Abstract
To enhance the photocatalytic performance of ZnO, the ZnO/g-C3N4 (ZCN) composite was prepared by ZnO and g-C3N4 under ball milling, and then the ternary graphene oxide (GO)/ZnO/g-C3N4 (GZCN) composite was achieved by using sonicating, stirring, and liquid phase evaporating. The photocatalytic performance was tested under UV light and natural solar light, respectively. The experimental results displayed that the GZCN composite revealed excellent photocatalytic performance under UV light and natural sunlight. When the ratio of ZnO to g-C3N4 is 1:0.2 and the mass fraction of graphene oxide is 0.25% in GZCN composite, the modified ZnO possesses optimal photocatalytic activity under UV light or natural solar light. RhB dye is degraded by 94% within 20 min under UV light, which is 3.41 times that of pure ZnO. Moreover, GZCN can degrade 88% of RhB in 60 min under natural sunlight. The enhancement for photocatalytic activity is attributed to the excellent conductivity of GO and heterojunction interaction between ZnO and g-C3N4, where the special electronic structure of g-C3N4 expands the spectral response range of ZnO and accelerates the transmission of photogenerated electrons and holes.
1. Introduction
ZnO photocatalyst has been extensively researched due to its wide band gap (3.37 eV), high exciton binding energy (60 meV), numerous morphologies, and due to the fact that it is cheap and non-toxic [1,2,3,4]. However, pure ZnO can only absorb near-ultraviolet light, has a very high combination rate of photogenerated electron–hole pairs, and displays a low photocatalytic activity. Accordingly, there have been a number of reports about enhancing the photocatalytic activity of ZnO, such as element doping, constructing heterojunctions [5,6,7], searching for suitable co-catalysts [8], and catalyzer carriers [9]. Among them, semiconductors coupling and element doping can change the semiconductor band structure by introducing impurity into ZnO to increase the solar energy utilization rate [10]. Furthermore, catalyst carrier is an effective method to improve the photocatalytic performance of ZnO, which has a large amount of impurities and defects compared with pure ZnO materials owing to reducing the recombination of photogenerated e−-h+ hole pairs [11].
In recent years, graphite-like carbon nitride (g-C3N4) as a metal-free polymer n-type semiconductor has become a research hotspot because of its narrow band gap (2.74 eV), high chemistry stability, and unique structure and electric properties [12]. It has been studied in a variety of fields such as photocatalytic water splitting [13,14,15], carbon dioxide photoreduction [16], disinfection [17], the degradation of pollutants [18,19], and the battery field [20]. Currently, g-C3N4 is being utilized as a reinforcement material to improve photocatalytic performance, which signifies a great achievement. For instance, He and his co-workers have successfully prepared ZnO/g-C3N4 composite with high photocatalytic activity compared to ZnO or g-C3N4 [21]. Tang et al. have prepared a wide-spectrum response high potential composite through constructing the ternary Ag/g-C3N4/NaTaO3 Z-scheme heterojunction [22].
Unfortunately, g-C3N4 has some serious drawbacks, such as poor conductivity, low surface area, small active sites, slow surface reaction kinetics, and moderate oxidation ability [23,24,25]. At this point, it is necessary to design a new material that has the ability to overcome those problems. Graphene has been widely used to improve the photocatalytic property of semiconductor oxide due to its unique electric properties and structure, large specific surface area, high thermal stability, and chemical stability [26,27,28,29,30]. Our previous report also illustrated that graphene can enhance the photocatalytic activity of ZnO nanomaterials [31]. Hence, using the excellent properties of graphene can neutralize the disadvantages of g-C3N4, which can further improve the photocatalytic performance of ZnO/g-C3N4 (ZCN) composite.
ZnO is most likely to be applied in practice because of its low cost, non-toxicity, and superior properties. However, few researchers have reported on the photocatalytic properties of ZnO under natural light. In this manuscript, graphene oxide (GO) and g-C3N4 are used as co-reinforcing materials for improving the photocatalytic of ZnO, and the ternary GO/ZnO/g-C3N4 (GZCN) composite was successfully obtained by the mechanical ball milling method, and the photocatalytic activity was studied under natural solar light and UV light. This work will promote the application of ZnO in sewage treatment and environmental protection.
