The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks
Abstract
1. Introduction
2. Subjects, Materials and Methods
2.1. Design of the Study
2.2. Inclusion Criteria
- I.
- Age range 18–80 years.
- II.
- Patients for whom multislice spiral computed tomography coronary angiography (MSCT-CA) is indicated:
- a.
- Patients with atypical anginal pain with low or intermediate CVR;
- b.
- Asymptomatic patients at high CVR;
- c.
- Patients at high CVR with indications for diagnostic coronary angiography who refused invasive tests;
- III.
- Written informed consent of the patient to participate in the study.
2.3. Exclusion Criteria
- I.
- Pregnancy and breastfeeding;
- II.
- Diabetes mellitus;
- III.
- Heart surgery or coronary interventions in anamnesis;
- IV.
- Severe heart failure (III–IV classes according to the classification of the New York Heart Association, NYHA);
- V.
- History of myocardial infarction;
- VI.
- Body mass index 35 kg/m2 or more;
- VII.
- The presence of any moderate or severe somatic pathology at the time of the study (including oncological diseases, impaired liver function, renal failure, systemic and inflammatory diseases, as well as any infections);
- VIII.
- Refusal of the patient from participation in the study;
- IX.
- Psychiatric disorders, including claustrophobia.
2.4. Study Duration
2.5. Description of the Intervention
2.6. Hemolysis Assessment of Plasma Samples
2.7. Plasma miRNA Isolation
2.8. cDNA Synthesis and Quantitative PCR (qPCR) for miRNA Detection
2.9. Statistical Data Analysis Methods
3. Results
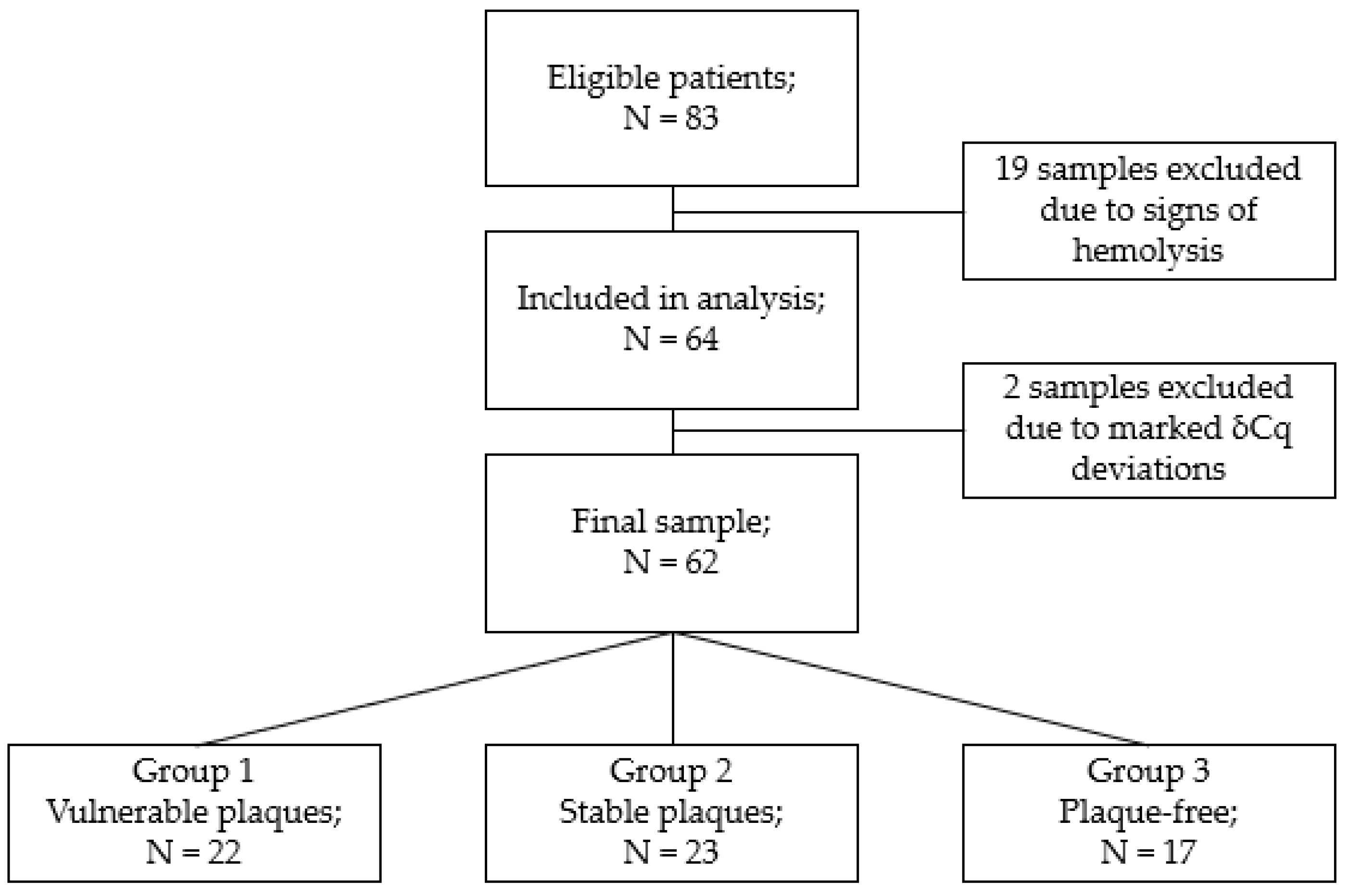
3.1. Study Participants
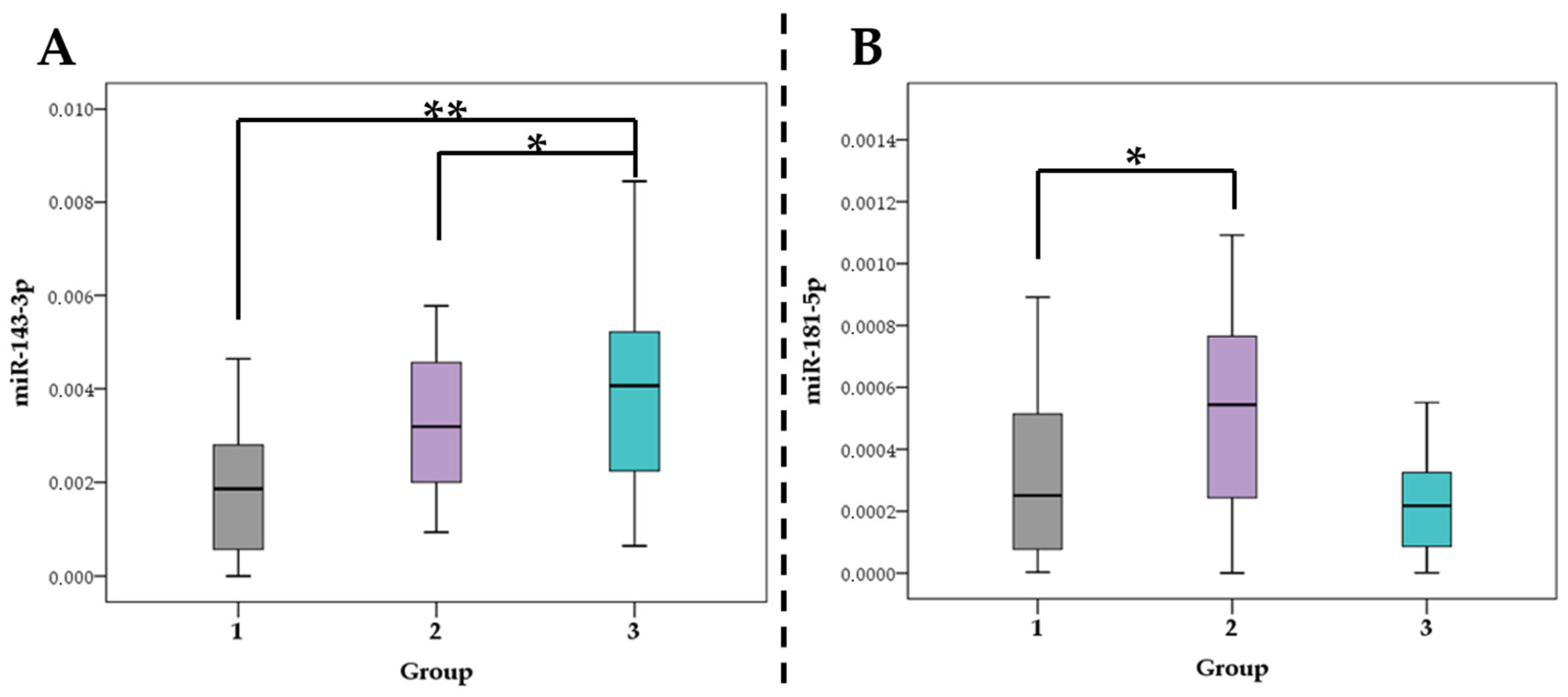
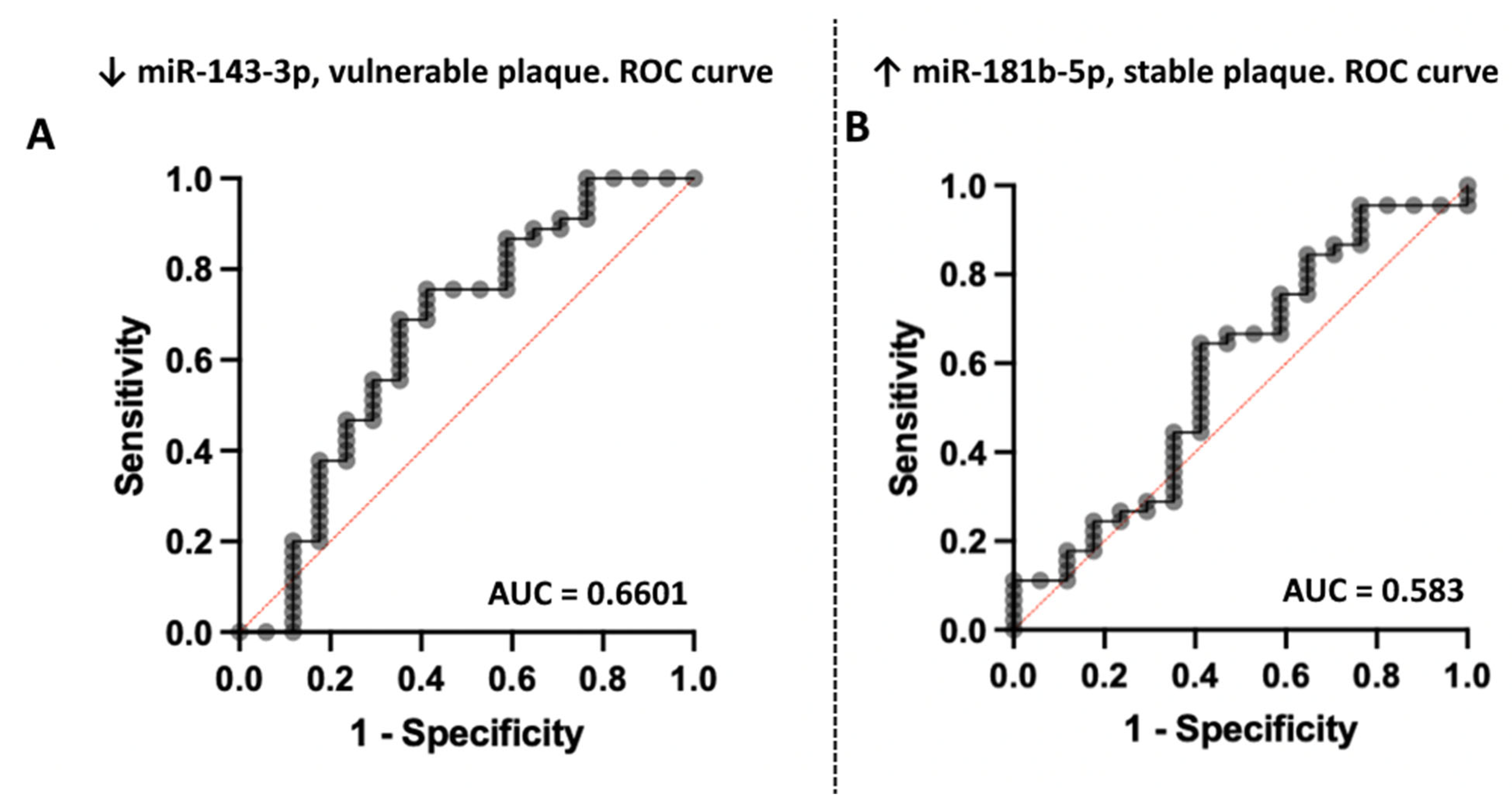
3.2. Association of miRNA Levels with the Type of Atherosclerotic Plaques
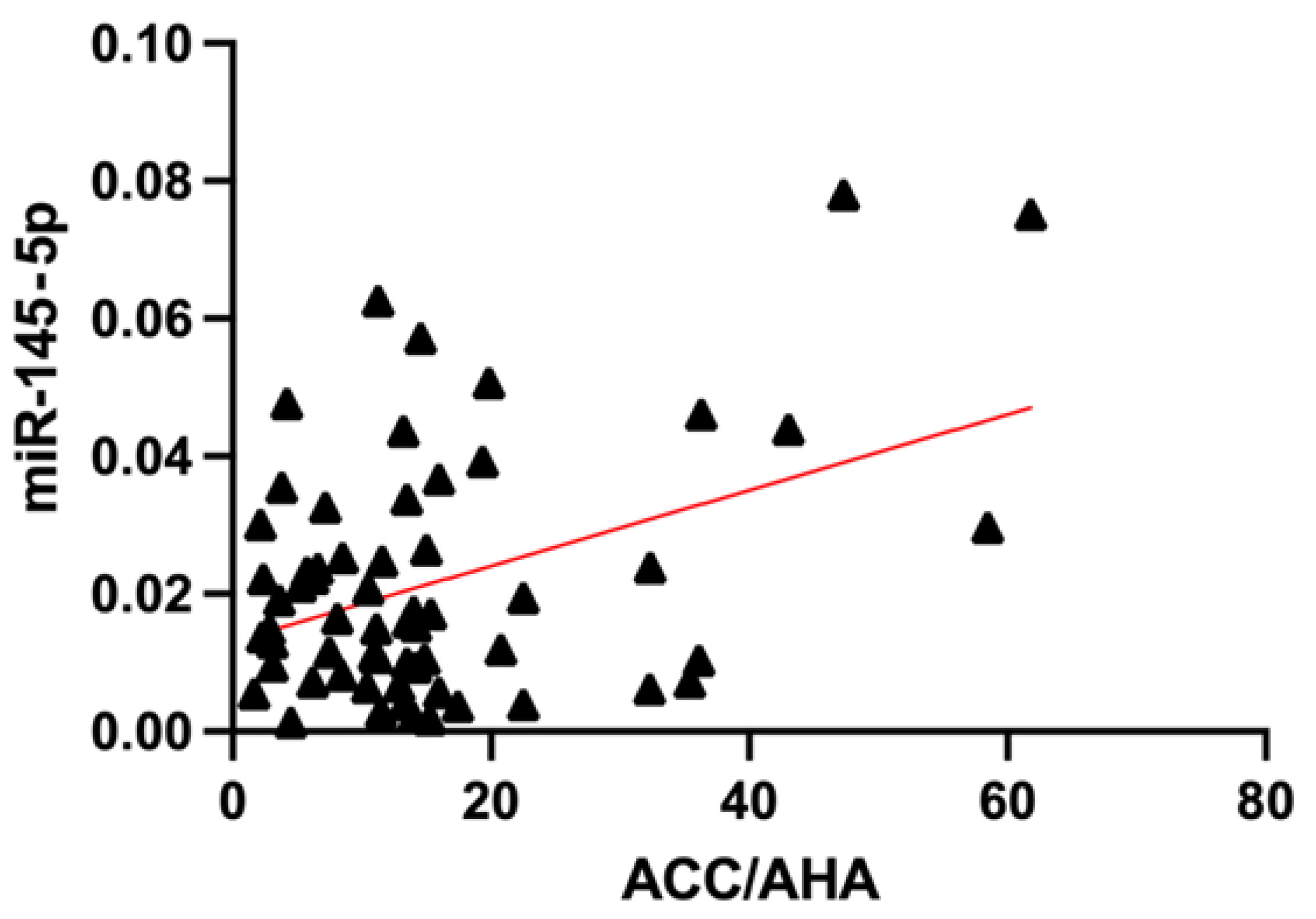
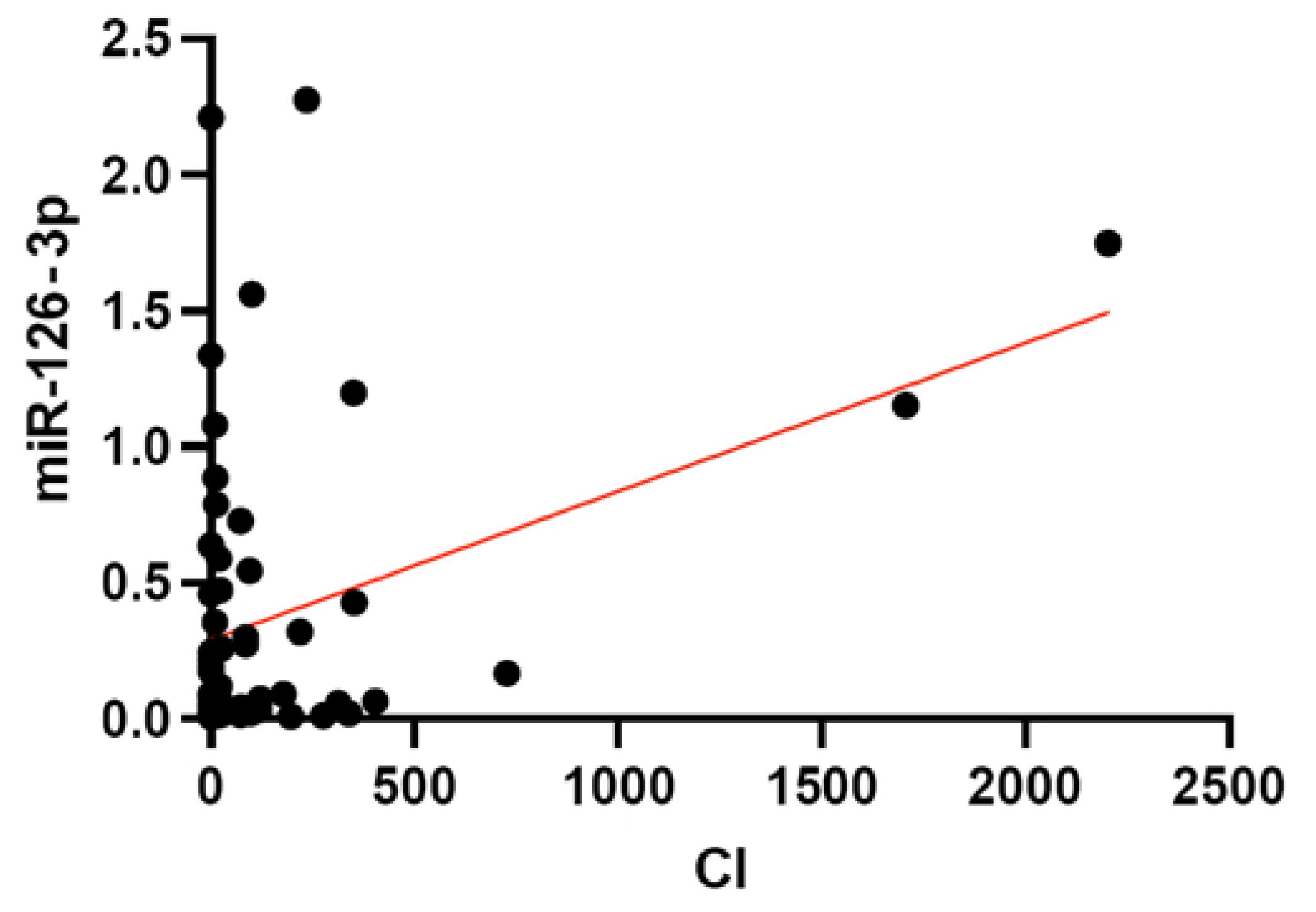
3.3. Association of miRNA Levels with CVR
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M.; et al. Risk Assessment for Cardiovascular Disease With Nontraditional Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Tian, Y. (Eds.) MicroRNA Detection and Pathological Functions; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-47292-7. [Google Scholar]
- Wei, Y.; Schober, A.; Weber, C. Pathogenic arterial remodeling: The good and bad of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1050–H1059. [Google Scholar] [CrossRef]
- Tabaei, S.; Tabaee, S.S. Implications for MicroRNA involvement in the prognosis and treatment of atherosclerosis. Mol. Cell. Biochem. 2021, 476, 1327–1336. [Google Scholar] [CrossRef]
- Siddeek, B.; Mauduit, C.; Yzydorczyk, C.; Benahmed, M.; Simeoni, U. At the heart of programming: The role of micro-RNAs. J. Dev. Orig. Health Dis. 2018, 9, 615–631. [Google Scholar] [CrossRef]
