Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies
Abstract
1. Introduction
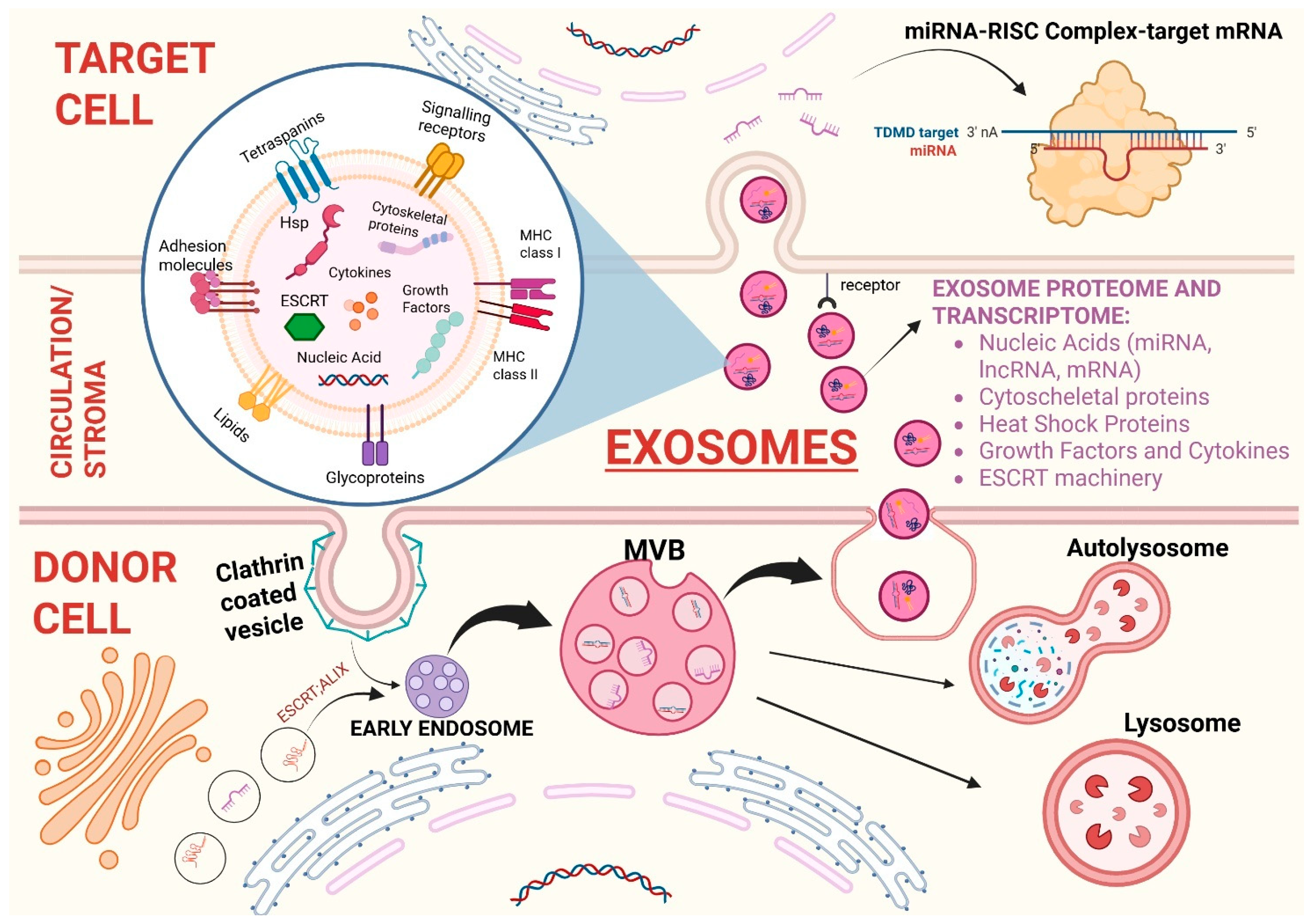
General Considerations on Exosomes
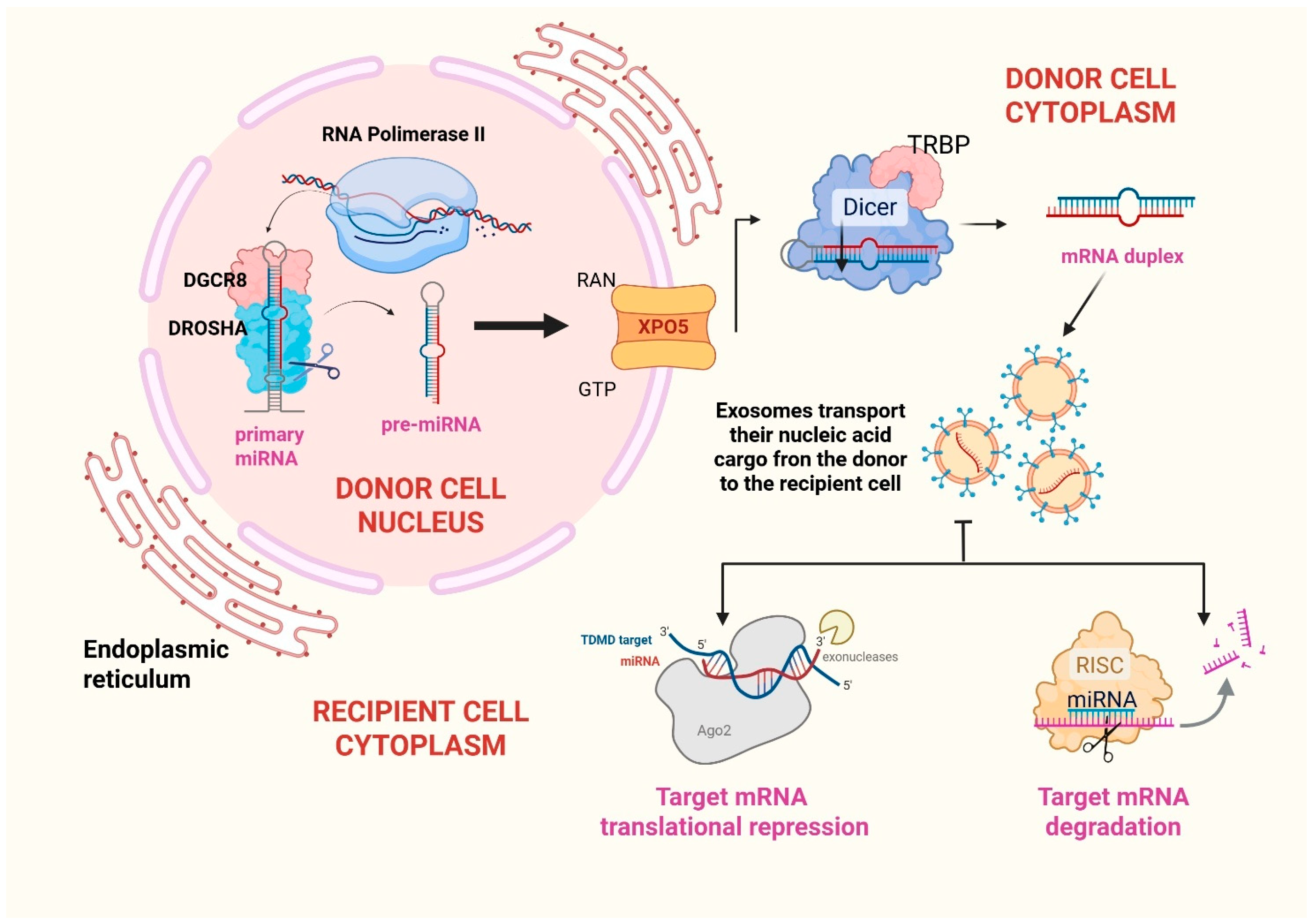
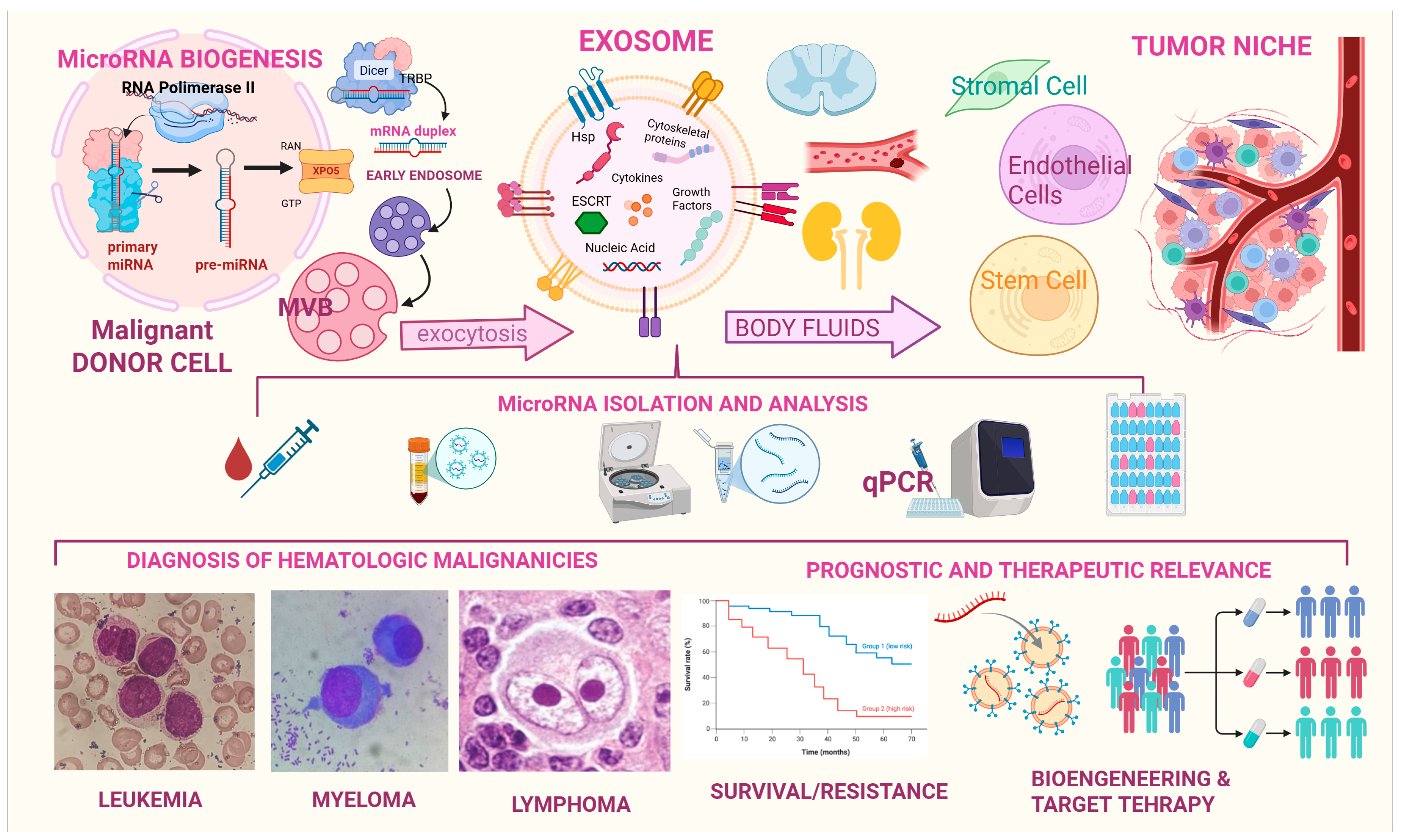
2. Biogenesis of Extracellular Vesicles and Their RNA Cargo
2.1. Exosomes
2.2. Tumor Derived Exosomes (TEXs)
2.3. MIRNAs
3. Bioengineered Exosomes for miRNA Targeted Delivery and Related Therapeutic Prospectives
4. Examining the Exosomal Mirnome in Hematological Malignancies
4.1. Hematological Malignancies of Lymphoid Lineage
4.1.1. Exosomal miRNAs and Multiple Myeloma (MM)
4.1.2. Exosomal miRNAs and Chronic Lymphoid Leukemia (CLL)
4.1.3. Exosomal miRNAs and Lymphomas
4.1.4. Exosomal miRNAs and Acute Lymphoblastic Leukemia (ALL)
4.2. Hematological Malignancies of Myeloid Lineage
4.2.1. Exosomal miRNAs and Chronic Myeloid Leukemia
4.2.2. Exosomal miRNAs and Myelodisplastic Syndromes
4.2.3. Exosomal miRNAs and Acute Myeloid Leukemia
4.2.4. Exosomes miRNAs and Systemic Mastocytosis (SM)
4.2.5. Exosomes miRNAs and BCR-ABL Negative Myeloproliferative Neoplasms (MPNs)
4.3. Exosomal miRNAs and Late-Onset Acute Graft Versus Host Disease (LA-GVHD)
5. An Overview on Mechanisms of Exosomal MiRNA Interference in the Hematologic Niche
6. Recommended Panels and Current Challenges
- TECHNICAL VARIABILITY IN ISOLATION METHODOLOGIES: Traditional ultracentrifugation has long been regarded as the gold standard for exosome isolation. Nevertheless, it suffers from poor reproducibility and contamination due to several intrinsic limitations. Specifically, this method relies on sequential high-speed spins to pellet EVs based on their density and size, yet these conditions often cause EV aggregation and co-isolation of non-vesicular contaminants such as protein polymers, viruses, and high-density lipoproteins. These issues are particularly pronounced in complex and viscous biological fluids like plasma. Furthermore, the intense centrifugal forces can damage vesicle integrity, potentially altering their morphology and biological activity. These limitations, along with time-consuming protocols and the requirement for expensive instrumentation, hinder the scalability of ultracentrifugation for routine clinical use or high-throughput applications [187]. Microfluidic platforms are miniaturized systems that manipulate fluids within chips containing microscopic channels and are used in exosome and miRNA research to rapidly isolate exosomes from small biological samples such as blood, plasma, or saliva [187,188]. In the context of exosome and miRNA research, these platforms provide a promising alternative to traditional isolation techniques. One of the most advanced modalities is acoustic-based microfluidic separation, which employs ultrasonic standing waves to exert differential acoustic radiation forces on particles based on their size, density, and compressibility. These devices typically consist of two modular zones: the first removes larger components (>1 µm), including cells and debris, while the second isolates EVs by filtering out larger microvesicles and apoptotic bodies, thereby enriching for exosomes (<200 nm). Moreover, the cutoff size for separation can be dynamically adjusted to achieve precise discrimination between EV subtypes. However, challenges remain, including limited standardization across platforms, which can affect reproducibility and downstream molecular profiling [187]. One major issue is the lack of standardization in chip design and materials, such as polydimethylsiloxane (PDMS), glass, or plastic, which affects how efficiently exosomes are captured from different bodily fluids. In addition, key settings like flow rate, electric or acoustic field strength, and channel size are often operator-dependent, making results difficult to reproduce across different laboratories. These design differences can also lead to the isolation of different EV types (e.g., more microvesicles vs. fewer exosomes), which alter downstream analyses like miRNA or protein profiling. Additionally, without agreed standards for measuring exosome purity or concentration, it is hard to apply these platforms consistently in clinical settings [189]. Furthermore, by analyzing the lipidomic and proteomic cargo of isolated exosomes, it results in a certain heterogeneity in exosomal cargo according to the isolation technique. For instance, techniques such as ultracentrifugation, size exclusion chromatography (SEC), and ultrafiltration differ significantly in their ability to retain or remove contaminating proteins, lipoproteins, and soluble factors. Ultracentrifugation often co-isolates protein aggregates, while SEC offers improved purity but may lose smaller vesicles or underrepresent certain subpopulations. This technical variability contributes to inconsistencies in downstream proteomic and lipidomic profiling, as certain proteins or lipids may be enriched or depleted depending on the method used [190].
- LACK OF A CONSENSUS ON EXOSOME CHARACTERIZATION STRATEGIES: Techniques such as Transmission Electron Microscopy (TEM) and Cryogenic Electron Microscopy (Cryo-EM) provide high-resolution imaging of exosomal morphology, yet they are limited by artifacts from sample preparation and low throughput; nanoparticle Tracking Analysis (NTA) and Tunable Resistive Pulse Sensing (TRPS) allow quantification of particle size and concentration but struggle to distinguish exosomes from contaminants. Surface plasmon resonance (SPR) and Surface-Enhanced Raman Spectroscopy (SERS) enable label-free, real-time cargo profiling but require sophisticated nanotechnology expertise. Flow Cytometry (FCM), especially when combined with imaging (Imaging Flow Cytometry, IFCM), facilitates multiparametric surface marker analysis but is hampered by sensitivity limitations for submicron particles. Asymmetric Flow Field-Flow Fractionation (AF4) has shown promise in distinguishing exosomal subpopulations but remains a niche technology [191]. At present, no single method fulfills all those critical criteria (high purity, yield, reproducibility, scalability, and affordability) needed for robust clinical employment.