2. Experimental Section
2.1. Preparation of GZCN Material
Zinc oxalate (ZnC2O4) was purchased from Zhengzhou Runbang Chemical Products Co., Ltd., Zhengzhou city, China. Urea was purchased from Shanghai Civic Chemical Co., Ltd., Shanghai city China, with a purity of 99%. Graphene oxide (GO) solution (0.5 g/L) was prepared by Hummers’ classic method.
GZCN composites were synthesized typically as follows: First, ZnO and g-C3N4 powder were attained by annealing zinc oxalate and urea at 550 °C, respectively, and the heating rate was 5 °C/min. Next, the ZnO/g-C3N4 (ZCN) powder was obtained by ball milling 5 g ZnO and 1 g g-C3N4 for 4 h at a speed of 150 r/min and then dried in an oven at 60 °C. To find the optimum ratio of ZnO to g-C3N4, ZCNx was prepared by the same method, x = 0.1, 0.2, 0.3. Next, 2 g ZCN was dispersed in 60 mL ethanol. Subsequently, 10 mL GO solution (0.5 g/L) was added into the suspension after sonicating for 30 min. The GZCN composites were successfully prepared via stirring in a water bath at 70 °C until the liquid phase evaporated. The xGZCN was synthesized through the same process to explore the best mass ratio of GO, x = 0.0125, 0.25, 0.0375, and 0.5.
2.2. Characterization
The X-ray diffractometer (XRD) measurement was performed by Philips PW1710 diffractometer (Made in Philips Co., Ltd., Amsterdam city, Netherlands) with Cu Ka1 radiation to analyze the crystal structures. The morphological analysis of different samples was observed by the NovaNano SEM 200 (FEI Co., Ltd., Hillsboro city, Oregon state, USA) and JEM-3010 Transmission Electron Microscope (TEM, JEOL Co., Ltd., Tokyo city, Japan). UV–vis diffuse reflection spectra were recorded on the TU-2550 spectrophotometer (SHIMADZU Co., Ltd., Tokyo, Japan).
The photocatalytic activity and cycle performance were evaluated by the degradation of RhB molecular under UV light and natural sunlight, and these specific steps were referred to in our previous works [32]. Briefly, 0.1 g sample was added into 200 mL RhB solution (1 × 10−5 mol·L−1), and the mixed solution was placed under a 100 W high-pressure mercury lamp (the light wavelength is 365 nm, and the distance is about 20 cm between lamp and liquid). The solution depth is approximately 10 cm. Moreover, 0.1 g sample was added into 300 mL RhB solution (1 × 10−5 mol·L−1), and then the mixed solution was irradiated under outdoor natural sunlight, for which the light strength is approximately 700 lux and solution depth is approximately 15 cm. The cycle stability of GZCN was tested at outdoor sunlight from 10 am to 14 pm.
Electrochemical impedance spectroscopy (EIS) was performed with the CHI 660 electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., Shanghai city, China). Ag/AgCl was used as the reference electrode, and Pt wire was used as the counter electrode. ITO conducting glass coated with sample (1 cm × 1 cm) was exploited as the working electrode, and the electrolyte solution was 0.5 mol/L Na2SO4 solution (100 mL).
3. Results and Discussion
The crystal structures of ZnO, g-C3N4, ZCN, and GZCN were characterized by X-ray diffraction. As shown in Figure 1, pure g-C3N4 exhibits two distinct peaks at 13.7° and 27.4°, corresponding to (100) and (002) plane, which can be indexed to the characteristic in-planar structural packing and inter-planar stacking peaks of the aromatic systems in g-C3N4 [33]. The diffraction peaks of ZnO are well matched with the hexagonal crystalline structure, and the high intensity demonstrated its crystallinity and purity. Except the same peaks with ZnO, it was found that a characteristic peak at 27.4° of CN also existed in the XRD patterns of ZCN and GZCN, demonstrating that g-C3N4 has been successfully introduced into industrial ZnO. However, the peak of GO could not be found in the pattern of GZCN, which may mean that the content of graphene oxide was too small or that the intensity of the peak was too low. Furthermore, the intensity of the diffraction peak for ZnO did not changed, meant that the crystallinity of ZnO has not changed after adding GO and g-C3N4.