- Kim, J.S.; Pak, K.; Goh, T.S.; Jeong, D.C.; Han, M.E.; Kim, J.; Oh, S.O.; Kim, C.D.; Kim, Y.H. Prognostic Value of MicroRNAs in Coronary Artery Diseases: A Meta-Analysis. Yonsei Med. J. 2018, 59, 495–500. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. Role of MicroRNAs in the Pathogenesis of Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 632392. [Google Scholar] [CrossRef]
- Keller, T.; Boeckel, J.-N.; Groß, S.; Klotsche, J.; Palapies, L.; Leistner, D.; Pieper, L.; Stalla, G.K.; Lehnert, H.; Silber, S.; et al. Improved risk stratification in prevention by use of a panel of selected circulating microRNAs. Sci. Rep. 2017, 7, 4511. [Google Scholar] [CrossRef]
- Zampetaki, A.; Willeit, P.; Tilling, L.; Drozdov, I.; Prokopi, M.; Renard, J.-M.; Mayr, A.; Weger, S.; Schett, G.; Shah, A.; et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Jiménez-Xarrié, E.; Camps-Renom, P.; Benitez, S. Search for Reliable Circulating Biomarkers to Predict Carotid Plaque Vulnerability. Int. J. Mol. Sci. 2020, 21, 8236. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Maffei, E. Cardiac CT for the detection of vulnerable plaque. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Perich, L.; Puigoriol-Illamola, D.; Bashir, S.; Terceño, M.; Silva, Y.; Gubern-Mérida, C.; Serena, J. Clinical Parameters and Epigenetic Biomarkers of Plaque Vulnerability in Patients with Carotid Stenosis. Int. J. Mol. Sci. 2022, 23, 5149. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Ronde, M.W.; Kok, M.G.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.; Creemers, E.E.; Pinto-Sietsma, S.-J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lee, C.; Song, J.; Lu, C.; Liu, J.; Cui, Y.; Liang, H.; Cao, C.; Zhang, F.; Chen, H. Circulating microRNAs as potential biomarkers for coronary plaque rupture. Oncotarget 2017, 8, 48145–48156. [Google Scholar] [CrossRef]
- Ternovoy, S.K.; Veselova, T.N. MSCT In Detection of Unstable Coronary Plaques. REJR 2014, 4, 7–13. [Google Scholar]
- Yoo, S.M.; Lee, H.Y.; Jin, K.N.; Chun, E.J.; Ann, F.A.; White, C.S. Current Concepts of Vulnerable Plaque on Coronary CT Angiography. Cardiovasc. Imaging Asia 2017, 1, 4–12. [Google Scholar] [CrossRef][Green Version]
- SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [CrossRef]
- SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [CrossRef]
