The Flower-Shaped Co (II) and Cu (II) Phthalocyanine Polymers as Highly Efficient and Stable Catalysts for Chemical Fixation of CO2 to Cyclic Carbonate
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Phthalonitrile
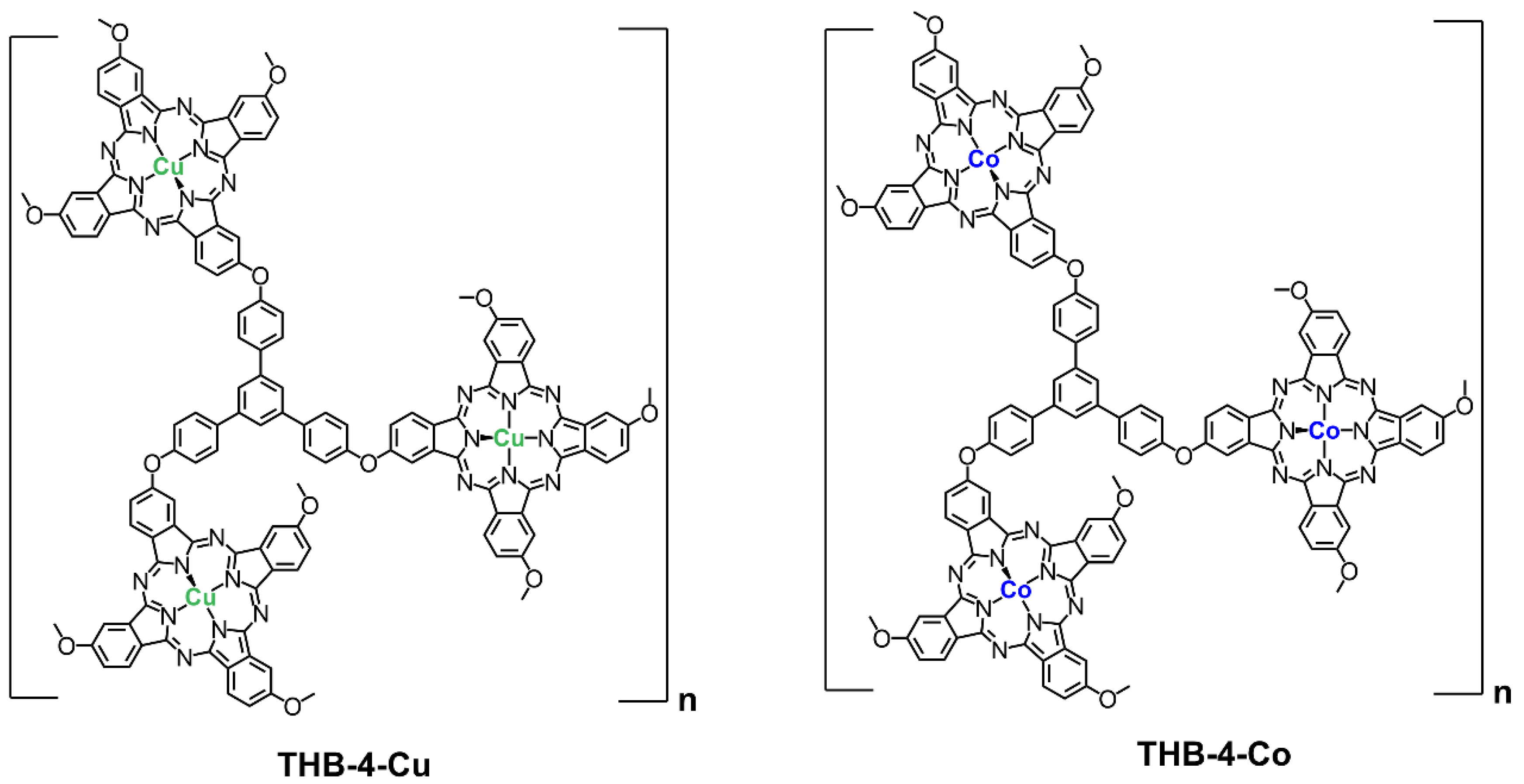
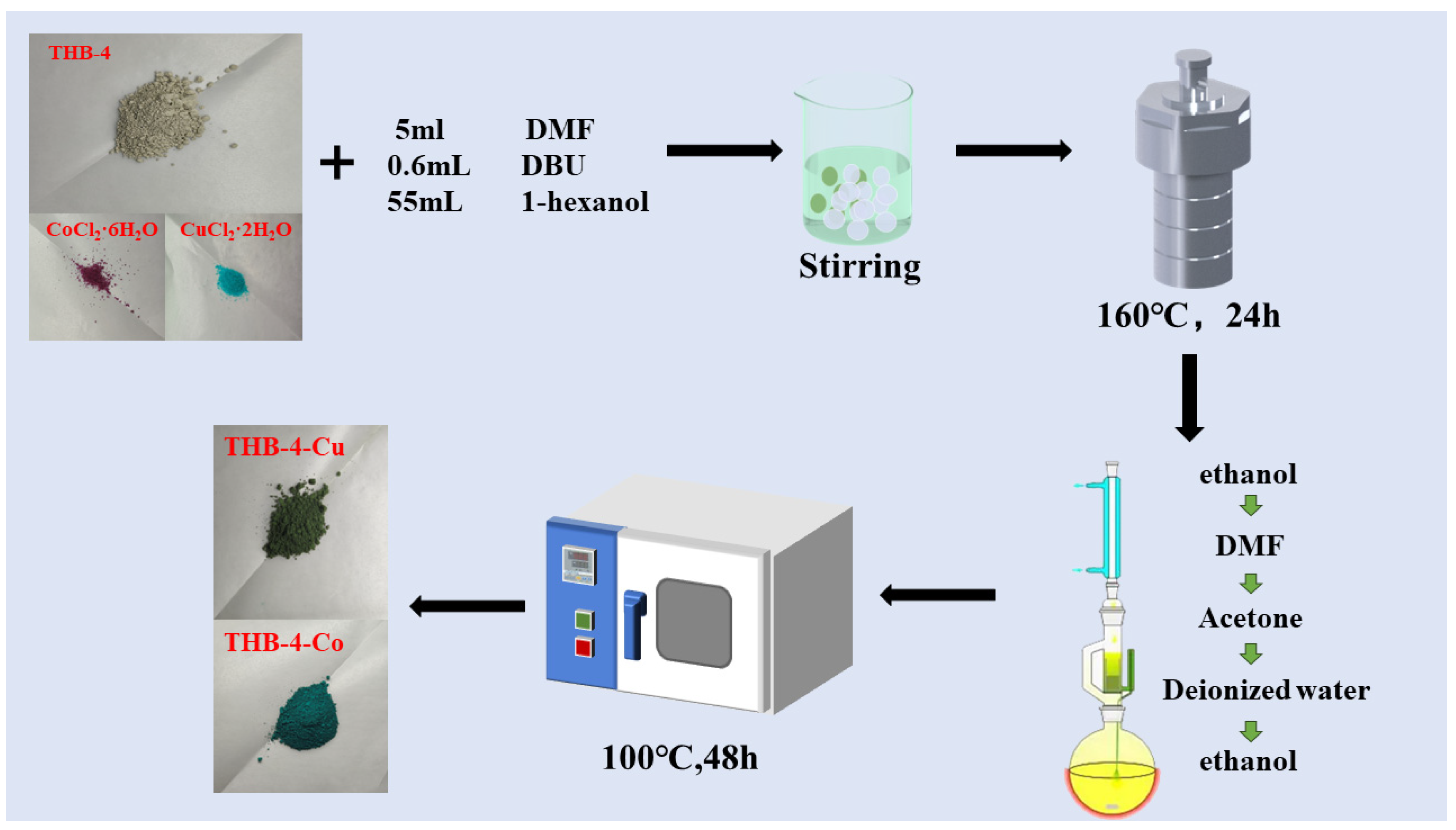
2.3. Synthesis of THB-4-M (M = Co, Cu)
2.4. Characterizations
3. Results and Discussion
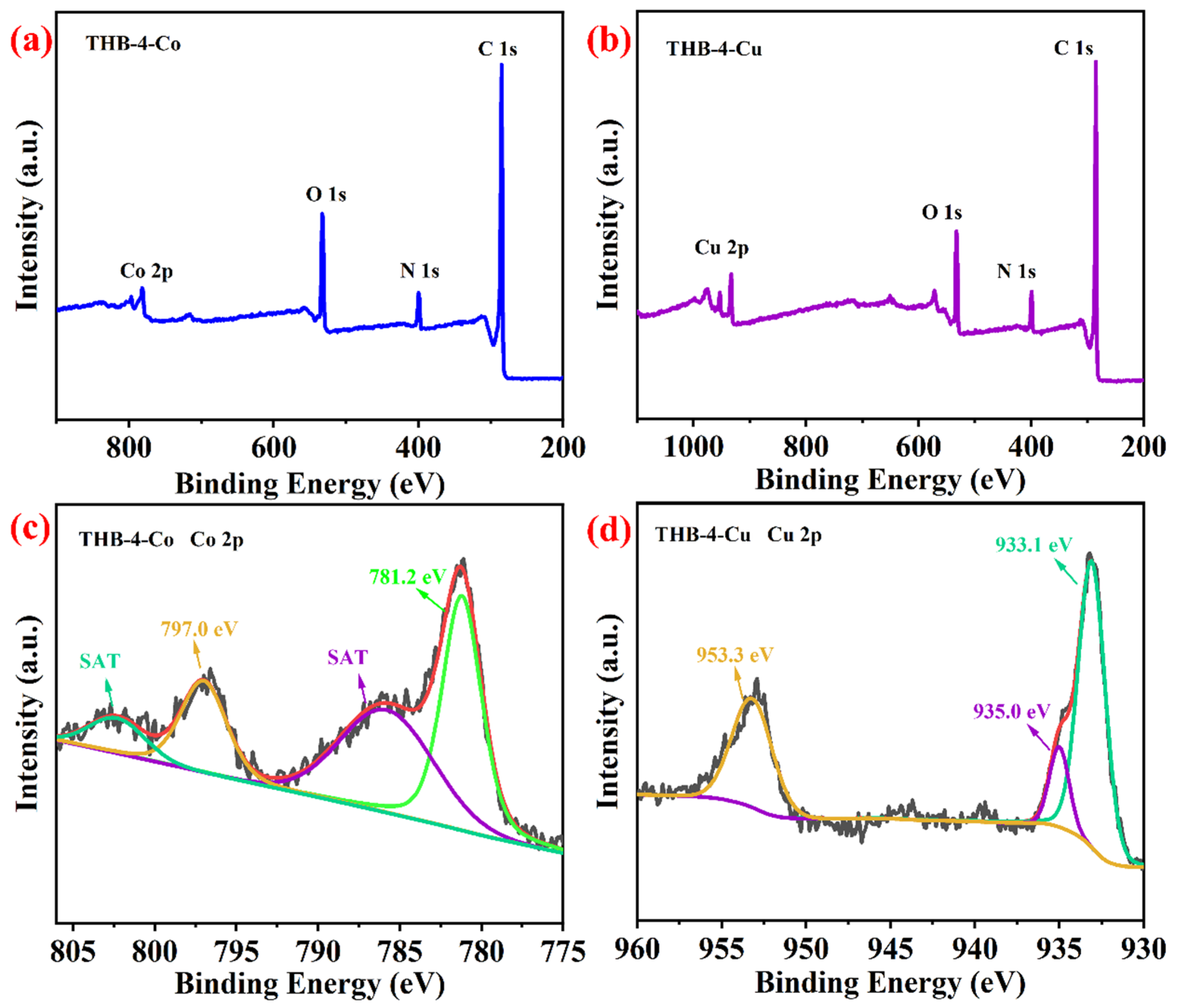
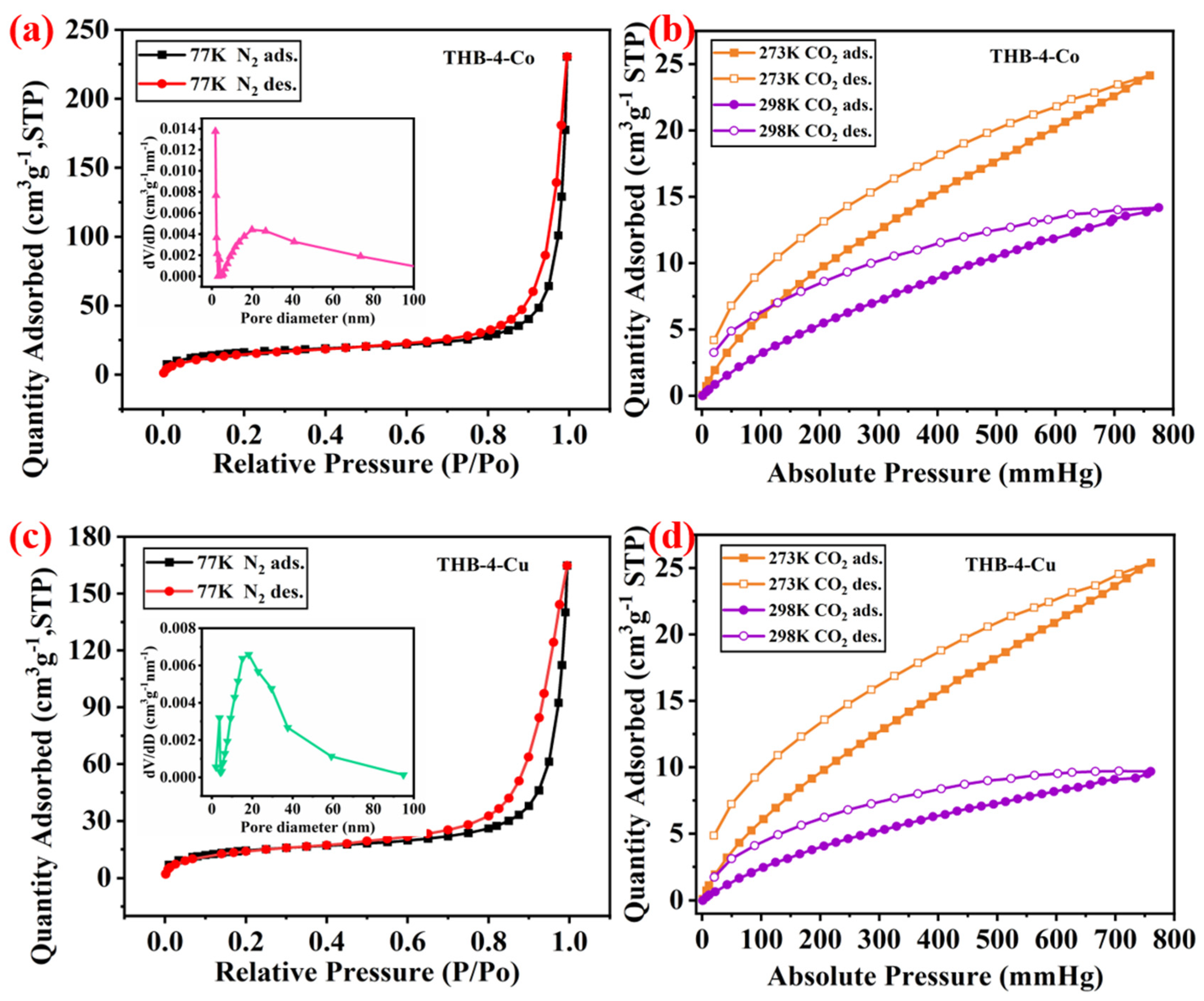
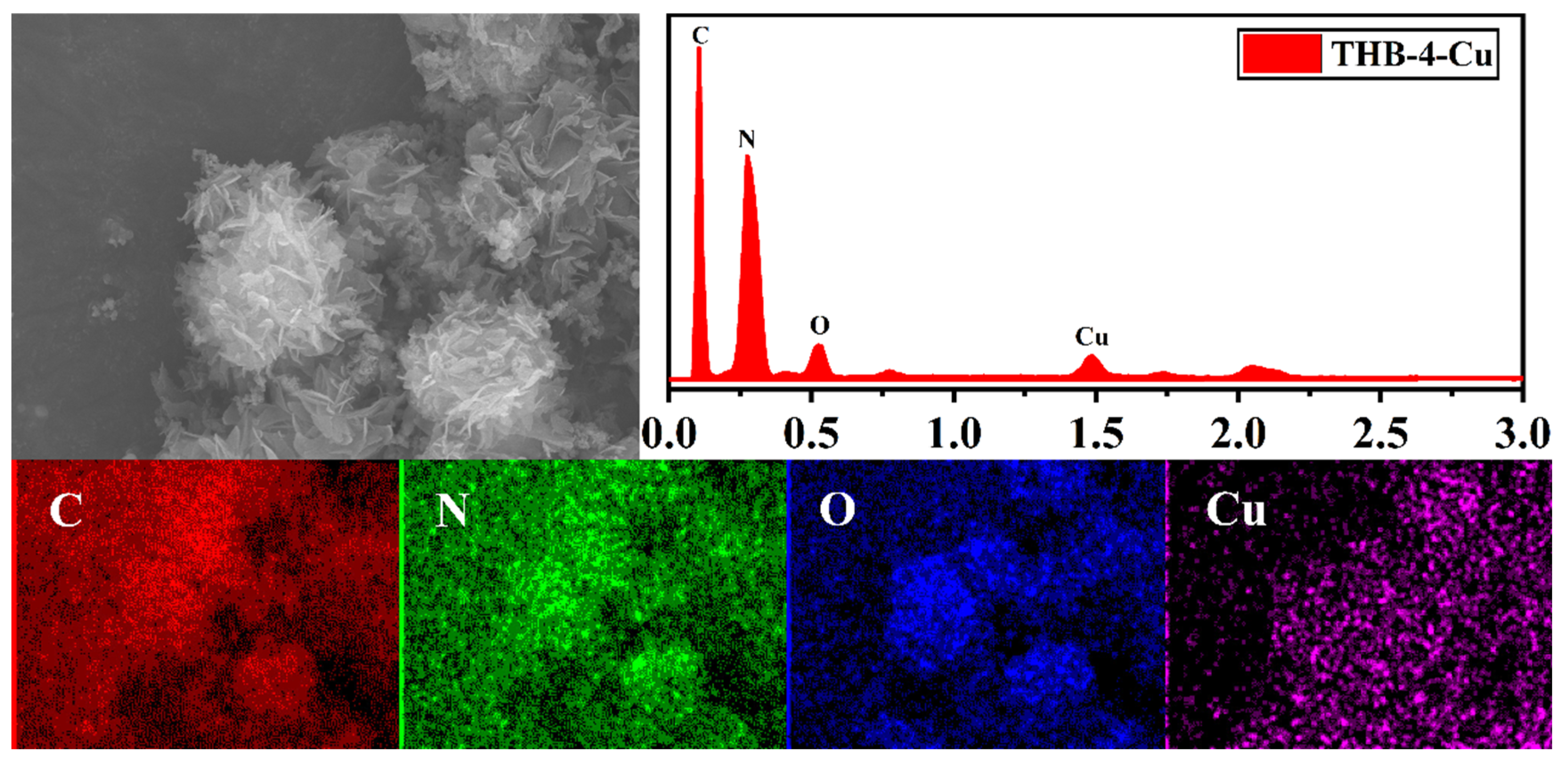
3.1. Characterization of Network Polymers
3.2. Cycloaddition of CO2 to Cyclic Carbonate
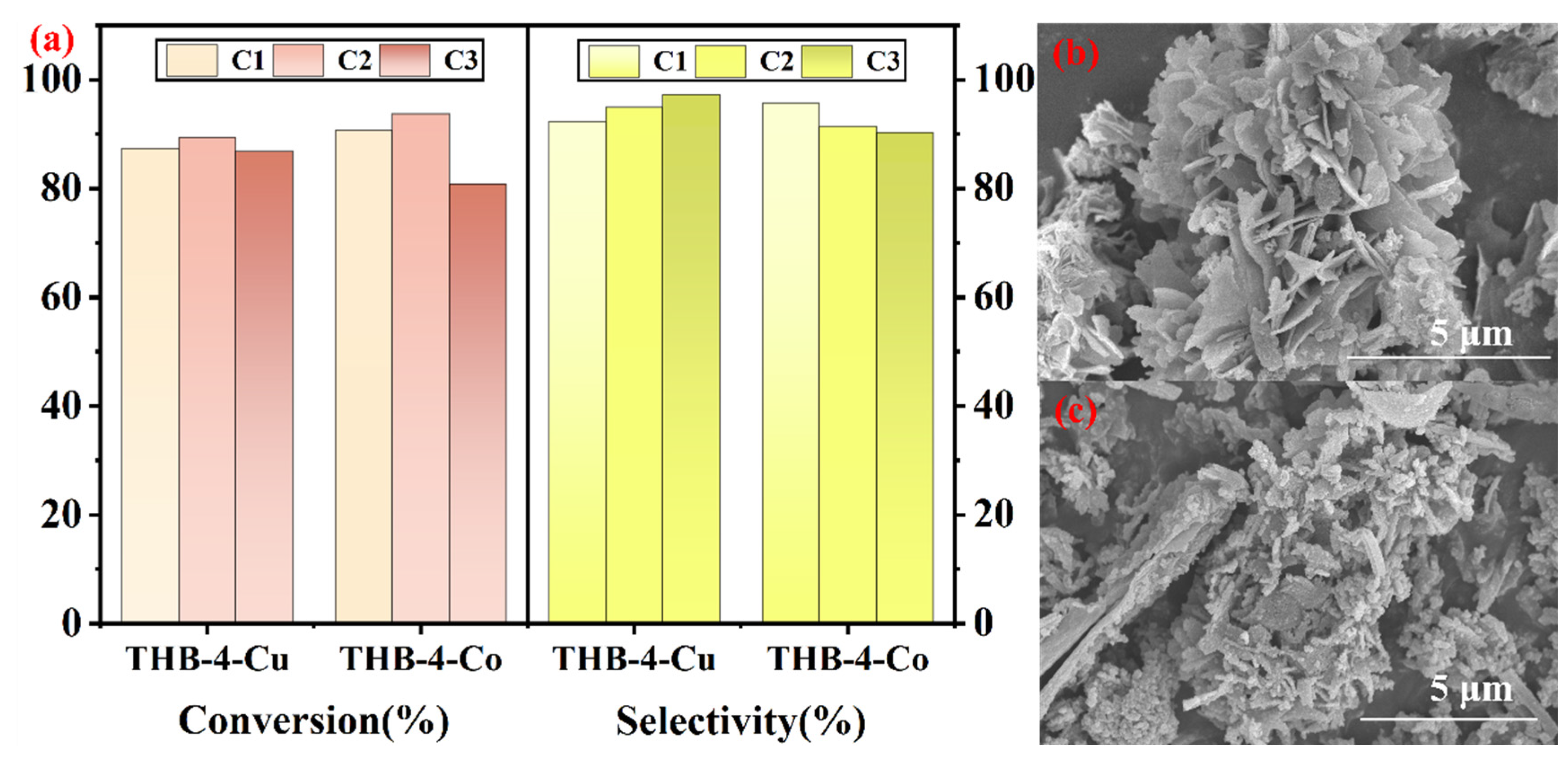
3.3. Catalyst Recycling Performance
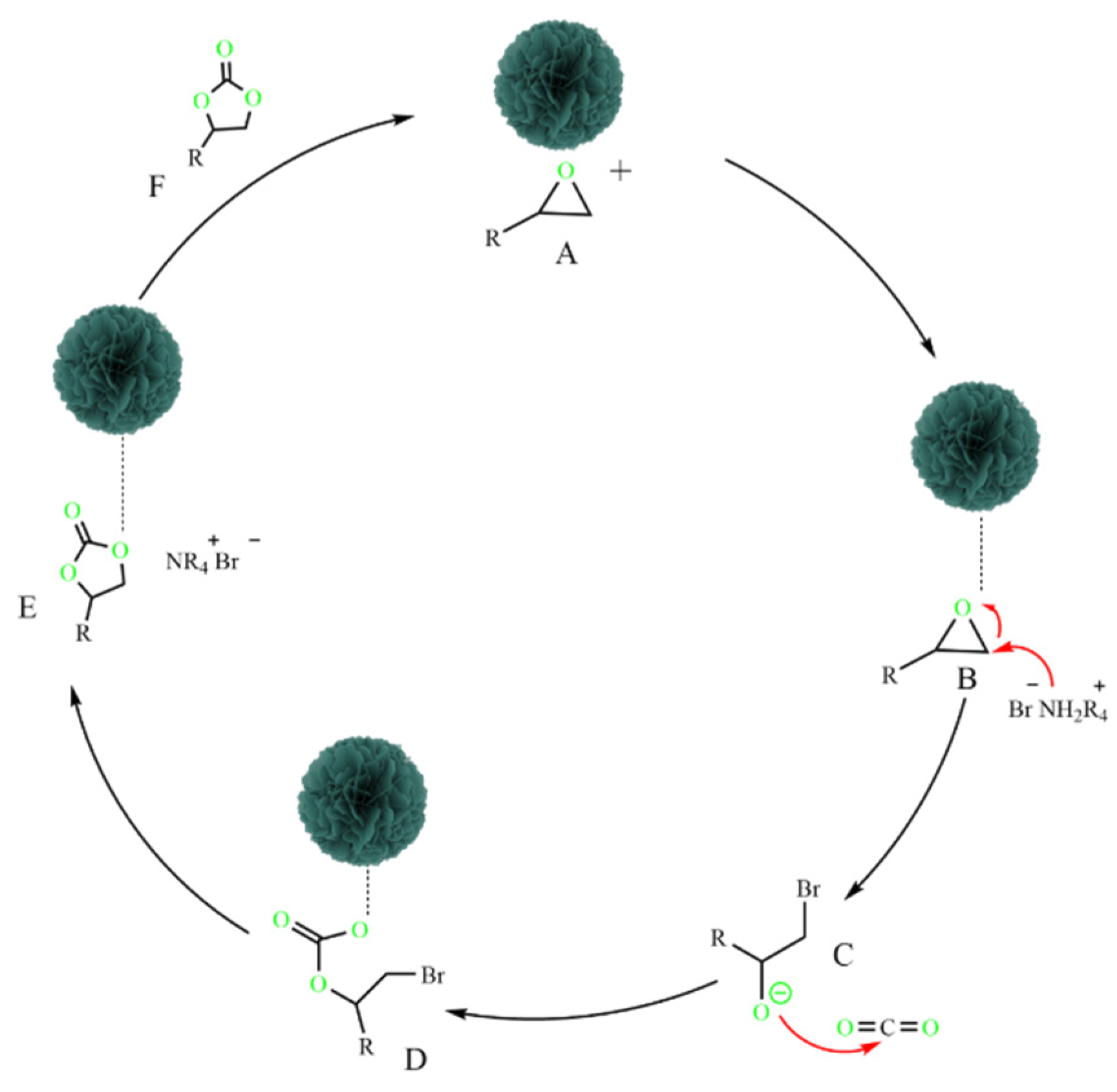
3.4. Possible Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.H.; Tian, C.; Jiang, X.W.; Xu, X.; Zhao, H.T.; Sun, J.Y.; Zhang, L.F.; Cheng, Z.P. Synthesis of water-soluble NIR macro-photocatalysts from polymerizable zinc phthalocyanine. Eur. Polym. J. 2023, 196, 112313. [Google Scholar] [CrossRef]
- Xu, R.; Li, B.L.; Ding, J.L.; Li, W.; Zhang, Y.H. Donor–acceptor conjugates-functionalized aluminum phthalocyanines: Photophysical and nonlinear optical properties. Eur. Polym. J. 2020, 134, 109813. [Google Scholar] [CrossRef]
- Wöhrle, D.; Kreienhoop, L.; Schnurpfeil, G.; Elbe, J.; Tennigkeit, B.; Hiller, S.; Schlettwein, D. Investigations of n/p-junction photovoltaic cells of perylenetetracarboxylic acid diimides and phthalocyanine. J. Mater. Chem. 1995, 5, 1819–1829. [Google Scholar] [CrossRef]
- Chen, X.N.; Zhang, J.L.; Wei, W.W.; Jiang, Z.H.; Zhang, Y.H. Synthesis and third-order optical nonlinearities of hyperbranched metal phthalocyanines. Eur. Polym. J. 2014, 53, 58–64. [Google Scholar] [CrossRef]
- Diaz Garcia, M.A.; Ledoux, I.; Duro, J.A.; Torres, T.; Agullo-Lopez, F.; Zyss, J. Third-order nonlinear optical properties of soluble octasubstituted metallophthalocyanines. J. Phys. Chem. 1994, 98, 8761–8764. [Google Scholar] [CrossRef]
- Li, B.W.; Wei, P.R.; Leon, A.D.; Frey, T.; Pentzer, E. Polymer composites with photo-responsive phthalocyanine for patterning in color and fluorescence. Eur. Polym. J. 2017, 89, 399–405. [Google Scholar] [CrossRef]