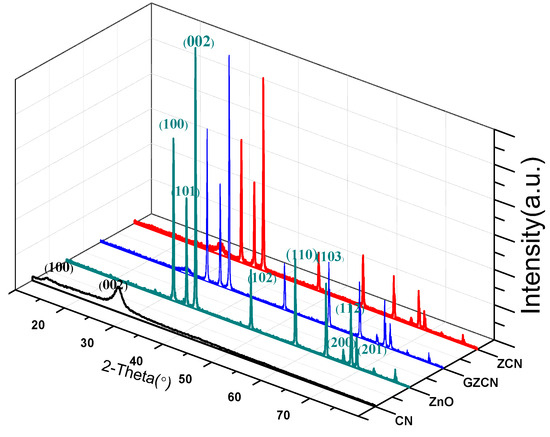
Figure 1.
XRD patterns of different samples.
Figure 2a,b show the morphology of the ZnO and GZCN samples, respectively. It is clear that ZnO exhibits irregular particle morphology. The morphology of GZCN displays that ZnO particles are dispersed in the fluffy g-C3N4 sheet surface, and there are a few ZnO agglomerations, as shown in Figure 2b. Figure 3a is the TEM image of GZCN; it is obvious that ZnO particles were embedded in the g-C3N4 nanosheet, but GO could not be found, which is attributed to the low content of GO. The selected area electron diffraction pattern (Figure 3b) verified that ZnO has good crystal characteristics containing multiple single diffraction spots. In order to confirm the distribution of elements in the composite, element mapping is characterized, and the results are shown in Figure 4. According to the element mapping, the black particles are ZnO, and the flake structure is g-C3N4, demonstrating that ZnO particles are loaded on the g-C3N4 sheet.
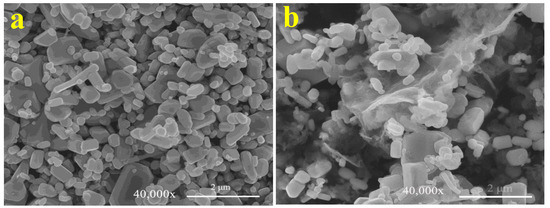
Figure 2.
SEM of pure ZnO (a) and GZCN (b).
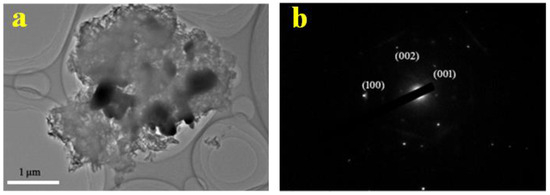
Figure 3.
TEM (a) and SAED pattern (b) of GZCN.
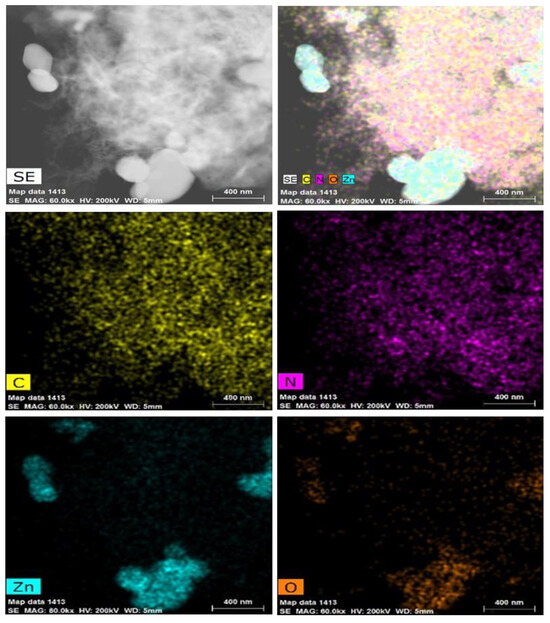
Figure 4.
TEM element mapping of GZCN hybrids.