- INTER-PATIENT VARIABILITY IN EXOSOMAL MIRNA EXPRESSION. Intrinsic interindividual variability and disease-independent factors can both impair the accuracy in the interpretation of circulating miRNAs and challenges efforts to establish universal reference ranges [192]. As widely explained in previous sections, the first cause of this variability is the tumor itself (and associated inflammation and immune deregulation) since miRNAs represent a specific neoplastic signature. In other words, they vary according to molecular subtypes and tumor features (e.g., oncogene overexpression, etc.). Secondly, specific signatures of circulating miRNA have also been associated with a variety of pathological conditions which can coexist with tumor as comorbidities, such as cardiovascular diseases, diabetes, liver pathologies, and sepsis [193]. In addition, other factors can influence the diversity of miRNAs levels in circulation: race, gender, lifestyle, drug assumption, smoking habits, diet, and physical activity. However, there are other variables which are more difficult to verify such as polymorphisms in miRNAs chromosome loci. An example is represented by copy number variations (CNVs) occurring in coding regions of the genome. As a result, they can deregulate certain miRNAs, alter their expression, and thus, contribute to the development of the disease [192]. Interestingly, even diet represents a variable. Several dietary constituents (resveratrol, curcumin, isoflavones, catechins, indoles, vitamins A and D) play a certain role in affecting miRNAs expression profile [194]. The rationale of the effect exerted by these substances may depend on homeostatic changes in circulating miRNA-containing vehicles (including exosomes) [195]. In addition to these considerations, it should be said that the amount of circulating miRNA may vary in the same patient over time. For example, it can be influenced by common medications (aspirin has shown to reduce miR-126 levels). Therefore, miRNAs appear as potentially useful parameters in pharmacodynamic studies [192].
- NORMALIZATION OF EXOSOMAL MIRNA QUANTIFICATION: A major translational barrier in exosome-based diagnostics is the lack of standardized normalization strategies for exosomal miRNA quantification. Although qRT-PCR remains the gold standard for miRNA detection due to its sensitivity and specificity, its reliability is highly dependent on reference miRNAs whose expression is stable across different biological conditions, disease states, and technical protocols. Current reference small RNAs such as U6 or RNU44, traditionally used in cellular RNA studies, have shown inconsistent expression in serum- or plasma-derived exosomes, particularly in pathological contexts like cancer or inflammation [196,197]. In an important effort to address this gap, Damanti et al. performed a systematic assessment of RNA-seq datasets and identified miR-26a-5p and miR-486-5p as promising endogenous reference candidates in pediatric hematological malignancies. Their validation across diverse disease subtypes of lymphomas and B-cell ALL demonstrated superior stability of miR-26a-5p, independent of disease status or exosome isolation method (ultracentrifugation vs. kit-based protocols). This interesting data positions miR-26a-5p as a bona fide universal calibrator for plasma exosomal miRNA studies. Conversely, miR-486-5p, while abundant and stable across disease groups, was highly susceptible to the choice of isolation technique, showing significant variation between protocols [15]. This observation is particularly relevant in multi-center or retrospective studies, where differences in isolation methods (e.g., ultracentrifugation, SEC, precipitation kits) are common and often unavoidable (as previously discussed). Therefore, using normalization controls that are method-sensitive could bias miRNA levels, leading to false-positive/negative biomarker signals. Critically, several issues remain unresolved as follows: (1) this study is limited to pediatric lymphoid malignancies and extrapolation to adult cohorts or myeloid neoplasms remains speculative; (2) the identified normalizers may not generalize across biofluid types (e.g., urine, CSF) or technical platforms (e.g., digital PCR, NGS). On a final analysis, the study provides valuable empirical evidence supporting miR-26a-5p as a technical normalizer, but broader standardization efforts (including reference selection) are still needed.
7. Recent Advances
- Ubiquitous tetraspanins such as CD9, CD63, and CD81 remain gold-standard markers used in affinity capture workflows, given their abundant expression on exosomal membranes across cell types. These membrane proteins are known to possess multiple functional roles in several biological processes, such as cell adhesion, fusion, signaling, and trafficking [198]. Apart from these classical markers, surface antigens such as epithelial cell adhesion molecule (EpCAM), EGFR, integrins (e.g., α6β4, αvβ5), and even PD-L1 have been used for immunoaffinity capture of tumor-derived exosomes, significantly improving specificity and reducing contamination from non-vesicular particles or lipoproteins [199]. A recent study introduced the “EVs on Demand” (EVOD) chip, designed to selectively capture cancer-related exosome subpopulations. The chip uses a chemical reaction between tetrazine-tagged antibodies (targeting EpCAM and EGFR) and a specially coated microfluidic surface to isolate exosomes. It successfully captured 76% more EGFR-positive exosomes from cancer patients compared to healthy individuals. However, this approach is still limited by high costs [200].
- Magnetic bead-based immunoaffinity enrichment is another separation method that has recently gained attention. This approach employs antibody-modified magnetic beads to capture exosomes. In a later passage, exosomes are separated by magnetic force. Furthermore, novel immuno-affinitive superparamagnetic nanoparticles (IS-NPs) have shown higher yield and increased purity than conventional separation methods. For example, in a study by Fang et al., superparamagnetic nanoparticles were combined with anti-CD63 antibodies through a molecular interaction between β-cyclodextrin (β-CD, a heptasaccharide derived from glucose) and 4-aminoazobenzene (AAB, an aromatic amine). This system achieved impressive results, with exosome capture and release efficiencies reaching 80% and 86.5%, respectively, in artificial model sample [201].
- Lipid-based separation techniques exploit the natural structure of exosome membranes, which are made of lipid bilayers. These lipids can interact with specially designed molecules to help isolate exosomes efficiently. In a study, Wan et al. created a special probe called a lipid nanoprobe to quickly extract exosomes from plasma. This probe includes a molecule called DSPE-PEG-biotin. More specifically, DSPE is a lipid that can insert itself into the exosome’s membrane through hydrophobic interactions; polyethylene glycol (PEG) is attached to DSPE to make the molecule soluble in water, preventing aggregation. Once the probe embeds into the exosome membrane, the biotin on its surface binds strongly to NeutrAvidin, which is coated on magnetic beads [202]. This interaction allows the exosomes to be pulled out quickly (just 15 min) and efficiently using a magnet, a much faster process than traditional isolation techniques.