- Wright, J.D.; Roisin, P.; Rigby, G.P. Crowned and liquid-crystalline phthalocyanines as gas-sensor materials. Sens. Actuators B 1993, 13, 276–280. [Google Scholar] [CrossRef]
- Huai, M.M.; Yin, Z.L.; Wei, F.Y.; Wang, G.W.; Xiao, L.; Lu, J.T.; Zhuang, L. Electrochemical CO2 reduction on heterogeneous cobalt phthalocyanine catalysts with different carbon supports. Chem. Phys. Lett. 2020, 754, 137655. [Google Scholar] [CrossRef]
- Zhou, R.; Josse, F.; Gopel, W.; Ozturk, Z.Z.; Bekaroğlu, O. Phthalocyanines as Sensitive Materials for Chemical Sensors. Appl. Organomet. Chem. 1996, 10, 557–577. [Google Scholar] [CrossRef]
- Mevlüde, C.; Tebello, N. Synthesis, characterization, and photophysical properties of novel ball-type dinuclear and mononuclear containing four 1,1’-binaphthyl-8,8’-diol bridged metallophthalocyanines with long triplet state lifetimes. Dalton Trans. 2011, 40, 5285–5290. [Google Scholar]
- Al-Raqa, S.Y.; Ghanem, B.S.; Kaya, E.N.; Durmus, M.; EI-Khouly, M.E. Symmetrical phthalocyanine bearing four triptycene moieties: Synthesis, photophysical and singlet oxygen generation. J. Porphyr. Phthalocyanines 2019, 23, 990–1000. [Google Scholar] [CrossRef]
- Li, T.F.; Peng, Y.X.; Li, K.; Zhang, R.; Zheng, L.R.; Xia, D.G.; Zuo, X. Enhanced activity and stability of binuclear iron (III) phthalocyanine on graphene nanosheets for electrocatalytic oxygen reduction in acid. J. Power Sources 2015, 293, 511–518. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.S.; Zhang, X.; Li, L.W.; Li, Y.Y.; Xu, H.M.; Li, X.X.; Yu, X.L.; Zhang, Z.S.; Liang, Y.Y.; et al. Highly selective and active CO2 reduction electro-catalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, X.M.; Liu, Q.; Li, Q.; Xu, X.; Kang, L.T.; Cao, Z.M.; Chai, G.L.; Chen, J.; Wang, Y.B.; et al. The synthesis and synergistic catalysis of iron phthalocyanine and its graphene-based axial complex for enhanced oxygen reduction. Nano Energy 2018, 46, 347–355. [Google Scholar] [CrossRef]
- Costa, Í.A.; Maciel, A.P.; Sales, M.J.A.; Rivera, L.M.R.; Soler, M.A.G.; Pereira-da-Silva, M.A.; Moreira, S.G.C.; Paterno, L.G. Photocatalytic Method for the Simultaneous Synthesis and Immobilization of Ag Nanoparticles onto Solid Substrates. J. Phys. Chem. C 2018, 122, 24110–24119. [Google Scholar] [CrossRef]