- D’Agostino, R.B.; Pencina, M.J.; Massaro, J.M.; Coady, S. Cardiovascular Disease Risk Assessment: Insights from Framingham. Glob. Heart 2013, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Huffman, M.D.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; Goff, D.C. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2017, 69, 1617–1636. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Appierto, V.; Callari, M.; Cavadini, E.; Morelli, D.; Daidone, M.G.; Tiberio, P. A lipemia-independent NanoDrop(®)-based score to identify hemolysis in plasma and serum samples. Bioanalysis 2014, 6, 1215–1226. [Google Scholar] [CrossRef]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, S.-P.; Zhao, Y.-H. MicroRNA-143/-145 in Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 531740. [Google Scholar] [CrossRef]
- Dégano, I.R.; Camps-Vilaró, A.; Subirana, I.; García-Mateo, N.; Cidad, P.; Muñoz-Aguayo, D.; Puigdecanet, E.; Nonell, L.; Vila, J.; Crepaldi, F.M.; et al. Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study. J. Clin. Med. 2020, 9, 1402. [Google Scholar] [CrossRef]
- Troidl, K.; Hammerschick, T.; Albarran-Juarez, J.; Jung, G.; Schierling, W.; Tonack, S.; Krüger, M.; Matuschke, B.; Troidl, C.; Schaper, W.; et al. Shear Stress-Induced miR-143-3p in Collateral Arteries Contributes to Outward Vessel Growth by Targeting Collagen V-α2. Arter. Thromb. Vasc. Biol. 2020, 40, e126–e137. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Vilades, D.; Martínez-Camblor, P.; Vea, À.; Nasarre, L.; Sanchez Vega, J.; Leta, R.; Carreras, F.; Llorente-Cortés, V. Circulating microRNAs in suspected stable coronary artery disease: A coronary computed tomography angiography study. J. Intern. Med. 2019, 286, 341–355. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef]
- Schober, A.; Weber, C. Mechanisms of MicroRNAs in Atherosclerosis. Annu. Rev. Pathol. 2016, 11, 583–616. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.F.; Beylerli, O.; Alyshov, A.B. MicroRNA regulatory mechanisms in atherosclerosis. Her. North West. State Med. Univ. Named II Mechnikov 2020, 12, 11–20. [Google Scholar] [CrossRef]
- Sun, P.; Li, L.; Liu, Y.-Z.; Li, G.-Z.; Xu, Q.-H.; Wang, M.; Gong, Y. MiR-181b regulates atherosclerotic inflammation and vascular endothelial function through Notch1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, G. Potential role of microRNA-181b on atherosclerosis. Zhonghua Xin Xue Guan Bing Za Zhi 2015, 43, 516–520. [Google Scholar] [PubMed]
- Di Gregoli, K.; Mohamad Anuar, N.N.; Bianco, R.; White, S.J.; Newby, A.C.; George, S.J.; Johnson, J.L. MicroRNA-181b Controls Atherosclerosis and Aneurysms Through Regulation of TIMP-3 and Elastin. Circ. Res. 2017, 120, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Song, M.A.; Choe, D.; Xiao, D.; Foster, G.; Zhang, L. Early Detection of Coronary Artery Disease by Micro-RNA Analysis in Asymptomatic Patients Stratified by Coronary CT Angiography. Diagnostics 2020, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Y.; Wu, Q.; Huang, L.; Xu, J.; Zeng, Q. Pathogenic role of microRNAs in atherosclerotic ischemic stroke: Implications for diagnosis and therapy. Genes Dis. 2021, 9, 682–696. [Google Scholar] [CrossRef]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.A.