H2 Adsorption on Small Pd-Ni Clusters Deposited on N-Doped Graphene: A Theoretical Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Properties of Pd4-nNin (n = 0–3) Clusters Embedded on PNG
3.2. H2 Adsorption on Pd4-nNin (n = 0–3) Clusters Embedded on PNG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sust. Energ. Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Nazir, H.; Louis, C.; Jose, S.; Prakash, J.; Muthuswamy, N.; Buan, M.E.; Kannan, A.M. Is the H2 economy realizable in the foreseeable future? Part I: H2 production methods. Int. J. Hydrogen Energy 2020, 45, 13777–13788. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sust. Energ. Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.G.; Cool, P.; Denayer, J.F. Hydrogen clathrates: Next generation hydrogen storage materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Jena, P. Materials for hydrogen storage: Past, present, and future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Zhu, M. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Tozzini, V.; Pellegrini, V. Prospects for hydrogen storage in graphene. Phys. Chem. Chem. Phys. 2013, 15, 80–89. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; García-Hilerio, B.; Montejo-Alvaro, F.; Gazga-Villalobos, A.; Rojas-Chávez, H.; Sánchez-Rodríguez, E.P. Density functional theory-based approaches to improving hydrogen storage in graphene-based materials. Molecules 2024, 29, 436. [Google Scholar] [CrossRef]
- Jukk, K.; Kongi, N.; Rauwel, P.; Matisen, L.; Tammeveski, K. Platinum nanoparticles supported on nitrogen-doped graphene nanosheets as electrocatalysts for oxygen reduction reaction. Electrocatalysis 2016, 7, 428–440. [Google Scholar] [CrossRef]
- Gracia-Espino, E.; Jia, X.; Wågberg, T. Improved oxygen reduction performance of Pt–Ni nanoparticles by adhesion on nitrogen-doped graphene. J. Phys. Chem. C 2014, 118, 2804–2811. [Google Scholar] [CrossRef]