The photocatalytic activity was evaluated by degrading organic dye (RhB) under ultraviolet light and natural solar light. Figure 5a shows the performance of ZCNx under UV light. t equals 0, indicating the signal for turning on/off the light. When t is less than 0, this indicates dark adsorption. C0 reveals the initial concentration of organic dyes, and C denotes the concentration of organic dyes at a certain moment. When the content of g-C3N4 increases, the photocatalytic activity first increases and then decreases. When the mass ratio of ZnO to g-C3N4 is 1:0.2, ZCN possess optimum photocatalytic activity, of which the degradation rate reached 99.7% within 40 min. Figure 5b depicts the effect of GO on photocatalytic performance, and GZCN showed excellent photocatalytic performance when the content of GO was 0.25 wt%. For a comparison of the different samples, the photocatalytic degradation under UV light and reaction rate constants k are depicted in Figure 6. As shown in Figure 6a, it is obvious that the blank test only shows a small amount of degradation for RhB. ZCN shows a better photocatalytic performance than ZnO, which also demonstrates that introducing g-C3N4 can well improve the photocatalytic activity of ZnO. The GZCN composite exhibits the highest photocatalytic activity when comparing ZnO and ZCN, and RhB dye is degraded by 94% within 20 min. Furthermore, the degradation rate was calculated as shown in Figure 6b. The k value of GZCN is 0.14318 min−1, in which it is 3.41 times faster than that of ZnO (0.04192 min−1). The result proved that introducing GO can further improve the photocatalytic performance of ZCN.
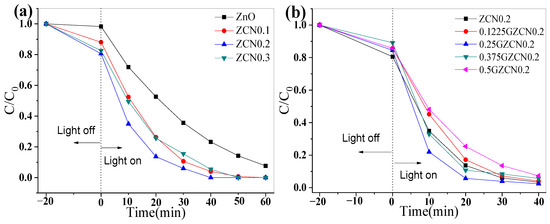
Figure 5.
Effect of component content on photocatalytic activity under UV light: (a) g-C3N4 and (b) GO.
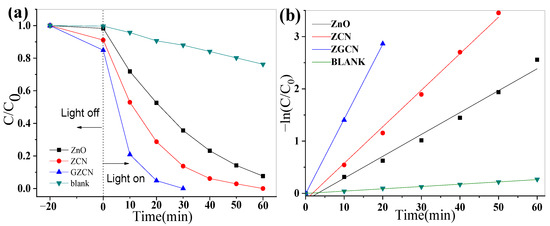
Figure 6.
Photocatalytic activity (a) and reaction rate constants k (b) of different samples under UV light.
The different samples’ visible activity was tested by decomposing RhB dye under natural solar light, as shown in Figure 7a. ZnO has a low photocatalytic activity, while the ZCN and GZCN composites exhibited a higher photocatalytic activity, demonstrating that the introduction of g-C3N4 and GO can enhance the natural light activity of industrial ZnO. Additionally, in order to evaluate stability, cycle tests were completed and are shown in Figure 7b. Figure 7b shows that the photocatalytic still has high activity after five cycles, meaning that GZCN has high photocatalytic stability.
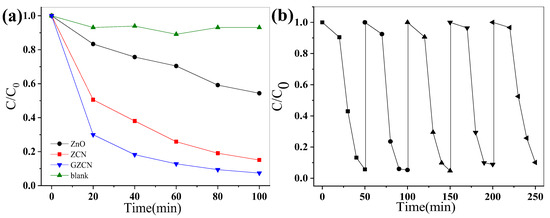
Figure 7.
The photocatalytic degradation of different samples (a) and photocatalytic stability of GZCN (b) under natural sunlight.
The above experiments demonstrated that ZnO has very high photocatalytic activity after being modified with GO and g-C3N4. Figure 8a displays the UV-vis diffuse reflectance spectra of different samples. All samples have a typical absorption in the UV light region with an edge of 400 nm. After compounding with g-C3N4, the absorption edge starts moving to the visible light region, for which there is a small enhancement for visible light absorption. Furthermore, it is not hard to find that the GZCN composite has a stronger absorption ability than the ZCN composite in the visible light region, implying that GO can enhance the light absorption ability of ZnO for visible light. According to the Kubelka–Munk formula [αhv = A(Eg−hv)n/2] [34], the band gap energies of ZnO, ZCN, and GZCN are 3.08 eV, 2.52 eV, and 2.43 eV, respectively (shown in Figure 8b). Notably, ZnO has a narrower band gap compared to the ordinary ZnO (3.20 eV), which can be caused by its impurities. Figure 9 shows the electrochemical impedance spectroscopy (EIS) of different materials. It is clear that ZCN has a larger slope compared to GZCN, illustrating that the conductivity of GZCN is higher than that of ZCN.
