Atomic-Scale Mechanisms of Catalytic Recombination and Ablation in Knitted Graphene Under Hyperthermal Atomic Oxygen Exposure
Abstract
1. Introduction
2. Model and Methods
2.1. Model Construction
2.2. Simulation Details
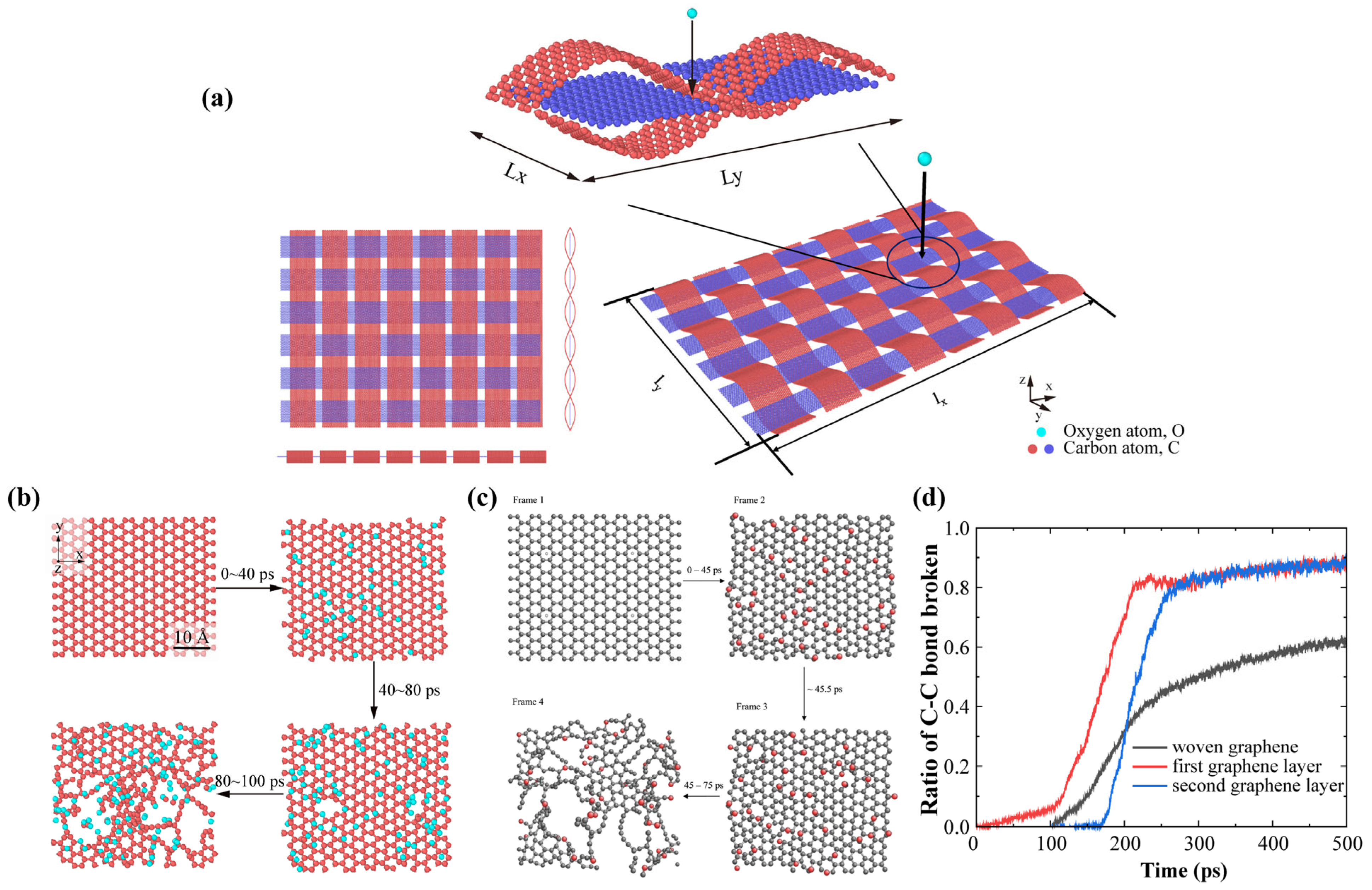
2.3. Model and Validation
3. Results and Discussion
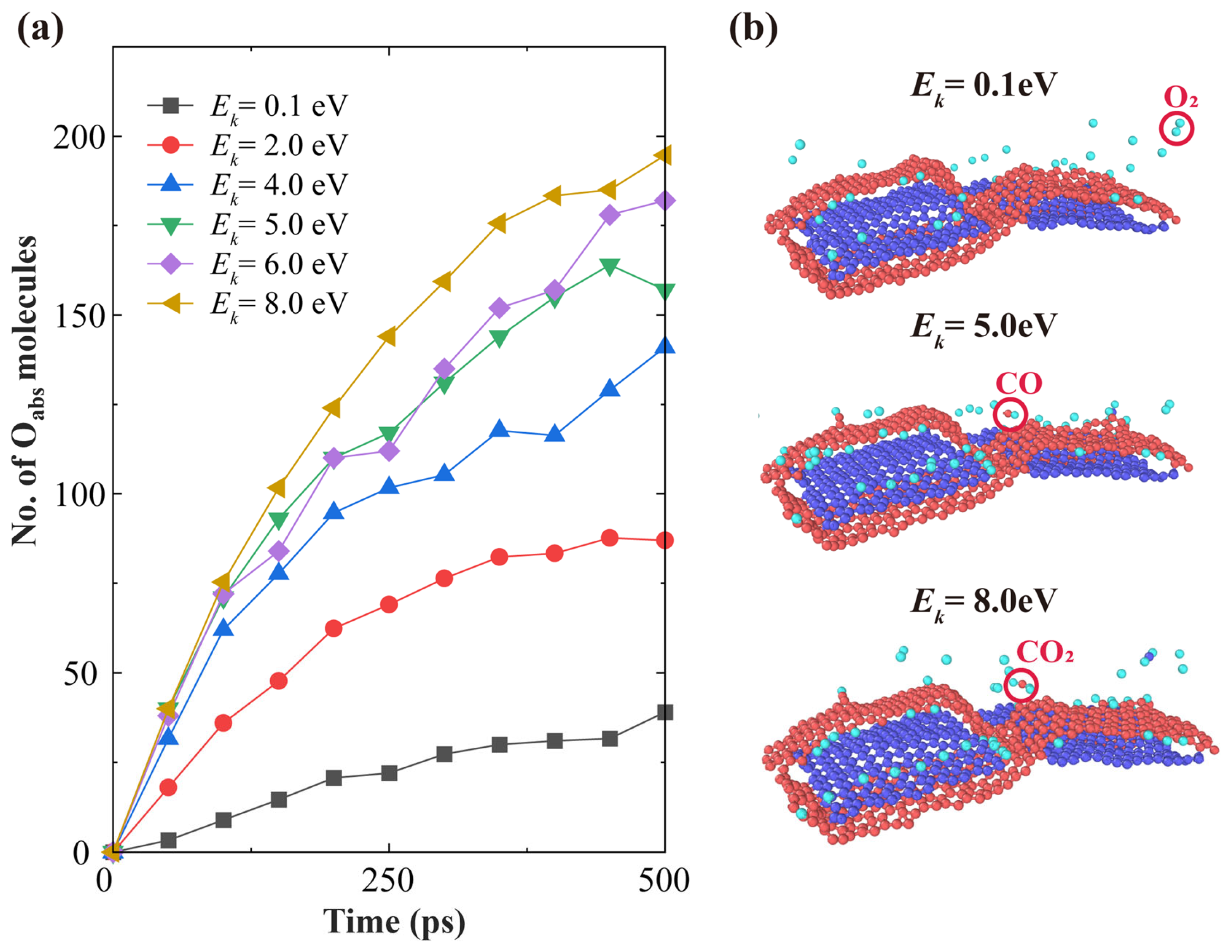
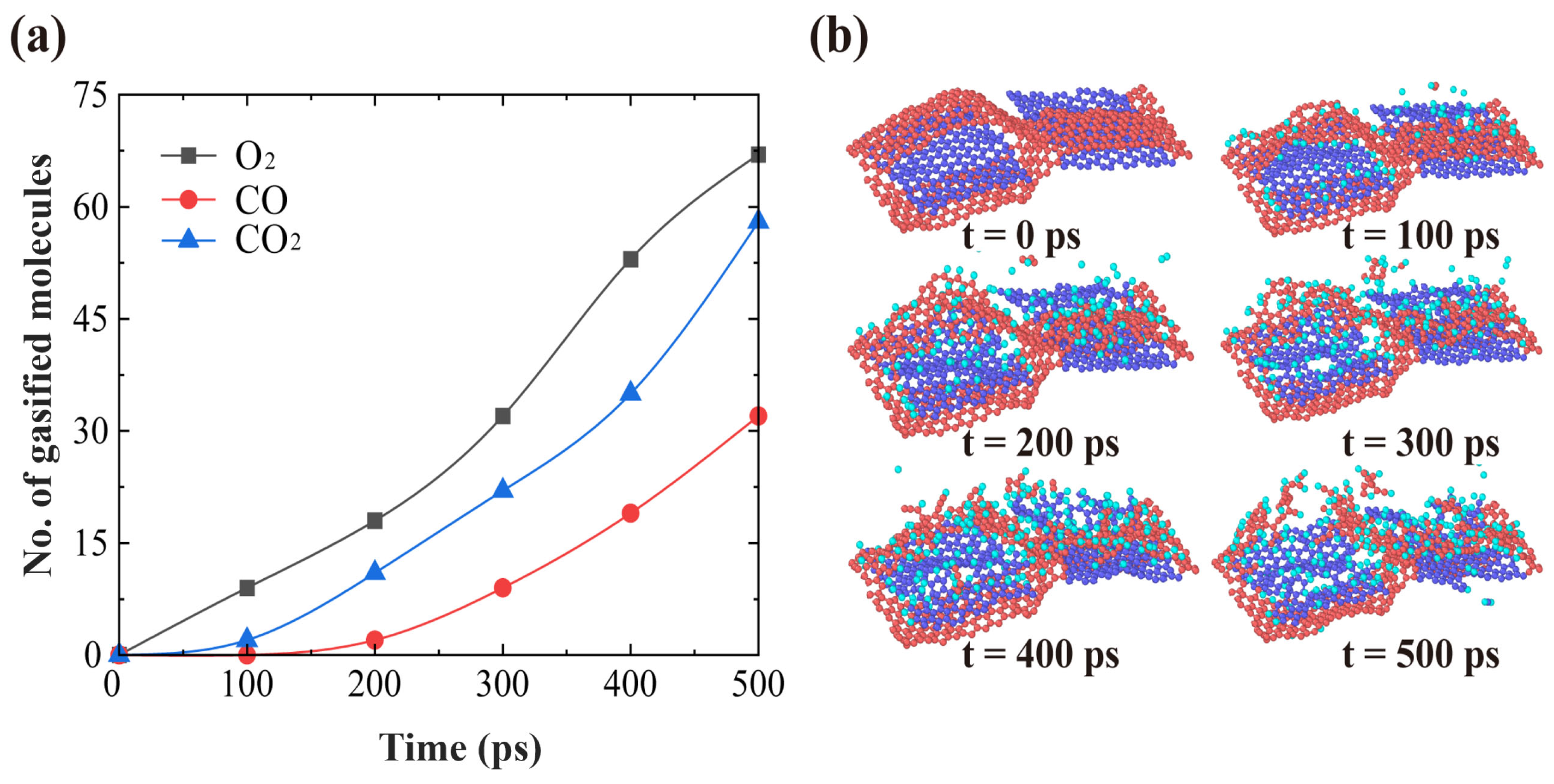
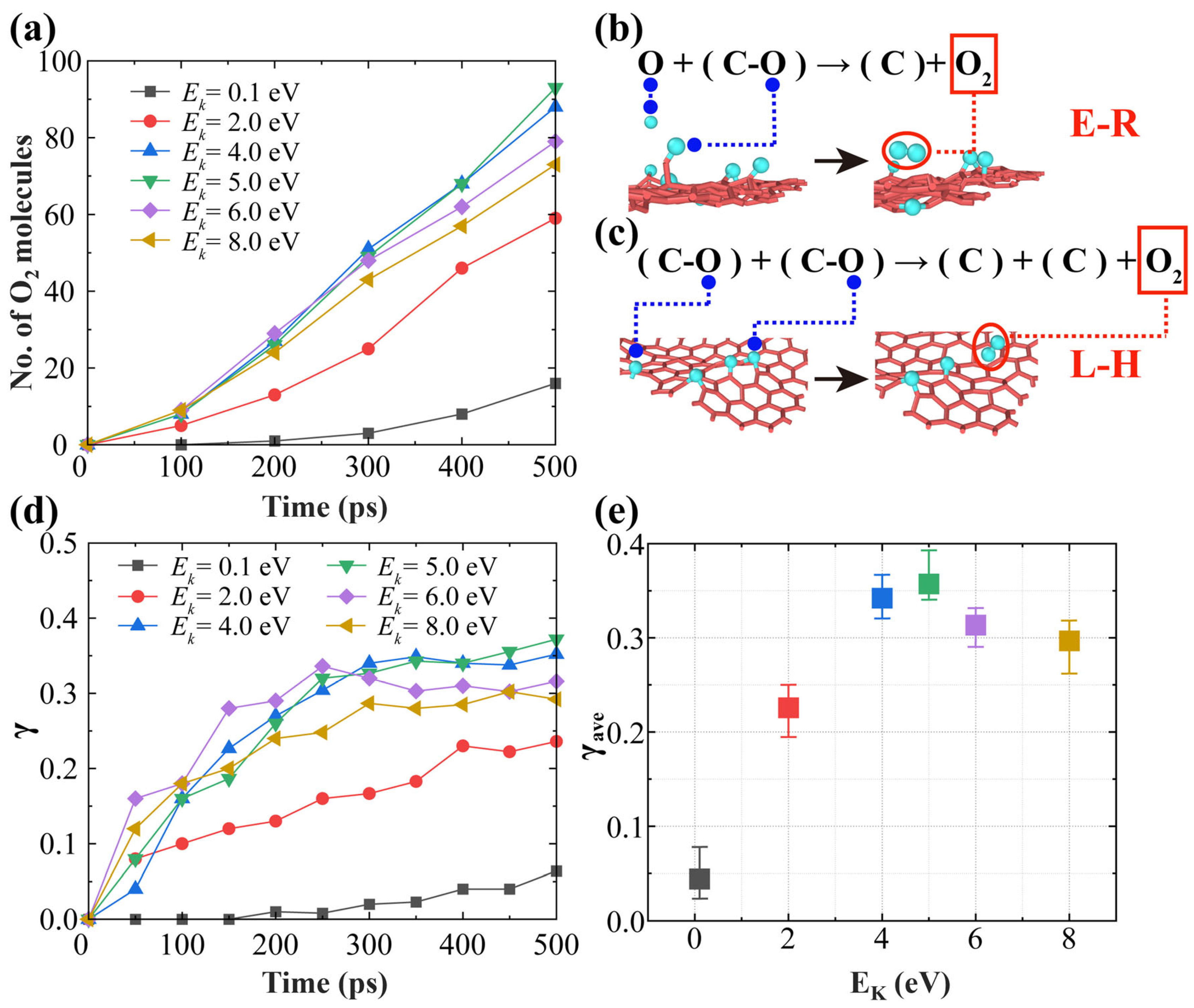
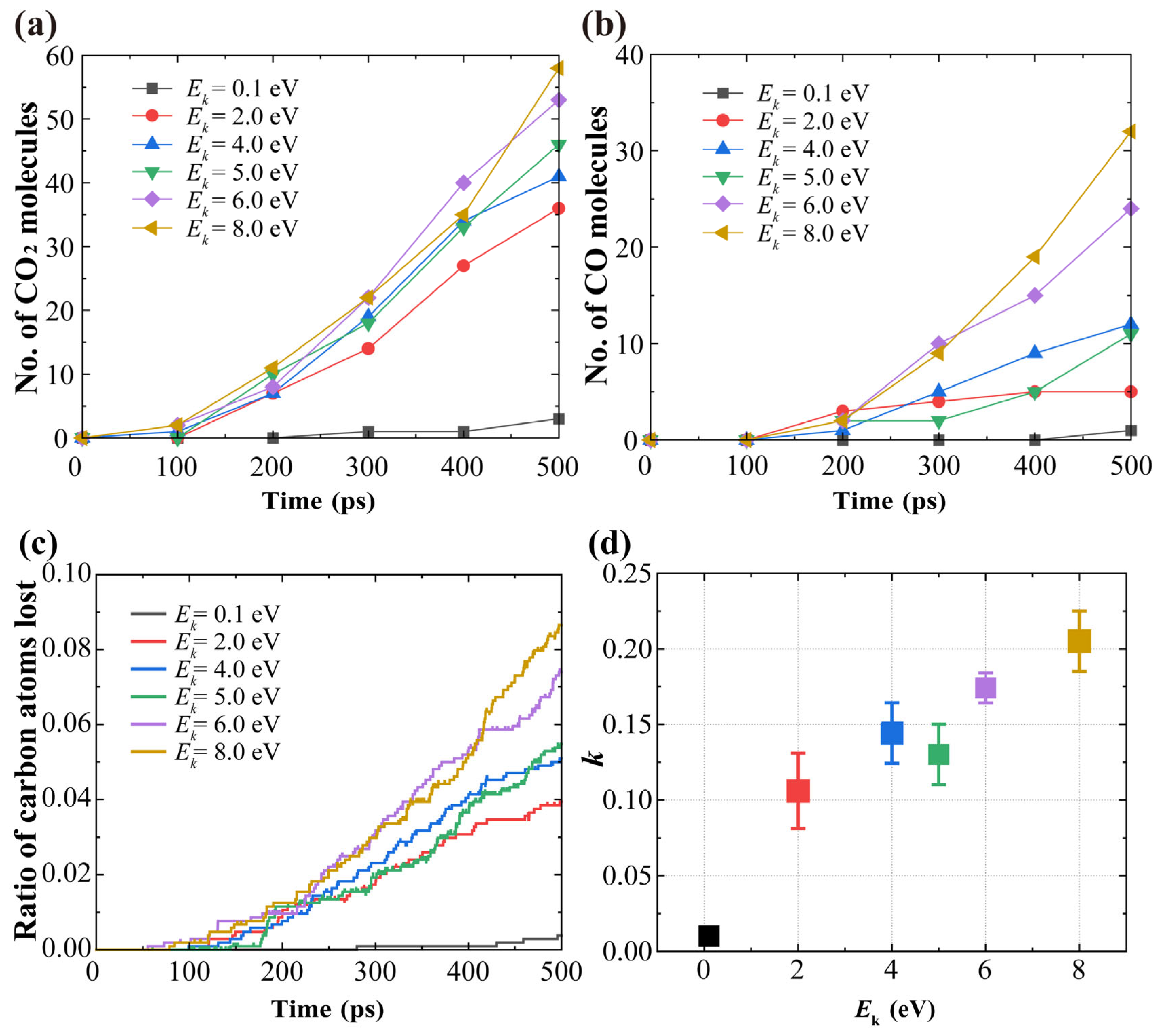
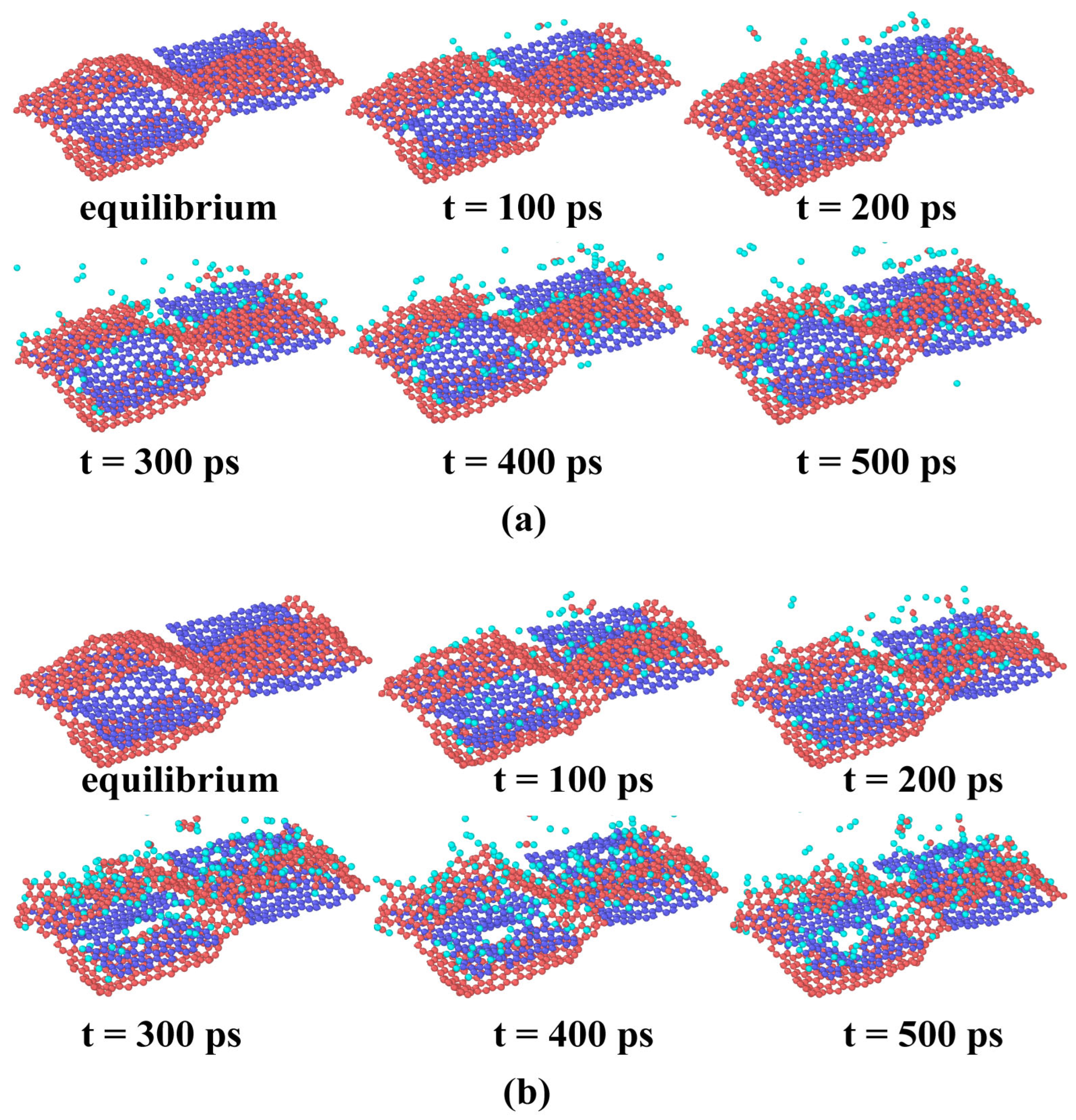
3.1. Effects of AO Incident Kinetic Energy on the Ablation of Knitted Graphene
3.2. Effects of AO Incident Angle on the Ablation of Knitted Graphene
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Singh, N.; Schwartzentruber, T. Nonequilibrium internal energy distributions during dissociation. Proc. Natl. Acad. Sci. USA 2018, 115, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hader, C.; Fasel, H.F. Direct numerical simulations of hypersonic boundary-layer transition for a flared cone: Fundamental breakdown. J. Fluid Mech. 2019, 869, 341–384. [Google Scholar] [CrossRef]
- Streicher, J.W.; Krish, A.; Hanson, R.K. Vibrational relaxation time measurements in shock-heated oxygen and air from 2000 K to 9000 K using ultraviolet laser absorption. Phys. Fluids 2020, 32, 086101. [Google Scholar] [CrossRef]