- Liu, D.; Long, Y.T. Superior catalytic activity of electrochemically reduced graphene oxide supported iron phthalocyanines toward oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 24063–24068. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.S.; Guo, J.; Feng, X.A.; Honsho, Y.; Guo, J.D.; Seki, S.; Maitarad, P.; Saeki, A.; Nagase, S.; Jiang, D.L. Synthesis of Metallophthalocyanine Covalent Organic Frameworks That Exhibit High Carrier Mobility and Photoconductivity. Angew. Chem. Int. Ed. 2011, 50, 1289–1293. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Warren, J.E.; Helliwell, M.; Allan, D.R.; McKeown, N.B. Heme-like coordination chemistry within nanoporous molecular crystals. Science 2010, 327, 1627–1630. [Google Scholar] [CrossRef]
- Li, J.T.; Guo, L.M.; Shi, J.L. Stepwise in situ synthesis and characterization of metallophthalocyanines@mesoporous matrix SBA-15 composites. Phys. Chem. Chem. Phys. 2010, 12, 5109–5114. [Google Scholar] [CrossRef]
- Lopez-Duarte, I.; Dieu, L.Q.; Dolamic, I.; Martinez-Diaz, M.V.; Torres, T.; Calzaferri, G.; Bruhwiler, D. On the significance of the anchoring group in the design of antenna materials based on phthalocyanine stopcocks and zeolite L. Chem. A Eur. J. 2011, 17, 1855–1862. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Kovalenko, K.A.; Chesalov, Y.A.; Mel’gunov, M.S.; Zaikovskii, V.I.; Kaichev, V.V.; Sorokin, A.B.; Kholdeeva, O.A.; Fedin, V.P. Iron tetrasulfophthalocyanine immobilized on metal organic framework MIL-101: Synthesis, characterization and catalytic properties. Dalton Trans. 2011, 40, 1441–1444. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Exploitation of Intrinsic Microporosity in Polymer-Based Materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Polymers of Intrinsic Microporosity (PIMs): Organic Materials for Membrane Separations, Heterogeneous Catalysis and Hydrogen Storage. ChemInform 2006, 35, 675–683. [Google Scholar]
- McKeown, N.B.; Makhseed, S.; Budd, P.M. Phthalocyanine-based nanoporous network polymers. Chem. Commun. 2002, 23, 2780–2781. [Google Scholar] [CrossRef]
- Maffei, A.V.; Budd, P.M.; McKeown, N.B. Adsorption studies of a microporous phthalocyanine network polymer. Langmuir: ACS J. Surf. Colloids 2006, 22, 4225–4229. [Google Scholar] [CrossRef]
