] | |
| Ro, Vt, L, Sv, I | ISO 7404-3-84 “Methods for the petrographic analysis of bituminous coal and anthracite—Part 3: Method of determining maceral group composition”; ISO 7404-5-85 “Methods for the petrographic analysis of coals—Part 5: Method of determining microscopically the reflectance of vitrinite” | [32,33] | |
| X, Y | State standard of Ukraine 7722:2015 “Hard coal. Method of Determining Plastometric Characteristics” | [34] | |
| RI | State standard of Ukraine 7723:2015 Coal. Determination of the sintering index by the Rog method | [35] | |
| Fmax, t1, t2, Δt | ISO-FDIS 10329 Coal—Determination of plastic properties—Constant-torque Gieseler plastometer method | [36] | |
| tI, tII, tIII, a, b | ISO 349:2020 Hard coal—Audibert–Arnu dilatometer test | [37] | |
| FSI | ISO 501:2012 Hard coal. Method for determining the free swelling index | [38] | |
| G | ISO 502:2025 Hard coal—Determination of caking power—Gray–King coke test | [39] | |
| Characteristics of coke | M25 M10 | ISO 556-80 “Coke (greater than 20 mm in size)—Determination of mechanical strength” | [40] |
| CRI CSR | SO 18894:2006 “Coke—Determination of coke reactivity index (CRI) and coke strength after reaction (CSR)” | [41] | |
| ρ | State standard of Ukraine 8831:2019 Coke. Method for the determination of the resistivity of coal coke powder | [42] |