- Colonna, G.; Bonelli, F.; Pascazio, G. Impact of fundamental molecular kinetics on macroscopic properties of high-enthalpy flows: The case of hypersonic atmospheric entry. Phys. Rev. Fluids 2019, 4, 033404. [Google Scholar] [CrossRef]
- Ma, Q.; Cui, J.; Li, Z.; Wang, Z. Second-order asymptotic algorithm for heat conduction problems of periodic composite materials in curvilinear coordinates. J. Comput. Appl. Math. 2016, 306, 87–115. [Google Scholar] [CrossRef]
- Candler, G.V. Rate effects in hypersonic flows. Annu. Rev. Fluid Mech. 2019, 51, 379–402. [Google Scholar] [CrossRef]
- Herault, J.; Facchini, G.; Le Bars, M. Erosion of a sharp density interface by a turbulent jet at moderate Froude and Reynolds numbers. J. Fluid Mech. 2018, 838, 631–657. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Wu, J.; Chen, S.; Lee, C.; Gad-el-Hak, M. Aerodynamic heating in transitional hypersonic boundary layers: Role of second-mode instability. Phys. Fluids 2018, 30, 011701. [Google Scholar] [CrossRef]
- Alba, C.R.; Greendyke, R.B.; Lewis, S.W.; Morgan, R.G.; McIntyre, T.J. Numerical modeling of earth reentry flow with surface ablation. J. Spacecr. Rocket. 2016, 53, 84–97. [Google Scholar] [CrossRef]
- Martin, A.; Cozmuta, I.; Wright, M.J.; Boyd, I.D. Kinetic rates for gas-phase chemistry of phenolic-based carbon ablator in atmospheric air. J. Thermophys. Heat Transf. 2015, 29, 222–240. [Google Scholar] [CrossRef]
- Lu, H.; Chen, W.; Yuan, L.; Liu, W. Ablation analysis and engineering calculation on carbon-based material of reentry vehicles. J. Ballist. 2008, 20, 107–110. [Google Scholar]
