Role of Non-Coding RNAs in White and Brown Adipose Tissue Differentiation and Development
Abstract
1. Introduction
2. White vs. Brown Adipose Tissue
2.1. White Adipose Tissue (WAT)
2.2. Brown Adipose Tissue (BAT)
3. Stem Cells and Adipose Tissue Plasticity
3.1. Adipose-Derived Stem Cells (ADSCs)
3.2. Adipogenic Differentiation Potential
4. Transcription Factors Involved in WAT and BAT Development
5. miRNAs as Key Genetic Regulators
5.1. miRNAs Involved in the Differentiation and Function of WAT
5.1.1. Positive WAT Regulators
5.1.2. Negative WAT Regulators
5.2. miRNAs Involved in the Differentiation and Function of BAT
5.2.1. Positive BAT Regulators
5.2.2. Negative BAT Regulators
6. Other Non-Coding RNA Molecules Involved in the Differentiation and Function of WAT and BAT
6.1. Long Non-Coding RNAs (lncRNAs)
6.2. Circular RNAs (circRNAs)
7. Future Perspectives of Non-Coding RNAs as Potential Therapeutic Targets
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romao, J.M.; Guan, L.L. Adipogenesis and Obesity. In MicroRNA in Regenerative Medicine; Sen, C.K., Ed.; Academic Press: Oxford, UK, 2015; Chapter 21; pp. 539–565. [Google Scholar]
- Shook, B.; Gonzalez, G.R.; Ebmeier, S.; Grisotti, G.; Zwick, R.; Horsley, V. The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches. Ann. Rev. Cell Dev. Biol. 2016, 32, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Esteve, R.M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Jeon, S.; Kim, I.; Na, Y.R.; Hong, K.Y.; Chang, H.; Kim, S.H.; Jeong, Y.J.; Chung, J.H.; Kim, S.W. Multiple Injections of Adipose-Derived Stem Cells Improve Graft Survival in Human-to-Rat Skin Xenotransplantation through Immune Modulation. Tissue Eng. Regen. Med. 2023, 20, 905–919. [Google Scholar] [CrossRef]
- Gavin, K.M.; Sullivan, T.M.; Maltzahn, J.K.; Jackman, M.R.; Libby, A.E.; MacLean, P.S.; Kohrt, W.M.; Majka, S.M.; Klemm, D.J. Hematopoietic Stem Cell-Derived Adipocytes Modulate Adipose Tissue Cellularity, Leptin Production and Insulin Responsiveness in Female Mice. Front. Endocrinol. 2022, 13, 844877. [Google Scholar] [CrossRef]
- Klein-Wieringa, I.R.; Andersen, S.N.; Kwekkeboom, J.C.; Giera, M.; de Lange-Brokaar, B.J.E.; van Osch, G.J.V.M.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Nelissen, R.G.H.H.; Pijl, H.; et al. Adipocytes Modulate the Phenotype of Human Macrophages through Secreted Lipids. J. Immunol. 2013, 191, 1356–1363. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2022, 204, 91–107. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Horwitz, A.; Birk, R. Adipose Tissue Hyperplasia and Hypertrophy in Common and Syndromic Obesity—The Case of BBS Obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Zhang, P.; Liu, W.; Kuang, S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J. Lipid. Res. 2013, 54, 2214–2224. [Google Scholar] [CrossRef]
- Park, H.; He, A.; Lodhi, I.J. Lipid Regulators of Thermogenic Fat Activation. Trends Endocrinol. Metab. 2019, 30, 710–723. [Google Scholar] [CrossRef]
- Oeckl, J.; Janovska, P.; Adamcova, K.; Bardova, K.; Brunner, S.; Dieckmann, S.; Ecker, J.; Fromme, T.; Funda, J.; Gantert, T.; et al. Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat. Mol. Metab. 2022, 61, 101499. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 1–26. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Song, W.; Jiang, X.; Wang, Y.; Li, C.; Yu, W.; He, Y. Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: Recent advances and perspectives. Stem Cell Res. Ther. 2024, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Rumiński, S.; Kalaszczyńska, I.; Długosz, A.; Lewandowska-Szumieł, M. Osteogenic differentiation of human adipose-derived stem cells in 3D conditions—Comparison of spheroids and polystyrene scaffolds. Eur. Cell Mater. 2019, 37, 382–401. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic differentiation of adipose derived stem cells on graphene-based mat. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 685–692. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Samuel, S.; Kurkalli, B.G.; Kumar, C.; Kumar, B.M.; Shetty, N.; Shetty, V.; Vishwanath, K. In vitro Comparison of Adipogenic Differentiation in Human Adipose-Derived Stem Cells Cultured with Collagen Gel and Platelet-Rich Fibrin. Indian J. Plast. Surg. 2021, 54, 278–283. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef]
- Sun, L.; Goff, L.A.; Trapnell, C.; Alexander, R.; Lo, K.A.; Hacisuleyman, E.; Sauvageau, M.; Tazon-Vega, B.; Kelley, D.R.; Hendrickson, D.G.; et al. Long noncoding RNAs regulate adipogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 3387–3392. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Li, S.; Wang, G.-X.; Yu, Q.; Lin, J.D. A Long Noncoding RNA Transcriptional Regulatory Circuit Drives Thermogenic Adipocyte Differentiation. Mol. Cell. 2014, 55, 372–382. [Google Scholar] [CrossRef]
- Gu, N.; You, L.; Shi, C.; Yang, L.; Pang, L.; Cui, X.; Ji, C.; Zheng, W.; Guo, X. Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol. Med. Rep. 2016, 14, 1180–1186. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, N.; Song, L.; Xie, H.; Zhao, C.; Li, S.; Zhao, W.; Zhao, Y.; Gao, C.; Xu, G. Adipokines and free fatty acids regulate insulin sensitivity by increasing microRNA-21 expression in human mature adipocytes. Mol. Med. Rep. 2017, 16, 2254–2258. [Google Scholar] [CrossRef][Green Version]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Skillington, J.; Derynck, R. Roles of Autocrine TGF-β Receptor and Smad Signaling in Adipocyte Differentiation. J. Cell Biol. 2000, 149, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, M.; Li, X.; Zheng, Y.; Zhang, K.; Liu, X.; Cai, B.; Yin, G. DNMT1-mediated methylation inhibits microRNA-214-3p and promotes hair follicle stem cell differentiate into adipogenic lineages. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Y.; Jin, C.; Li, X.; Jia, L.; Li, W. Long Non-coding RNA H19 Inhibits Adipocyte Differentiation of Bone Marrow Mesenchymal Stem Cells through Epigenetic Modulation of Histone Deacetylases. Sci. Rep. 2016, 6, 28897. [Google Scholar] [CrossRef]