| Coking Period, Hours | Temperatures in the Control Verticals, °C | Coke Cake Axis Temperature, °C | |
|---|---|---|---|
| Machine Side | Coke Side | ||
| 15.5 | 1320 | 1350 | 1100 |
| Sample | Rank of Coal DSTU 3472-2015 | Proximate Analysis, % | Plastometric Parameters, mm | ||||
|---|---|---|---|---|---|---|---|
| Ad | Sdt | Vd | Vdaf | X | Y | ||
| A | G | 9.4 ± 0.2 | 0.32 ± 0.05 | 37.3 ± 0.3 | 41.2 ± 0.3 | 35 ± 3 | 14 ± 1 |
| B (coal USA) | Zh | 6.9 ± 0.2 | 1.03 ± 0.05 | 30.8 ± 0.3 | 33.1 ± 0.3 | 17 ± 3 | 20 ± 1 |
| C | Zh | 9.1 ± 0.2 | 0.54 ± 0.05 | 29.8 ± 0.3 | 32.7 ± 0.3 | 31 ± 3 | 19 ± 1 |
| D | K/KO/KZh | 12.2 ± 0.2 | 0.67 ± 0.05 | 21.5 ± 0.3 | 24.5 ± 0.3 | 28 ± 3 | 15 ± 1 |
| E | K | 8.8 ± 0.2 | 0.72 ± 0.05 | 26.6 ± 0.3 | 29.2 ± 0.3 | 17 ± 3 | 12 ± 1 |
| F | K | 12.8 ± 0.2 | 1.75 ± 0.05 | 24.0 ± 0.3 | 27.5 ± 0.3 | 36 ± 3 | 14 ± 1 |
| G | PS | 9.9 ± 0.2 | 0.40 ± 0.05 | 19.0 ± 0.3 | 21.1 ± 0.3 | 16 ± 3 | 11 ± 1 |
| H | K | 8.6 ± 0.2 | 0.41 ± 0.05 | 18.2 ± 0.3 | 20.0 ± 0.3 | 25 ± 3 | 11 ± 1 |
| Base batch | - | 10.4 ± 0.2 | 0.83 ± 0.05 | 26.5 ± 0.3 | 29.6 ± 0.3 | 34 ± 3 | 13 ± 1 |
| Sample | Petrographic Composition (Without Mineral Impurities), % | Mean Vitrinite Reflection Coefficient, % | ||||