- Advanced microchip technologies such as nanoplasmonic exosome assay (nPLEX) have significantly improved the sensitivity and speed of exosome detection. This technology employs nanohole arrays embedded in a thin gold film, which are functionalized with antibodies targeting exosome surface markers (e.g., CD63 and EpCAM). The metal nanohole array supports surface plasmon resonance (SPR), a phenomenon where the light energy provided by laser or LED causes electrons on the metal surface to oscillate in resonance. These oscillations are sensitive to changes on the surface of the array, such as when exosomes bind to the antibodies anchored within the holes. The binding causes a shift in the resonance signal (e.g., change in transmitted light intensity or wavelength), which is measured by a sensor. By monitoring these optical changes, scientists can detect and quantify very small amounts of target molecules (thousands/µL) without the need to attach any additional markers (like fluorescent dyes, radioactive isotopes, or enzymes) to the particles they are trying to detect [203].
- A novel digital microfluidic (DMF) platform was developed for automated, rapid, and low-volume EVs pretreatment. The system combines a reusable DMF chip with a magnetic particle-based protocol, enabling complete exosome isolation and miRNA extraction within 20–30 min from as little as 20–40 μL of plasma. This DMF system operates by manipulating droplets on an electrode array chip, allowing for precise, programmable control of EVs isolation, washing, and lysis steps. The method was validated using clinical plasma samples from patients with non-small cell lung cancer (NSCLC). RT-qPCR analysis revealed that EV-derived miR-486-5p and miR-21-5p were effective biomarkers for NSCLC, and the results were consistent with those obtained using a commercial exosome RNA extraction kit. Importantly, the platform achieves over 77% isolation efficiency and is cost-effective due to chip reusability. This advance demonstrates strong potential for standardized, scalable EV-miRNA–based liquid biopsy applications in cancer diagnostics [204].
- Droplet digital PCR (ddPCR) is a highly sensitive technique that improves nucleic acid detection by dividing the PCR reaction mixture into thousands of tiny droplets. Typically, each droplet contains either zero or one copy of the target gene. After PCR, droplets are classified as positive or negative based on their fluorescence signal, and the concentration of the target molecule is calculated using the Poisson distribution and the ratio of positive droplets. Compared to traditional qPCR, ddPCR offers greater sensitivity and accuracy, particularly for detecting low-abundance targets such as urinary exosomal miRNAs. Recent studies highlight its utility for liquid biopsy in cancer diagnostics. For example, exosomal miR-15a-5p, measured by ddPCR, was found to effectively distinguish endometrial cancer patients from healthy individuals [205]. Therefore, ddPCR has shown to improve sensitivity and reproducibility in miRNAs detection from limited blood volumes. For this reason, it appears particularly suited to monitoring minimal residual disease (MRD) or detecting low-expression miRNAs in blood cancers, where early relapse detection is critical [206].
- The CRISPR/Cas system, widely known for its role in gene editing, has recently been adapted for the highly sensitive and specific detection of exosomal miRNAs. A notable example is the RACE (rolling circle amplification–Assisted CRISPR/Cas9 Cleavage) method developed by Wang et al., which combines nucleic acid amplification with CRISPR/Cas-based cleavage. In this approach, a specially designed DNA padlock probe (a short single-stranded DNA molecule) binds to the target miRNA, accurately identifying even single-nucleotide differences. This probe is then circularized. This circular DNA is amplified through rolling circle amplification (RCA), producing long single-stranded DNA (ssDNA) that contains multiple repeats of the target sequence along with PAM motifs (short, specific DNA sequences) necessary for Cas9 recognition. The CRISPR/Cas9 complex then cleaves these amplified sequences. Simultaneously, a TaqMan probe is added to the reaction and binds the target DNA. When Cas9 cuts the target DNA, the probe is cleaved, producing a measurable fluorescence signal that indicates the presence of the miRNA [207]. This method is highly sensitive even for small differences in miRNA sequence and is able to detect multiple miRNAs in a single test.
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vyas, N.; Dhawan, J. Exosomes: Mobile Platforms for Targeted and Synergistic Signaling across Cell Boundaries. Cell. Mol. Life Sci. 2017, 74, 1567–1576. [Google Scholar] [CrossRef]
- Martinelli, N.; Moruzzi, S.; Udali, S.; Castagna, A.; Di Santo, L.; Ambrosani, F.; Baroni, M.; Pattini, P.; Pizzolo, F.; Ruzzenente, A.; et al. Tissue Factor Pathway-Related Biomarkers in Liver Cancer: Activated Factor VII–Antithrombin Complex and Tissue Factor MRNA Levels Are Associated with Mortality. Res. Pr. Thromb. Haemost. 2024, 8, 102310. [Google Scholar] [CrossRef] [PubMed]
- Trams, E.G.; Lauter, C.J.; Norman, S., Jr.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Et. Biophys. Acta (BBA)—Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular Organelles Important in Intercellular Communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Ludlow, J.W. Exosomes for Repair, Regeneration and Rejuvenation. Expert. Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-G.; Grizzle, W.E. Exosomes. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Choi, D.; Lee, T.H.; Spinelli, C.; Chennakrishnaiah, S.; D’Asti, E.; Rak, J. Extracellular Vesicle Communication Pathways as Regulatory Targets of Oncogenic Transformation. Semin. Cell Dev. Biol. 2017, 67, 11–22. [Google Scholar] [CrossRef]
- Reddy, S.D.N.; Gajula, R.P.; Pakala, S.B.; Kumar, R. MicroRNAs and Cancer Therapy: The next Wave or Here to Stay? Cancer Biol. Ther. 2010, 9, 479–482. [Google Scholar] [CrossRef][Green Version]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Nedaeinia, R.; Manian, M.; Jazayeri, M.H.; Ranjbar, M.; Salehi, R.; Sharifi, M.; Mohaghegh, F.; Goli, M.; Jahednia, S.H.; Avan, A.; et al. Circulating Exosomes and Exosomal MicroRNAs as Biomarkers in Gastrointestinal Cancer. Cancer Gene Ther. 2017, 24, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Damanti, C.C.; Gaffo, E.; Lovisa, F.; Garbin, A.; Di Battista, P.; Gallingani, I.; Tosato, A.; Pillon, M.; Carraro, E.; Mascarin, M.; et al. MiR-26a-5p as a Reference to Normalize MicroRNA QRT-PCR Levels in Plasma Exosomes of Pediatric Hematological Malignancies. Cells 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, M.; Zeng, S.; Wang, L.; Liu, P.; Xiao, X.; Liu, Y. Exosome-Derived MiRNAs as Predictive Biomarkers for Diffuse Large B-Cell Lymphoma Chemotherapy Resistance. Epigenomics 2019, 11, 35–51. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. Horizontal Transfer of MicroRNAs: Molecular Mechanisms and Clinical Applications. Protein Cell 2012, 3, 28–37. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extracellular Communication during Cancer Progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Nolte-’t Hoen, E.N.M.; Buermans, H.P.J.; Waasdorp, M.; Stoorvogel, W.; Wauben, M.H.M.; ’t Hoen, P.A.C. Deep Sequencing of RNA from Immune Cell-Derived Vesicles Uncovers the Selective Incorporation of Small Non-Coding RNA Biotypes with Potential Regulatory Functions. Nucleic Acids Res. 2012, 40, 9272–9285. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental PH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Luciani, F.; Spada, M.; De Milito, A.; Molinari, A.; Rivoltini, L.; Montinaro, A.; Marra, M.; Lugini, L.; Logozzi, M.; Lozupone, F.; et al. Effect of Proton Pump Inhibitor Pretreatment on Resistance of Solid Tumors to Cytotoxic Drugs. JNCI J. Natl. Cancer Inst. 2004, 96, 1702–1713. [Google Scholar] [CrossRef]
- Kim, J.W.; Wieckowski, E.; Taylor, D.D.; Reichert, T.E.; Watkins, S.; Whiteside, T.L. Fas Ligand-Positive Membranous Vesicles Isolated from Sera of Patients with Oral Cancer Induce Apoptosis of Activated T Lymphocytes. Clin. Cancer Res. 2005, 11, 1010–1020. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of Biologically Active and Morphologically Intact Exosomes from Plasma of Patients with Cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Et. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.-P.; Goh, B.-C. Exosome-Mediated Metastasis: From Epithelial–Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune Modulation of T-Cell and NK (Natural Killer) Cell Activities by TEXs (Tumour-Derived Exosomes). Biochem. Soc. Trans. 2013, 41, 245–251. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High Serum Levels of Extracellular Vesicles Expressing Malignancy-Related Markers Are Released in Patients with Various Types of Hematological Neoplastic Disorders. Tumor Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for MicroRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Wallace, J.A.; O’Connell, R.M. MicroRNAs and Acute Myeloid Leukemia: Therapeutic Implications and Emerging Concepts. Blood 2017, 130, 1290–1301. [Google Scholar] [CrossRef]
- Weiss, C.N.; Ito, K. A Macro View of MicroRNAs: The Discovery of MicroRNAs and Their Role in Hematopoiesis and Hematologic Disease. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–175. [Google Scholar]
- Landskroner-Eiger, S.; Moneke, I.; Sessa, W.C. MiRNAs as Modulators of Angiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a006643. [Google Scholar] [CrossRef]