- Seeliger, C.; Krauss, T.; Honecker, J.; Mengel, L.A.; Buekens, L.; Mesas-Fernández, A.; Skurk, T.; Claussnitzer, M.; Hauner, H. miR-375 is cold exposure sensitive and drives thermogenesis in visceral adipose tissue derived stem cells. Sci. Rep. 2022, 12, 9557. [Google Scholar] [CrossRef]
- Ng, R.; Hussain, N.A.; Zhang, Q.; Chang, C.; Li, H.; Fu, Y.; Cao, L.; Han, W.; Stunkel, W.; Xu, F. miRNA-32 Drives Brown Fat Thermogenesis and Trans-activates Subcutaneous White Fat Browning in Mice. Cell Rep. 2017, 19, 1229–1246. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhou, X.; Hu, S.; Ge, L.; Sun, J.; Li, P.; Long, K.; Jin, L.; Tang, Q.; et al. Comprehensive Analysis of mRNA and lncRNA Transcriptomes Reveals the Differentially Hypoxic Response of Preadipocytes During Adipogenesis. Front. Genet. 2020, 11, 845. [Google Scholar] [CrossRef]
- Caca, J.; Bartelt, A.; Egea, V. Hypoxia Regulates Brown Adipocyte Differentiation and Stimulates miR-210 by HIF-1α. Int. J. Mol. Sci. 2024, 26, 117. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Ad. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, Y.; Hu, X.; Peng, X.; Wei, H.; Peng, J.; Jiang, S. Activation of PPARγ2 by PPARγ1 through a functional PPRE in transdifferentiation of myoblasts to adipocytes induced by EPA. Cell Cycle 2015, 14, 1830–1841. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, J.; Choi, J. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, J.; Yoshida, Y.; Kominato, Y.; Auron, P.E. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 2011, 54, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Zahnow, C.A. CCAAT/enhancer-binding protein β: Its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev. Mol. Med. 2009, 11, e12. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2014, 290, 755–761. [Google Scholar] [CrossRef]
- Cha, H.C.; Oak, N.R.; Kang, S.; Tran, T.A.; Kobayashi, S.; Chiang, S.H.; Tenen, D.G.; MacDougald, O.A. Phosphorylation of CCAAT/Enhancer-binding Protein α Regulates GLUT4 Expression and Glucose Transport in Adipocytes. J. Biol. Chem. 2008, 283, 18002–18011. [Google Scholar] [CrossRef]
- Rosen, E.; Eguchi, J.; Xu, Z. Transcriptional targets in adipocyte biology. Expert Opin. Ther. Targets 2009, 13, 975–986. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Shen, S.-H.; Singh, S.P.; Raffaele, M.; Waldman, M.; Hochhauser, E.; Ospino, J.; Arad, M.; Peterson, S.J. Adipocyte-Specific Expression of PGC1α Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion. Antioxidants 2022, 11, 1147. [Google Scholar] [CrossRef]
- Chi, J.; Cohen, P. The Multifaceted Roles of PRDM16: Adipose Biology and Beyond. Trends Endocrinol. Metab. 2016, 27, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yang, M.; Han, Y.; Zhao, H.; Sun, L. PRDM16 Regulating Adipocyte Transformation and Thermogenesis: A Promising Therapeutic Target for Obesity and Diabetes. Front. Pharmacol. 2022, 13, 870250. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Fang, Z.; Du, R.; Edwards, A.; Flemington, E.K.; Zhang, K. The Sequence Structures of Human MicroRNA Molecules and Their Implications. PLoS ONE 2013, 8, e54215. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Q.; Yu, J.; Rao, Y.; Kou, Z.; Fang, G.; Shi, X.; Liu, W.; Han, H. A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front. Genet. 2020, 11, 278. [Google Scholar] [CrossRef]
- van Rooij, E. The Art of MicroRNA Research. Circ. Res. 2011, 108, 219–234. [Google Scholar] [CrossRef]
- Zaragosi, L.-E.; Wdziekonski, B.; Brigand, K.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011, 12, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 Regulates Runx2 Protein Expression and Mesenchymal Progenitor Cell Differentiation. Stem Cells 2009, 28, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Hamam, D.; Ali, D.; Vishnubalaji, R.; Hamam, R.; Al-Nbaheen, M.; Chen, L.; Kassem, M.; Aldahmash, A.; Alajez, N.M. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Dis. 2014, 5, e1499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Wang, S.; Wei, J.; Fan, J.; Li, J.; Han, Q.; Liao, L.; Shao, C.; Zhao, R.C. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013, 10, 313–324. [Google Scholar] [CrossRef]
- Jeong, B.-C.; Kang, I.-H.; Hwang, Y.-C.; Kim, S.-H.; Koh, J.-T. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014, 5, e1532. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W.; He, M.; Wang, H.; Wang, W.; Yu, S.; Bian, X.-W.; Zhou, J.; Lin, M.C.M.; Lu, G.; et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.C.; Wang, J.; Kong, J.; Qi, Y.; Quigg, R.J.; Li, X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc. Natl. Acad. Sci. USA 2008, 105, 2889–2894. [Google Scholar] [CrossRef]
- Chen, L.; Cui, J.; Hou, J.; Long, J.; Li, C.; Liu, L. A Novel Negative Regulator of Adipogenesis: MicroRNA-363. Stem Cells 2014, 32, 510–520. [Google Scholar] [CrossRef]
- Cioffi, M.; Vallespinos-Serrano, M.; Trabulo, S.M.; Fernandez-Marcos, P.J.; Firment, A.N.; Vazquez, B.N.; Vieira, C.R.; Mulero, F.; Camara, J.A.; Cronin, U.P.; et al. MiR-93 Controls Adiposity via Inhibition of Sirt7 and Tbx3. Cell Rep. 2015, 12, 1594–1605. [Google Scholar] [CrossRef]