- Makhseed, S.; Al-Kharafi, F.; Samuel, J.; Ateya, B. Catalytic oxidation of sulphide ions using a novel microporous cobalt phthalocyanine network polymer in aqueous solution. Catal. Commun. 2009, 10, 1284–1287. [Google Scholar] [CrossRef]
- Mackintosh, H.J.; Budd, P.M.; McKeown, N.B. Catalysis by microporous phthalocyanine and porphyrin network polymers. J. Mater. Chem. 2008, 18, 573–578. [Google Scholar] [CrossRef]
- Tamura, R.; Kawata, T.; Hattori, Y.; Kobayashi, N.; Kimura, M. Catalytic oxidation of thiols within cavities of phthalocyanine network polymers. Macromolecules 2017, 50, 7978–7983. [Google Scholar] [CrossRef]
- Boroujeni, M.B.; Laeini, M.S.; Nazeri, M.T.; Shaabani, A. A novel and green in situ strategy for the synthesis of metallophthalocyanines on chitosan and investigation their catalytic activity in the CO2 fixation. Catal. Lett. 2019, 149, 2089–2097. [Google Scholar] [CrossRef]
- Hashem, M.; Bezzu, C.G.; Kariuki, B.M.; McKeown, N.B. Enhancing the rigidity of a network polymer of intrinsic microporosity by the combined use of phthalocyanine and triptycene components. Polym. Chem. 2011, 2, 2190–2193. [Google Scholar] [CrossRef]
- Ding, S.M.; Sun, L.; Ma, X.H.; Cheng, D.; Wu, S.H.; Zeng, R.; Deng, S.J.; Chen, C.; Zhang, N. Microporous polymeric spheres as highly efficient and metal-free catalyst for the cycloaddition of CO2 to cyclic organic carbonates at ambient conditions. Catal. Lett. 2020, 150, 2970–2977. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Wang, X.; Wu, Q.; Li, Y.J.; Luo, Q.X.; Mao, X.L.; Cai, Y.J.; Liu, X.; Liang, R.P.; Qiu, J.D. Rapid charge transfer enabled by noncovalent interaction through guest insertion in supercapacitors based on covalent organic frameworks. Angew. Chem. Int. Ed. 2023, 62, e202313970. [Google Scholar] [CrossRef]
- Zhang, W.W.; Ping, R.; Lu, X.Y.; Shi, H.B.; Liu, F.S.; Ma, J.J.; Liu, M.S. Rational design of Lewis acid-base bifunctional nanopolymers with high performance on CO2/epoxide cycloaddition without a cocatalyst. Chem. Eng. J. 2023, 451, 138715–138724. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Zeng, Z.X.; Pan, W.G. Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2023, 68, 102355–102370. [Google Scholar] [CrossRef]