- Kim, J.; Yao, F.; Xiao, Z.; Sun, Y.; Ma, L. MicroRNAs and Metastasis: Small RNAs Play Big Roles. Cancer Metastasis Rev. 2018, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Natarajan, R. MicroRNAs in Diabetic Nephropathy: Functions, Biomarkers, and Therapeutic Targets. Ann. N. Y. Acad. Sci. 2015, 1353, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered Exosomes for Targeted Co-Delivery of MiR-21 Inhibitor and Chemotherapeutics to Reverse Drug Resistance in Colon Cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science (1979) 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Zhong, Z.; Qiu, Y.; Liu, S.; Wu, H.; Tang, X.; Chen, C.; Fu, Y.; Chen, Q.; et al. 3D HESC Exosomes Enriched with MiR-6766-3p Ameliorates Liver Fibrosis by Attenuating Activated Stellate Cells through Targeting the TGFβRII-SMADS Pathway. J. Nanobiotechnol. 2021, 19, 437. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic Acid Delivery for Therapeutic Applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Chen, H.; Yao, H.; Chi, J.; Li, C.; Liu, Y.; Yang, J.; Yu, J.; Wang, J.; Ruan, Y.; Pi, J.; et al. Engineered Exosomes as Drug and RNA Co-Delivery System: New Hope for Enhanced Therapeutics? Front. Bioeng. Biotechnol. 2023, 11, 1254356. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Liang, X.; Cao, B.; Wang, S.; Jiang, J.; Luo, H.; He, S.; Lang, J.; Zhu, G. ΓδTDEs: An Efficient Delivery System for MiR-138 with Anti-Tumoral and Immunostimulatory Roles on Oral Squamous Cell Carcinoma. Mol. Ther. Nucleic Acids 2019, 14, 101–113. [Google Scholar] [CrossRef]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3- Receptor Targeted Exosomes Inhibit in Vitro and in Vivo Chronic Myelogenous Leukemia Cell Growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of SiRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA Drug Delivery Using Red Blood Cell Extracellular Vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, W.; Li, M.; Zheng, A. Exosome-Based Carrier for RNA Delivery: Progress and Challenges. Pharmaceutics 2023, 15, 598. [Google Scholar] [CrossRef]
- Kato, H.; Hashimoto, Y.; Hatayama, Y.; Shimohiro, H.; Motokura, T. Serum Levels of Vault RNA Significantly Varied in Patients with Haematological Malignancies. Mol. Med. Rep. 2023, 28, 190. [Google Scholar] [CrossRef]
- Afsar, S.; Syed, R.U.; Khojali, W.M.A.; Masood, N.; Osman, M.E.; Jyothi, J.S.; Hadi, M.A.; Khalifa, A.A.S.; Aboshouk, N.A.M.; Alsaikhan, H.A.; et al. Non-Coding RNAs in BRAF-Mutant Melanoma: Targets, Indicators, and Therapeutic Potential. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 297–317. [Google Scholar] [CrossRef]
- Fazio, M.; Del Fabro, V.; Parrinello, N.L.; Allegra, A.; Markovic, U.; Botta, C.; Accardi, F.; Vincelli, I.D.; Leotta, S.; Elia, F.; et al. Multiple Myeloma in 2023 Ways: From Trials to Real Life. Curr. Oncol. 2023, 30, 9710–9733. [Google Scholar] [CrossRef]
- Fazio, M.; Sorbello, C.M.C.; Del Fabro, V.; Romano, A.; Cannizzaro, M.T.; Parrinello, N.L.; Esposito, B.; Frazzetto, S.; Elia, F.; Di Raimondo, F.; et al. IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects. Hematol. Rep. 2024, 16, 541–551. [Google Scholar] [CrossRef]
- Palumbo, F.; Orofino, A.; Del Fabro, V.; Fazio, M.; Elia, F.; Esposito, B.; Frazzetto, S.; Romano, A.; Di Raimondo, F.; Conticello, C. P60 evolving outcomes of extramedullary disease in multiple myeloma: 20-years single center experience. Hemasphere 2023, 7, 43–44. [Google Scholar] [CrossRef]
- Di Noto, G.; Bugatti, A.; Zendrini, A.; Mazzoldi, E.L.; Montanelli, A.; Caimi, L.; Rusnati, M.; Ricotta, D.; Bergese, P. Merging Colloidal Nanoplasmonics and Surface Plasmon Resonance Spectroscopy for Enhanced Profiling of Multiple Myeloma-Derived Exosomes. Biosens. Bioelectron. 2016, 77, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Di Gioacchino, M.; Tonacci, A.; Petrarca, C.; Musolino, C.; Gangemi, S. Multiple Myeloma Cell-Derived Exosomes: Implications on Tumorigenesis, Diagnosis, Prognosis and Therapeutic Strategies. Cells 2021, 10, 2865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Geng, C.; Zhou, H.; Gao, W.; Chen, W. Serum Exosomal MicroRNAs as Novel Biomarkers for Multiple Myeloma. Hematol. Oncol. 2019, 37, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Han, G.; Liu, Y.; Jiang, H.; He, Q. MiRNA-20a-5p Promotes the Growth of Triple-Negative Breast Cancer Cells through Targeting RUNX3. Biomed. Pharmacother. 2018, 103, 1482–1489. [Google Scholar] [CrossRef]
- Bao, F.; Zhang, L.; Pei, X.; Lian, C.; Liu, Y.; Tan, H.; Lei, P. MiR-20a-5p Functions as a Potent Tumor Suppressor by Targeting PPP6C in Acute Myeloid Leukemia. PLoS ONE 2021, 16, e0256995. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Jin, Y.; Yang, Y.; Shi, J.; Chen, F.; Han, S.; Chu, P.; Lu, J.; Wang, H.; et al. MiR-20a-5p Suppresses Tumor Proliferation by Targeting Autophagy-Related Gene 7 in Neuroblastoma. Cancer Cell Int. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; He, X. Advances in the Role of MicroRNAs Associated with the PI3K/AKT Signaling Pathway in Lung Cancer. Front. Oncol. 2023, 13, 1279822. [Google Scholar] [CrossRef]
- Li, H.; Huhe, M.; Lou, J. MicroRNA-103a-3p Promotes Cell Proliferation and Invasion in Non-Small-Cell Lung Cancer Cells through Akt Pathway by Targeting PTEN. Biomed. Res. Int. 2021, 2021, 7590976. [Google Scholar] [CrossRef]
- Puła, A.; Robak, T.; Dróżdż, I.; Stawiski, K.; Rycerz, A.; Misiewicz, M.; Robak, P. Circulating Serum MicroRNAs as Biomarkers of Drug Resistance in Multiple Myeloma Patients Treated with Bortezomib-Based Regimens—Pilot Study. Leuk. Lymphoma 2024, 65, 257–264. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, D.; Qiu, T.; Li, J.; Liu, Z. MicroRNA-103a Curtails the Stemness of Non-Small Cell Lung Cancer Cells by Binding OTUB1 via the Hippo Signaling Pathway. Technol. Cancer Res. Treat. 2020, 19, 1533033820971643. [Google Scholar] [CrossRef]
- Peng, Y.; Song, X.; Lan, J.; Wang, X.; Wang, M. Bone Marrow Stromal Cells Derived Exosomal MiR-10a and MiR-16 May Be Involved in Progression of Patients with Multiple Myeloma by Regulating EPHA8 or IGF1R/CCND1. Medicine 2021, 100, e23447. [Google Scholar] [CrossRef] [PubMed]
- Sedlarikova, L.; Bollova, B.; Radova, L.; Brozova, L.; Jarkovsky, J.; Almasi, M.; Penka, M.; Kuglík, P.; Sandecká, V.; Stork, M.; et al. Circulating Exosomal Long Noncoding RNA PRINS—First Findings in Monoclonal Gammopathies. Hematol. Oncol. 2018, 36, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Umezu, T.; Ohyashiki, K. Exosomes Promote Bone Marrow Angiogenesis in Hematologic Neoplasia. Curr. Opin. Hematol. 2016, 23, 268–273. [Google Scholar] [CrossRef]
- Allegra, A.; Ettari, R.; Innao, V.; Bitto, A. Potential Role of MicroRNAs in Inducing Drug Resistance in Patients with Multiple Myeloma. Cells 2021, 10, 448. [Google Scholar] [CrossRef]
- Gkioka, A.-I.; Tsota, M.; Koudouna, A.; Gkiokas, A.; Mitropoulou, C.-A.; Palaiokrassa, A.; Alexandropoulos, A.; Papadatou-Gigante, M.; Bartzi, V.; Tryfou, T.-M.; et al. Circulating MiR-16 and MiR-21 Levels in Multiple Myeloma: Prognostic Significance of Survival and Response to Lenalidomide Treatment. Int. J. Mol. Sci. 2024, 25, 6065. [Google Scholar] [CrossRef]
- Růžičková, T.; Vlachová, M.; Pečinka, L.; Brychtová, M.; Večeřa, M.; Radová, L.; Ševčíková, S.; Jarošová, M.; Havel, J.; Pour, L.; et al. Detection of Early Relapse in Multiple Myeloma Patients. Cell Div. 2025, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, L.; Xiang, B.; Zhu, H.; Wu, Y.; Chen, M.; Guan, P.; Zou, X.; Valencia, C.A.; Dong, B.; et al. Potential Role of Exosome-Associated MicroRNA Panels and in Vivo Environment to Predict Drug Resistance for Patients with Multiple Myeloma. Oncotarget 2016, 7, 30876–30891. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Du, C.; Yu, Z.; Yang, Y.; Xu, J.; Wei, X.; Zhan, F.; Lai, Y.; Qiu, L.; et al. Therapeutic Effects of Oligo-Single-Stranded DNA Mimicking of Hsa-MiR-15a-5p on Multiple Myeloma. Cancer Gene Ther. 2020, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Sciaccotta, R.; Gangemi, S.; Penna, G.; Giordano, L.; Pioggia, G.; Allegra, A. Potential New Therapies “ROS-Based” in CLL: An Innovative Paradigm in the Induction of Tumor Cell Apoptosis. Antioxidants 2024, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Nano, E.; Reggiani, F.; Amaro, A.A.; Monti, P.; Colombo, M.; Bertola, N.; Ferrero, F.; Fais, F.; Bruzzese, A.; Martino, E.A.; et al. MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study. Noncoding RNA 2024, 10, 46. [Google Scholar] [CrossRef]
- Moussay, E.; Wang, K.; Cho, J.-H.; van Moer, K.; Pierson, S.; Paggetti, J.; Nazarov, P.V.; Palissot, V.; Hood, L.E.; Berchem, G.; et al. MicroRNA as Biomarkers and Regulators in B-Cell Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 2011, 108, 6573–6578. [Google Scholar] [CrossRef]
- Xu, S.; He, L.; Chen, Y.; Lin, T.; Tang, L.; Wu, Y.; He, Y.; Sun, X. Clinical Implications of MiR-195 in Cancer: Mechanisms, Potential Applications, and Therapeutic Strategies. J. Cancer Res. Clin. Oncol. 2025, 151, 148. [Google Scholar] [CrossRef]
- Farahat, N.M.G.; Elkaffash, D.M.N.E.D.; Alghandour, A.H.; Swelem, R.S.; Abo El-Wafa, R.A.H. Study of MicroRNA Profile as a Molecular Biomarker in Egyptian Chronic Lymphocytic Leukemia. Indian. J. Hematol. Blood Transfus. 2019, 35, 89–99. [Google Scholar] [CrossRef]
- Dehkordi, K.A.; Chaleshtori, M.H.; Sharifi, M.; Jalili, A.; Fathi, F.; Roshani, D.; Nikkhoo, B.; Hakhamaneshi, M.S.; Sani, M.R.M.; Ganji-Arjenaki, M. Inhibition of MicroRNA MiR-222 with LNA Inhibitor Can Reduce Cell Proliferation in B Chronic Lymphoblastic Leukemia. Indian. J. Hematol. Blood Transfus. 2017, 33, 327–332. [Google Scholar] [CrossRef]
- Vargova, K.; Pesta, M.; Obrtlikova, P.; Dusilkova, N.; Minarik, L.; Vargova, J.; Berkova, A.; Zemanova, Z.; Michalova, K.; Spacek, M.; et al. MiR-155/MiR-150 Network Regulates Progression through the Disease Phases of Chronic Lymphocytic Leukemia. Blood Cancer J. 2017, 7, e585. [Google Scholar] [CrossRef] [PubMed]