- Wei, J.; Li, H.; Wang, S.; Li, T.; Fan, J.; Liang, X.; Li, J.; Han, Q.; Zhu, L.; Fan, L.; et al. let-7 Enhances Osteogenesis and Bone Formation While Repressing Adipogenesis of Human Stromal/Mesenchymal Stem Cells by Regulating HMGA2. Stem Cells Dev. 2014, 23, 1452–1463. [Google Scholar] [CrossRef]
- Price, N.L.; Holtrup, B.; Kwei, S.L.; Wabitsch, M.; Rodeheffer, M.; Bianchini, L.; Suárez, Y.; Fernández-Hernando, C. SREBP-1c/MicroRNA 33b Genomic Loci Control Adipocyte Differentiation. Mol. Cell Biol. 2016, 36, 1180–1193. [Google Scholar] [CrossRef]
- Chen, L.; Dai, Y.; Ji, C.; Yang, L.; Shi, C.; Xu, G.; Pang, L.; Huang, F.; Zhang, C.; Guo, X. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell Endocrinol. 2014, 393, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, X.; Jia, Y.; Li, R.; Zhao, R. Microvesicle-Shuttled miR-130b Reduces Fat Deposition in Recipient Primary Cultured Porcine Adipocytes by Inhibiting PPAR-γ Expression. J. Cell Physiol. 2014, 229, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, X.; Jia, Y.; Li, Y.; Chen, R.; Wang, M.; Cai, D.; Zhao, R. Intravenous injection of microvesicle-delivery miR-130b alleviates high-fat diet-induced obesity in C57BL/6 mice through translational repression of PPAR-γ. J. Biomed. Sci. 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Bian, C.; Zhou, H.; Huang, S.; Wang, S.; Liao, L.; Zhao, R.C. MicroRNA hsa-miR-138 Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells Through Adenovirus EID-1. Stem Cells Dev. 2011, 20, 259–267. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. miR-21 Regulates Adipogenic Differentiation Through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Yi, C.; Xie, W.; Li, F.; Lv, Q.; He, J.; Wu, J.; Gu, D.; Xu, N.; Zhang, Y. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011, 585, 3303–3309. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, M.; Tong, M.; Yang, L.; Pang, L.; Chen, L.; Xu, G.; Chi, X.; Hong, Q.; Ni, Y.; et al. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci. Rep. 2015, 5, 9930. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Y.; Niu, Y.; Chen, W.; Wang, Q.; Xiao, S.; Li, A.; Xie, Y.; Li, J.; Zhao, X.; et al. A deep investigation into the adipogenesis mechanism: Profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/β-catenin signaling pathway. BMC Genom. 2010, 11, 320. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Chen, L.; Chen, Y.; Wu, M.; Zhang, Y.; Yu, K.; Huang, Z.; Qin, L.; Mo, D. MicroRNA-344 inhibits 3T3-L1 cell differentiation via targeting GSK3β of Wnt/β-catenin signaling pathway. FEBS Lett. 2014, 588, 429–435. [Google Scholar] [CrossRef]
- Chen, H.; Mo, D.; Li, M.; Zhang, Y.; Chen, L.; Zhang, X.; Li, M.; Zhou, X.; Chen, Y. miR-709 inhibits 3T3-L1 cell differentiation by targeting GSK3β of Wnt/β-catenin signaling. Cell Signal. 2014, 26, 2583–2589. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Jeon, T.I.; Ha, T.Y. Micro RNA -146b promotes adipogenesis by suppressing the SIRT 1- FOXO 1 cascade. EMBO Mol. Med. 2013, 5, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.-Y.; Wen, G.-B.; Feng, S.-D.; Tuo, Q.-H.; Ou, H.-S.; Yao, C.H.; Zhu, B.-Y.; Gao, Z.-P.; Zhang, L.; Liao, D.-F. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin. Exp. Pharmacol. Physiol. 2011, 38, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Guan, L.; Qi, Q.; Shu, G.; Jiang, Q.; Yuan, L.; Xi, Q.; Zhang, Y. MiRNA-181a Regulates Adipogenesis by Targeting Tumor Necrosis Factor-α (TNF-α) in the Porcine Model. PLoS ONE 2013, 8, e71568. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.C.; Jensen, C.H.; Schneider, M.; Nossent, A.Y.; Eskildsen, T.; Hansen, J.L.; Teisner, B.; Sheikh, S.P. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes. Exp. Cell Res. 2010, 316, 1681–1691. [Google Scholar] [CrossRef]
- Skårn, M.; Namløs, H.M.; Noordhuis, P.; Wang, M.-Y.; Meza-Zepeda, L.A.; Myklebost, O. Adipocyte Differentiation of Human Bone Marrow-Derived Stromal Cells Is Modulated by MicroRNA-155, MicroRNA-221, and MicroRNA-222. Stem Cells Dev. 2012, 21, 873–883. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Wu, J. TNFα-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem. Biophys. Res. Commun. 2011, 414, 618–624. [Google Scholar] [CrossRef]
- Huang, S.; Wang, S.; Bian, C.; Yang, Z.; Zhou, H.; Zeng, Y.; Li, H.; Han, Q.; Zhao, R.C. Upregulation of miR-22 Promotes Osteogenic Differentiation and Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells by Repressing HDAC6 Protein Expression. Stem Cells Dev. 2012, 21, 2531–2540. [Google Scholar] [CrossRef]
- Ji, H.-L.; Song, C.-C.; Li, Y.-F.; He, J.-J.; Li, Y.-L.; Zheng, X.-L.; Yang, G.-S. miR-125a inhibits porcine preadipocytes differentiation by targeting ERRα. Mol. Cell Biochem. 2014, 395, 155–165. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nakajima, I.; Chikuni, K.; Kojima, M.; Awata, T.; Mikawa, S. MicroRNA-33b downregulates the differentiation and development of porcine preadipocytes. Mol. Biol. Rep. 2014, 41, 1081–1090. [Google Scholar] [CrossRef]