|---|---|---|---|---|---|---|
| Vt | Sv | I | L | Σ FC | Ro | |
| A | 85 ± 4 | - | 12 ± 2 | 3 ± 2 | 12 | 0.69 ± 0.02 |
| B (coal USA) | 73 ± 5 | - | 22 ± 5 | 5 ± 2 | 22 | 1.02 ± 0.02 |
| C | 79 ± 5 | - | 20 ± 5 | 1 ± 2 | 20 | 0.94 ± 0.02 |
| D | 91 ± 4 | - | 8 ± 2 | 1 ± 2 | 8 | 1.06 ± 0.02 |
| E | 89 ± 4 | 1 ± 2 | 8 ± 2 | 2 ± 2 | 9 | 1.31 ± 0.02 |
| F | 76 ± 5 | 3 ± 2 | 21 ± 5 | - | 23 | 1.14 ± 0.02 |
| G | 89 ± 4 | 1 ± 2 | 10 ± 2 | - | 11 | 1.53 ± 0.02 |
| H | 38 ± 6 | 2 ± 2 | 60 ± 6 | - | 62 | 1.28 ± 0.02 |
| Base batch | 74 ± 5 | 1 ± 2 | 22 ± 5 | 3 ± 2 | 23 | 1.07 ± 0.02 |
| Sample | Rank of Coal DSTU 3472-2015 | Plastometric Parameter, mm, Y мм/(Rog’s Index, Units) | Free Swelling Index | Plastic–Viscous Properties According to Gieseler | Dilation According to Odbert-Arnou, °C | Sintering According to Gray–King, GK | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y/(RI) | FSI | t1, °C | tmax, °C | th, °C | ∆t, °C | Fmax, ddpm | t1 | tII | tIII | a, % | b, % | |||
| Coal USA | Zh | 20/80 | 6 1/2 | 370 | 435 | 480 | 110 | >2800 | 360 | 415 | 475 | 29 | 191 | G8 |
| Batch | - | 13/58 | 5 | 370 | 430 | 475 | 105 | 692 | 380 | 430 | 460 | 23 | 18 | G4 |
| Batch | Granulometric Composition, % | Proximate Analysis, % | Sintering According to Rog, Units | ||||