- Sanoj, P.; Kandasubramanian, B. Hybrid carbon-carbon ablative composites for thermal protection in aerospace. J. Compos. 2014, 2014, 825607. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Li, K.; Zhu, G.; Hu, B.; Liu, Y.; Ji, J. Carbon nanotube reinforced ablative material for thermal protection system with superior resistance to high-temperature dense particle erosion. Aerosp. Sci. Technol. 2020, 106, 106234. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2019, 120, 1250–1349. [Google Scholar] [CrossRef] [PubMed]
- Uyanna, O.; Najafi, H. Thermal protection systems for space vehicles: A review on technology development, current challenges and future prospects. Acta Astronaut. 2020, 176, 341–356. [Google Scholar] [CrossRef]
- Wang, L.; Tian, W.; Chen, L.; Guo, Y. Investigation of carbon-carbon nozzle throat erosion in a solid rocket motor under acceleration conditions. Int. J. Aeronaut. Space Sci. 2021, 22, 42–51. [Google Scholar] [CrossRef]
- Li, K.-Z.; Jing, X.; Qian-gang, F.; He-jun, L.; Ling-jun, G. Effects of porous C/C density on the densification behavior and ablation property of C/C–ZrC–SiC composites. Carbon 2013, 57, 161–168. [Google Scholar] [CrossRef]
- Brunier-Coulin, F.; Cuéllar, P.; Philippe, P. Erosion onset of a cohesionless granular medium by an immersed impinging round jet. Phys. Rev. Fluids 2017, 2, 034302. [Google Scholar] [CrossRef]