- Nyokong, T. Coord. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocya-nines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Canlica, M.; Booysen, I.N.; Nyokong, T. Syntheses, electrochemical and spectroelectrochemical properties of novel ball-type and mononuclear Co(II) phthalocyanines substituted at the peripheral and non-peripheral positions with binaphthol groups. Polyhedron 2011, 30, 508–514. [Google Scholar] [CrossRef]
- Wagh, G.D.; Akamanchi, K.G. Sulfated tungstate catalyzed synthesis of C3-symmetric 1,3,5-triaryl benzenes under solvent-free condition. Tetrahedron Lett. 2017, 58, 3032–3036. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ohno, E. Synthesis of cobalt phthalocyanine derivatives and their cyclic voltammograms. Dye. Pigment. 1997, 35, 375–386. [Google Scholar] [CrossRef]
- Achar, B.N.; Lokesh, K.S. Studies on polymorphic modifications of copper phthalocyanine. J. Solid State Chem. 2004, 177, 1987–1993. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.F.; Huang, X.L.; Liao, Y.N.; Chen, J.Z. Bifunctional catalyst of a metallophthalocyanine-carbon nitride hybrid for chemical fixation of CO2 to cyclic carbonate. RSC Adv. 2016, 6, 2810–2818. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Tahir, H.B.; Abdullah, R.M.; Aziz, S.B. The H+ ion transport study in polymer blends incorporated with ammonium nitrate: XRD, FTIR, and electrical characteristics. Results Phys. 2022, 42, 105960. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, M.; Liu, H.; Dai, L.M.; Wang, F. A facile route to bimetal and nitrogen-codoped 3D porous graphitic carbon networks for efficient oxygen reduction. Small 2016, 12, 4193–4199. [Google Scholar] [CrossRef]
- Samal, P.P.; Deskshinamoorthy, A.; Arunachalam, S.; Vijayaraghavan, S.; Krishnamurty, S. Free base phthalocyanine coating as a superior corrosion inhibitor for copper surfaces: A combined experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129138. [Google Scholar] [CrossRef]
- Ivanco, J.; Toader, T.; Firsov, A.; Brzhezinskaya, M.; Sperling, M.; Braun, W.; Zaha, D.R.Z. Indium on a copper phthalocyanine thin film: Not a reactive system. Phys. Rev. B 2010, 81, 115325. [Google Scholar] [CrossRef]