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci. Transl. Med. 2020, 12, eaaz2294. [Google Scholar] [CrossRef]
- Hao, X.-Z.; Fan, H.-M. Identification of miRNAs as atherosclerosis biomarkers and functional role of miR-126 in atherosclerosis progression through MAPK signalling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2725–2733. [Google Scholar]
- Mondadori dos Santos, A.; Metzinger, L.; Haddad, O.; M’baya-Moutoula, E.; Taïbi, F.; Charnaux, N.; Massy, Z.A.; Hlawaty, H.; Metzinger-Le Meuth, V. miR-126 Is Involved in Vascular Remodeling under Laminar Shear Stress. BioMed Res. Int. 2015, 2015, 497280. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Dimmeler, S. MicroRNA-126 in atherosclerosis. Arter. Thromb. Vasc. Biol. 2014, 34, e15–e16. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, M.; Guzik, T.J.; Słodowski, W.; Pelvan, A.; Becker, J.; Halle, M.; Buchwald, A.B.; Channon, K.M.; Hecker, M. Shear stress insensitivity of endothelial nitric oxide synthase expression as a genetic risk factor for coronary heart disease. Circ. Res. 2004, 95, 841–847. [Google Scholar] [CrossRef]
- Zeng, P.; Yang, J.; Liu, L.; Yang, X.; Yao, Z.; Ma, C.; Zhu, H.; Su, J.; Zhao, Q.; Feng, K.; et al. ERK1/2 inhibition reduces vascular calcification by activating miR-126-3p-DKK1/LRP6 pathway. Theranostics 2021, 11, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, M.; Jin, C.; Sun, Y.; Xu, D.; Lin, Y. Expression of inflammatory factors and oxidative stress markers in serum of patients with coronary heart disease and correlation with coronary artery calcium score. Exp. Ther. Med. 2020, 20, 2127–2133. [Google Scholar] [CrossRef]
- Elbaz, M.; Faccini, J.; Laperche, C.; Grousset, E.; Roncalli, J.; Ruidavets, J.-B.; Vindis, C. Identification of a miRNA Based-Signature Associated with Acute Coronary Syndrome: Evidence from the FLORINF Study. J. Clin. Med. 2020, 9, 1674. [Google Scholar] [CrossRef]
- Zeller, T.; Keller, T.; Ojeda, F.; Reichlin, T.; Twerenbold, R.; Tzikas, S.; Wild, P.S.; Reiter, M.; Czyz, E.; Lackner, K.J.; et al. Assessment of microRNAs in patients with unstable angina pectoris. Eur. Heart J. 2014, 35, 2106–2114. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, C.; Pei, H.; Duan, G.; Huang, L.; Li, L. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2015, 15, 51. [Google Scholar] [CrossRef]
