1. Introduction
Activated carbon is considered a widely versatile adsorbent platform and constitutes the primary class of physical adsorbent used in industry, after activated alumina, with a total market of USD 4.4 billion in 2023. Activated carbon materials are widely used in gas- and liquid-phase adsorption processes (e.g., water treatment, food and beverages, pharmaceuticals, etc.). The widespread use of activated carbon materials is based on their widely developed porous structure and tailored surface chemistry [
1]. While carbon materials exhibit an excellent performance for high-boiling-point molecules (e.g., volatile organic compounds), these materials fail for low-boiling-point probes (e.g., toxic or odorous gases) due to the small adsorption potential and low packing density in narrow pores, as well as the absence of condensation processes (supercritical conditions) [
2,
3,
4]. These limitations can be overcome either by using high pressure (e.g., methane storage) or through the incorporation of an impregnant able to selectively trap the target compound (e.g., the removal of toxic gases) [
1,
5,
6]. One of these gases is hydrogen sulfide (H
2S). Hydrogen sulfide is a component in hydrocarbon sources (e.g., natural gas, biogas, syngas, etc.), and must be selectively removed to mitigate associated side effects such as corrosion in industrial facilities, poison for catalysts, environmental pollution, and health effects [
7]. Among technological approaches, adsorption using impregnated carbon materials is one of the most promising alternatives due to the low cost and high efficiency of the process (unfortunately, unmodified carbon materials exhibit a poor adsorption performance) [
8,
9]. The development of high-surface-area carbon materials as a support for the active phase (e.g., either metal oxides or caustic impregnants) is of paramount importance to provide high dispersion, proper diffusion, and consequently, an optimized performance [
10,
11]. Impregnation with sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, potassium iodide, and potassium iodate has been widely described in the literature for H
2S removal [
11,
12,
13,
14,
15]. These studies have anticipated the crucial role of the alkali impregnant in the removal process, although it is associated with severe blocking effects after deposition and/or after the H
2S adsorption process, with the associated formation of bulky carbonates or sulfates. Another limitation arises when comparing activated carbons from different origins due to the concomitant effect of a different porosity (promoting H
2S physisorption), and the associated differences in the characteristics of the alkaline species deposited (promoting H
2S chemisorption/reaction) [
16]. The presence of these variables simultaneously prevents proper evaluation of the real role exerted by the alkali impregnant, triggering important questions regarding the role of alkali content, effect of humidity, and effect of the intrinsic porosity of the carbon material, among others. Furthermore, a proper evaluation of the exhausted/used catalysts is needed to provide more insight about the reaction mechanism for NaOH-impregnated carbons (e.g., formation of elemental sulfur, sulfur dioxide, sulfates, or even sulfuric acid).
With these premises in mind, the objective of this study is the development of novel H2S adsorbents using sodium hydroxide (NaOH) as an impregnant and a common activated carbon material as a support. The activated carbon selected is characterized by a highly developed porous structure (large BET surface area) and a perfectly tailored micro-/mesoporous network to minimize blocking effects. This material will be used as a support for different NaOH loadings to be applied under dry and moist conditions under industrially relevant H2S streams.