|---|---|---|---|---|---|---|---|
| Class, mm | Yield, % | Wa | Ad | Vd | Vdaf | RI | |
| >10 | 3.2 | 1.4 | 17.5 | 26.4 | 32.0 | 45 | |
| 10–6 | 5.5 | 1.6 | 11.0 | 26.3 | 29.5 | 53 | |
| 6–3 | 8.6 | 2.0 | 9.4 | 28.7 | 31.7 | 48 | |
| 3–2 | 11.6 | 1.8 | 9.3 | 27.5 | 30.4 | 53 | |
| 2–1 | 11.5 | 1.7 | 8.4 | 27.3 | 29.8 | 66 | |
| 1–0.5 | 14.0 | 2.2 | 8.5 | 26.7 | 29.2 | 65 | |
| 0.5–0.25 | 13.0 | 2.0 | 8.1 | 27.5 | 29.9 | 65 | |
| <0.25 | 32.6 | 2.1 | 12.8 | 25.0 | 28.6 | 56 | |
| Total batch (actual) | 100.0 | 1.7 | 10.4 | 26.5 | 29.6 | 58 | |
| Total batch (calculated) | 100.0 | 2.0 | 10.4 | 26.5 | 29.6 | 58 | |
| Fractional composition of the batch (class > 1 mm) | |||||||
| Fraction < 1400 kg/m3 | 78.7 | 2.1 | 6.4 | 29.3 | 31.3 | 60 | |
| Fraction > 1400 kg/m3 | 21.3 | 2.2 | 28.4 | 19.6 | 27.4 | 21 | |
| Class, mm | Yield of Grain Size, % | Petrographic Composition (Without Mineral Impurities), % | Mean Vitrinite Reflection Coefficient, % | Stages of Vitrinite Metamorphism, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.50– | 0.66– | 0.90– | 1.20– | 1.40– | 1.70– | 2.60 | ||||||||
| 0.65 | 0.89 | 1.19 | 1.39 | 1.69 | 2.59 | And More | ||||||||
| Coal Ranks, Which Roughly Correspond to The Stages of Vitrinite Metamorphism | ||||||||||||||