- Manoharan, P.; Basford, J.E.; Pilcher-Roberts, R.; Neumann, J.; Hui, D.Y.; Lingrel, J.B. Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Krüppel-like factor 2 (KLF2)-deficient macrophages. J. Biol. Chem. 2014, 289, 31638–31646. [Google Scholar] [CrossRef]
- Deng, P.; Chen, L.; Liu, Z.; Ye, P.; Wang, S.; Wu, J.; Yao, Y.; Sun, Y.; Huang, X.; Ren, L.; et al. MicroRNA-150 Inhibits the Activation of Cardiac Fibroblasts by Regulating c-Myb. Cell. Physiol. Biochem. 2016, 38, 2103–2122. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, P.; Wang, S.; Wu, J.; Sun, Y.; Zhang, A.; Ren, L.; Cheng, C.; Huang, X.; Wang, K.; et al. MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circ. Cardiovasc. Genet. 2015, 8, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, B.; Hong, X.; Shen, Z.; Sui, L.; Wang, S. microRNA-9 Inhibits Vulnerable Plaque Formation and Vascular Remodeling via Suppression of the SDC2-Dependent FAK/ERK Signaling Pathway in Mice With Atherosclerosis. Front. Physiol. 2020, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.-H.; Cheng, W.-L.; Wang, H.; Gao, M.; Qin, J.-J.; Zhang, Y.; Li, X.; Zhu, X.; Xia, H.; She, Z.-G. Reduced atherosclerosis lesion size, inflammatory response in miR-150 knockout mice via macrophage effects. J. Lipid Res. 2018, 59, 658–669. [Google Scholar] [CrossRef]
- Qin, B.; Shu, Y.; Xiao, L.; Lu, T.; Lin, Y.; Yang, H.; Lu, Z. MicroRNA-150 targets ELK1 and modulates the apoptosis induced by ox-LDL in endothelial cells. Mol. Cell. Biochem. 2017, 429, 45–58. [Google Scholar] [CrossRef]
- Rayner, K.J.; Hennessy, E.J. Extracellular communication via microRNA: Lipid particles have a new message. J. Lipid Res. 2013, 54, 1174–1181. [Google Scholar] [CrossRef]
- Luo, N.; Garvey, W.T.; Wang, D.-Z.; Fu, Y. MicroRNA-150 Regulates Lipid Metabolism and Inflammatory Response. J. Metab. Syndr. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Rozhkov, A.N.; Shchekochikhin, D.Y.; Baulina, N.M.; Matveeva, N.A.; Favorova, O.O.; Akselrod, A.S.; Tebenkova, E.S.; Gognieva, D.G.; Kopylov, P.Y. Analysis of circulating miRNA levels in coronary heart disease patients with varying degrees of cardiovascular complications risk. correlations with the MSCT-CA data. Ann. RAMS 2020, 75, 283–291. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wang, H.-Y.J.; Liao, Y.-C.; Tsai, P.-C.; Chen, K.-C.; Cheng, H.-Y.; Lin, R.-T.; Juo, S.-H.H. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012, 95, 517–526. [Google Scholar] [CrossRef]