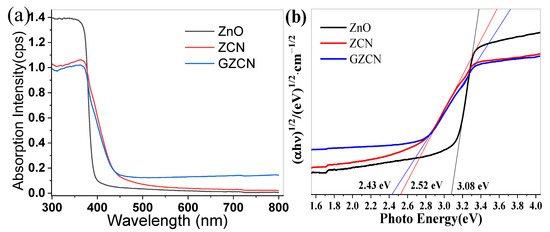
Figure 8.
UV–vis diffuse reflectance spectra (a) and Kubelka–Munk transformation (b) of ZCN and GZCN.
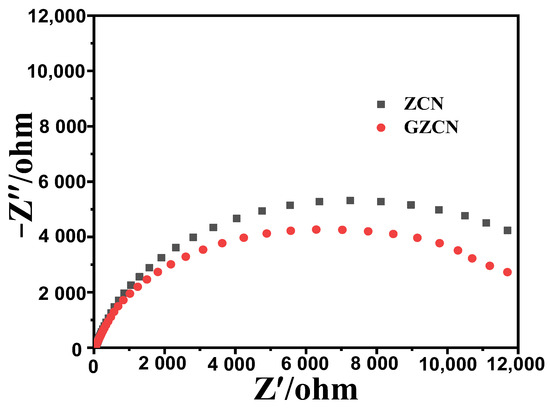
Figure 9.
The Nyquist plots of ZCN and GZCN.
A possible enhancement mechanism of photocatalytic property was proposed, as shown in Figure 10. The pure ZnO is supported on g-C3N4 sheet, and a small amount of graphene oxide is dispersed on the g-C3N4 nanosheet or pure ZnO. The introduction of g-C3N4 not only enhances its absorption in the visible region but can also form a heterojunction with pure ZnO, reducing the composite probability of photogenerated electron–hole pairs. The photogenerated electrons transfer from the conduction band of g-C3N4 to the conduction band of ZnO, while the holes migrate from the valence band of ZnO to the valence band of g-C3N4, inhibiting the recombination of photogenerated electron–hole pairs and improving the photocatalytic activity of ZnO [35]. In addition, owing to its excellent conductivity ability, graphene oxide (GO) can reduce the electrical resistance of the composite, promoting the mobility rate of photogenerated electron–hole pairs, leading to a further increase in the photocatalytic activity of ZCN.
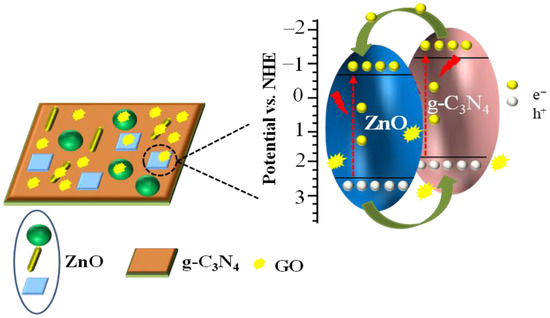
Figure 10.
The possible enhancement mechanism of GO and g-C3N4 for industrial ZnO.
4. Conclusions
A GZCN composite with high activity, visible light response, and high stability was prepared using a low-cost method. Utilizing g-C3N4 and grapheme as co-modifiers can significantly enhance the photocatalytic performance of pure ZnO. The photocatalytic activity of GZCN reaches optimization when the ratio of ZnO to g-C3N4 is 1:0.2 and the mass ratio of graphene oxide is 0.25%. Compared to industrial ZnO, the photocatalytic degrading process of GZCN was accelerated by 2.41 times under UV light irradiation. Moreover, the GZCN composite exhibited an excellent photocatalytic performance in sunlight irradiation, which can degrade 88% of RhB in 60 min. The main reason for this improvement is that the GZCN composite displayed good ability in the absorption of visible light and high stability. More importantly, this material is inexpensive and can be prepared on a large scale, which can hopefully be applied in practice.
Author Contributions
H.C.: Investigation, Data Curation, Writing—Original Draft. S.C.: Data Curation, Writing—Original Draft. Q.F.: Investigation, Data Curation, Writing—Original Draft. C.C.: Writing—Review and Editing, Writing—Original Draft, Supervision, Funding Acquisition, Formal Analysis, Data Curation. All authors have read and agreed to the published version of this manuscript.