- Shmatko, V.A.; Yalovega, G.E.; Myasiedova, T.N.; Brzhezinskaya, M.M.; Shtekhin, I.E.; Petrov, V.V. Influence of the surface morphology and structure on the gas-sorption properties of SiO2CuOx nanocomposite materials: X-ray spectroscopy investigations. Phys. Solid State 2015, 57, 399. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Myasoedova, T.N.; Shmatko, V.A.; Brzhezinskaya, M.M.; Popv, Y.V. Influence of Cu/Sn mixture on the shape and structure of crystallites in copper-containing films: Morphologicaland X-ray spectroscopy studies. Appl. Surf. Sci. 2016, 372, 93–99. [Google Scholar] [CrossRef]
- Saptal, V.; Shinde, D.B.; Banerjee, R.; Bhanage, B.M. State-of-the-art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones. Catal. Sci. Technol. 2016, 6, 6152–6158. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Liu, G.; Luo, X.; Wu, F. Cobalt phthalocyanine-based nanodots as efficient catalysts for chemical conversion of CO2 under ambient conditions. J. Mater. Sci. 2021, 56, 10990–10999. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.; Liu, X.; Zou, K.; Deng, W. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960. [Google Scholar] [CrossRef]
- He, L.; Natha, J.K.; Lin, Q. Robust multivariate metal-porphyrin frameworks for efficient ambient fixation of CO2 to cyclic carbonates. Chem. Commun. 2019, 55, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, M.Y.; Jiang, F.L.; Su, W.P.; Hong, M.C. Stable porphyrin Zr and Hf metal–organic frameworks featuring 2.5 nm cages: High surface areas, SCSC transformations and catalyses. Chem. Sci. 2015, 6, 3466–3470. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Wojtas, L.; Ma, S.Q. A porous metal–metalloporphyrin framework featuring high-density active sites for chemical fixation of CO2 under ambient conditions. Chem. Commun. 2014, 50, 5316–5318. [Google Scholar] [CrossRef]
- Gao, W.Y.; Chen, Y.; Niu, Y.H.; Williams, K.; Cash, L.; Perez, P.J.; Wpjtas, L.; Cai, J.F.; Chen, Y.S.; Ma, S.Q. Crystal engineering of an nbo topology metal–organic framework for chemical fixation of CO2 under ambient conditions. Angew. Chem. 2014, 53, 2615–2619. [Google Scholar] [CrossRef]