- Murray, V.J.; Smoll, E.J., Jr.; Minton, T.K. Dynamics of graphite oxidation at high temperature. J. Phys. Chem. C 2018, 122, 6602–6617. [Google Scholar] [CrossRef]
- Prakash, R.; Le Page, L.; McQuellin, L.; Gai, S.; O’Byrne, S. Direct simulation Monte Carlo computations and experiments on leading-edge separation in rarefied hypersonic flow. J. Fluid Mech. 2019, 879, 633–681. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shioya, T.; Satoh, K. Degradation of carbon-based materials due to impact of high-energy atomic oxygen. Int. J. Impact Eng. 2003, 28, 1–11. [Google Scholar] [CrossRef]
- Roy, S.S.; Safron, N.S.; Wu, M.-Y.; Arnold, M.S. Evolution, kinetics, energetics, and environmental factors of graphene degradation on silicon dioxide. Nanoscale 2015, 7, 6093–6103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, P.; Yao, S.; Fei, F. Multiscale investigation of Kolmogorov flow: From microscopic molecular motions to macroscopic coherent structures. Phys. Fluids 2019, 31, 082008. [Google Scholar] [CrossRef]
- Xing, S.; Wu, W.; Wang, Y.; Bao, J.; Pei, S.-S. Kinetic study of graphene growth: Temperature perspective on growth rate and film thickness by chemical vapor deposition. Chem. Phys. Lett. 2013, 580, 62–66. [Google Scholar] [CrossRef]