3. Results and Discussion
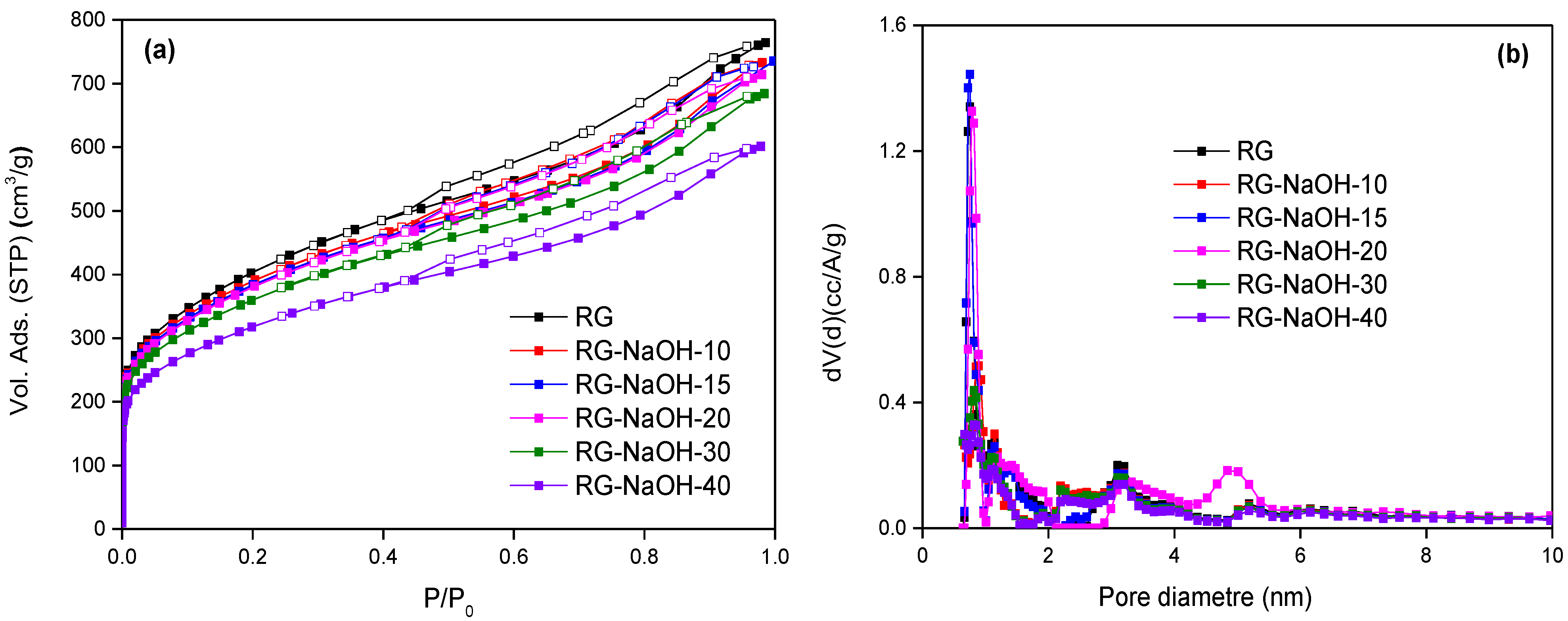
The textural properties of the original and NaOH-loaded samples are evaluated using nitrogen adsorption measurements at cryogenic temperatures.
Figure 1a confirms the micro-/mesoporous nature of the original RG activated carbon with a specific BET surface area of 1425 m
2/g. Impregnation with NaOH gives rise to a decrease in the nitrogen adsorption capacity, mainly in the microporous region. As shown in
Figure 1a and
Table S1, the amount of nitrogen adsorbed decreases with the amount of NaOH incorporated, down to a BET surface area of 1140 m
2/g for sample RG-NaOH-40 (~20% decrease). This decrease in textural properties after NaOH incorporation does not necessarily reflect the presence of blocking effects by NaOH, but can also be attributed to the additional weight of the impregnated samples. Indeed, the normalization of the nitrogen adsorption isotherms per unit mass of activated carbon (
Figure S1) confirms the absence of severe blocking effects by NaOH, i.e., the deposition of NaOH on the surface of the high-surface-area RG activated carbon creates a thin alkaline film with basic properties, with minimal porosity loss. The formation of an extended interfacial layer while preserving most of the excellent textural properties (micro- and mesoporous structure) in a high-surface-area porous system is an ideal scenario to obtain optimized H
2S adsorbents. Pore size distribution estimated from the nitrogen adsorption data (
Figure 1b) confirms the combined presence of narrow and wide micropores in the evaluated carbons, together with some significant contributions of mesopores around 3–5 nm, with small differences between the samples. The total amount of NaOH incorporated is roughly estimated from the weight increase after impregnation. The real NaOH content ranges from 3.7 wt.% for sample RG-NaOH-10 up to 13.4 wt.% for sample RG-NaOH-40 (
Table S1). These results anticipate that the total amount of NaOH that can be incorporated in a high-surface-area carbon material, such as RG, is limited, even at extremely high solution concentrations (nominal value of 40 wt.% vs. real value of 13.4 wt.% for sample RG-NaOH-40).