- Ji, D.F.; Lu, X.B.; He, R. Syntheses of cyclic carbonates from carbon dioxide and epoxides with metal phthalocyanines as catalyst. Appl. Catal. A Gen. 2000, 203, 329–333. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Nguyen, N.; Ma, S.Q. Flexibility matters: Cooperative active sites in covalent organic framework and threaded ionic polymer. J. Am. Chem. Soc. 2016, 138, 15790–15796. [Google Scholar] [CrossRef]
- Martίn, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Wang, J.Q.; Kun, D.; Cheng, W.G.; Sun, J.; Zhang, S.J. Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides. Catal. Sci. Technol. 2012, 2, 1480–1484. [Google Scholar] [CrossRef]
- Lan, J.W.; Qu, Y.; Ping, X.; Sun, J.M. Novel HBD-Containing Zn (dobdc) (datz) as efficiently heterogeneous catalyst for CO2 chemical conversion under mild conditions. Green Energy Environ. 2021, 6, 66–74. [Google Scholar] [CrossRef]
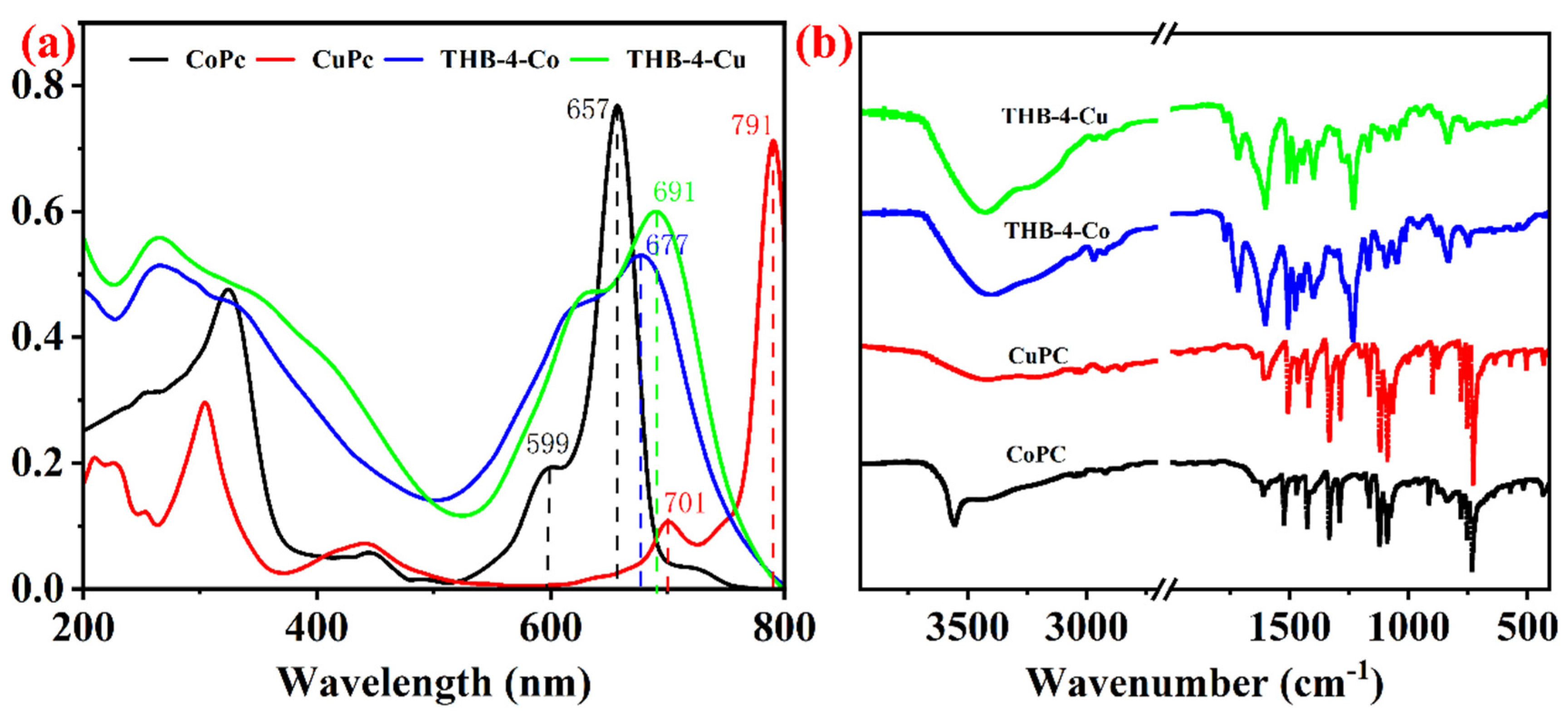



| Entry | MPcs | Absorption/nm | IR Peaks/cm−1 |
|---|---|---|---|
| 1 | CoPc | 599, 657 a | 3068, 2856, 1610, 1470, 1390 |
| 2 | THB-4-Co | 626, 677 | |
| 3 | CuPc | 701, 791 b | |
| 4 | THB-4-Cu | 629, 691 |
| Entry | MPcs | SBET (m2·g−1) | Vpore (cm3·g−1) | Dpore (nm) | CO2 Capacities (273 K, cm3·g−1) | CO2 Capacities (298 K, cm3·g−1) | ICP |
|---|---|---|---|---|---|---|---|
| 1 | THB-4-Co | 71.14 | 0.36 | 24.07 | 24.14 | 14.17 | 7.73 |
| 2 | THB-4-Cu | 114.25 | 0.25 | 19.71 | 25.40 | 9.68 | 8.52 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalysts | P CO2 (bar) | T (°C) | t (h) | Conv. (%) | TON (a) | Ref. |
| 1 | Co/POP-TPP/TBAB | 1 | 29 | 24 | 95.6 | 442 | [48] |
| 2 | CoPc/g-C3N4 | 30 | 130 | 24 | 97.6 | 296 | [40] |
| 3 | CoPc-NDs/TBAB | 1 | 25 | 24 | 88.0 | 1708 | [49] |
| 4 | Co-CMP/TBAB | 3 | 100 | 1 | 98.1 | 201 | [50] |
| 5 | FTPFs-Cu-Nb-Ni/TBAB | 1 | r.t. | 48 | 93.0 | 186 | [51] |
| 6 | CuPc@CS/TBAB | 1 | 80 | 4.5 | 95.0 | 270 | [29] |
| 7 | FJI-H7(Cu)/TBAB | 1 | 25 | 60 | 66.5 | 332.5 | [29] |
| 8 | MMPF-9(Cu)/TBAB | 1 | 25 | 48 | 87.4 | NM (b) | [52] |
| 9 | MMCF-2(Cu)/TBAB | 1 | r.t. | 48 | 88.5 | NM | [53] |
| 10 | CoPc/TBAB | / (c) | 140 | 5 | 5.1 | NM | [54] |
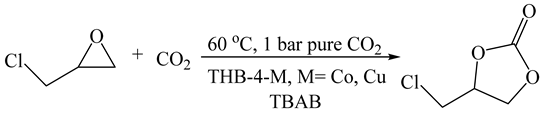
| 11 | THB-4-Co/TBAB | 1 | 60 | 24 | 90.3 | 674 | This work (d) |
| 12 | THB-4-Cu/TBAB | 1 | 60 | 24 | 91.6 | 676 | This work |
| Entry | Epoxide | Co-Cat (a) | T (°C) | t (h) | Conv. (%) (b) | Selec. (%) (b) | TON (c) |