- Bras, J.P.; Silva, A.M.; Calin, G.A.; Barbosa, M.A.; Santos, S.G.; Almeida, M.I. miR-195 inhibits macrophages pro-inflammatory profile and impacts the crosstalk with smooth muscle cells. PLoS ONE 2017, 12, e0188530. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Liao, X.; Castillero, E.; Kennel, P.J.; Brunjes, D.L.; Franz, M.; Möbius-Winkler, S.; Drosatos, K.; George, I.; et al. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation 2018, 137, 2052–2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, D.; Shi, H.; Zhang, Y.; Li, H.; Han, Q. MiR-195-5p Promotes Cardiomyocyte Hypertrophy by Targeting MFN2 and FBXW7. BioMed Res. Int. 2019, 2019, 1580982. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kim, H.W.; Matsu-ura, K.; Wang, Y.-G.; Xu, M.; Ashraf, M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells 2016, 34, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yao, J.; Xie, H.; Wang, C.; Chen, J.; Wei, K.; Ji, Y.; Liu, L. MicroRNA-195-5p Downregulation Inhibits Endothelial Mesenchymal Transition and Myocardial Fibrosis in Diabetic Cardiomyopathy by Targeting Smad7 and Inhibiting Transforming Growth Factor Beta 1-Smads-Snail Pathway. Front. Physiol. 2021, 12, 709123. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Wang, Y.-S.; Hsu, P.-Y.; Chen, C.-Y.; Liao, Y.-C.; Juo, S.-H.H. miR-195 Has a Potential to Treat Ischemic and Hemorrhagic Stroke through Neurovascular Protection and Neurogenesis. Mol. Ther. Methods Clin. Dev. 2019, 13, 121–132. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, L.; You, F.; Zhou, J.; Ma, Y.; Yang, F.; Tao, L. MiR-145-5p regulates hypoxia-induced inflammatory response and apoptosis in cardiomyocytes by targeting CD40. Mol. Cell. Biochem. 2017, 431, 123–131. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Chen, M.; Chen, L.; Zhang, B.; Wu, T.; Guo, R. miRNA-145 inhibits VSMC proliferation by targeting CD40. Sci. Rep. 2016, 6, 35302. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, B.; Fan, S.; Pu, Y.; Xia, H.; Xu, L. MicroRNA-145 Protects against Myocardial Ischemia Reperfusion Injury via CaMKII-Mediated Antiapoptotic and Anti-Inflammatory Pathways. Oxid. Med. Cell. Longev. 2019, 2019, 8948657. [Google Scholar] [CrossRef]
- Li, L.-L.; Mao, C.-D.; Wang, G.-P.; Wang, N.; Xue, A.-G. MiR-145-5p alleviates hypoxia/reoxygenation- induced cardiac microvascular endothelial cell injury in coronary heart disease by inhibiting Smad4 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5008–5017. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arter. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M.; et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012, 126, S81–S90. [Google Scholar] [CrossRef]