- Labruquère, S.; Bourrat, X.; Pailler, R.; Naslain, R. Structure and oxidation of C/C composites: Role of the interface. Carbon 2001, 39, 971–984. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Zhang, Z. Damage evolution simulation and laser ablation mechanisms of C/SiC composites under in high-speed airflow environment. Int. J. Therm. Sci. 2023, 194, 108596. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D.; Guo, Z.; Zhang, Z.; Cui, M.; Xu, C. SiBCN-precursor-derived gradient oxidation protective ceramic coating for C/C composites. Surf. Coat. Technol. 2018, 350, 101–109. [Google Scholar] [CrossRef]
- Chen, X.; Munjiza, A.; Zhang, K.; Wen, D. Molecular dynamics simulation of heat transfer from a gold nanoparticle to a water pool. J. Phys. Chem. C 2014, 118, 1285–1293. [Google Scholar] [CrossRef]
- Andric, N.; Jenny, P. Molecular dynamics investigation of energy transfer during gas-surface collisions. Phys. Fluids 2018, 30, 077104. [Google Scholar] [CrossRef]
- Yao, G.; Zhao, J.; Ramisetti, S.B.; Wen, D. Atomistic molecular dynamic simulation of dilute poly (acrylic acid) solution: Effects of simulation size sensitivity and ionic strength. Ind. Eng. Chem. Res. 2018, 57, 17129–17141. [Google Scholar] [CrossRef]
- Zhang, J.; John, B.; Pfeiffer, M.; Fei, F.; Wen, D. Particle-based hybrid and multiscale methods for nonequilibrium gas flows. Adv. Aerodyn. 2019, 1, 12. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, G.; Wen, D. Salinity-dependent alterations of static and dynamic contact angles in oil/brine/calcite systems: A molecular dynamics simulation study. Fuel 2020, 272, 117615. [Google Scholar] [CrossRef]