- Ortiz-Vázquez, E.A.; Montejo-Alvaro, F.; Cruz-Martínez, H.; Calaminici, P. Theoretical study of PdNi and PdCu clusters embedded on graphene modified by monovacancy and nitrogen doping. J. Comput. Chem. 2024, 45, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.P.; Vargas-Hernández, C.N.; Cruz-Martínez, H.; Medina, D.I. Stability, magnetic, energetic, and reactivity properties of icosahedral M@Pd12 (M = Fe, Co, Ni, and Cu) core-shell nanoparticles supported on pyridinic N3-doped graphene. Solid State Sci. 2021, 112, 106483. [Google Scholar] [CrossRef]
- Jalili, S.; Goliaei, E.M.; Schofield, J. Silver cluster supported on nitrogen-doped graphene as an electrocatalyst with high activity and stability for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 14522–14533. [Google Scholar] [CrossRef]
- Martínez-Espinosa, J.A.; Cruz-Martínez, H.; Calaminici, P.; Medina, D.I. Structures and properties of Co13−xCux (x = 0–13) nanoclusters and their interaction with pyridinic N3-doped graphene nanoflake. Phys. E 2021, 134, 114858. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Chen, G.; Zhao, J. Theoretical insights into the energetics and electronic properties of MPt12 (M = Fe, Co, Ni, Cu, and Pd) nanoparticles supported by N-doped defective graphene. Appl. Surf. Sci. 2017, 397, 199–205. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; López-Sosa, L.; Calaminici, P. Stability and catalytic properties of Pt–Ni clusters supported on pyridinic N-doped graphene nanoflakes: An auxiliary density functional theory study. Theor. Chem. Acc. 2022, 141, 46. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Martínez-Espinosa, J.A.; Rojas-Chávez, H.; Navarro-Ibarra, D.C.; Cruz-Martínez, H.; Medina, D.I. CO2 Adsorption over 3d Transition-Metal Nanoclusters Supported on Pyridinic N3-Doped Graphene: A DFT Investigation. Materials 2022, 15, 6136. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.; Sansores, E. heoretical study of hydrogen adsorption on nitrogen doped graphene decorated with palladium clusters. Int. J. Hydrogen Energy 2014, 39, 6558–6566. [Google Scholar] [CrossRef]
- Rangel, E.; Sansores, E.; Vallejo, E.; Hernández-Hernández, A.; López-Pérez, P.A. Study of the interplay between N-graphene defects and small Pd clusters for enhanced hydrogen storage via a spill-over mechanism. Phys. Chem. Chem. Phys. 2016, 18, 33158–33170. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Fan, X.; Pan, R.; An, Y. A first-principles study of Sc-decorated graphene with pyridinic-N defects for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 3106–3113. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; Pronsato, M.E. DFT study of Rh and Ti dimers decorating N-doped pyridinic and pyrrolic graphene for molecular and dissociative hydrogen adsorption. Appl. Surf. Sci. 2019, 464, 243–254. [Google Scholar] [CrossRef]
- Singla, M.; Jaggi, N. Enhanced hydrogen sensing properties in copper decorated nitrogen doped defective graphene nanoribbons: DFT study. Phys. E 2021, 131, 114756. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Guerra-Cabrera, W.; Flores-Rojas, E.; Ruiz-Villalobos, D.; Rojas-Chávez, H.; Peña-Castañeda, Y.A.; Medina, D.I. Pt-free metal nanocatalysts for the oxygen reduction reaction combining experiment and theory: An overview. Molecules 2021, 26, 6689. [Google Scholar] [CrossRef]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-based nanomaterials for electro-catalytic oxygen reduction in fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 14596–14627. [Google Scholar] [CrossRef]