- He, H.; Cai, M.; Zhu, J.; Xiao, W.; Liu, B.; Shi, Y.; Yang, X.; Liang, X.; Zheng, T.; Hu, S.; et al. miR-148a-3p promotes rabbit preadipocyte differentiation by targeting PTEN. In Vitro Cell Dev. Biol. Anim. 2018, 54, 241–249. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Zhu, J.; Huang, K.; Wang, Y. MiR-26b-5p regulates the preadipocyte differentiation by targeting FGF21 in goats. In Vitro Cell Dev. Biol. Anim. 2021, 57, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Yan, R.; Xu, X.; Gao, L.; Mei, J.; Liu, J.; Wang, X.; Zhang, J.; Wu, P.; et al. miR-377-3p regulates adipogenic differentiation of human bone marrow mesenchymal stem cells by regulating LIFR. Mol. Cell Biochem. 2018, 449, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ye, Y.; Chen, Z.; Xiao, T.; Liu, W.; Hu, F. MicroRNA 182 is a Novel Negative Regulator of Adipogenesis by Targeting CCAAT/Enhancer-Binding Protein α. Obesity 2020, 28, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, A.Y.; Lee, H.W.; Son, Y.H.; Lee, G.Y.; Lee, J.-Y.; Lee, Y.S.; Kim, J.B. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARγ expression. Biochem. Biophys. Res. Commun. 2010, 392, 323–328. [Google Scholar] [CrossRef]
- Kang, M.; Yan, L.-M.; Zhang, W.-Y.; Li, Y.-M.; Tang, A.-Z.; Ou, H.-S. Role of microRNA-21 in regulating 3T3-L1 adipocyte differentiation and adiponectin expression. Mol. Biol. Rep. 2013, 40, 5027–5034. [Google Scholar] [CrossRef]
- Song, G.; Xu, G.; Ji, C.; Shi, C.; Shen, Y.; Chen, L.; Zhu, L.; Yang, L.; Zhao, Y.; Guo, X. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene 2013, 533, 481–487. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Wang, X.-B.; Zhao, H.; Zhou, C.-N. MicroRNA-9-5p promotes osteoporosis development through inhibiting osteogenesis and promoting adipogenesis via targeting Wnt3a. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 456–463. [Google Scholar]
- Zhang, X.; Chang, A.; Li, Y.; Gao, Y.; Wang, H.; Ma, Z.; Li, X.; Wang, B. miR-140-5p regulates adipocyte differentiation by targeting transforming growth factor-β signaling. Sci. Rep. 2015, 5, 18118. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chu, X.; Wang, M.; Xin, Y.; Liu, S. MiR-146a-5p, targeting ErbB4, promotes 3T3-L1 preadipocyte differentiation through the ERK1/2/PPAR-γ signaling pathway. Lipids Health Dis. 2022, 21, 54. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, F.; Wang, G.; Wang, J.; Zheng, F.; Guan, X.; Chang, A.; Zhang, X.; Dai, C.; Li, S.; et al. miR-20a regulates adipocyte differentiation by targeting lysine-specific demethylase 6b and transforming growth factor-β signaling. Int. J. Obes. 2015, 39, 1282–1291. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Zhang, Z.; Liu, G.; Sun, S.; Sun, C. miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biol. Chem. 2015, 396, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, J.; Ye, L.; Chen, Y.; Cui, J.; Tian, W.; Li, C.; Liu, L. MicroRNA-143 Regulates Adipogenesis by Modulating the MAP2K5–ERK5 Signaling. Sci. Rep. 2014, 4, 3819. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, M.A.; Haridas, P.A.N.; Mysore, R.; Wabitsch, M.; Fischer-Posovszky, P.; Olkkonen, V.M. miR-107 inhibits CDK6 expression, differentiation, and lipid storage in human adipocytes. Mol. Cell Endocrinol. 2019, 479, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Deng, Y.; Hu, X.; Ren, H.; Zhu, J.; Fu, S.; Xie, J.; Peng, Y. miR-128-3p regulates 3T3-L1 adipogenesis and lipolysis by targeting Pparg and Sertad2. J. Physiol. Biochem. 2018, 74, 381–393. [Google Scholar] [CrossRef]
- Walden, T.B.; Timmons, J.A.; Keller, P.; Nedergaard, J.; Cannon, B. Distinct expression of muscle-specific MicroRNAs (myomirs) in brown adipocytes. J. Cell Physiol. 2009, 218, 444–449. [Google Scholar] [CrossRef]
- Arias, N.; Aguirre, L.; Fernández-Quintela, A.; González, M.; Lasa, A.; Miranda, J.; Macarulla, M.T.; Portillo, M.P. MicroRNAs involved in the browning process of adipocytes. J. Physiol. Biochem. 2015, 72, 509–521. [Google Scholar] [CrossRef]
- Karbiener, M.; Pisani, D.F.; Frontini, A.; Oberreiter, L.M.; Lang, E.; Vegiopoulos, A.; Mössenböck, K.; Bernhardt, G.A.; Mayr, T.; Hildner, F.; et al. MicroRNA-26 Family Is Required for Human Adipogenesis and Drives Characteristics of Brown Adipocytes. Stem Cells 2014, 32, 1578–1590. [Google Scholar] [CrossRef]
- Døssing, K.B.V.; Binderup, T.; Kaczkowski, B.; Jacobsen, A.; Rossing, M.; Winther, O.; Federspiel, B.; Knigge, U.; Kjær, A.; Friis-Hansen, L. Down-Regulation of miR-129-5p and the let-7 Family in Neuroendocrine Tumors and Metastases Leads to Up-Regulation of Their Targets Egr1, G3bp1, Hmga2 and Bach1. Genes 2014, 6, 1–21. [Google Scholar] [CrossRef]
- Fu, X.; Jin, L.; Han, L.; Yuan, Y.; Mu, Q.; Wang, H.; Yang, J.; Ning, G.; Zhou, D.; Zhang, Z. miR-129-5p Inhibits Adipogenesis through Autophagy and May Be a Potential Biomarker for Obesity. Int. J. Endocrinol. 2019, 2019, 5069578. [Google Scholar] [CrossRef]
- Oliverio, M.; Schmidt, E.; Mauer, J.; Baitzel, C.; Hansmeier, N.; Khani, S.; Konieczka, S.; Pradas-Juni, M.; Brodesser, S.; Van, T.-M.; et al. Dicer1–miR-328–Bace1 signalling controls brown adipose tissue differentiation and function. Nat. Cell Biol. 2016, 18, 328–336. [Google Scholar] [CrossRef]
- Pan, D.; Mao, C.; Quattrochi, B.; Friedline, R.H.; Zhu, L.J.; Jung, D.Y.; Kim, J.K.; Lewis, B.; Wang, Y.-X. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun. 2014, 5, 4725. [Google Scholar] [CrossRef] [PubMed]
- Kulyté, A.; Lorente-Cebrián, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Rydén, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, M.; Townsend, K.L.; Huang, T.; An, D.; Lin, Y.; Xue, R.; Schulz, T.J.; Winnay, J.N.; Mori, M.A.; et al. Micro RNA -455 regulates brown adipogenesis via a novel HIF 1an-AMPK-PGC 1α signaling network. EMBO Rep. 2015, 16, 1378–1393. [Google Scholar] [CrossRef]
- Sun, L.; Trajkovski, M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism 2014, 63, 272–282. [Google Scholar] [CrossRef]