- Dubois, K.; Tannoury, M.; Bauvois, B.; Susin, S.A.; Garnier, D. Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies? Cancers 2023, 15, 2307. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Böttcher, M.; Qorraj, M.; Fabri, M.; Jitschin, S.; Dindorf, J.; Busch, L.; Jitschin, R.; Mackensen, A.; Mougiakakos, D. CLL-Cell-Mediated MDSC Induction by Exosomal MiR-155 Transfer Is Disrupted by Vitamin D. Leukemia 2017, 31, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.; Crompot, E.; Meuleman, N.; Bron, D.; Lagneaux, L.; Stamatopoulos, B. Importance of Crosstalk Between Chronic Lymphocytic Leukemia Cells and the Stromal Microenvironment: Direct Contact, Soluble Factors, and Extracellular Vesicles. Front. Oncol. 2020, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Rubbi, C.; Liu, L.; Slupsky, J.R.; Kalakonda, N. CLL Exosomes Modulate the Transcriptome and Behaviour of Recipient Stromal Cells and Are Selectively Enriched in MiR-202-3p. PLoS ONE 2015, 10, e0141429. [Google Scholar] [CrossRef]
- Nisticò, N.; Maisano, D.; Iaccino, E.; Vecchio, E.; Fiume, G.; Rotundo, S.; Quinto, I.; Mimmi, S. Role of Chronic Lymphocytic Leukemia (CLL)-Derived Exosomes in Tumor Progression and Survival. Pharmaceuticals 2020, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Autore, F.; Ramassone, A.; Stirparo, L.; Pagotto, S.; Fresa, A.; Innocenti, I.; Visone, R.; Laurenti, L. Role of MicroRNAs in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2023, 24, 12471. [Google Scholar] [CrossRef]
- Mohseni, A.; Salehi, F.; Rostami, S.; Hadiloo, K.; Hashemi, M.; Baridjavadi, Z.; Ahangari, F.; Karami, N.; Samani, F.; Tahmasebi, S.; et al. Harnessing the Power of Exosomes for Diagnosis, Prognosis, and Treatment of Hematological Malignancies. Stem Cell Res. Ther. 2025, 16, 6. [Google Scholar] [CrossRef]
- van Eijndhoven, M.A.J.; Zijlstra, J.M.; Groenewegen, N.J.; Drees, E.E.E.; van Niele, S.; Baglio, S.R.; Koppers-Lalic, D.; van der Voorn, H.; Libregts, S.F.W.M.; Wauben, M.H.M.; et al. Plasma Vesicle MiRNAs for Therapy Response Monitoring in Hodgkin Lymphoma Patients. JCI Insight 2016, 1, e89631. [Google Scholar] [CrossRef] [PubMed]
- Chawra, H.S.; Agarwal, M.; Mishra, A.; Chandel, S.S.; Singh, R.P.; Dubey, G.; Kukreti, N.; Singh, M. MicroRNA-21′s Role in PTEN Suppression and PI3K/AKT Activation: Implications for Cancer Biology. Pathol. Res. Pr. 2024, 254, 155091. [Google Scholar] [CrossRef]
- Hering, C.; Conover, G.M. Advancing Ischemic Stroke Prognosis: Key Role of MiR-155 Non-Coding RNA. Int. J. Mol. Sci. 2025, 26, 3947. [Google Scholar] [CrossRef] [PubMed]
- Ofori, K.; Bhagat, G.; Rai, A.J. Exosomes and Extracellular Vesicles as Liquid Biopsy Biomarkers in Diffuse Large B-cell Lymphoma: Current State of the Art and Unmet Clinical Needs. Br. J. Clin. Pharmacol. 2021, 87, 284–294. [Google Scholar] [CrossRef]
- Lin, J.; Ding, S.; Xie, C.; Yi, R.; Wu, Z.; Luo, J.; Huang, T.; Zeng, Y.; Wang, X.; Xu, A.; et al. MicroRNA-4476 Promotes Glioma Progression through a MiR-4476/APC/β-Catenin/c-Jun Positive Feedback Loop. Cell Death Dis. 2020, 11, 269. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Huang, Z.; Keramat, S.; Izadirad, M.; Li, Y.-D.; Bo, L.; Gharehbaghian, A.; Chen, Z.-S. The Roles of Exosomal MicroRNAs in Diffuse Large B-Cell Lymphoma: Diagnosis, Prognosis, Clinical Application, and Biomolecular Mechanisms. Front. Oncol. 2022, 12, 904637. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Hekmatirad, S.; Mofarahe, Z.S.; Asghari, M.H. Exosomal MicroRNA Panels as Biomarkers for Hematological Malignancies. Curr. Probl. Cancer 2021, 45, 100726. [Google Scholar] [CrossRef] [PubMed]
- Punnachet, T.; Chattipakorn, S.C.; Chattipakorn, N.; Kumfu, S. Critical Role of Extracellular Vesicles in Diffuse Large B-Cell Lymphoma; Pathogenesis, Potential Biomarkers, and Targeted Therapy—A Narrative Review. Biomedicines 2024, 12, 2822. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Xu, H.; Wang, X.; Gu, T.; Dong, H.; Chang, H.; Pang, L. Identification of MiR-451 Target Genes as Prognostic Markers in Diffuse Large B-Cell Lymphoma. Expert. Rev. Hematol. 2024, 1–12. [Google Scholar] [CrossRef]
- Lee, A.A.; Godwin, A.K.; Abdelhakim, H. The Multifaceted Roles of Extracellular Vesicles for Therapeutic Intervention with Non-Hodgkin Lymphoma. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 329–343. [Google Scholar] [CrossRef]
- Cao, D.; Cao, X.; Jiang, Y.; Xu, J.; Zheng, Y.; Kang, D.; Xu, C. Circulating Exosomal MicroRNAs as Diagnostic and Prognostic Biomarkers in Patients with Diffuse Large B-cell Lymphoma. Hematol. Oncol. 2022, 40, 172–180. [Google Scholar] [CrossRef]
- Ferrajoli, A.; Shanafelt, T.D.; Ivan, C.; Shimizu, M.; Rabe, K.G.; Nouraee, N.; Ikuo, M.; Ghosh, A.K.; Lerner, S.; Rassenti, L.Z.; et al. Prognostic Value of MiR-155 in Individuals with Monoclonal B-Cell Lymphocytosis and Patients with B Chronic Lymphocytic Leukemia. Blood 2013, 122, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Goldschmidt, N.; Bardugo, A.; Gur-Wahnon, D.; Ben-Dov, I.Z.; Avni, B. Plasma MicroRNA Profiling: Exploring Better Biomarkers for Lymphoma Surveillance. PLoS ONE 2017, 12, e0187722. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The MiR-17/92 Cluster: A Comprehensive Update on Its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Tenhaken, V.; Seternes, O.-M.; Cascorbi, I.; Bruckmueller, H. The MAP Kinase Negative Regulator DUSP2 (Dual Specificity Phosphatase 2) Is Controlled by Oncogenic MicroRNA Cluster MiR-17-92, MiR-106a-363 and MiR-106b-25. BMC Cancer 2025, 25, 1020. [Google Scholar] [CrossRef]
- Caner, V.; Cetin, G.O.; Hacioglu, S.; Baris, I.C.; Tepeli, E.; Turk, N.S.; Bagci, G.; Yararbas, K.; Cagliyan, G. The MiRNA Content of Circulating Exosomes in DLBCL Patients and in Vitro Influence of DLBCL-Derived Exosomes on MiRNA Expression of Healthy B-Cells from Peripheral Blood. Cancer Biomark. 2021, 32, 519–529. [Google Scholar] [CrossRef]
- Kantarjian, H.; Jabbour, E. Adult Acute Lymphoblastic Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2025, 100, 1205–1231. [Google Scholar] [CrossRef] [PubMed]
- Foà, R. Ph-Positive Acute Lymphoblastic Leukemia—25 Years of Progress. N. Engl. J. Med. 2025, 392, 1941–1952. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Ruzibakieva, M.; Gupta, R.; Malviya, J.; Toama, M.A.; Hjazi, A.; Alkhayyat, M.R.R.; Alsaab, H.O.; Hadi, A.; Alwaily, E.R. Detailed Role of MicroRNA-mediated Regulation of PI3K/AKT Axis in Human Tumors. Cell Biochem. Funct. 2024, 42, e3904. [Google Scholar] [CrossRef]
- Kansha, T.; Ma, X.; Wang, H.; Yu, X.; Song, Y.; Guo, Z.; Song, J.; Xue, L.; Yang, J. Exosomal PD-L1 Detection in Cancer Predictive Biomarker for Response to Immune Checkpoint Blockade Therapy. Front. Immunol. 2025, 16, 1603855. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, S.; Peng, H.; Cheng, Z.; Zhang, G.; Wang, Z. B-Cell Acute Lymphoblastic Leukemia-Related MicroRNAs: Uncovering Their Diverse and Special Roles. Am. J. Cancer Res. 2021, 11, 1104–1120. [Google Scholar] [PubMed]
- Zhu, S.; Xing, C.; Li, R.; Cheng, Z.; Deng, M.; Luo, Y.; Li, H.; Zhang, G.; Sheng, Y.; Peng, H.; et al. Proteomic Profiling of Plasma Exosomes from Patients with B-Cell Acute Lymphoblastic Leukemia. Sci. Rep. 2022, 12, 11975. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Wu, Z.; Chen, K.; Xu, X.; Ma, W.; Chen, B.; Jin, L.; Guan, M. Exosomal MiR-200c and MiR-141 as Cerebrospinal Fluid Biopsy Biomarkers for the Response to Chemotherapy in Primary Central Nervous System Lymphoma. Discov. Oncol. 2023, 14, 205. [Google Scholar] [CrossRef]
- Péterffy, B.; Nádasi, T.J.; Krizsán, S.; Horváth, A.; Márk, Á.; Barna, G.; Timár, B.; Almási, L.; Müller, J.; Csanádi, K.; et al. Digital PCR-Based Quantification of MiR-181a in the Cerebrospinal Fluid Aids Patient Stratification in Pediatric Acute Lymphoblastic Leukemia. Sci. Rep. 2024, 14, 28556. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.-Q.; Zhang, P.; Huang, L.-B.; Zheng, Y.-S.; Wu, J.; Zhou, H.; Qu, L.-H.; Xu, L.; Chen, Y.-Q. MicroRNA Patterns Associated with Clinical Prognostic Parameters and CNS Relapse Prediction in Pediatric Acute Leukemia. PLoS ONE 2009, 4, e7826. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Vaiselbuh, S.R. Silencing of Exosomal MiR-181a Reverses Pediatric Acute Lymphocytic Leukemia Cell Proliferation. Pharmaceuticals 2020, 13, 241. [Google Scholar] [CrossRef]
- Yan, W.; Song, L.; Wang, H.; Yang, W.; Hu, L.; Yang, Y. Extracellular Vesicles Carrying MiRNA-181b-5p Affects the Malignant Progression of Acute Lymphoblastic Leukemia. J. Transl. Med. 2021, 19, 511. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Song, J.; Wang, L.; Guo, P.; Cao, J. MiRNA-181b-5p Modulates Cell Proliferation, Cell Cycle, and Apoptosis by Targeting SSX2IP in Acute Lymphoblastic Leukemia. Turk. J. Hematol. 2022, 39, 160–169. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-W.; Jung, J.-H.; Hur, W.; Park, J.; Shin, H.; Choi, B.; Jeong, H.; Kim, D.S.; Yu, E.S.; Lee, S.R.; et al. The Potential of Exosomes Derived from Chronic Myelogenous Leukaemia Cells as a Biomarker. Anticancer. Res. 2018, 38, 3935–3942. [Google Scholar] [CrossRef]
- Mineo, M.; Taverna, S.; Flugy, A.; Leo, G.D.; Alessandro, R.; Kohn, E.C. Abstract 4372: Chronic Myeloid Leukemia (CML) Exosomes Promote Angiogenesis in a Src-Dependent Fashion In Vitro and In Vivo. Cancer Res. 2012, 72, 4372. [Google Scholar] [CrossRef]
- Jia, Q.; Sun, H.; Xiao, F.; Sai, Y.; Li, Q.; Zhang, X.; Yang, S.; Wang, H.; Wang, H.; Yang, Y.; et al. MiR-17-92 Promotes Leukemogenesis in Chronic Myeloid Leukemia via Targeting A20 and Activation of NF-ΚB Signaling. Biochem. Biophys. Res. Commun. 2017, 487, 868–874. [Google Scholar] [CrossRef]