- Geudtner, G.; Calaminici, P.; Carmona-Espíndola, J.; del Campo, J.M.; Domínguez-Soria, V.D.; Moreno, R.F.; Gamboa, G.U.; Goursot, A.; Köster, A.M.; Salahub, D.R.; et al. DeMon2k. WIREs Comput. Mol. Sci. 2012, 2, 548–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W. Comment on “Generalized gradient approximation made simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Mintmire, J.W.; Dunlap, B.I. Fitting the Coulomb potential variationally in linear-combination-of-atomic-orbitals density-functional calculations. Phys. Rev. A 1982, 25, 88. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Calaminici, P.; Janetzko, F.; Köster, A.M.; Mejia-Olvera, R.; Zuniga-Gutierrez, B. Density functional theory optimized basis sets for gradient corrected functionals: 3d transition metal systems. J. Chem. Phys. 2007, 126, 044108. [Google Scholar] [CrossRef] [PubMed]
- Köster, A.M.; Reveles, J.U.; del Campo, J.M. Calculation of exchange-correlation potentials with auxiliary function densities. J. Chem. Phys. 2004, 121, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Reveles, J.U.; Köster, A.M. Geometry optimization in density functional methods. J. Comput. Chem. 2004, 25, 1109–1116. [Google Scholar] [CrossRef]
- Cervantes-Flores, A.; Cruz-Martínez, H.; Solorza-Feria, O.; Calaminici, P. A first-principles study of NinPdn (n = 1–5) clusters. J. Mol. Model. 2017, 23, 161. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; López-Sosa, L.; Solorza-Feria, O.; Calaminici, P. First-principles investigation of adsorption and dissociation of molecular oxygen on pure Pd, Ni-doped Pd and NiPd alloy clusters. Int. J. Hydrogen Energy 2017, 42, 30310–30317. [Google Scholar] [CrossRef]
- Roy, G.; Chattopadhyay, A.P. The reactivity of CO on bimetallic Ni3M clusters (M = Sc, Ti, V, Cr, Mn, Fe, Co, Cu, Rh, Ru, Ag, Pd and Pt) by density functional theory. New J. Chem. 2019, 43, 11363–11373. [Google Scholar] [CrossRef]