- Ding, G.; Yu, J.; Bi, J.; Qi, H.; Di, W.; Wu, L.; Wang, L.; Juanmin, Z.; Shan, L.; Zhang, F.; et al. Glucocorticoids Transcriptionally Regulate miR-27b Expression Promoting Body Fat Accumulation Via Suppressing the Browning of White Adipose Tissue. Diabetes 2015, 64, 393–404. [Google Scholar]
- Fu, T.; Seok, S.; Choi, S.; Huang, Z.; Suino-Powell, K.; Xu, H.E.; Kemper, B.; Kemper, J.K. MicroRNA 34a Inhibits Beige and Brown Fat Formation in Obesity in Part by Suppressing Adipocyte Fibroblast Growth Factor 21 Signaling and SIRT1 Function. Mol. Cell Biol. 2014, 34, 4130–4142. [Google Scholar] [CrossRef]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.-X.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a Regulates Adipocyte Browning In Vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012, 14, 1330–1335. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, H.; Alexander, R.; Patterson, H.C.; Gu, M.; Lo, K.A.; Xu, D.; Goh, V.J.; Nguyen, L.N.; Chai, X.; et al. MicroRNAs Are Required for the Feature Maintenance and Differentiation of Brown Adipocytes. Diabetes 2014, 63, 4045–4056. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Xiao, T.; Yin, B.; He, L.; Meng, W.; Dong, M.; Liu, F. miR-30 Promotes Thermogenesis and the Development of Beige Fat by Targeting RIP140. Diabetes 2015, 64, 2056–2068. [Google Scholar] [CrossRef]
- Sun, L.; Xie, H.; Mori, M.A.; Alexander, R.; Yuan, B.; Hattangadi, S.M.; Liu, Q.; Kahn, C.R.; Lodish, H.F. Mir193b–365 is essential for brown fat differentiation. Nat. Cell Biol. 2011, 13, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential Role for miR-196a in Brown Adipogenesis of White Fat Progenitor Cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Pasut, A.; Soleimani, V.D.; Bentzinger, C.F.; Antoun, G.; Thorn, S.; Seale, P.; Fernando, P.; van Ijcken, W.; Grosveld, F.; et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013, 17, 210–224. [Google Scholar] [CrossRef]
- Chen, Y.; Siegel, F.; Kipschull, S.; Haas, B.; Fröhlich, H.; Meister, G.; Pfeifer, A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 2013, 4, 1769–1813. [Google Scholar] [CrossRef]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Ge, X.; Sathiakumar, D.; Lua, B.J.G.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2016, 41, 137–148. [Google Scholar] [CrossRef]
- Huang, R.; Shi, C.; Liu, G. Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation. Open Life Sci. 2023, 18, 20220552. [Google Scholar] [CrossRef]
- Wei, N.; Wang, Y.; Xu, R.-X.; Wang, G.-Q.; Xiong, Y.; Yu, T.-Y.; Yang, G.-S.; Pang, W.-J. PU.1 antisense lncRNA against its mRNA translation promotes adipogenesis in porcine preadipocytes. Anim. Genet. 2015, 46, 133–140. [Google Scholar] [CrossRef]
- Liu, S.; Xu, R.; Gerin, I.; Cawthorn, W.P.; MacDougald, O.A.; Chen, X.-W.; Saltiel, A.R.; Koenig, R.J.; Xu, B. SRA Regulates Adipogenesis by Modulating p38/JNK Phosphorylation and Stimulating Insulin Receptor Gene Expression and Downstream Signaling. PLoS ONE 2014, 9, e95416. [Google Scholar] [CrossRef]
- Yi, X.; He, Z.; Tian, T.; Kou, Z.; Pang, W. LncIMF2 promotes adipogenesis in porcine intramuscular preadipocyte through sponging MiR-217. Anim. Biotechnol. 2023, 34, 268–279. [Google Scholar] [CrossRef]
- Huang, Y.; Jin, C.; Zheng, Y.; Li, W.; Zhang, S.; Zhang, Y.; Jia, L.; Li, W. Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose-derived stem cells via histone modification of FABP4. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Zhang, P.; Wang, Y.; Xu, Y.; Zhang, Z.; Ma, W.; Xu, B.; Xia, Q.; Du, Q. Long non-coding RNA slincRAD functions in methylation regulation during the early stage of mouse adipogenesis. RNA Biol. 2019, 16, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Liu, L.; Li, H.; Sun, Y.; Luo, H.; Li, T.; Wang, S.; Dalton, S.; Zhao, R.C.; Wu, D. Long Noncoding RNA ADINR Regulates Adipogenesis by Transcriptionally Activating C/EBPα. Stem Cell Rep. 2015, 5, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Sun, Y.; Qimuge, N.; Wang, G.; Wang, Y.; Chu, G.; Yu, T.; Yang, G.; Pang, W. Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating Adiponectin mRNA translation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 420–432. [Google Scholar] [CrossRef]
- Shen, L.; Han, J.; Wang, H.; Meng, Q.; Chen, L.; Liu, Y.; Feng, Y.; Wu, G. Cachexia-related long noncoding RNA, CAAlnc1, suppresses adipogenesis by blocking the binding of HuR to adipogenic transcription factor mRNAs. Int. J. Cancer 2019, 145, 1809–1821. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Cai, H.; Sun, Y.; Plath, M.; Li, C.; Lan, X.; Lei, C.; Lin, F.; Bai, Y.; et al. Long non-coding RNA ADNCR suppresses adipogenic differentiation by targeting miR-204. Biochim. Biophys. Acta 2016, 1859, 871–882. [Google Scholar] [CrossRef]
- Li, C.; Xiao, Y.; Yang, M.; Su, T.; Sun, X.; Guo, Q.; Huang, Y.; Luo, X. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J. Clin. Investig. 2018, 128, 5251–5266. [Google Scholar] [CrossRef]
- Wu, C.; Fang, S.; Zhang, H.; Li, X.; Du, Y.; Zhang, Y.; Lin, X.; Wang, L.; Ma, X.; Xue, Y.; et al. Long noncoding RNA XIST regulates brown preadipocytes differentiation and combats high-fat diet induced obesity by targeting C/EBPα. Mol. Med. 2022, 28, 6. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, J.R.; Bai, Z.; Xu, D.; Yuan, B.; Lo, K.A.; Yoon, M.J.; Lim, Y.C.; Knoll, M.; Slavov, N.; Chen, S.; et al. De Novo Reconstruction of Adipose Tissue Transcriptomes Reveals Long Non-coding RNA Regulators of Brown Adipocyte Development. Cell Metab. 2015, 21, 764–776. [Google Scholar] [CrossRef]