| Vt | Sv | I | L | Σ FC | Ro | DG | G | Zh | K | PS | T | A | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| >10; 10–6; 6–3 | 17.3 | 61 | 1 | 34 | 4 | 35 | 1.11 | 4 | 25 | 55 | 3 | 4 | 4 | 5 |
| 3–2; 2–1 | 23.1 | 70 | 1 | 28 | 1 | 29 | 1.08 | 4 | 23 | 58 | 6 | 5 | 1 | 3 |
| 1–0.5; 0.5–0.25; <0.25 | 59.6 | 72 | 1 | 25 | 2 | 26 | 1.10 | 4 | 14 | 61 | 11 | 6 | 3 | 1 |
| Total batch (actual) | 100.0 | 74 | 1 | 23 | 3 | 23 | 1.07 | 4 | 20 | 59 | 7 | 5 | 2 | 2 |
| Total batch (calculated) | 100.0 | 73 | 1 | 24 | 2 | 24 | 1.10 | 4 | 18 | 59 | 8 | 5 | 3 | 3 |
| Fractional composition of the batch (class > 1 mm) | ||||||||||||||
| Fraction < 1400 kg/m3 | 78.7 | 79 | - | 18 | 3 | 18 | 0.98 | 8 | 31 | 48 | 6 | 4 | 3 | - |
| Fraction > 1400 kg/m3 | 21.3 | 54 | 2 | 39 | 5 | 41 | 1.21 | 2 | 16 | 57 | 8 | 7 | 4 | 6 |
| Component Composition of the Batches, % | ||||
|---|---|---|---|---|
| Base Batch | Batch 1 | Batch 2 | Batch 3 | |
| A | 5 | 10 | - | 10 |
| B (coal USA) | 30 | 30 | 30 | 30 |
| C | 8 | 10 | 10 | 10 |
| D | 35 | 40 | 40 | 40 |
| E | - | 10 | 10 | - |
| F | 12 | - | - | - |
| H | 10 | - | - | - |
| G | - | - | 10 | 10 |
| 100.00 | 100.00 | 100.0 | 100.0 | |
| Sample | Proximate Analysis, % | Plastometric Parameters, mm | ||||
|---|---|---|---|---|---|---|
| Ad | Sdt | Vd | Vdaf | X | Y | |