The Raman spectra of the modified carbon materials shown in
Figure 2a are dominated by two intense bands at ~1350 cm
−1 and 1600 cm
−1, which are attributed to D and G contributions, respectively. More specifically, the D band corresponds to the A
1g breathing mode symmetry, which is associated with the carbon atoms having a disordered sp
3 configuration, while the G band is associated with the E
2g mode symmetry, corresponding to the sp
2 carbon system, and results from the in-plane bond stretching of the C-C bond in graphitic materials [
17]. The I
D/I
G ratio (an indirect measure of structural disorder) is rather similar among the samples, thus suggesting that NaOH incorporation does not modify the carbon matrix. The inset reflects the new Raman shifts developed after NaOH incorporation, attributed to O-H vibrational modes from NaOH or H
2O in the range of 2000–3500 cm
−1. XRD patterns for the pristine activated carbon (
Figure 2b) reflect characteristic broad diffraction peaks at 23° and 43°, attributed to the (022) and (100) planes from the graphitic microdomains in the amorphous activated carbon structure. The incorporation of NaOH does not produce any modification in the XRD pattern, except for in samples RG-NaOH-30 and RG-NaOH-40, with new diffraction peaks appearing in the 2-theta range of 30–50°. These new peaks do not correspond to NaOH, but rather to Na
2CO
3 microcrystals formed on the carbon surface (main peaks at 30.2°, 34°, 35.2°, and 38°) [
18,
19]. These results anticipate that the thermal treatment at 70 °C performed after impregnation with NaOH promoted the carbonation reaction with ambient CO
2 to Na
2CO
3 [
20]. However, the presence of amorphous NaOH cannot be ruled out.
For a better understanding of the morphology and microstructure of the NaOH-modified carbon materials, the samples are evaluated using FE-SEM. Representative images (
Figure 3) show the granular shape of the pristine RG activated carbon and the formation of white needles in the modified samples due to the crystallization of Na
2CO
3, in close agreement with the XRD patterns. The good dispersion of the Na
2CO
3 nano-crystallites on the carbon structure and the absence of appreciable aggregation (or blocking) constitute
a priori an excellent platform for the design of efficient H
2S adsorbents. Mapping analysis (
Figure S2) confirms the excellent dispersion of sodium atoms in the evaluated surfaces.
In general, hydrogen sulfide removal using activated carbon materials involves several steps, i.e., the adsorption of H
2S on the carbon surface, the dissolution and dissociation of H
2S on the surface water film (under humid conditions), facilitating the formation of HS
− and H
+, the oxidation of HS
− with adsorbed oxygen, and the formation of elemental sulfur or sulfur dioxide (depending on the surface characteristics). The additional oxidation of SO
2 to H
2SO
4 can take place in the presence of water and metal impurities that promote catalytic oxidation [
14]. Therefore, the removal mechanism combining physical adsorption and a subsequent oxidation step can be summarized as follows:
An important factor that plays a significant role in the desulfurization process is the surface pH of the carbon before and after impregnation. A low pH of the carbon surface is expected to suppress H
2S dissociation and the creation of HS
− ions so that only physical adsorption can occur. Under these conditions, sulfur is easily oxidized to SO
2 and converted to H
2SO
4 in small pores [
14]. On the contrary, a basic pH promotes these dissociation processes to produce HS
− or S
2−, with HS
− being oxidized to polymeric sulfur with a chain or ring-like morphology (S
n). The pH values obtained in this study are reported in
Table 1. While the pristine carbon has a neutral pH, loading NaOH gives rise to a significant increase in the pH up to values of around 10.3–10.6, independently of the amount of NaOH incorporated.
The pKa constants for H2S are 7.2 and 13.9 for the first and second dissociation, respectively. Based on the pH values obtained, a high concentration of HS− ions is expected under the experimental conditions tested in this study, including the possibility to make polysulfides.
The thermal behavior of the NaOH-loaded samples is evaluated using thermogravimetric analysis.
Figure 4 shows the TG profiles for the different samples in the temperature range of 25–1000 °C under a nitrogen atmosphere. The TG profiles exhibit an initial weight loss at low temperatures (around 80–100 °C) due to the removal of moisture. Above 100 °C, the TG profile remains relatively stable up to 700–800 °C, where a significant weight loss is observed (up to 1000 °C). The magnitude of this weight loss at high temperatures scales up with the amount of NaOH incorporated (5 wt.% for RG; 12 wt.% for RG-NaOH-10; 13 wt.% for RG-NaOH-15; 15 wt.% for RG-NaOH-20; 18 wt.% for RG-NaOH-30; and 20 wt.% for RG-NaOH-40), most probably associated with the high-temperature decomposition of the alkali carbonate (Na
2CO
3) to CO
2 and Na
2O. Na
2CO
3 decomposition is traditionally associated with a large CO
2 evolution starting at 780 °C and reaching the maximum at 850 °C, in perfect agreement with our TG measurements [
21].