- Bai, Z.; Chai, X.; Yoon, M.J.; Kim, H.-J.; LO, K.A.; Zhang, Z.; Xu, D.; Siang, D.T.C.; Walet, A.C.E.; Xu, S.; et al. Dynamic transcriptome changes during adipose tissue energy expenditure reveal critical roles for long noncoding RNA regulators. PLoS Biol. 2017, 15, e2002176. [Google Scholar] [CrossRef]
- Mi, L.; Zhao, X.-Y.; Li, S.; Yang, G.; Lin, J.D. Conserved function of the long noncoding RNA Blnc1 in brown adipocyte differentiation. Mol. Metab. 2017, 6, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yue, F.; Jia, Z.; Gao, Y.; Jin, W.; Hu, K.; Zhang, Y.; Zhu, D.; Yang, G.; Kuang, S. A novel brown adipocyte-enriched long non-coding RNA that is required for brown adipocyte differentiation and sufficient to drive thermogenic gene program in white adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; You, L.; Li, Y.; Zhu, L.; Zhang, F.; Xie, K.; Cao, Y.; Ji, C.; Guo, X. A transcribed ultraconserved noncoding RNA, uc.417, serves as a negative regulator of brown adipose tissue thermogenesis. FASEB J. 2016, 30, 4301–4312. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Ponjavic, J.; Ponting, C.P.; Lunter, G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007, 17, 556–565. [Google Scholar] [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2016, 541, 228–232. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Y.; Cheng, P.; Wu, C. Long non-coding RNAs with peptide-encoding potential identified in esophageal squamous cell carcinoma: KDM4A-AS1-encoded peptide weakens cancer cell viability and migratory capacity. Mol Oncol. 2023, 17, 1419–1436. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, Y.; Sun, M.; Huang, X.; Zhang, H.; Fu, Z.; Wang, J.; Zhang, S.; Lian, C.; Tang, B.; et al. LncRNA DGCR5-encoded polypeptide RIP aggravates SONFH by repressing nuclear localization of β-catenin in BMSCs. Cell Rep. 2023, 42, 112969. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: LncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Borkiewicz, L.; Kalafut, J.; Dudziak, K.; Przybyszewska-Podstawka, A.; Telejko, I. Decoding LncRNAs. Cancers 2021, 13, 2643. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, X.; Su, D.; Jiang, T.; Zhan, S.; Zhong, T.; Guo, J.; Cao, J.; Li, L.; Zhang, H.; et al. A Novel LncRNA MSTRG.310246.1 Promotes Differentiation and Thermogenesis in Goat Brown Adipocytes. Genes 2023, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yang, Q.Y.; Hu, Y.; Liu, X.D.; de Avila, J.M.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance. Nat. Commun. 2021, 12, 6845. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Gu, M.; Peng, Y. lncRNA TUG1 promotes the brown remodeling of white adipose tissue by regulating miR 204 targeted SIRT1 in diabetic mice. Int. J. Mol. Med. 2020, 46, 2225–2234. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Sun, J.-Y.; Liu, Y.; Liu, Z.-Y.; Huang, Y.; Xiao, Y.; Su, T. lncRNA Hnscr Regulates Lipid Metabolism by Mediating Adipocyte Lipolysis. Endocrinology 2023, 164, bqad147. [Google Scholar] [CrossRef]
- Cooper, D.; Carter, G.; Li, P.; Patel, R.; Watson, J.; Patel, N. Long Non-Coding RNA NEAT1 Associates with SRp40 to Temporally Regulate PPARγ2 Splicing during Adipogenesis in 3T3-L1 Cells. Genes 2014, 5, 1050–1063. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.; Fan, L.; Deng, L.; Fan, J.; Li, D.; Li, H.; Zhao, R.C. Lnc13728 facilitates human mesenchymal stem cell adipogenic differentiation via positive regulation of ZBED3 and downregulation of the WNT/β-catenin pathway. Stem Cell Res. Ther. 2021, 12, 176. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Zhang, X.; Chen, J.; Li, M.; Liu, L. The novel long noncoding RNA lncRNA-Adi regulates adipogenesis. Stem Cells Transl. Med. 2020, 9, 1053–1067. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; He, X.; Zhang, S.; Wang, K.; Wu, H.; Chen, L. LncRNA TINCR/miR-31-5p/C/EBP-α feedback loop modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells. Stem Cell Res. 2018, 32, 35–42. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Gao, W.; Han, J.; Yuan, R.; Zhang, M.; Ge, Z. LncRNA HCG11 Inhibits Adipocyte Differentiation in Human Adipose-Derived Mesenchymal Stem Cells by Sponging miR-204-5p to Upregulate SIRT1. Cell Transplant. 2020, 29, 0963689720968090. [Google Scholar] [CrossRef]
- Hong, P.; Wang, D.; Wu, Y.; Zhang, Q.; Liu, P.; Pan, J.; Yu, M.; Tian, W. A novel long noncoding RNA AK029592 contributes to thermogenic adipocyte differentiation. Stem Cells Transl. Med. 2024, 13, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, L.; Gu, H.; Liang, X.; Meng, X.; Gao, J.; Xu, Y.; Nuermaimaiti, N.; Guan, Y. Ad36 promotes differentiation of hADSCs into brown adipocytes by up-regulating LncRNA ROR. Life Sci. 2020, 265, 118762. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Jaijyan, D.K.; Yang, S.; Zeng, M.; Pei, S.; Zhu, H. Functions of Circular RNA in Human Diseases and Illnesses. NonCoding RNA 2023, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Tan, C.; Liu, X. Circular RNAs: A new frontier in the study of human diseases. J. Med. Genet. 2016, 53, 359–365. [Google Scholar] [CrossRef]
- Zhou, M.; Li, S.; Huang, C. Physiological and pathological functions of circular RNAs in the nervous system. Neural Regen. Res. 2023, 19, 342–349. [Google Scholar] [CrossRef]