- Charreau, B. Secretome and Tunneling Nanotubes: A Multilevel Network for Long Range Intercellular Communication between Endothelial Cells and Distant Cells. Int. J. Mol. Sci. 2021, 22, 7971. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.; Regazzo, G.; De Dominici, M.; Sacconi, A.; Pelosi, A.; Korita, E.; Marchesi, F.; Pisani, F.; Magenta, A.; Lulli, V.; et al. Transcriptional Activation of the MiR-17-92 Cluster Is Involved in the Growth-Promoting Effects of MYB in Human Ph-Positive Leukemia Cells. Haematologica 2019, 104, 82–92. [Google Scholar] [CrossRef]
- Peters, J.; Bollaert, E.; Cloos, A.-S.; Claus, M.; Essaghir, A.; Lenglez, S.; Saussoy, P.; Dachy, G.; Autin, P.; Demoulin, J.-B.; et al. MiR-92a-1-5p Contributes to Cellular Proliferation and Survival in Chronic Myeloid Leukemia and Its Inhibition Enhances Imatinib Efficacy. Noncoding RNA Res. 2025, 14, 14–24. [Google Scholar] [CrossRef]
- Palazzo, C.; Mastrantonio, R.; Gioelli, N.; Testa, E.; Recco, F.; Lucchetti, D.; Villari, G.; D’Alessio, A.; Sgambato, A.; Mignone, F.; et al. Neuropilin1-Dependent Paracrine Signaling of Cancer Cells Mediated by MiRNA Exosomal Cargo. Cell Commun. Signal. 2025, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, O.; Mierzejewski, B.; Brzoska, E. MicroRNA-126: A Key Regulator of Angiogenesis, Inflammation, and Tumorigenesis—Exploring Its Multifaceted Functions in Vascular Health and Cancer. Biochim. Et. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167984. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, Y.; Hu, C.; Zhang, A.; Ren, Y.; Xu, X. MicroRNA-221 Sensitizes Chronic Myeloid Leukemia Cells to Imatinib by Targeting STAT5. Leuk. Lymphoma 2019, 60, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Rostampour, S.; Eslami, F.; Babaei, E.; Mostafavi, H.; Mahdavi, M. An Active Compound from the Pyrazine Family Induces Apoptosis by Targeting the Bax/Bcl2 and Survivin Expression in Chronic Myeloid Leukemia K562 Cells. Anticancer. Agents Med. Chem. 2024, 24, 203–212. [Google Scholar] [CrossRef]
- Smith, M.R.; Satter, L.R.F.; Vargas-Hernández, A. STAT5b: A Master Regulator of Key Biological Pathways. Front. Immunol. 2023, 13, 1025373. [Google Scholar] [CrossRef]
- Ai, K.; Chen, M.; Liang, Z.; Ding, X.; Gao, Y.; Zhang, H.; Wu, S.; He, Y.; Li, Y. Inhibition of Tumoral VISTA to Overcome TKI Resistance via Downregulation of the AKT/MTOR and JAK2/STAT5 Pathways in Chronic Myeloid Leukemia. Biomol. Ther. 2024, 32, 582–600. [Google Scholar] [CrossRef]
- Bourgeais, J.; Ishac, N.; Medrzycki, M.; Brachet-Botineau, M.; Desbourdes, L.; Gouilleux-Gruart, V.; Pecnard, E.; Rouleux-Bonnin, F.; Gyan, E.; Domenech, J.; et al. Oncogenic STAT5 Signaling Promotes Oxidative Stress in Chronic Myeloid Leukemia Cells by Repressing Antioxidant Defenses. Oncotarget 2017, 8, 41876–41889. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Giudice, V.; Banaszak, L.G.; Gutierrez-Rodrigues, F.; Kajigaya, S.; Panjwani, R.; Ibanez, M.d.P.F.; Rios, O.; Bleck, C.K.; Stempinski, E.S.; Raffo, D.Q.; et al. Circulating Exosomal MicroRNAs in Acquired Aplastic Anemia and Myelodysplastic Syndromes. Haematologica 2018, 103, 1150–1159. [Google Scholar] [CrossRef]
- Aghaei-Zarch, S.M. Crosstalk between MiRNAs/LncRNAs and PI3K/AKT Signaling Pathway in Diabetes Mellitus: Mechanistic and Therapeutic Perspectives. Noncoding RNA Res. 2024, 9, 486–507. [Google Scholar] [CrossRef]
- Guo, B.; Gu, J.; Zhuang, T.; Zhang, J.; Fan, C.; Li, Y.; Zhao, M.; Chen, R.; Wang, R.; Kong, Y.; et al. MicroRNA-126: From Biology to Therapeutics. Biomed. Pharmacother. 2025, 185, 117953. [Google Scholar] [CrossRef]
- Meunier, M.; Laurin, D.; Park, S. Extracellular Vesicles and MicroRNA in Myelodysplastic Syndromes. Cells 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, V.; Koumpis, E.; Hatzimichael, E. The Role of Non-Coding RNAs in Myelodysplastic Neoplasms. Cancers 2023, 15, 4810. [Google Scholar] [CrossRef] [PubMed]
- Chee, L.; Koldej, R.; Thio, N.; Ludford-Menting, M.; Fox, L.; Blombery, P.; Ritchie, D. MicroRNA Profiling in Aplastic Anemia Reveals Similarities between Secondary Myelodysplastic Syndromes Arising from Clonal Progression and de Novo MDS. Front. Hematol. 2023, 2, 1184962. [Google Scholar] [CrossRef]
- Kang, S.-H.; Choi, J.-S. MicroRNA-661 Upregulation in Myelodysplastic Syndromes Induces Apoptosis through P53 Activation and Associates with Decreased Overall Survival. Leuk. Lymphoma 2019, 60, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Walker, C.S.; Agarwal, P.; Vick, E.; Sampson, A.; Choi, K.; Niederkorn, M.; Ishikawa, C.; Hueneman, K.; Varney, M.; et al. Inactivation of P53 Provides a Competitive Advantage to Del(5q) Myelodysplastic Syndrome Hematopoietic Stem Cells during Inflammation. Haematologica 2023, 108, 2715–2729. [Google Scholar] [CrossRef]
- Mongiorgi, S.; De Stefano, A.; Ratti, S.; Indio, V.; Astolfi, A.; Casalin, I.; Pellagatti, A.; Paolini, S.; Parisi, S.; Cavo, M.; et al. A MiRNA Screening Identifies MiR-192-5p as Associated with Response to Azacitidine and Lenalidomide Therapy in Myelodysplastic Syndromes. Clin. Epigenetics 2023, 15, 27. [Google Scholar] [CrossRef]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2025, 100, 860–891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Sun, K.; Chng, W.-J. The Emerging Roles of Exosomes in Leukemogeneis. Oncotarget 2016, 7, 50698–50707. [Google Scholar] [CrossRef]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia Cell to Endothelial Cell Communication via Exosomal MiRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Yang, Y.; Zhou, S.; Zhang, P.; Feng, T. The Role of Exosomal LncRNAs in Cancer Biology and Clinical Management. Exp. Mol. Med. 2021, 53, 1669–1673. [Google Scholar] [CrossRef]
- Xiao, Q.; Lin, C.; Peng, M.; Ren, J.; Jing, Y.; Lei, L.; Tao, Y.; Huang, J.; Yang, J.; Sun, M.; et al. Circulating Plasma Exosomal Long Non-Coding RNAs LINC00265, LINC00467, UCA1, and SNHG1 as Biomarkers for Diagnosis and Treatment Monitoring of Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 1033143. [Google Scholar] [CrossRef]
- Ma, L.; Kuai, W.-X.; Sun, X.-Z.; Lu, X.-C.; Yuan, Y.-F. Long Noncoding RNA LINC00265 Predicts the Prognosis of Acute Myeloid Leukemia Patients and Functions as a Promoter by Activating PI3K-AKT Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7867–7876. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Q.; Zhu, K.; Zhu, J.; Li, J.; Yuan, Y.; Zhang, P.; Zhou, L.; Liu, L. LncRNA LINC00265/MiR-485-5p/IRF2-Mediated Autophagy Suppresses Apoptosis in Acute Myeloid Leukemia Cells. Am. J. Transl. Res. 2020, 12, 2451–2462. [Google Scholar] [PubMed]
- Lu, J.; Wu, X.; Wang, L.; Li, T.; Sun, L. Long Noncoding RNA LINC00467 Facilitates the Progression of Acute Myeloid Leukemia by Targeting the MiR-339/SKI Pathway. Leuk. Lymphoma 2021, 62, 428–437. [Google Scholar] [CrossRef]
- Liang, Y.; Li, E.; Zhang, H.; Zhang, L.; Tang, Y.; Wanyan, Y. Silencing of LncRNA UCA1 Curbs Proliferation and Accelerates Apoptosis by Repressing SIRT1 Signals by Targeting MiR-204 in Pediatric AML. J. Biochem. Mol. Toxicol. 2020, 34, e22435. [Google Scholar] [CrossRef]
- Bao, X.-L.; Zhang, L.; Song, W.-P. LncRNA SNHG1 Overexpression Regulates the Proliferation of Acute Myeloid Leukemia Cells through MiR-488-5p/NUP205 Axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5896–5903. [Google Scholar] [CrossRef]
- Lin, X.; Ling, Q.; Lv, Y.; Ye, W.; Huang, J.; Li, X.; Guo, Q.; Wang, J.; Li, Z.; Jin, J. Plasma Exosome-Derived MicroRNA-532 as a Novel Predictor for Acute Myeloid Leukemia. Cancer Biomark. 2020, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, X.; Zheng, J.; Yin, R.; Li, Y.; Wu, X.; Xu, L.; Jin, Z. Utilizing Exosomes as Sparking Clinical Biomarkers and Therapeutic Response in Acute Myeloid Leukemia. Front. Immunol. 2024, 14, 1315453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Deng, T.; Wang, D.; Xiao, Y. Elevated Serum Exosomal MiR-125b Level as a Potential Marker for Poor Prognosis in Intermediate-Risk Acute Myeloid Leukemia. Acta Haematol. 2018, 140, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, X.; Wu, J.; Xiao, R.; Liu, J. High Serum Extracellular Vesicle MiR-10b Expression Predicts Poor Prognosis in Patients with Acute Myeloid Leukemia. Cancer Biomark. 2019, 27, 1–9. [Google Scholar] [CrossRef]
- Hornick, N.I.; Huan, J.; Doron, B.; Goloviznina, N.A.; Lapidus, J.; Chang, B.H.; Kurre, P. Serum Exosome MicroRNA as a Minimally-Invasive Early Biomarker of AML. Sci. Rep. 2015, 5, 11295. [Google Scholar] [CrossRef]
- Barrera-Ramirez, J.; Lavoie, J.R.; Maganti, H.B.; Stanford, W.L.; Ito, C.; Sabloff, M.; Brand, M.; Rosu-Myles, M.; Le, Y.; Allan, D.S. Micro-RNA Profiling of Exosomes from Marrow-Derived Mesenchymal Stromal Cells in Patients with Acute Myeloid Leukemia: Implications in Leukemogenesis. Stem Cell Rev. Rep. 2017, 13, 817–825. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Z.; Zhou, Y.; Ni, J.; Zhu, J.; Fan, X.; Chen, X.; Liu, Y.; Li, Z.; Zhou, H. MicroRNA-1246-Containing Extracellular Vesicles from Acute Myeloid Leukemia Cells Promote the Survival of Leukemia Stem Cells via the LRIG1-Meditated STAT3 Pathway. Aging 2021, 13, 13644–13662. [Google Scholar] [CrossRef]
- Sharma, S.; Patnaik, S.K.; Thomas Taggart, R.; Kannisto, E.D.; Enriquez, S.M.; Gollnick, P.; Baysal, B.E. APOBEC3A Cytidine Deaminase Induces RNA Editing in Monocytes and Macrophages. Nat. Commun. 2015, 6, 6881. [Google Scholar] [CrossRef]
- Kumar, B.; Garcia, M.; Weng, L.; Jung, X.; Murakami, J.L.; Hu, X.; McDonald, T.; Lin, A.; Kumar, A.R.; DiGiusto, D.L.; et al. Acute Myeloid Leukemia Transforms the Bone Marrow Niche into a Leukemia-Permissive Microenvironment through Exosome Secretion. Leukemia 2018, 32, 575–587. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic Mastocytosis in Adults: 2023 Update on Diagnosis, Risk Stratification and Management. Am. J. Hematol. 2023, 98, 1097–1116. [Google Scholar] [CrossRef]