- Valentini, P.; Schwartzentruber, T.E.; Bender, J.D.; Nompelis, I.; Candler, G.V. Direct molecular simulation of nitrogen dissociation based on an ab initio potential energy surface. Phys. Fluids 2015, 27, 086102. [Google Scholar] [CrossRef]
- Hahn, S.H.; Van Duin, A.C. Surface reactivity and leaching of a sodium silicate glass under an aqueous environment: A ReaxFF molecular dynamics study. J. Phys. Chem. C 2019, 123, 15606–15617. [Google Scholar] [CrossRef]
- Furman, D.; Wales, D.J. A well-behaved theoretical framework for ReaxFF reactive force fields. J. Chem. Phys. 2020, 153, 021102. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Ashraf, C.; Van Duin, A.C. Extension of the ReaxFF combustion force field toward syngas combustion and initial oxidation kinetics. J. Phys. Chem. A 2017, 121, 1051–1068. [Google Scholar] [CrossRef]
- Newsome, D.A.; Sengupta, D.; Foroutan, H.; Russo, M.F.; Van Duin, A.C. Oxidation of silicon carbide by O2 and H2O: A ReaxFF reactive molecular dynamics study, Part I. J. Phys. Chem. C 2012, 116, 16111–16121. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; Van Duin, A.C.; Ganesh, P. Development of a ReaxFF potential for carbon condensed phases and its application to the thermal fragmentation of a large fullerene. J. Phys. Chem. A 2015, 119, 571–580. [Google Scholar] [CrossRef]