| Base batch | 10.4 | 0.83 | 26.5 | 29.6 | 34 | 13 |
| Batch 1 | 9.7 | 0.65 | 28.0 | 30.9 | 27 | 16 |
| Batch 2 | 9.9 | 0.65 | 26.1 | 28.8 | 25 | 16 |
| Batch 3 | 9.8 | 0.61 | 27.2 | 30.0 | 27 | 16 |
| Sample | Petrographic Composition (Without Mineral Impurities), % | Mean Vitrinite Reflection Coefficient, % | ||||
|---|---|---|---|---|---|---|
| Vt | Sv | I | L | Σ FC | Ro | |
| Base batch | 74 | 1 | 22 | 3 | 23 | 1.07 |
| Batch 1 | 79 | - | 20 | 1 | 20 | 1.00 |
| Batch 2 | 78 | 1 | 19 | 2 | 20 | 1.10 |
| Batch 3 | 80 | 1 | 18 | 3 | 18 | 1.05 |
| Sample | Proximate Analysis, % | Ashing Coefficient | Desulphurization Coefficient | CRI, % | CSR, % | ρ, Om·cm | ||
|---|---|---|---|---|---|---|---|---|
| Ad | Std | Vdaf | ||||||
| Base batch | 12.9 | 0.61 | 0.9 | 1.303 | 0.806 | 38.3 ± 3.5 | 40.5 ± 3.5 | 0.0597 |
| Batch 1 | 12.9 | 0.53 | 0.6 | 1.330 | 0.815 | 36.5 ± 3.5 | 44.0 ± 3.5 | 0.0568 |
| Batch 2 | 12.6 | 0.52 | 0.5 | 1.299 | 0.800 | 36.1 ± 3.5 | 44.9 ± 3.5 | 0.0562 |
| Batch 3 | 12.8 | 0.53 | 1.1 | 1.312 | 0.869 | 35.2 ± 3.5 | 48.8 ± 3.5 | 0.0547 |
| Sample | Yield and Strength of Coke, % | ||
|---|---|---|---|
| Yield | M25 | M10 | |
| Base batch | 76.31 | 92.1 ± 3 | 7.3 ± 1 |
| Batch 1 | 75.26 | 92.3 ± 3 | 7.5 ± 1 |
| Batch 2 | 76.60 | 92.4 ± 3 | 7.0 ± 1 |