- Hoque, P.; Romero, B.; Akins, R.E.; Batish, M. Exploring the Multifaceted Biologically Relevant Roles of circRNAs: From Regulation, Translation to Biomarkers. Cells 2023, 12, 2813. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Shen, X.; Tang, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. CircRNF111 Contributes to Adipocyte Differentiation by Elevating PPARγ Expression via miR-27a-3p. Epigenetics 2022, 18, 2145058. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Y.; Mao, R.; Yang, H.; Zhang, Y.; Zhang, Y.; Guo, P.; Zhan, D.; Zhang, T. Circular RNA SAMD4A controls adipogenesis in obesity through the miR-138-5p/EZH2 axis. Theranostics 2020, 10, 4705–4719. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; Wu, J.; Zhang, L.; Lee, S.; Shin, D.-J.; Tran, M.; Wang, L. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology 2018, 67, 1768–1783. [Google Scholar] [CrossRef]
- Zhu, Y.; Gui, W.; Lin, X.; Li, H. Knock-down of circular RNA H19 induces human adipose-derived stem cells adipogenic differentiation via a mechanism involving the polypyrimidine tract-binding protein 1. Exp. Cell Res. 2019, 387, 111753. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Li, R.; Gong, T.; Li, H.; Zhao, X.; Cao, G.; Li, M.; Li, B.; Yang, Y.; Guo, X. CircMEF2C(2, 3) modulates proliferation and adipogenesis of porcine intramuscular preadipocytes by miR-383/671-3p/MEF2C axis. iScience 2024, 27, 109710. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Ji, M.; An, J.; Zhao, T.; Yang, Y.; Cai, C.; Gao, P.; Cao, G.; Guo, X.; et al. CircHOMER1 inhibits porcine adipogenesis via the miR-23b/SIRT1 axis. FASEB J. 2023, 37, e22828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Jiang, T.; Zhan, S.; Zhong, T.; Guo, J.; Cao, J.; Li, L.; Zhang, H.; Wang, L. Circular RNA circZEB1 regulates goat brown adipocytes differentiation and thermogenesis through miR-326–3p. Small Rumin. Res. 2023, 218, 106884. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Xia, T.; Liu, C.; Sun, C. circNrxn2 Promoted WAT Browning via Sponging miR-103 to Relieve Its Inhibition of FGF10 in HFD Mice. Mol. Ther. Nucleic Acids 2019, 17, 551–562. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Q.; Li, Z.; Yang, Q.; Liu, Y.; Du, Z.; Zhang, G.; Song, Y. Circular RNA CDR1as promotes adipogenic and suppresses osteogenic differentiation of BMSCs in steroid-induced osteonecrosis of the femoral head. Bone 2020, 133, 115258. [Google Scholar] [CrossRef]
- Song, X.-H.; He, N.; Xing, Y.-T.; Jin, X.-Q.; Li, Y.-W.; Liu, S.-S.; Gao, Z.-Y.; Guo, C.; Wang, J.-J.; Huang, Y.-Y.; et al. A Novel Age-Related Circular RNA Circ-ATXN2 Inhibits Proliferation, Promotes Cell Death and Adipogenesis in Rat Adipose Tissue-Derived Stromal Cells. Front. Genet. 2021, 12, 761926. [Google Scholar] [CrossRef]
- Jiang, R.; Li, H.; Yang, J.; Shen, X.; Song, C.; Yang, Z.; Wang, X.; Huang, Y.-Z.; Lan, X.; Lei, C.; et al. circRNA Profiling Reveals an Abundant circFUT10 that Promotes Adipocyte Proliferation and Inhibits Adipocyte Differentiation via Sponging let-7. Mol. Ther. Nucleic Acids 2020, 20, 491–501. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, H.; Zhao, D.; Liang, Y.; Qiao, L.; Liu, J.; Pan, Y.; Yang, K.; Liu, W. circINSR Inhibits Adipogenic Differentiation of Adipose-Derived Stromal Vascular Fractions through the miR-152/MEOX2 Axis in Sheep. Int. J. Mol. Sci. 2023, 24, 3501. [Google Scholar] [CrossRef]
- Zhang, P.; Sheng, M.-X.; Du, C.; Chao, Z.; Xu, H.; Cheng, X.; Li, C.; Xu, Y. Assessment of CircRNA Expression Profiles and Potential Functions in Brown Adipogenesis. Front. Genet. 2021, 12, 769690. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Deng, Y.; Li, Z.; Tang, A. CircRNA-mediated regulation of brown adipose tissue adipogenesis. Front. Nutr. 2022, 9, 926024. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sainz, E.; Milagro, F.I.; Aranaz, P.; Riezu-Boj, J.I.; Batrow, P.-L.; Contu, L.; Gautier, N.; Amri, E.-Z.; Mothe-Satney, I.; Lorente-Cebrián, S. Human miR-1 Stimulates Metabolic and Thermogenic-Related Genes in Adipocytes. Int. J. Mol. Sci. 2024, 26, 276. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Mandal, M.; Rakib, A.; Bajwa, A.; Singh, U.P. miR-10a-3p modulates adiposity and suppresses adipose inflammation through TGF-β1/Smad3 signaling pathway. Front. Immunol. 2023, 14, 1213415. [Google Scholar] [CrossRef]
- Meuth, V.M.-L.; Metzinger, L. The Roles of MicroRNAs in Obesity: Emphasizing Links with Chronic Kidney Disease and Cardiovascular Disorders. Obesities 2023, 3, 243–252. [Google Scholar] [CrossRef]
- Kim, M.; Zhang, X. The Profiling and Role of miRNAs in Diabetes Mellitus. J. Diabetes Clin. Res. 2019, 1, 5–23. [Google Scholar]
- Palihaderu, P.; Mendis, B.; Premarathne, J.; Dias, W.; Yeap, S.K.; Ho, W.Y.; Dissanayake, A.; Rajapakse, I.; Karunanayake, P.; Senarath, U.; et al. Therapeutic Potential of miRNAs for Type 2 Diabetes Mellitus: An Overview. Epigenet. Insights 2022, 15, 25168657221130041. [Google Scholar] [CrossRef]
- Grueter, C.E.; Van Rooij, E.; Johnson, B.A.; DeLeon, S.M.; Sutherland, L.B.; Qi, X.; Gautron, L.; Elmquist, J.K. A Cardiac MicroRNA Governs Systemic Energy Homeostasis by Regulation of MED13. Cell 2012, 149, 671–683. [Google Scholar] [CrossRef]
- Lhamyani, S.; Gentile, A.-M.; Giráldez-Pérez, R.M.; Feijóo-Cuaresma, M.; Romero-Zerbo, S.Y.; Clemente-Postigo, M.; Zayed, H.; Oliva-Olivera, W.; Bermúdez-Silva, F.J.; Salas, J.; et al. miR-21 mimic blocks obesity in mice: A novel therapeutic option. Mol. Ther. Nucleic Acids 2021, 26, 401–416. [Google Scholar] [CrossRef]
- Frost, R.J.A.; Olson, E.N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef]