- Fazio, M.; Vetro, C.; Markovic, U.; Duminuco, A.; Parisi, M.; Maugeri, C.; Mauro, E.; Parrinello, N.L.; Stagno, F.; Villari, L.; et al. A Case of High-risk AML in a Patient with Advanced Systemic Mastocytosis. Clin. Case Rep. 2023, 11, e7134. [Google Scholar] [CrossRef]
- Kim, D.-K.; Bandara, G.; Cho, Y.-E.; Komarow, H.D.; Donahue, D.R.; Karim, B.; Baek, M.-C.; Kim, H.M.; Metcalfe, D.D.; Olivera, A. Mastocytosis-Derived Extracellular Vesicles Deliver MiR-23a and MiR-30a into Pre-Osteoblasts and Prevent Osteoblastogenesis and Bone Formation. Nat. Commun. 2021, 12, 2527. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A. Classification of Myelodysplastic, Myeloproliferative, and Myelodysplastic/Myeloproliferative Neoplasms: The Past, Present, and Future. Am. J. Hematol. 2025, 100, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Hu, B.; Wang, P.; Wang, W.; Liu, J. Post-Transcriptional Regulation of Erythropoiesis. Blood Sci. 2023, 5, 150–159. [Google Scholar] [CrossRef]
- Kretov, D.A.; Folkes, L.; Mora-Martin, A.; Walawalkar, I.A.; Imrat; Syedah, N.; Vanuytsel, K.; Moxon, S.; Murphy, G.J.; Cifuentes, D. The MiR-144/Hmgn2 Regulatory Axis Orchestrates Chromatin Organization during Erythropoiesis. Nat. Commun. 2024, 15, 3821. [Google Scholar] [CrossRef]
- Wagiu Basrowi, R.; Sundjaya, T.; Pratiwi, D.; Amalia, N.; Tandi, Y.Y.P.; Syafa’atulloh, M.Y.; Utomo, G.N.P.; Albarok, M.A.R.; Nurkolis, F. Harnessing the Power of Proteins in Modulation of MiRNAs for Targeting Iron Deficiency Anemia: Opinion for Future Implications and Strategies. Front. Nutr. 2025, 12, 1535498. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Tozzi, L.; Bogani, C.; Iacobucci, I.; Ponziani, V.; Martinelli, G.; Bosi, A.; Vannucchi, A.M. Overexpression of MicroRNA-16-2 Contributes to the Abnormal Erythropoiesis in Polycythemia Vera. Blood 2011, 117, 6923–6927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruchova, H.; Merkerova, M.; Prchal, J.T. Aberrant Expression of MicroRNA in Polycythemia Vera. Haematologica 2008, 93, 1009–1016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.-C.; You, J.-Y.; Lung, J.; Huang, C.-E.; Chen, Y.-Y.; Leu, Y.-W.; Ho, H.-Y.; Li, C.-P.; Lu, C.-H.; Lee, K.-D.; et al. Aberrant Let7a/HMGA2 Signaling Activity with Unique Clinical Phenotype in JAK2-Mutated Myeloproliferative Neoplasms. Haematologica 2017, 102, 509–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lan, L.; Wang, X.; Zhang, S.; Zeng, D.; Mo, L.; Feng, Q.; Li, J.; Zhu, C. MiR-223-3p Regulates Erythropoiesis by Targeting TGFBR3/Smad Signaling Pathway in Hemoglobin H-Constant Spring Disease. Ann. Med. 2025, 57, 2530690. [Google Scholar] [CrossRef]
- Zhan, H.; Cardozo, C.; Yu, W.; Wang, A.; Moliterno, A.R.; Dang, C.V.; Spivak, J.L. MicroRNA Deregulation in Polycythemia Vera and Essential Thrombocythemia Patients. Blood Cells Mol. Dis. 2013, 50, 190–195. [Google Scholar] [CrossRef][Green Version]
- Guglielmelli, P.; Tozzi, L.; Pancrazzi, A.; Bogani, C.; Antonioli, E.; Ponziani, V.; Poli, G.; Zini, R.; Ferrari, S.; Manfredini, R.; et al. MicroRNA Expression Profile in Granulocytes from Primary Myelofibrosis Patients. Exp. Hematol. 2007, 35, 1708.e1–1708.e12. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, S.; Huang, E.; Gnatenko, D.; Bahou, W.F.; Zhu, W. Integrated Micro/Messenger RNA Regulatory Networks in Essential Thrombocytosis. PLoS ONE 2018, 13, e0191932. [Google Scholar] [CrossRef]
- Albano, F.; Anelli, L.; Zagaria, A.; Coccaro, N.; Casieri, P.; Minervini, A.; Specchia, G. SETBP1 and MiR_4319 Dysregulation in Primary Myelofibrosis Progression to Acute Myeloid Leukemia. J. Hematol. Oncol. 2012, 5, 48. [Google Scholar] [CrossRef]
- Zhang, Z.; Ran, Y.; Shaw, T.; Peng, Y. MicroRNAs 10a and 10b Regulate the Expression of Human Platelet Glycoprotein Ibα for Normal Megakaryopoiesis. Int. J. Mol. Sci. 2016, 17, 1873. [Google Scholar] [CrossRef]
- Slezak, S.; Jin, P.; Caruccio, L.; Ren, J.; Bennett, M.; Zia, N.; Adams, S.; Wang, E.; Ascensao, J.; Schechter, G.; et al. Gene and MicroRNA Analysis of Neutrophils from Patients with Polycythemia Vera and Essential Thrombocytosis: Down-Regulation of Micro RNA-1 and -133a. J. Transl. Med. 2009, 7, 39. [Google Scholar] [CrossRef]
- Guo, L.; Huang, W.; Chen, B.; Jebessa Bekele, E.; Chen, X.; Cai, B.; Nie, Q. Gga-Mir-133a-3p Regulates Myoblasts Proliferation and Differentiation by Targeting PRRX1. Front. Genet. 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Azizi, S.G.; Soleimani, M.; Farshi, Y.; Kashani Khatib, Z. The Role of MicroRNAs in Myeloproliferative Neoplasia. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 172–185. [Google Scholar] [PubMed]
- Coccaro, N.; Tota, G.; Zagaria, A.; Anelli, L.; Specchia, G.; Albano, F. SETBP1 Dysregulation in Congenital Disorders and Myeloid Neoplasms. Oncotarget 2017, 8, 51920–51935. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Umezu, T.; Saitoh, Y.; Gotoh, M.; Akahane, D.; Kobayashi, C.; Ohyashiki, J.H.; Ohyashiki, K. Exosomal MiRNA Signatures for Late-Onset Acute Graft-Versus-Host Disease in Allogenic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2018, 19, 2493. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal MiR-135b Shed from Hypoxic Multiple Myeloma Cells Enhances Angiogenesis by Targeting Factor-Inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes Derived from Hypoxic Leukemia Cells Enhance Tube Formation in Endothelial Cells. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in Extracellular Vesicles: Sorting Mechanisms, Diagnostic Value, Isolation, and Detection Technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Nie, C.; Shaw, I.; Chen, C. Application of Microfluidic Technology Based on Surface-Enhanced Raman Scattering in Cancer Biomarker Detection: A Review. J. Pharm. Anal. 2023, 13, 1429–1451. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tiwari, P.K.; Kashyap, V.; Kumar, S. Proteomics of Extracellular Vesicles: Recent Updates, Challenges and Limitations. Proteomes 2025, 13, 12. [Google Scholar] [CrossRef]
- Dilsiz, N. A Comprehensive Review on Recent Advances in Exosome Isolation and Characterization: Toward Clinical Applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, P.; Callari, M.; Angeloni, V.; Daidone, M.G.; Appierto, V. Challenges in Using Circulating MiRNAs as Cancer Biomarkers. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Marabita, F.; de Candia, P.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of Circulating MicroRNA Expression Data Obtained by Quantitative Real-Time RT-PCR. Brief. Bioinform. 2016, 17, 204–212. [Google Scholar] [CrossRef]
- Becker, N.; Lockwood, C.M. Pre-Analytical Variables in MiRNA Analysis. Clin. Biochem. 2013, 46, 861–868. [Google Scholar] [CrossRef]
- Witwer, K.W. XenomiRs and MiRNA Homeostasis in Health and Disease. RNA Biol. 2012, 9, 1147–1154. [Google Scholar] [CrossRef]
- Benz, F.; Roderburg, C.; Vargas Cardenas, D.; Vucur, M.; Gautheron, J.; Koch, A.; Zimmermann, H.; Janssen, J.; Nieuwenhuijsen, L.; Luedde, M.; et al. U6 Is Unsuitable for Normalization of Serum MiRNA Levels in Patients with Sepsis or Liver Fibrosis. Exp. Mol. Med. 2013, 45, e42. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Ciuffi, S.; Brandi, M.L. Human Circulating MiRNAs Real-Time QRT-PCR-Based Analysis: An Overview of Endogenous Reference Genes Used for Data Normalization. Int. J. Mol. Sci. 2019, 20, 4353. [Google Scholar] [CrossRef]
- Hsu, M.-T.; Wang, Y.-K.; Tseng, Y.J. Exosomal Proteins and Lipids as Potential Biomarkers for Lung Cancer Diagnosis, Prognosis, and Treatment. Cancers 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-T.; Hadlock, T.; Jolly, S.; Nagrath, S. Extracellular Vesicles on Demand (EVOD) Chip for Screening and Quantification of Cancer-Associated Extracellular Vesicles. Biosens. Bioelectron. 2020, 168, 112535. [Google Scholar] [CrossRef]
- Cai, S.; Luo, B.; Jiang, P.; Zhou, X.; Lan, F.; Yi, Q.; Wu, Y. Immuno-Modified Superparamagnetic Nanoparticles via Host–Guest Interactions for High-Purity Capture and Mild Release of Exosomes. Nanoscale 2018, 10, 14280–14289. [Google Scholar] [CrossRef]
- Wan, Y.; Cheng, G.; Liu, X.; Hao, S.-J.; Nisic, M.; Zhu, C.-D.; Xia, Y.-Q.; Li, W.-Q.; Wang, Z.-G.; Zhang, W.-L.; et al. Rapid Magnetic Isolation of Extracellular Vesicles via Lipid-Based Nanoprobes. Nat. Biomed. Eng. 2017, 1, 0058. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.; Bathini, S.; Badilescu, S.; Ghosh, A.; Packirisamy, M. Microfluidic Platforms for the Isolation and Detection of Exosomes: A Brief Review. Micromachines 2022, 13, 730. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Shen, C.; Li, C.; Xu, X.; Li, Q.; Wu, Z.; Ma, H.; Chen, F.; Mao, H. Rapid Automated Extracellular Vesicle Isolation and MiRNA Preparation on a Cost-Effective Digital Microfluidic Platform. Anal. Chim. Acta 2024, 1296, 342337. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-Derived Exosomal MiR-15a-5p as a Promising Diagnostic Biomarker for Early Detection of Endometrial Carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef]
- Rausch, C.; Rothenberg-Thurley, M.; Buerger, S.A.; Tschuri, S.; Dufour, A.; Neusser, M.; Schneider, S.; Spiekermann, K.; Metzeler, K.H.; Ziemann, F. Double Drop-Off Droplet Digital PCR. J. Mol. Diagn. 2021, 23, 975–985. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Chen, X.; Qiu, X.; Qing, G.; Zhang, H.; Zhang, L.; Hu, X.; He, Z.; Zhong, D.; et al. Rolling Circular Amplification (RCA)-Assisted CRISPR/Cas9 Cleavage (RACE) for Highly Specific Detection of Multiple Extracellular Vesicle MicroRNAs. Anal Chem 2020, 92, 2176–2185. [Google Scholar] [CrossRef]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Farina, M.; Bosio, K.; Di Lucanardo, A.; Leoni, A.; Re, F.; Polverelli, N.; Turra, A.; Morello, E.; Accorsi Buttini, E.; et al. Feasibility of Leukemia-Derived Exosome Enrichment and Co-Isolated DsDNA Sequencing in Acute Myeloid Leukemia Patients: A Proof of Concept for New Leukemia Biomarkers Detection. Cancers 2022, 14, 4504. [Google Scholar] [CrossRef] [PubMed]