| Batch 3 | 75.82 | 92.7 ± 3 | 6.9 ± 1 |
| № | Mathematical Equations | Statistical Assessment | |
|---|---|---|---|
| r | D, % | ||
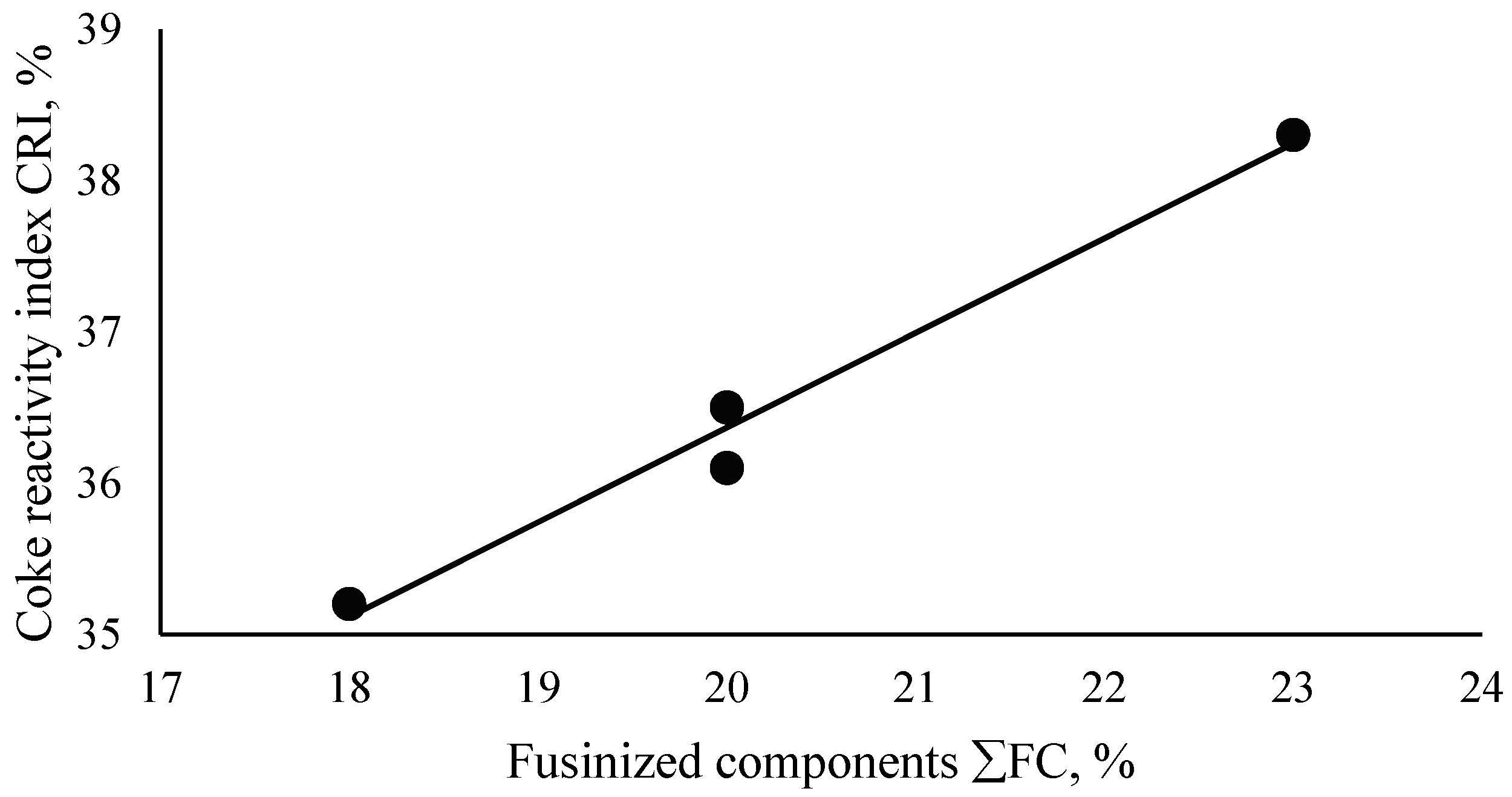
| (3) | CRI = 0.6255·ΣFC + 23.859 | 0.99 | 98 |
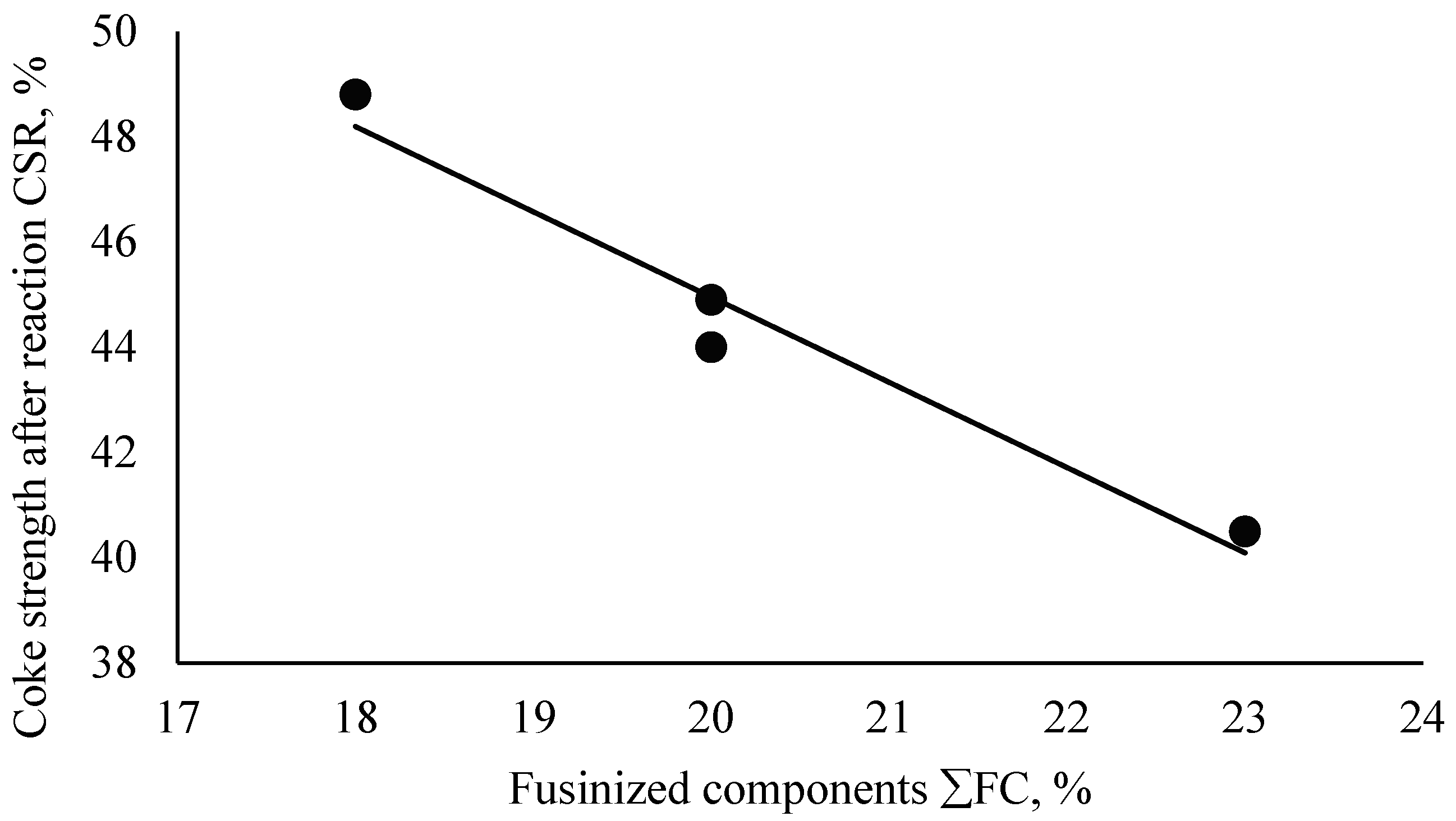
| (4) | CSR = −1.6196·ΣFC + 77.347 | 0.98 | 95.86 |
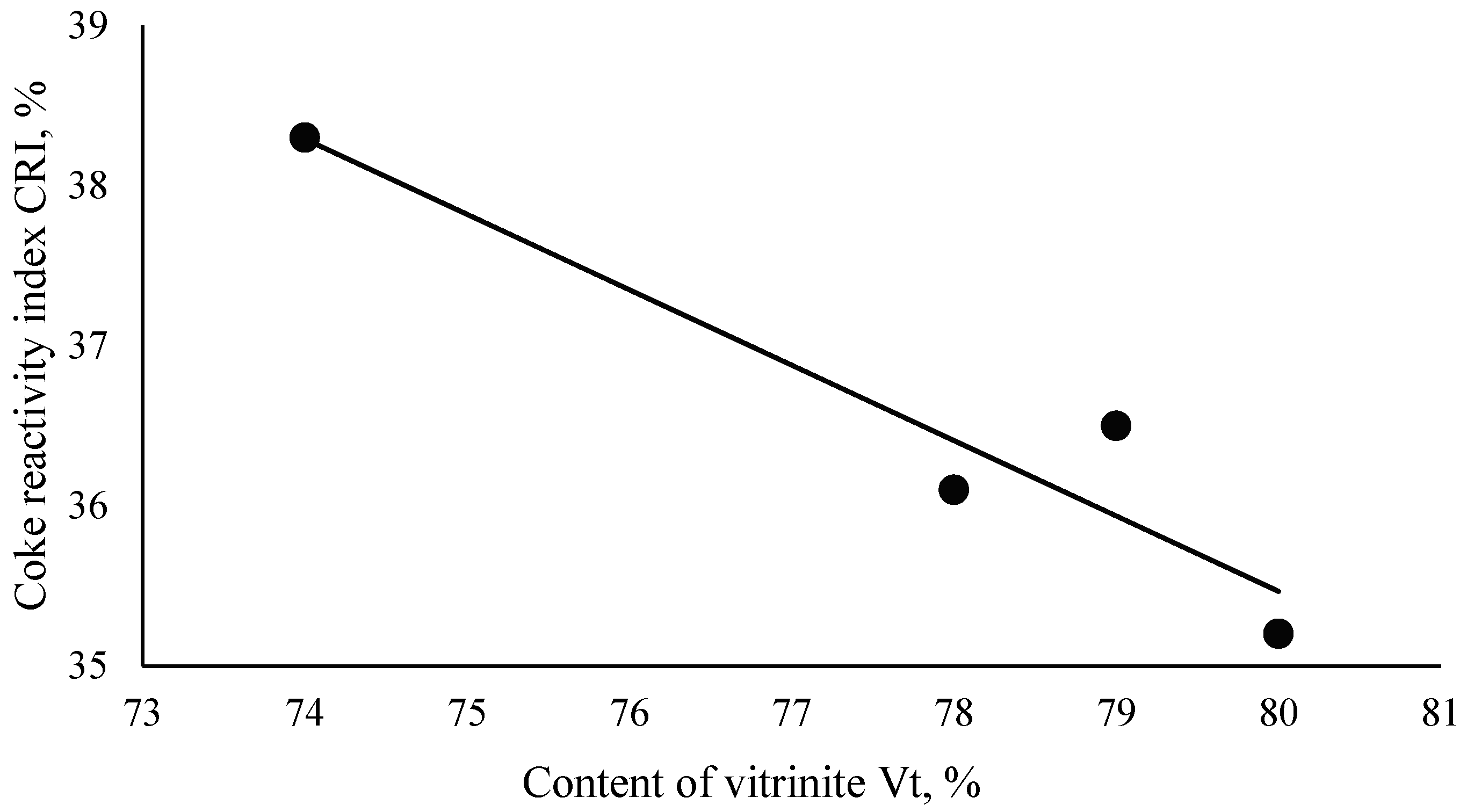
| (5) | CRI = −0.4711·Vt + 73.152 | 0.95 | 90.51 |
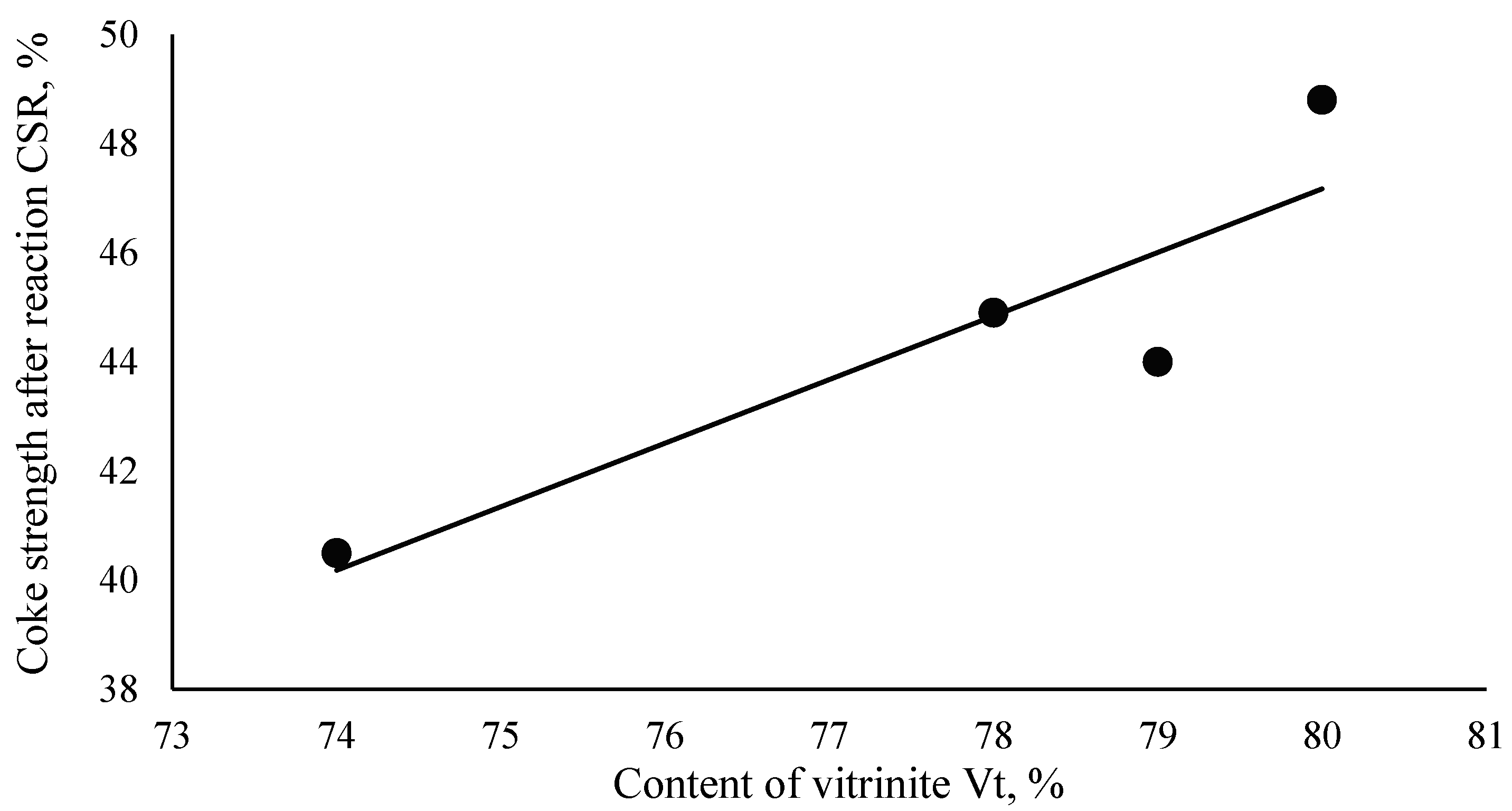
| (6) | CSR = 1.1639·Vt − 45.94 | 0.9 | 80.56 |
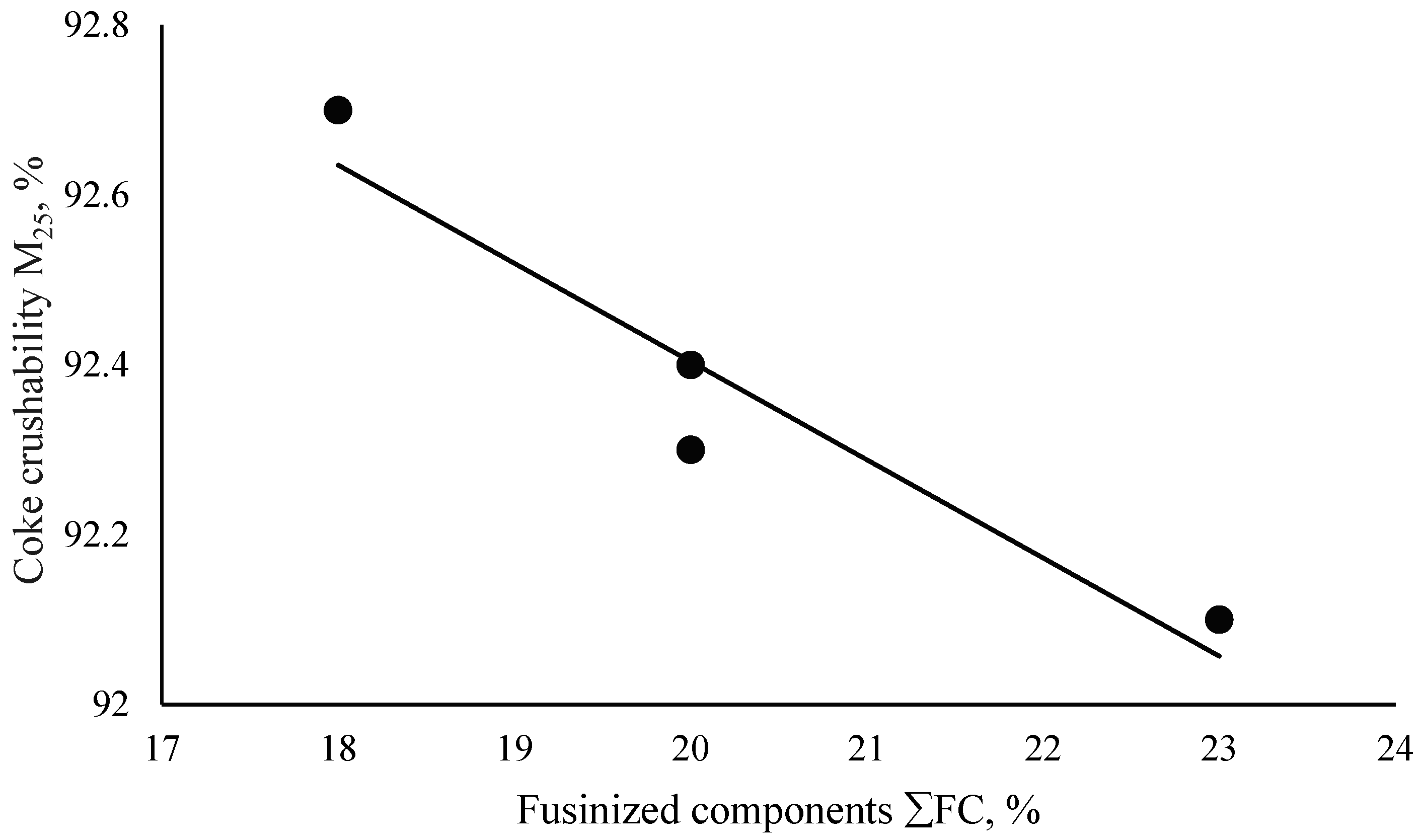
| (7) | M25 = −0.1157·ΣFC + 94.718 | 0.95 | 91.01 |
| Parameter | Base Batch | Batch 1 | Batch 2 | Batch 3 | |
|---|---|---|---|---|---|
| Reflection coefficient Ro, % | Vt | 1.07 | 1.00 | 1.10 | 1.05 |
| Sv | 1.28 | - | 1.32 | 1.26 | |
| I | 1.98 | 1.85 | 2.04 | 1.94 | |
| L | 0.58 | 0.54 | 0.59 | 0.57 | |
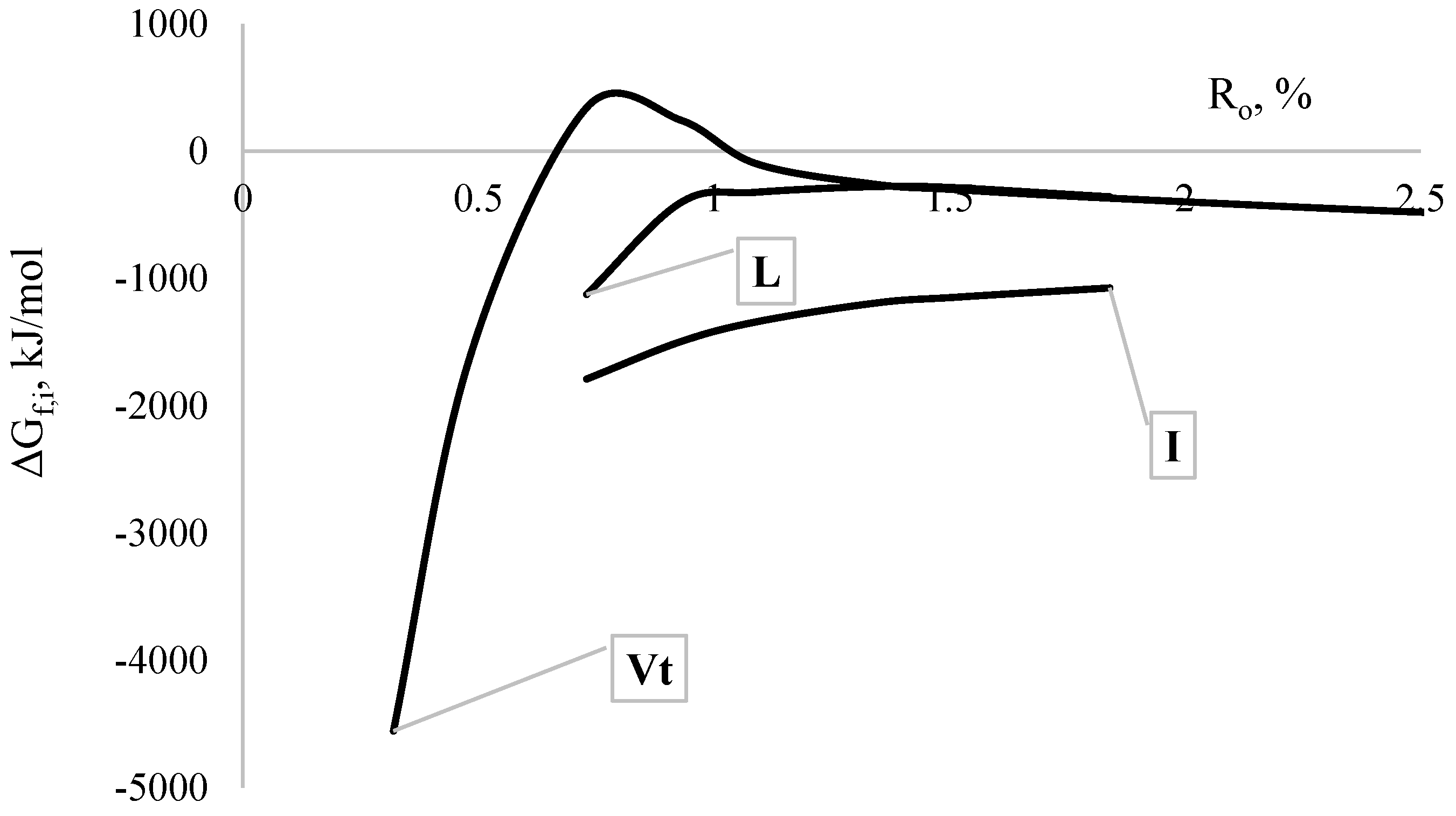
| ΔGf,i, kJ/mol | Vt | −64.9 | 71.8 | −112.5 | −10.7 |
| Sv | −1227.2 | - | −1203.8 | −1239.0 | |
| I | −1039.0 | −1069.6 | −1026.6 | −1047.7 | |
| L | −1665.4 | −1810.0 | −1614.8 | −1712.4 | |
| ΔGf,total, kJ/mol | −338.8 | −175.3 | −327.2 | −260.