- Domancich, N.F.; Ferullo, R.M.; Castellani, N.J. Interaction of aluminum dimer with defective graphene. Comput. Theor. Chem. 2015, 1059, 27–34. [Google Scholar] [CrossRef]
- Nieman, R.; Aquino, A.J.; Hardcastle, T.P.; Kotakoski, J.; Susi, T.; Lischka, H. Structure and electronic states of a graphene double vacancy with an embedded Si dopant. J. Chem. Phys. 2017, 147, 194702. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Teillet-Billy, D.; Rougeau, N.; Sidis, V.; Morisset, S.; Allouche, A. Stability and magnetism of hydrogen dimers on graphene. Phys. Rev. B 2008, 78, 085417. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H. Density functional investigation of mercury and arsenic adsorption on nitrogen doped graphene decorated with palladium clusters: A promising heavy metal sensing material in farmland. Appl. Surf. Sci. 2017, 399, 55–66. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Rojas-Chávez, H.; Román-Doval, R.; Mtz-Enriquez, A.I.; Cruz-Martínez, H.; Medina, D.I. Stability of Pd clusters supported on pristine, B-doped, and defective graphene quantum dots, and their reactivity toward oxygen adsorption: A DFT analysis. Solid State. Sci. 2019, 93, 55–61. [Google Scholar] [CrossRef]
- Rêgo, C.R.; Tereshchuk, P.; Oliveira, L.N.; Da Silva, J.L. Graphene-supported small transition-metal clusters: A density functional theory investigation within van der Waals corrections. Phys. Rev. B 2017, 95, 235422. [Google Scholar] [CrossRef]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecular Structure: IV. Constants of Diatomic Molecules; Van Nostrand Reinhold Inc.: New York, NY, USA, 1979. [Google Scholar]
- Jin, X.; Qi, P.; Yang, H.; Zhang, Y.; Li, J.; Chen, H. Enhanced hydrogen adsorption on Li-coated B12C6N6. J. Chem. Phys. 2016, 145, 164301. [Google Scholar] [CrossRef]