The performance of the NaOH-loaded samples in the removal of H
2S was tested under dry and wet conditions. To this end, breakthrough column experiments were performed using a simulated industrial stream containing 800 ppm H
2S, 2.5% O
2, and 50% CO
2.
Figure 5a reports the breakthrough column performances for all the NaOH-loaded samples under humid conditions (60% relative humidity) at 25 °C. The performance of the pristine activated carbon is very limited under these experimental conditions, with a total uptake as low as 2 mg/g. The result achieved with sample RG anticipates that the presence of a highly developed porous structure and a large pore volume in the microporous range is not sufficient to reach a high H
2S adsorption capacity [
14,
22]. In other words, the role of micropores and mesopores in the H
2S removal process, if any, must be very limited. On the contrary, samples modified with NaOH exhibit a large improvement in removal performance. For all samples, the breakthrough profile at saturation exhibits a sharp increase, characteristic of acid–base reaction processes, i.e., once all basic sites are saturated, H
2S breaks the column sharply. As expected, the breakthrough time and, indirectly, the total removal capacity scale-up with the amount of NaOH incorporated up to an optimum value for sample RG-NaOH-30. Larger NaOH contents become detrimental for the total removal efficiency, most probably due to the growth of large Na
2CO
3 nanocrystals (less optimized dispersion). The total removal capacity measured up to the breakthrough point is reported in
Figure 5b. Highly dispersed Na
2CO
3 nanocrystals in RG activated carbon give rise to a large improvement in the H
2S removal capacity, e.g., a more than one hundred times (100×) improvement between unmodified RG and sample RG-NaOH-10. Several repetitions are performed to evaluate the reproducibility of the obtained results, with the standard deviation among measurements being very small for all samples evaluated. This is somehow expected, because NaOH impregnation must be relatively homogeneously dispersed, giving rise to an optimized performance. At this point, it is important to highlight that the total removal capacity for samples RG-NaOH-30 and RG-NaOH-40 is highly above the threshold value of 600 mg/g. Sample RG-NaOH-30 even reaches values close to 800 mg/g. To our knowledge, this is one of the best adsorption values reported in the literature for H
2S removal using either catalytic or acid–base adsorbents [
23]. The excellent performance achieved with these samples must be attributed to the presence of highly dispersed NaOH/Na
2CO
3 nanocrystals on the surface of a highly activated carbon material, such as RG. Previous studies described in the literature using Fe@carbon-rich nanoparticles reported exceptional values slightly above 600 mg/g under similar experimental conditions [
23]. The value obtained with sample RG-NaOH-30 highly surpasses the performance of catalytic Fe@carbon nanoparticles and constitutes a four-fold increase compared to commercial H
2S adsorbents (chemically impregnated adsorbents) such as ADDSORB
TM VA3 and ADDSORB
TM VA6, with total values of 177 and 204 mg/g, respectively, under the same experimental conditions (in agreement with the values reported in the technical sheets) [
23]. The obtained values constitute a four-fold increase compared to the theoretical predictions for MOFs (e.g., MIL-101) in the H
2S process, and are well above the performance of metal oxides and zeolites [
24,
25]. Overall, these results confirm that the newly designed NaOH-impregnated carbons exhibit exceptional behavior for H
2S removal. Taking into account the stoichiometry of the acid–base reaction process, the obtained results are twelve times above the stoichiometric value for the real amount of NaOH incorporated. This observation reflects the autocatalytic behavior of the reaction products, as described in the literature [
14].
One of the critical parameters in the removal of H
2S is the presence or absence of humidity and the potential formation of a water film on the carbon surface. To evaluate the effect of relative humidity on the H
2S removal performance, sample RG-NaOH-30 is tested under different moisture conditions. As can be appreciated in
Figure 6, moisture content is critical for the removal process, with the total amount adsorbed ranging from 307 mg/g at 40% RH up to 800 mg/g at 60% relative humidity. Unexpectedly, a larger humidity content becomes detrimental for H
2S adsorption performance, with a significant decrease (~30% reduction). In any case, the breakthrough tests exhibit a similar profile shape, independently of the moisture concentration.