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: Novel targets in essential hypertension. J. Hum. Hypertens. 2014, 28, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Mandolini, C.; Marcantonio, P.; de Nardis, V.; Bucci, M.; Paganelli, C.; Magnacca, F.; Ucchino, S.; Mastroiacovo, D.; Desideri, G.; et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin. Ther. Targets 2013, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Liu, D.; Zhu, W.; Su, Z.; Zhang, L.; Zhou, C.; Li, P. Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p Are Novel Biomarkers for Diagnosis of Acute Myocardial Infarction. Front. Physiol. 2019, 10, 123. [Google Scholar] [CrossRef]
| Assay Name | Assay ID | Mature miRNA Sequence | Type of miRNA |
|---|---|---|---|
| hsa-miR-16-5p | 477860_mir | UAGCAGCACGUAAAUAUUGGCG | Normalization control |
| hsa-miR-23a-3p | 478532_mir | AUCACAUUGCCAGGGAUUUCC | Hemolysis assessment |
| hsa-miR-451a | 478107_mir | AAACCGUUACCAUUACUGAGUU | Hemolysis assessment |
| hsa-miR-126-3p | 477887_mir | UCGUACCGUGAGUAAUAAUGCG | Candidate to atherosclerosis |
| hsa-miR-126-5p | 477888_mir | CAUUAUUACUUUUGGUACGCG | Candidate to atherosclerosis |
| hsa-miR-143-3p | 477912_mir | UGAGAUGAAGCACUGUAGCUC | Candidate to atherosclerosis |
| hsa-miR-145-5p | 477916_mir | GUCCAGUUUUCCCAGGAAUCCCU | Candidate to atherosclerosis |
| hsa-miR-146a-5p | 478399_mir | UGAGAACUGAAUUCCAUGGGUU | Candidate to atherosclerosis |
| hsa-miR-150-5p | 477918_mir | UCUCCCAACCCUUGUACCAGUG | Candidate to atherosclerosis |
| hsa-miR-181b-5p | 478583_mir | AACAUUCAUUGCUGUCGGUGGGU | Candidate to atherosclerosis |
| hsa-miR-195-5p | 477957_mir | UAGCAGCACAGAAAUAUUGGC | Candidate to atherosclerosis |
| hsa-miR-205-5p | 477967_mir | UCCUUCAUUCCACCGGAGUCUG | Candidate to atherosclerosis |
| hsa-miR-21-5p | 477975_mir | UAGCUUAUCAGACUGAUGUUGA | Candidate to atherosclerosis |
| hsa-miR-223-3p | 477983_mir | UGUCAGUUUGUCAAAUACCCCA | Candidate to atherosclerosis |
| hsa-miR-29b-3p | 478369_mir | UAGCACCAUUUGAAAUCAGUGUU | Candidate to atherosclerosis |
| hsa-miR-92a-3p | 477827_mir | UAUUGCACUUGUCCCGGCCUGU | Candidate to atherosclerosis |
| Parameter a | Group 1 b | Group 2 c | Group 3 d | SD | ANOVA |
|---|---|---|---|---|---|
| Age (years) | 62.18 | 67.09 | 65.94 | 9.70 | 0.217 |
| BMI (kg/m2) | 30.04 | 27.69 | 28.36 | 4.41 | 0.152 |
| ATP III | 9.77 | 10.16 | 8.14 | 6.64 | 0.622 |
| ACC/AHA | 12.02 | 17.04 | 17.38 | 13.06 | 0.333 |
| MESA | 9.26 | 14.52 | 3.62 | 7.13 | <0.001 |
| Agatson index | 45.50 | 336.78 | 0.00 | 362.83 | 0.003 |
| Glucose (mmol/L) | 5.81 | 5.16 | 5.18 | 0.78 | 0.007 |
| Creatinine (µmol/L) | 88.42 | 87.73 | 86.46 | 15.14 | 0.925 |
| eGFRCKD-EPI (mL/min/1.73 m2) | 69.43 | 64.90 | 67.14 | 15.06 | 0.609 |
| Cholesterol (mmol/L) | 5.94 | 5.34 | 5.73 | 1.28 | 0.287 |
| Triglycerides (mmol/L) | 1.92 | 1.38 | 1.39 | 0.77 | 0.030 |
| LDL (mmol/L) | 3.78 | 3.33 | 3.61 | 1.16 | 0.424 |