|---|---|---|---|---|---|---|---|
| 1 | 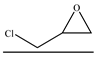 | TBAB | 60 | 24 | 90.3 | 90.5 | 674 |
| 2 | TBAI | 89.9 | 95.6 | 709 | |||
| 3 | TBAC | 78.7 | 73.3 | 476 | |||
| 4 | TEBAC | 88.0 | 70.3 | 510 | |||
| 5 | CTAC | 79.0 | 69.2 | 451 | |||
| 6 | CTAB | 94.9 | 81.2 | 635 | |||
| 7 |  | TBAB | 60 | 6 | 69.5 | 69.3 | 397 |
| 8 | 12 | 83.9 | 90.5 | 626 | |||
| 9 | 24 | 90.3 | 90.5 | 674 | |||
| 10 | 48 | 98.0 | 89.1 | 720 | |||
| 11 | 90 | 12 | 99.0 | 92.6 | 756 | ||
| 12 | 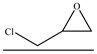 | TBAB 50 mg | 60 | 24 | 90.3 | 90.5 | 674 |
| 13 | TBAB 30 mg | 83.6 | 87.3 | 601 | |||
| 14 | TBAB 10 mg | 73.6 | 78.4 | 475 | |||
| 15 | 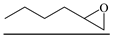 | TBAB | 90 | 24 | 82.4 | 90.3 | 613 |
| 16 | 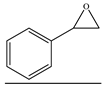 | TBAB | 90 | 24 | 90.1 | 92.6 | 697 |
| 17 |  | TBAB | 90 | 24 | 49.9 | 35.5 | 146 |
| 18 | 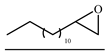 | TBAB | 90 | 24 | 65.7 | 88.4 | 239 |
| 19 | 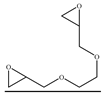 | TBAB | 60 | 24 | 69.7 | 50.4 | 142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shao, S.; Han, X.; Zhou, B.; Han, Y.; Dong, X.; Yu, S. The Flower-Shaped Co (II) and Cu (II) Phthalocyanine Polymers as Highly Efficient and Stable Catalysts for Chemical Fixation of CO2 to Cyclic Carbonate. C 2024, 10, 74. https://doi.org/10.3390/c10030074
Zhou Y, Shao S, Han X, Zhou B, Han Y, Dong X, Yu S. The Flower-Shaped Co (II) and Cu (II) Phthalocyanine Polymers as Highly Efficient and Stable Catalysts for Chemical Fixation of CO2 to Cyclic Carbonate. C. 2024; 10(3):74. https://doi.org/10.3390/c10030074
Chicago/Turabian StyleZhou, Yuyang, Shengyu Shao, Xiang Han, Baocheng Zhou, Yifeng Han, Xiaoping Dong, and Sanchuan Yu. 2024. "The Flower-Shaped Co (II) and Cu (II) Phthalocyanine Polymers as Highly Efficient and Stable Catalysts for Chemical Fixation of CO2 to Cyclic Carbonate" C 10, no. 3: 74. https://doi.org/10.3390/c10030074
APA StyleZhou, Y., Shao, S., Han, X., Zhou, B., Han, Y., Dong, X., & Yu, S. (2024). The Flower-Shaped Co (II) and Cu (II) Phthalocyanine Polymers as Highly Efficient and Stable Catalysts for Chemical Fixation of CO2 to Cyclic Carbonate. C, 10(3), 74. https://doi.org/10.3390/c10030074