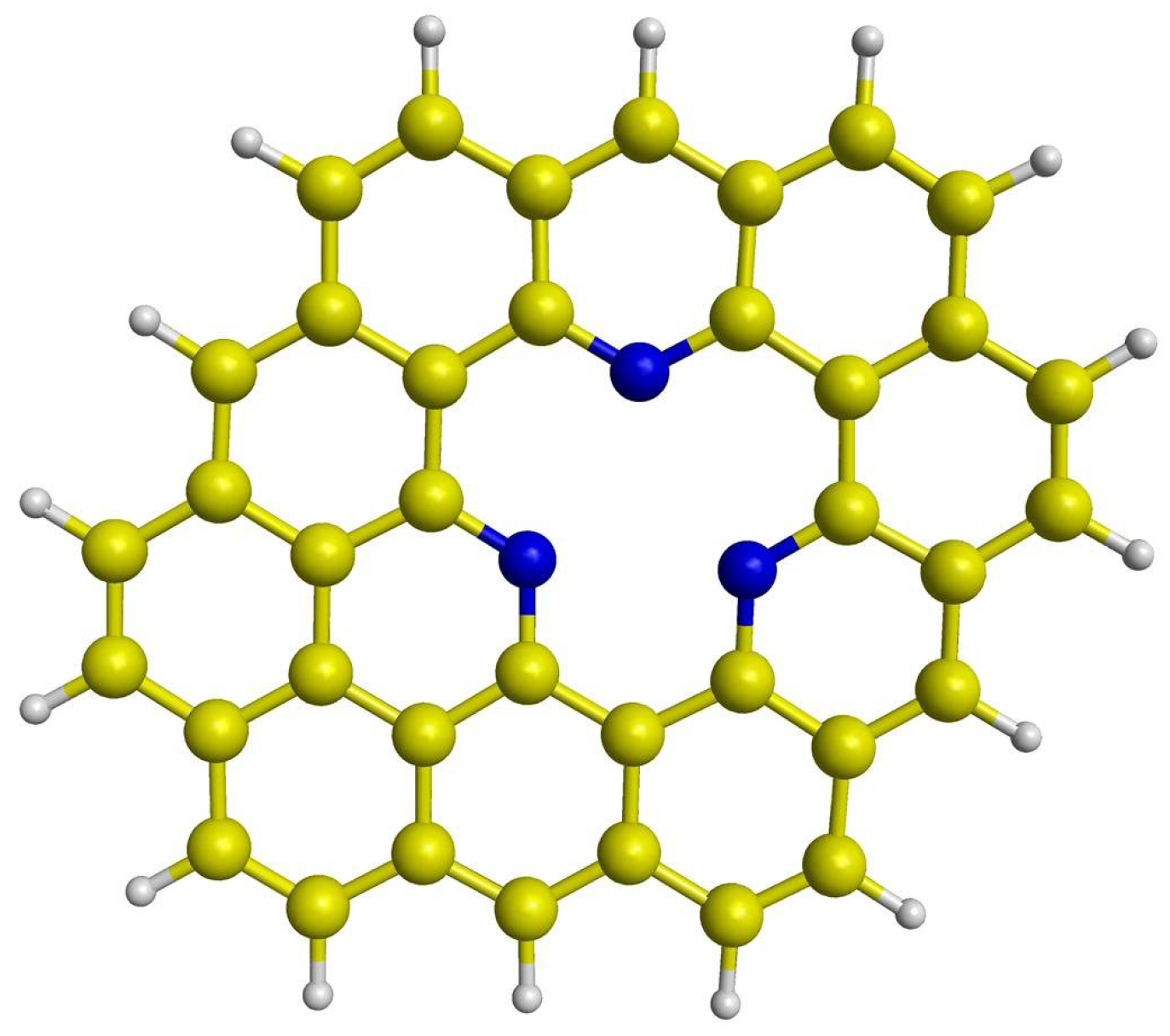

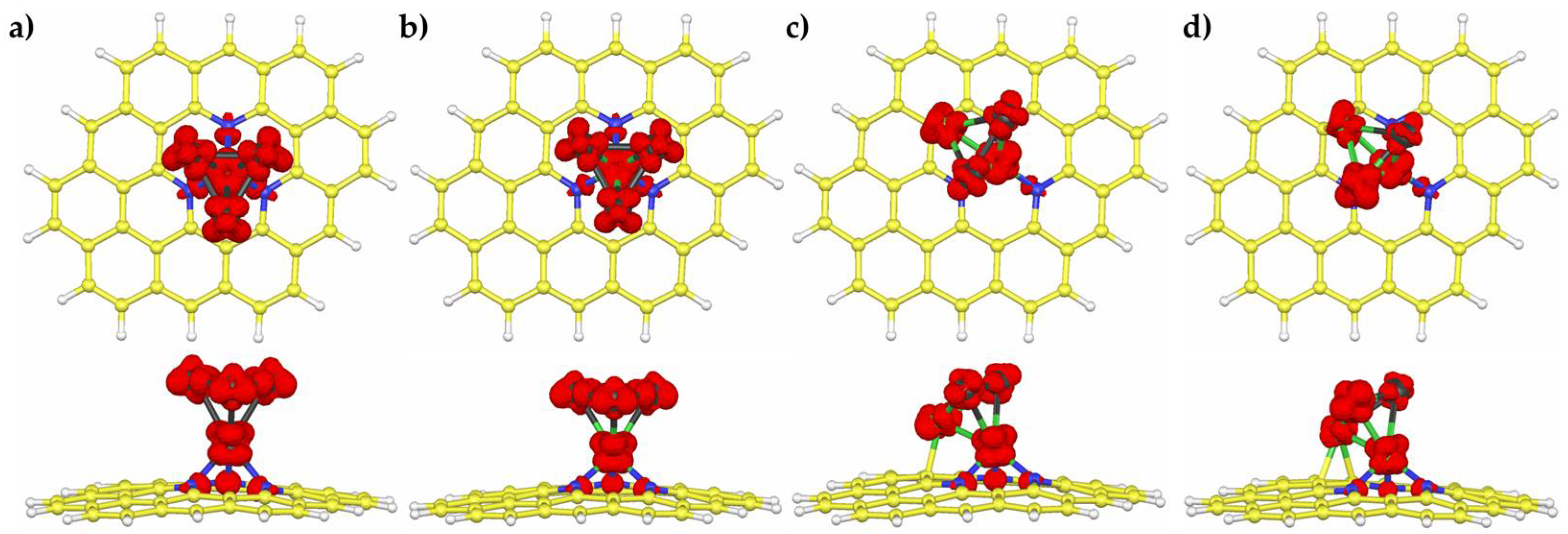
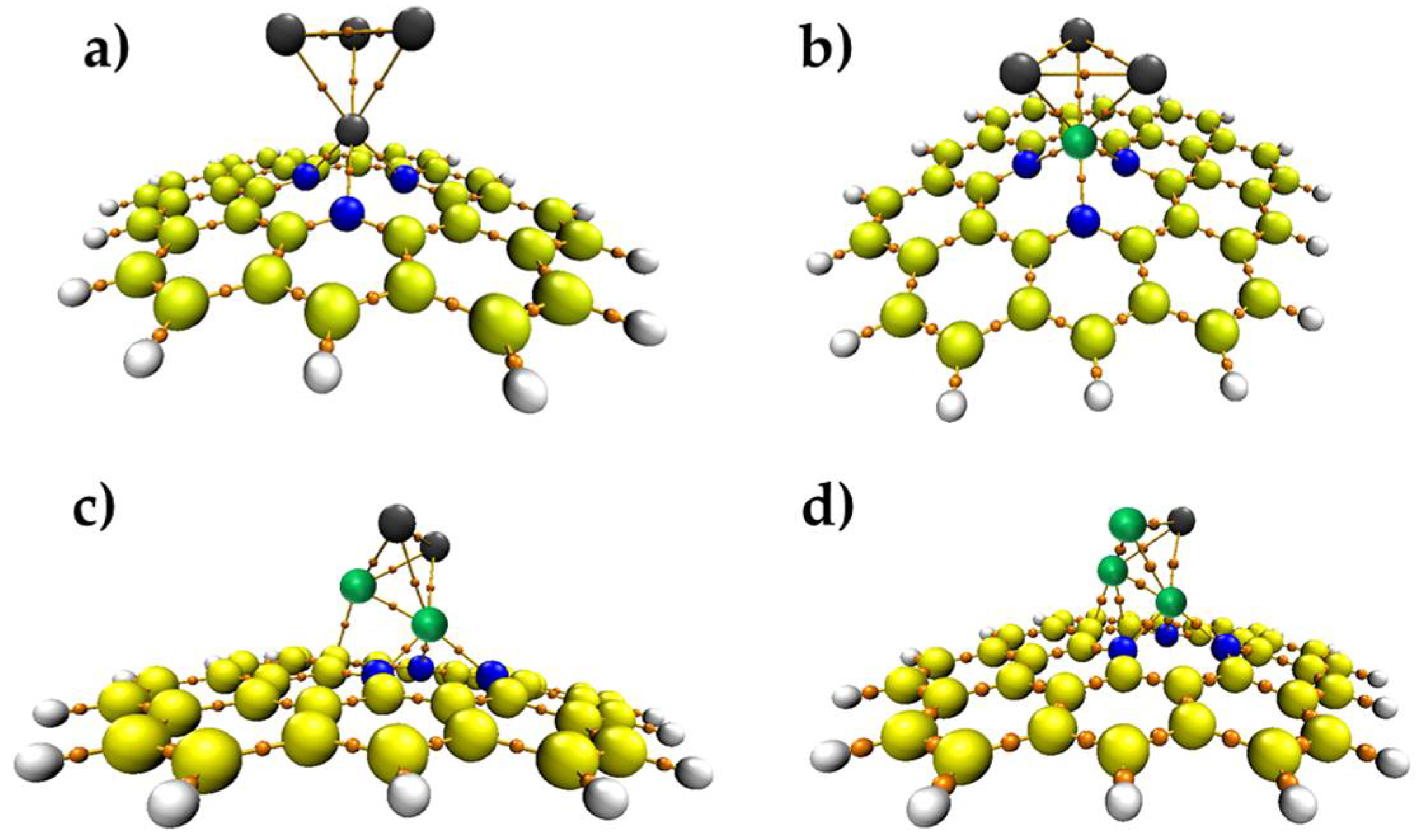
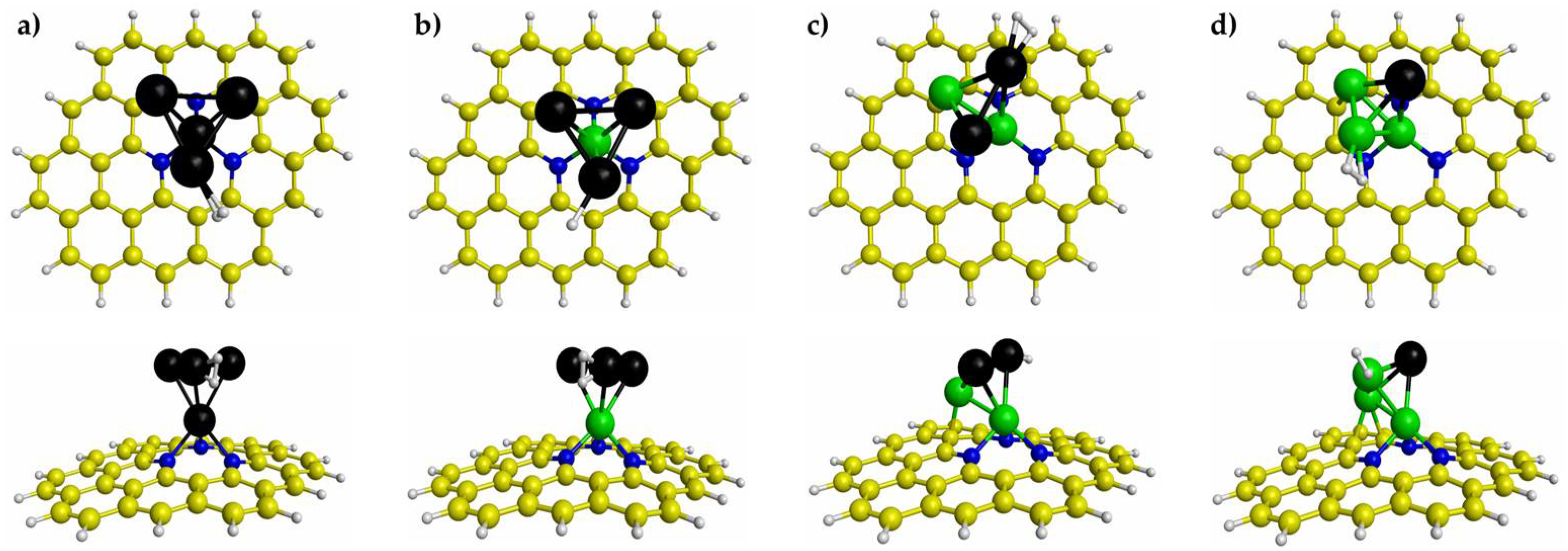
| Pd4/PNG | Pd3Ni1/PNG | Pd2Ni2/PNG | Pd1Ni3/PNG | |
|---|---|---|---|---|
| Spin multiplicities | 4 | 4 | 4 | 4 |
| BCPs | 3 | 3 | 4 | 5 |
| Eint (eV) | −2.74 | −4.37 | −5.00 | −5.50 |
| Bader charges (e) | 0.39 | 0.54 | 0.65 | 0.74 |
| HOMO–LUMO gap (eV) | 1.0 | 0.91 | 0.71 | 0.69 |
| Pd4/PNG | Pd3Ni1/PNG | Pd2Ni2/PNG | Pd1Ni3/PNG | |
|---|---|---|---|---|
| Eads (eV) | −0.29 | −0.31 | −0.39 | −0.37 |
| H-H bond lengths (Å) | 0.83 | 0.83 | 0.84 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Hilerio, B.; Santiago-Silva, L.; Vásquez-García, A.; Gomez-Sanchez, A.; Franco-Luján, V.A.; Cruz-Martínez, H. H2 Adsorption on Small Pd-Ni Clusters Deposited on N-Doped Graphene: A Theoretical Study. C 2024, 10, 73. https://doi.org/10.3390/c10030073
García-Hilerio B, Santiago-Silva L, Vásquez-García A, Gomez-Sanchez A, Franco-Luján VA, Cruz-Martínez H. H2 Adsorption on Small Pd-Ni Clusters Deposited on N-Doped Graphene: A Theoretical Study. C. 2024; 10(3):73. https://doi.org/10.3390/c10030073
Chicago/Turabian StyleGarcía-Hilerio, Brenda, Lidia Santiago-Silva, Adriana Vásquez-García, Alejandro Gomez-Sanchez, Víctor A. Franco-Luján, and Heriberto Cruz-Martínez. 2024. "H2 Adsorption on Small Pd-Ni Clusters Deposited on N-Doped Graphene: A Theoretical Study" C 10, no. 3: 73. https://doi.org/10.3390/c10030073
APA StyleGarcía-Hilerio, B., Santiago-Silva, L., Vásquez-García, A., Gomez-Sanchez, A., Franco-Luján, V. A., & Cruz-Martínez, H. (2024). H2 Adsorption on Small Pd-Ni Clusters Deposited on N-Doped Graphene: A Theoretical Study. C, 10(3), 73. https://doi.org/10.3390/c10030073