| HDL (mmol/L) | 1.33 | 1.42 | 1.56 | 0.38 | 0.162 |
| VLDL (mmol/L) | 0.84 | 0.58 | 0.66 | 0.39 | 0.192 |
| Parameter, % a | Group 1 b | Group 2 c | Group 3 d | χ2 | P |
|---|---|---|---|---|---|
| Female sex | 59.09 | 82.61 | 76.47 | 3.30 | 0.192 |
| Angina atypical | 45.45 | 34.78 | 0.00 | 10.20 | 0.006 |
| Hypertension | 95.45 | 82.61 | 88.24 | 1.86 | 0.395 |
| Hypertension 3 grade | 50.00 | 43.48 | 41.18 | 0.34 | 0.842 |
| Positive stress-test | 40.91 | 60.87 | 23.53 | 5.64 | 0.060 |
| EF > 50% | 90.91 | 95.65 | 88.24 | 1.37 | 0.679 |
| Paroxysmal AFib | 9.09 | 13.04 | 41.18 | 3.29 | 0.027 |
| Statins | 36.36 | 73.91 | 41.18 | 8.23 | 0.025 |
| Smoking | 18.18 | 13.04 | 5.88 | 2.19 | 0.524 |
| ACE inhibitors | 31.82 | 34.78 | 23.53 | 5.21 | 0.739 |
| ARBs | 36.36 | 52.17 | 29.41 | 6.85 | 0.312 |
| Beta blockers | 72.73 | 52.17 | 47.06 | 7.13 | 0.211 |
| Calcium channels blockers | 36.36 | 39.13 | 11.76 | 5.21 | 0.137 |
| Oral anticoagulants | 13.64 | 4.35 | 35.29 | 2.74 | 0.029 |
| Acetylsalicylic acid | 31.82 | 47.83 | 35.29 | 6.58 | 0.514 |
| ACC/AHA >10% | ACC/AHA >7.5% | |||
|---|---|---|---|---|
| p-value | Adjusted p-value | p-value | Adjusted p-value | |
| miRNA 126-5p | 0.003 | 0.0412 | 0.110 | 0.4773 |
| miRNA 21-5p | 0.014 | 0.1453 | 0.024 | 0.3054 |
| miRNA 146a-5p | 0.019 | 0.1746 | 0.041 | 0.3949 |
| miRNA 92a-3p | 0.013 | 0.1453 | 0.094 | 0.4773 |
| miRNA 150-5p | <0.001 | 0.0149 | 0.025 | 0.3054 |
| miRNA 181b-5p | 0.023 | 0.1889 | 0.121 | 0.4773 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozhkov, A.N.; Shchekochikhin, D.Y.; Ashikhmin, Y.I.; Mitina, Y.O.; Evgrafova, V.V.; Zhelankin, A.V.; Gognieva, D.G.; Akselrod, A.S.; Kopylov, P.Y. The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks. Non-Coding RNA 2022, 8, 47. https://doi.org/10.3390/ncrna8040047
Rozhkov AN, Shchekochikhin DY, Ashikhmin YI, Mitina YO, Evgrafova VV, Zhelankin AV, Gognieva DG, Akselrod AS, Kopylov PY. The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks. Non-Coding RNA. 2022; 8(4):47. https://doi.org/10.3390/ncrna8040047
Chicago/Turabian StyleRozhkov, Andrey N., Dmitry Yu. Shchekochikhin, Yaroslav I. Ashikhmin, Yulia O. Mitina, Veronika V. Evgrafova, Andrey V. Zhelankin, Daria G. Gognieva, Anna S. Akselrod, and Philippe Yu. Kopylov. 2022. "The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks" Non-Coding RNA 8, no. 4: 47. https://doi.org/10.3390/ncrna8040047
APA StyleRozhkov, A. N., Shchekochikhin, D. Y., Ashikhmin, Y. I., Mitina, Y. O., Evgrafova, V. V., Zhelankin, A. V., Gognieva, D. G., Akselrod, A. S., & Kopylov, P. Y. (2022). The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks. Non-Coding RNA, 8(4), 47. https://doi.org/10.3390/ncrna8040047