- Poovathingal, S.; Schwartzentruber, T.E.; Srinivasan, S.G.; Van Duin, A.C. Large scale computational chemistry modeling of the oxidation of highly oriented pyrolytic graphite. J. Phys. Chem. A 2013, 117, 2692–2703. [Google Scholar] [CrossRef]
- Majumder, M.; Gibson, K.; Sibener, S.; Hase, W.L. Chemical dynamics simulations and scattering experiments for O2 collisions with graphite. J. Phys. Chem. C 2018, 122, 16048–16059. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, F.C.; Park, H.S.; Wu, H.A. Defecting controllability of bombarding graphene with different energetic atoms via reactive force field model. J. Appl. Phys. 2013, 114, 054313. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, L.; Liu, L. Bombarding graphene with oxygen ions: Combining effects of incident angle and ion energy to control defect generation. J. Phys. Chem. C 2015, 119, 26793–26802. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.K.; Pang, M.; Chen, T.; Du, D.; Chen, K.Q. Thermal rectification in asymmetric graphene/hexagonal boron nitride van der Waals heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 15517–15526. [Google Scholar] [CrossRef]
- Dressler, R.A. Chemical Dynamics in Extreme Environments; World Scientific: Singapore, 2001. [Google Scholar]
- Srinivasan, S.G.; van Duin, A.C. Molecular-dynamics-based study of the collisions of hyperthermal atomic oxygen with graphene using the ReaxFF reactive force field. J. Phys. Chem. A 2011, 115, 13269–13280. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Kim, I.; Park, G. Experimental study of oxygen catalytic recombination on a smooth surface in a shock tube. Appl. Therm. Eng. 2019, 156, 678–691. [Google Scholar] [CrossRef]
- Vinogradov, N.A.; Schulte, K.; Ng, M.L.; Mikkelsen, A.; Lundgren, E.; Martensson, N.; Preobrajenski, A. Impact of atomic oxygen on the structure of graphene formed on Ir (111) and Pt (111). J. Phys. Chem. C 2011, 115, 9568–9577. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Zhu, Y.; Zhang, D.; Wei, N. Atomic-Scale Mechanisms of Catalytic Recombination and Ablation in Knitted Graphene Under Hyperthermal Atomic Oxygen Exposure. C 2025, 11, 67. https://doi.org/10.3390/c11030067
Pan Y, Zhu Y, Zhang D, Wei N. Atomic-Scale Mechanisms of Catalytic Recombination and Ablation in Knitted Graphene Under Hyperthermal Atomic Oxygen Exposure. C. 2025; 11(3):67. https://doi.org/10.3390/c11030067
Chicago/Turabian StylePan, Yating, Yunpeng Zhu, Donghui Zhang, and Ning Wei. 2025. "Atomic-Scale Mechanisms of Catalytic Recombination and Ablation in Knitted Graphene Under Hyperthermal Atomic Oxygen Exposure" C 11, no. 3: 67. https://doi.org/10.3390/c11030067
APA StylePan, Y., Zhu, Y., Zhang, D., & Wei, N. (2025). Atomic-Scale Mechanisms of Catalytic Recombination and Ablation in Knitted Graphene Under Hyperthermal Atomic Oxygen Exposure. C, 11(3), 67. https://doi.org/10.3390/c11030067