| Disease | Principal miRNA | Principal Biological Function | Key Targets/Pathways | Clinical Role |
|---|---|---|---|---|
| MM | miR-20a-5p | Promotes chemoresistance | RUNX3, Rab27B, ATG7 | Early disease progression marker |
| miR-103a-3p | Promotes progression | PTEN/PI3k-Akt | Biomarker of MGUS → MM shift | |
| miR-4505 miR-10a | Enhances tumor aggressiveness Pro-oncogenic | Cytokine network EPAH8/SEMA5A | Prognostic marker Favors proliferation and metastasis | |
| miR-16 | Regulates IGF1R signaling | IGF1R/CCND1/ELAVL1 | Low levels linked to poor prognosis | |
| PRINS (lncRNA) | Reflects chromosomal instability | del(13)(q14), t(4;14) | Distinguishes MM from healthy donors | |
| miR-135b miR-21 | Pro-angiogenic in hypoxic BM niche Counteract apoptosis | FIH-1 Rhob | Target for anti-angiogenic therapy Induces steroid-resistance | |
| miR-15a-5p | Tumor suppressor | CCND1, BCL2 | Reduced in bortezomib-resistant MM | |
| miR-17-5p | Both: tumor suppressor/onco-miR | HBP1, AIB1, E2F1 | Reduced in bortezomib-resistant MM | |
| miR-18a | Drives extramedullary disease | Dicer, HIF-1α | Marker of aggressive disease | |
| CLL | miR-15a, miR-16 | Induce apoptosis | BCL2 | Loss correlates with poor prognosis |
| miR-155 | Promotes MDSCs | PI3K/Akt, PD-L1 | Marker of immune escape/resistance | |
| miR-150 | Regulates T-cell differentiation | MYB, NOTCH | Prognostic marker/identifies CLL | |
| miR-223 | Immune suppression | STAT3 | Downregulated in case of progression | |
| miR-202-3p | Activates Hedgehog pathway | SMO | Marker for immune escape | |
| miR-195 | Correlates with time-to-treatment | CCND1 | Early detection biomarker | |
| HL | miR-24-3p | Regulates cell cycle | CDK6, SMAD4 | Diagnostic/reduced after treatment |
| miR-127-3p | Regulates cell cycle | CDK6, SMAD4 | Diagnostic/reduced after treatment | |
| miR-21-5p | Tumor growth/immune evasion | PTEN, SOCS1 | Marker for disease activity and relapse | |
| miR-155-5p | Tumor growth/immune evasion | PTEN, SOCS1 | Marker for disease activity and relapse | |
| Let-7a-5p | Tumor suppressor | RAS, HMGA2 | Monitoring disease response | |
| DLBCL | miR-379-5p | Promotes survival/chemoresistance | BCL2, LIN28B | Early diagnostic marker |
| miR-135a-3p | Promotes survival/chemoresistance | BCL2, LIN28B | Early diagnostic marker | |
| miR-99a-5p | Negatively targets apoptosis | mTOR, IGF-1R | Marker of chemoresistance | |
| miR-125b-5p | Negatively targets apoptosis | mTOR, IGF-1R | Predicts shorter PFS | |
| miR-451a | Suppresses invasion | MIF | Low levels linked to poor prognosis | |
| miR-155 | Regulates immune cell function | SHIP1, PU.1 | Marker of refractory-relapsed disease | |
| miR-20a | Regulates immune cell function | SHIP1, PU.1 | Associated with higher mortality rate | |
| miR-106a/b | Enhances cell survival and invasion | E2F1, TGFβ | Associated with higher mortality rate | |
| ALL | miR-181a | Induces CNS involvement | MCL-1, BCL2 | CNS relapse biomarker |
| miR-181b-5p | Suppresses apoptosis | PCNA, Ki-67 | Potential therapeutic target |
| Disease | Principal miRNA | Principal Biological Function | Key Targets/Pathways | Clinical Role |
|---|---|---|---|---|
| CML | miR-92a | Modulates angiogenesis | Integrin α5 | Biomarker for vascular remodeling |
| miR-210 | Promotes VEGF-mediated angiogenesis | EPHRIN-A3 | Potential therapeutic target | |
| miR-126 | Affects adhesion/migration of CML cells | CXCL12, VCAM1 | Modulator of leukemic niche | |
| MDS | miR-196a-5p | Regulates hematopoiesis/therapy resistance | HOXA, DNMT1, PTEN | Associated with progression to AML |
| miR-126-5p | Regulates hematopoiesis/therapy resistance | PTTG3P | Diagnostic/predictive of AZA response | |
| miR-192-5p let-7a mir-196-5b | Targets BCL2 and suppresses proliferation Associated with higher-risk karyotype Drives aberrant proliferation | BCL2 RAS/MYC CDKN1B | Predictor of AZA/LENA therapy success Favors proliferation Associated with progression to AML | |
| AML | miR-150 | Modulate apoptosis and proliferation | PKCα, FOXO4 | Early detection marker |
| miR-155 | Promotes leukemogenesis | SHIP1/SOCS1 | Early detection marker | |
| miR-10b | Enhances tumor invasiveness | HOXD10, RhoC | Poor prognosis biomarker (shorter OS) | |
| miR-125b | Promotes chemoresistance Inhibits differentiation | p53, BAK1, CBFβ TET2 | Associated with relapse and mortality Associated with relapse and mortality | |
| miR-532 | Improves overall survival | Unknown | Positive prognostic marker | |
| miR-1246 miR-425-5p | Induces survival and colony formation Tumor suppressor | LRIG1; JAK/STAT 23 genes (e.g., APOBEC3A) | Detection/aggressive disease marker Poor prognosis when deregulated | |
| SM | miR-23a | Inhibits osteogenesis | RUNX2, SMAD1, SMAD5 | Potential targets for reversing bone loss |
| miR-30a | Same as above | Same as above | Same as above | |
| PV | miR-451, 144 | Promote erythroid maturation | MYC/COX10; RAB14/CAP1 | Enhance erythropoiesis |
| miR-150 | Suppresses erythroid differentiation | c-MYB inhibition | Contributes to clonal expansion | |
| miR-16-2 | Promotes erythropoiesis | (Independent of JAK/STAT) | Supports erythroid colony formation | |
| let-7a, miR-182 | Regulate hematopoietic proliferation | PI3K/AKT | Their dysregulation alters hematopoiesis | |
| miR-143, 145, 223 | Modulate platelet differentiation | PI3K/AKT | Contribute to aberrant hematopoiesis | |
| ET | miR-34a | Induces apoptosis via p53/SIRT1 axis | p53/SIRT1 | Loss favors megakaryocytic hyperplasia |
| miR-342, 326 | Regulate inflammatory responses | NOTCH, PI3K/AKT | Loss favors meyeloproliferation | |
| miR-105, 149, 147 | Modulate inflammatory pathways | PI3K/AKT, NF-κB | Dysregulation promotes proliferation | |
| miR-10a | Regulate megakaryocyte development | MPL/JAK2 | Favors megakaryocytic expansion | |
| miR-150 miR-133a | Favors megakaryocytic lineage Tumor suppressor/regulate differentiation | c-MYB inhibition Cyclin D1; ERK1/2 | Thrombocytosis and risk of thrombosis Downregulation leads to proliferation | |
| PMF | miR-31, 150, 95 | Downregulated: impairs differentiation | Myb; PI3K/AKT-TGFβ | Associated with marrow fibrosis |
| miR-190 | Upregulated: pro-survival function | Unknown | Cellular persistence in fibrotic BM | |
| miR-223, 146b | Regulate megakaryocyte proliferation | NF-κB, JAK/STAT | Contribute to fibrosis and splenomegaly | |
| miR-4319 | Downregulated: SETBP1 upregulation | SETBP1 pathway | Associated with progression to AML |
| Disease | Micro-RNA | Main Function | Key Targets | Role as Biomarker |
|---|---|---|---|---|
| AML | miR-150 | Modulates apoptosis, differentiation, tumor growth | PKCα, FOXO4, iASSP, | Distinguishes AML from CML, ALL, MDS |
| EIF4B, TET3 | ||||
| miR-155 | Regulates proliferation and apoptosis via PI3K/Akt signaling | PU.1, SHIP1 | Indicates progression/refractoriness | |
| miR-1246 | Promotes angiogenesis and tumor aggressiveness | PML, ALDH1, SOX2 | Indicates resistance/invasiveness | |
| MM | miR-17-5p | Tumor suppressor and oncogene (context-dependent) | HBP1, AIB1, E2F1 | Reduced in bortezomib-resistant MM |
| miR-20a-5p | Promotes proliferation, chemoresistance | RUNX3, Rab27B, | Reduced in bortezomib-resistant MM | |
| Smad4, ATG7 | ||||
| miR-15a-5p | Tumor suppressor, regulates apoptosis | CCND1, BCL2, | Associated with CHT resistance | |
| BDNF, CXCL10 | ||||
| miR-16-5p | Tumor suppressor, modulates TGF-β | Smad3 | Associated with CHT resistance | |
| let-7b | Tumor suppressor, regulates cell cycle and resistance | CCND1, MTDH, CALU, | Associated with poor prognosis | |
| ERK, AURKB | in NDMM | |||
| miR-18a | Promotes growth and EMD, regulates miRNA biogenesis | Dicer, HIF-1α, | Indicates progression | |
| ATXN1, STK4 | ||||
| WM | miR-192-5p | Promotes proliferation, survival, metastasis | SEMA3A, XIAP, YY1, | Indicates progression |
| USP1, PI3K/Akt | ||||
| miR-320b | Tumor suppressor, reduces migration and invasion | c-Myc, CD71, TRIAP1, | Downregulated in aggressive forms | |
| Wnt/β-catenin, MMP2/9 | ||||
| let-7d | Dual role: regulates RAS, stem cell aging, stromal signaling | RAS, HMGA2, P16, | Useful to stratify high-risk patients | |
| COL3A1, CCL7 | ||||
| DLBCL | miR-99a-5p | Regulates tumor growth and survival | SMARCA5, SMARC1, | Indicates progression and useful to |
| mTOR, IGF-1R, FGFR3 | distinguish disease subtypes | |||
| miR-125b-5p | Tumor suppressor but also downregulates p53 | BCL-2, LIN28B, p53 | Useful to define subtype and risk level |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, M.; Stagno, F.; Penna, G.; Mirabile, G.; Allegra, A. Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies. Non-Coding RNA 2025, 11, 64. https://doi.org/10.3390/ncrna11050064
Fazio M, Stagno F, Penna G, Mirabile G, Allegra A. Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies. Non-Coding RNA. 2025; 11(5):64. https://doi.org/10.3390/ncrna11050064
Chicago/Turabian StyleFazio, Manlio, Fabio Stagno, Giuseppa Penna, Giuseppe Mirabile, and Alessandro Allegra. 2025. "Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies" Non-Coding RNA 11, no. 5: 64. https://doi.org/10.3390/ncrna11050064
APA StyleFazio, M., Stagno, F., Penna, G., Mirabile, G., & Allegra, A. (2025). Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies. Non-Coding RNA, 11(5), 64. https://doi.org/10.3390/ncrna11050064