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnichenko, D.; Shmeltser, K.; Kormer, M.; Bannikov, L.; Nedbailo, S.; Miroshnychenko, M.; Mukina, N.; Shved, M. Formation of Improved Metallurgical Properties and Carbon Structure of Coke by Optimizing the Composition of Petrographically Heterogeneous Interbasin Coal Batches. C 2025, 11, 69. https://doi.org/10.3390/c11030069
Miroshnichenko D, Shmeltser K, Kormer M, Bannikov L, Nedbailo S, Miroshnychenko M, Mukina N, Shved M. Formation of Improved Metallurgical Properties and Carbon Structure of Coke by Optimizing the Composition of Petrographically Heterogeneous Interbasin Coal Batches. C. 2025; 11(3):69. https://doi.org/10.3390/c11030069
Chicago/Turabian StyleMiroshnichenko, Denis, Kateryna Shmeltser, Maryna Kormer, Leonid Bannikov, Serhii Nedbailo, Mykhailo Miroshnychenko, Natalya Mukina, and Mariia Shved. 2025. "Formation of Improved Metallurgical Properties and Carbon Structure of Coke by Optimizing the Composition of Petrographically Heterogeneous Interbasin Coal Batches" C 11, no. 3: 69. https://doi.org/10.3390/c11030069
APA StyleMiroshnichenko, D., Shmeltser, K., Kormer, M., Bannikov, L., Nedbailo, S., Miroshnychenko, M., Mukina, N., & Shved, M. (2025). Formation of Improved Metallurgical Properties and Carbon Structure of Coke by Optimizing the Composition of Petrographically Heterogeneous Interbasin Coal Batches. C, 11(3), 69. https://doi.org/10.3390/c11030069