Funding
This work is supported by the Hunan Provincial Natural Science Foundation of China (No.2021JJ50016) and the Scientific Research Fund of Hunan Provincial Education Department (No.20A015).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hakki, H.K.; Sillanpää, M. Comprehensive analysis of photocatalytic and photoreactor challenges in photocatalytic wastewater treatment: A case study with ZnO photocatalyst. Mater. Sci. Semicond. Process. 2024, 181, 108592. [Google Scholar] [CrossRef]
- Bhapkar, A.R.; Bhame, S. A review on ZnO and its modifications for photocatalytic degradation of prominent textile effluents: Synthesis, mechanisms, and future directions. J. Environ. Chem. Eng. 2024, 12, 112553. [Google Scholar] [CrossRef]
- Deng, X.; Chen, C.; Huang, Y.; Zeng, B. Au nanoparticles modified three-dimensional ZnO sheet@ZnO nanorod hybrids for enhancing the photocatalytic H2 evolution ability. Vacuum 2025, 238, 114247. [Google Scholar] [CrossRef]
- Zeng, B.; Ning, X.; Li, L. Fabrication and photocatalytic performance of highly active exposed facets ZnO hexagonal cap/Ti3C2 MXene composites. J. Alloys Compd. 2023, 963, 171309. [Google Scholar] [CrossRef]
- Mora, J.R.; Flores-Carrasco, G.; Juárez, H.; Pacio, M.; Olvera, M.d.l.L.; Rabanal, M.E. Ce-doped ZnO nanonails synthesized by a simple thermal evaporation method for photocatalytic degradation. Opt. Mater. 2024, 157, 116156. [Google Scholar] [CrossRef]
- Chen, C.S.; Huang, Y. Preparation and photocatalytic H2 Production property of a novel three-dimensional ZnO/ZnS nanosheet@ZnO/ZnS nanotube hybrid with multiple interfaces heterostructure. Surf. Interfaces 2025, 65, 106264. [Google Scholar] [CrossRef]
- Ali, S.A.; Majumdar, S.; Chowdhury, P.K.; Alhokbany, N.; Ahmad, T. Photoinduced hole trapping in MoSe2–MoS2 nanoflowers/ZnO nanosheets S-Scheme conduit for ultrafast charge transfer during hydrogen evolution. ACS Appl. Energy Mater. 2024, 7, 2881–2895. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, H.; Gao, N.; Fang, Y.; Xie, X.; Fang, Y.; Chen, G. Novel N-ZnO/p-BN adsorption-photocatalytic composites with interfacial bonding for efficient synergistic degradation of pollutants in water. Appl. Surf. Sci. 2024, 677, 161060. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, C.S. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, L.; Zhong, J. Sm-doped ZnO with enhanced photocatalytic performance toward destruction of RhB. Chem. Phys. Lett. 2024, 844, 141299. [Google Scholar] [CrossRef]
- Fareed, I.; Farooq, M.H.; Khan, M.D.; Yunas, M.F.; Sandali, Y.; Ali, Z.; Tanveer, M.; Butt, F.K. Comprehensive investigations into the synergy of S-scheme heterojunction between nitrogen-doped ZnO nano-rods and g-C3N4 nanosheets for improved photocatalytic degradation. Mater. Chem. Phys. 2024, 316, 129062. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, Z.; Li, T.; Lv, Z. Research progress on g-C3N4-based materials for efficient tetracyclines photodegradation in wastewater: A review. J. Water Process Eng. 2024, 66, 105941. [Google Scholar] [CrossRef]
- Shi, X.; Fujitsuka, M.; Lou, Z.; Zhang, P.; Majima, T. In situ nitrogen-doped hollow-TiO2/g-C3N4 composite photocatalysts with efficient charge separation boosting water reduction under visible light. J. Mater. Chem. A. 2017, 5, 9671–9681. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; et al. Construction of MnO2/Monolayer g-C3N4 with Mn vacancies for Z-scheme overall water splitting. Appl. Catal. B Environ. 2019, 241, 452–460. [Google Scholar] [CrossRef]