To obtain some insights into the reaction mechanism, used samples (after H
2S) were evaluated using FE-SEM (samples labeled RG-NaOH-XX-af).
Figure 7 displays the morphology of the adsorbents after being exposed to saturation with hydrogen sulfite. Nicely, the FE-SEM images show the formation of large micron-size crystals, more clearly visible in the samples with a high NaOH content. The presence of perfectly defined microcrystals must be related to the reaction between NaOH and H
2S, most probably associated with the formation of sodium sulfate crystals (Na
2SO
4) and/or S
n crystals. The formation of sodium sulfate could be due to the interaction between SO
2 and NaOH or Na
2CO
3, through the following reactions:
The formation of S
n will be associated with the following reactions:
The predominant gemstone-like shape of the observed crystals suggests the presence of sulfur (Sn) crystals, rather than Na2SO4 (more needle-like crystals will be formed). Furthermore, the associated formation of NaOH upon NaHS oxidation will explain the large efficiency of the designed sorbents, well above the stoichiometric values.
Post mortem sorbents were also evaluated using X-ray diffraction. The XRD patterns described in
Figure S3 keep the broad bands at 23° and 45° due to the graphitic structure. Contrary to the fresh samples, the XRD peaks corresponding to Na
2CO
3 vanish after H
2S exposure and new bands emerge at 22.5°, 23.6°, 24.7°, 31°, and 32.7°. Although these bands could be attributed to Na
2SO
4, they better fit with the fingerprint of S
8 crystals formed on the material surface [
26,
27]. These peaks are more clearly appreciated for sample RG-NaOH-40-af.
Used adsorbents were also evaluated using thermogravimetric analyses (
Figure S4). The TG profiles of the NaOH-loaded samples are characterized by a prominent peak at ca. 360–400 °C and some minor contributions at 700–800 °C. The main contribution at low temperatures is not present in the original samples and, consequently, must be derived from the H
2S removal process. Furthermore, the main contribution presents a small shoulder at lower temperatures (~265 °C), only visible in the samples loaded with NaOH. Previous studies described in the literature have anticipated that the desorption of oxidized sulfur species (SO
2, H
2SO
4, etc.) takes place at lower temperatures (<300 °C), while higher temperatures (400–500 °C) correspond to the desorption of elemental sulfur [
14,
22]. The increased magnitude of the desorption peak at 360–400 °C with the amount of NaOH incorporated is in close agreement with the improved adsorption performance for samples with high caustic loadings and the higher S
8 formation yield. Furthermore, the shoulder at 265 °C due to oxidized sulfur is rather small and similar among all the samples evaluated, thus confirming that the formation of SO
2 and H
2SO
4 must be quite restricted under the experimental conditions tested. High-temperature peaks (>700 °C) must be associated with other sulfur species, most probably located in small micropores. The pH of the used samples (
Table 1) after the H
2S breakthrough experiment ranges from 3.5 to 8.7, depending on the NaOH concentration, thus confirming the preferential formation of sulfur. However, the formation of some residual highly acidic sulfur species (e.g., H
2SO
4) cannot be completely ruled out, in agreement with the TG measurements.
To further clarify the reaction mechanism and identify the nature of the observed crystals, the used adsorbents are evaluated using X-ray photoelectron spectroscopy (XPS).
Figure 8 shows representative spectra for the used sample RG-NaOH-30-af in the C1s, O1s, S2p, and Na1s regions. Quantitative analysis of the different materials is reported in
Table S2. As expected, the used sorbents exhibit a large sulfur content ranging from 8 at.% for sample RG-NaOH-10 up to 17–18 at.% for samples RG-NaOH-30/-40. A closer look at the XPS spectra in the S2p region (
Figure 8) clearly identifies two predominant peaks attributed to sulfur (S
8), with binding energies at 164.0 and 165.3 eV, while the contribution of sulfates (above 168 eV) is very small [
23,
28,
29]. These results confirm that the nature of the perfectly shaped microcrystals observed using the FE-SEM images of the used adsorbents corresponds to S
8 gemstone-like crystals, and not to Na
2SO
4.