- Xu, Y.; He, X.; Zhong, H.; Singh, D.J.; Zhang, L.; Wang, R. Solid salt confinement effect: An effective strategy to fabricate high crystalline polymer carbon nitride for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 246, 349–355. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, W.; Liu, Z.; Dong, H.; Yan, Y.; Huo, P. Construction of an attapulgite intercalated mesoporous g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. J. Photoch. Photobio. A. 2018, 359, 102–110. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. Ternary g-C3N4/ZnO/AgCl nanocomposites: Synergistic collaboration on visible-light-driven activity in photodegradation of an organic pollutant. Appl. Surf. Sci. 2015, 358, 261–269. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Guan, W.; Huang, H.; Liu, Y. Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol. 2017, 173, 295–303. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Q.; Han, J.; Tao, Y.; Liu, D.; Zhang, C.; Lv, W.; Yang, Q.H. Catalyzing polysulfide conversion by g-C3N4 in a graphene network for long-life lithium-sulfur batteries. Nano Res. 2018, 11, 3480–3489. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168–169, 1–8. [Google Scholar] [CrossRef]
- Tang, L.; Feng, C.; Deng, Y.; Zeng, G.; Wang, J.; Liu, Y.; Feng, H.; Wang, J. Enhanced photocatalytic activity of ternary Ag/g-C3N4/NaTaO3 photocatalysts under wide spectrum light radiation: The high potential band protection mechanism. Appl. Catal. B Environ. 2018, 230, 102–114. [Google Scholar] [CrossRef]
- Gao, M.; Chen, C.; Sun, S.; Shi, H.; Zhang, X.; Zhao, C.; Li, G.; Mu, J.; Sun, J. Photocatalytic removal of rhodamine B using a novel g–C3N4–organic solid waste biochar composite: Pathway, key factors, and mechanism. Opt. Mater. 2024, 156, 116001. [Google Scholar] [CrossRef]
- Bahiraei, H.; Azarakhsh, S.; Ghasemi, S. Ternary CoFe2O4/g-C3N4/ZnO heterostructure as an efficient and magnetically separable visible-light photocatalyst: Characterization, dye purification, and mechanism. Ceram. Int. 2023, 49, 21050–21059. [Google Scholar] [CrossRef]
- Škuta, R.; Kostura, B.; Ritz, M.; Foniok, K.; Pavlovský, J.; Matýsek, D. Comparing the photocatalytic performance of GO/ZnO and g-C3N4/ZnO composites prepared using metallurgical waste as a source of zinc. Inorg. Chem. Commun. 2023, 152, 110728. [Google Scholar] [CrossRef]
- Wang, P.; Song, B.; Zhao, G. Novel two-dimensional ZnO materials for enhanced photocatalytic hydrogen evolution performance. Appl. Surf. Sci. 2025, 697, 163068. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.; Zhou, Y. Photoelectron marginalization effect in ZnO/WO3/graphene-like composites: Study of alternating strong-low photocatalytic hydrogen production performance and mechanism. J. Alloys Compd. 2024, 1009, 176824. [Google Scholar]
- Xue, B.; Zou, Y. High photocatalytic activity of ZnO–graphene composite. J. Colloid Interf. Sci. 2018, 529, 306–313. [Google Scholar] [CrossRef]
- Khan, H. Graphene based semiconductor oxide photocatalysts for photocatalytic hydrogen (H2) production, a review. Int. J. Hydrogen Energy 2024, 84, 356–371. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Dabur, V.A.; Rachmantyo, R.; Judawisastra, H.; Hu, C.; Wibowo, A. Carbon nitride- and graphene-based materials for the photocatalytic degradation of emerging water pollutants. Mater. Adv. 2024, 5, 2668–2688. [Google Scholar]
- Liu, J.; Xia, Q.; Chen, C. Graphene oxide enhanced the photocatalytic performance of one-dimensional porous carbon/ZnO hybrids. Vacuum 2024, 228, 113528. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Fang, Q.; Chen, X.; Liu, T.; Zhang, M. Self-assembly synthesis of CuO/ZnO hollow microspheres and their photocatalytic performance under natural sunlight. Vacuum 2020, 174, 109198. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Cui, W.; Dong, F.; Zhang, Y. Band structure engineering and efficient charge transport in oxygen substituted g-C3N4 for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 230, 115–124. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.O.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B Environ. 2018, 239, 334–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).