- Li, S.; Qian, T.; Wang, X.; Liu, J.; Gu, X. Noncoding RNAs and Their Potential Therapeutic Applications in Tissue Engineering. Engineering 2017, 3, 3–15. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Huang, Q.; Yang, J.; Li, B.; Ma, K.; Wei, Q.; Wang, Y.; Su, J.; Sun, M.; et al. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct. Target Ther. 2023, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, Y.; Xue, H.; Liu, G.; Wang, N.; Shao, Z. MiR-21 regulating PVT1/PTEN/IL-17 axis towards the treatment of infectious diabetic wound healing by modified GO-derived biomaterial in mouse models. J. Nanobiotechnol. 2020, 20, 309. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, K.; Lin, H.; Tao, E.; Xia, W.; Wang, F.; Mao, C.; Feng, Y. Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front. Bioeng Biotechnol. 2023, 11, 1129538. [Google Scholar] [CrossRef] [PubMed]

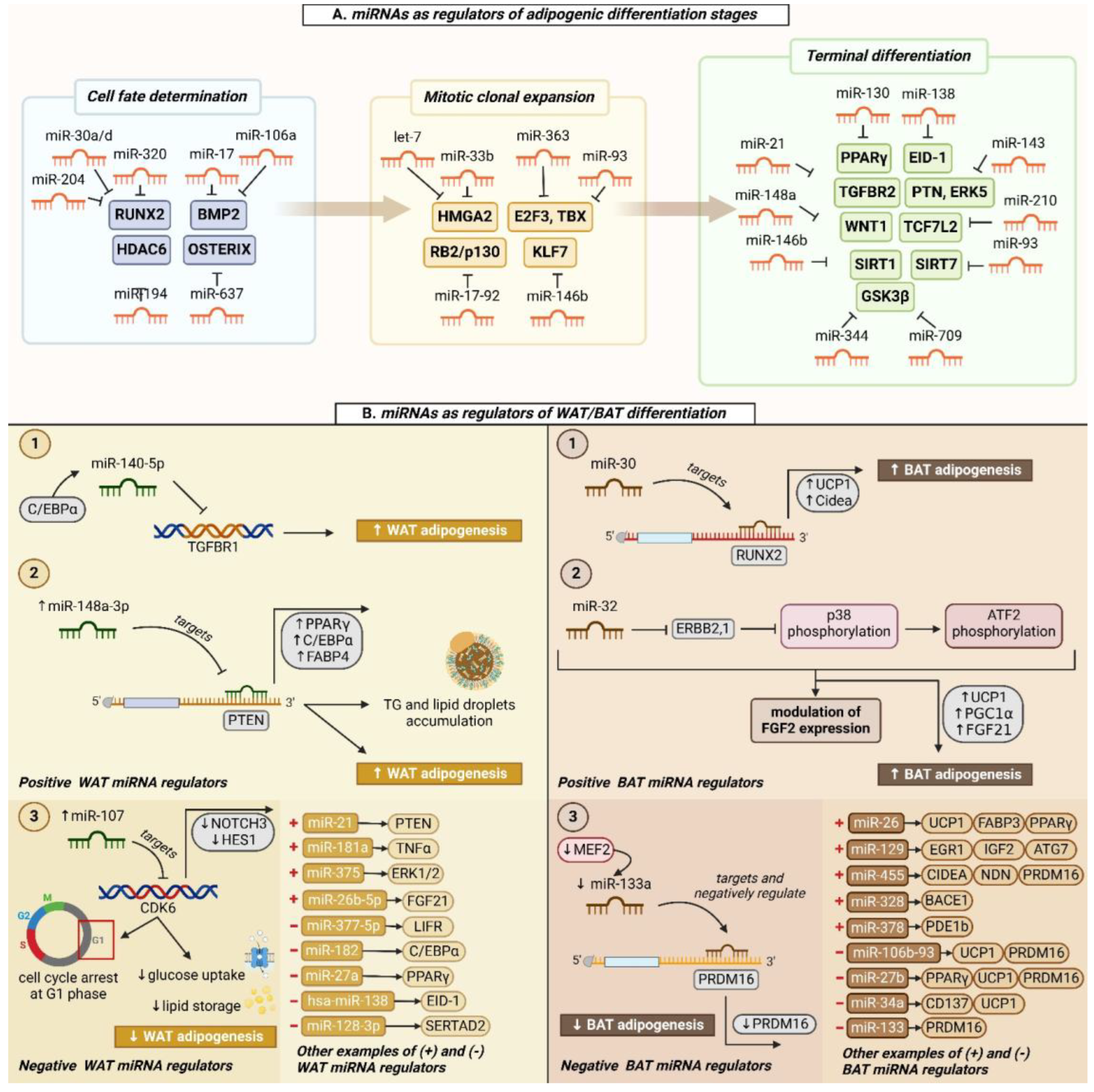

| Differentiation Stage | Key miRNA Regulators | Target Gene(s) | Outcome | Reference(s) |
|---|---|---|---|---|
| Cell fate determination | miR-30a/d, miR-204, miR-320 | RUNX2 | promote adipogenesis | [62,63,64] |
| miR-17, miR-106a | BMP2 | promote adipogenesis | [65] | |
| miR-194 | HDAC6 | inhibits adipogenesis | [66] | |
| miR-637 | Osterix | promotes adipogenesis | [67] | |
| Clonal expansion | miR-17-92 cluster | RB2/p130 | promotes adipogenesis | [68] |
| miR-363, miR-93 | E2F3, TBX3 | inhibit adipogenesis | [69,70] | |
| let-7 | HMGA2 | inhibits adipogenesis | [71] | |
| miR-33b | HMGA2 | inhibits adipogenesis | [72] | |
| miR-146b | KLF7 | promotes adipogenesis | [73] | |
| Terminal differentiation | miR-130 | PPARγ | inhibits adipogenesis | [74,75] |
| hsa-miR-138 | EID-1 | inhibits adipogenesis | [76] | |
| miR-21 | TGFBR2 | promotes adipogenesis | [77] | |
| miR-143 | PTN, ERK5 | promotes adipogenesis | [78] | |
| miR-148a | WNT1 | promotes adipogenesis | [78,79] | |
| miR-210 | TCF712 | promotes adipogenesis | [80] | |
| miR-344, miR-709 | GSK3β | inhibit adipogenesis | [81,82] | |
| miR-146b | SIRT1 | promotes adipogenesis | [83] | |
| miR-93 | SIRT7 | inhibits adipogenesis | [70] |
| miRNA | Function(s) | Target Gene(s) | Reference(s) | |
|---|---|---|---|---|
| Positive regulators of brown adipogenesis | miR-26 | impairs the browning process upon its repression regulates the expression of metalloproteinase ADAM17 | UCP1, PPARγ, FABP3, ADRB1, PRDM16 | [109] |
| miR-129 | regulates thermogenesis and energy expenditure represents a potential obesity biomarker | IGF2, EGR1, ATG7 | [110,111] | |
| miR-328 | favors BAT differentiation and increases C/EBPβ, UCP1 levels, and oxygen consumption impairs muscle progenitor commitment by regulating the switch between myogenic and brown adipogenic lineages | BACE1 | [112] | |
| miR-378 | regulates cAMP turnover in BAT and enhances brown adipocyte differentiation stimulates lipolysis | PDE1b | [113,114] | |
| miR-455 | enhances thermogenic capacity in response to cold controls brown adipogenesis represents a potential therapeutic target for human metabolic disorders | HIF1an, Cidea, RUNX1t1, NDN PPARγ, C/EBPα, and C/EBPδ, UCP1, PRDM16 | [115] | |
| Negative regulators of brown adipogenesis | miR-27b | increases expression of specific BAT markers (such as UCP1, PRMD16, PGC1α) upon its knockdown reduces energy expenditure and increases fat accumulation | PPARδ, prohibitin, PRDM16, UCP1 | [116,117] |
| miR-34a | reduces adiposity, improves serum levels, and increases oxidative function upon its blockage | CD137, UCP1 | [118] | |
| miR-106b-93 cluster | plays a role in energy homeostasis increased expression in obesity | UCP1, PRDM16, Cidea, PPARα, PPARγ, PGC1α, FABP4, adiponectin | [70] | |
| miR-133 | inhibits BAT differentiation | PRDM16 | [119,120] |
| miRNA | Function(s) | Target Gene(s) | Reference(s) | |
|---|---|---|---|---|
| Positive regulators of white adipogenesis | miR-148a-3p | promotes WAT differentiation upregulates mRNA and protein levels of PPARγ, C/EBPα, and FABP4 enhances intracellular triglyceride content contains a functional CREB domain | PTEN, WNT1 | [79,92] |
| miR-181a | promotes adipocyte differentiation accelerates the accumulation of lipid droplets and the synthesis of triglycerides represses TNFα function (a cytokine involved in regulating lipogenesis) | TNFα | [85] | |
| miR-375 | enhances adipogenic differentiation increases mRNA levels of C/EBPα, PPARγ2 induces the accumulation of Ap2 and triglyceride suppresses ERK1/2 phosphorylation levels | ERK1/2 | [84] | |
| miR-26b-5p | promotes adipocyte differentiation increases levels of lipid deposition and adipogenic-related marker genes dowregulates significantly FGF21 mRNA expression | FGF21 | [93] | |
| Negative regulators of white adipogenesis | miR-377-3p | decreases adipogenic differentiation downregulates expression of key-adipogenic markers (such as PPARγ, C/EBPα, and AP2) reduces intracellular lipid droplet accumulation | LIFR | [94] |
| miR-182 | impairs WAT differentiation suppresses the synthesis of lipid droplets inhibits the expression of adipogenic-related markers (such as C/EBPβ, PPARγ, adiponectin, and SREBP1) represses glucocorticoid-induced expression of C/EBPα | C/EBPα | [95] | |
| miR-27a | inhibits adipocyte differentiation by reducing the expression of key adipogenic regulator PPARγ decreases lipid accumulation represses mRNA expression levels of AP2, LPL, CD36, and adiponectin | PPARγ | [96] | |
| hsa-miR-138 | inhibits adipogenic differentiation reduces lipid droplet synthesis and accumulation decreases the expression of C/EBPα, PPARγ, LPL, adiponectin, and FABP4 | EID-1 | [76] |
| lncRNA | Function(s) | Target miRNA/Gene(s) | Reference | |
|---|---|---|---|---|
| Positive regulators of white adipogenesis | lncRNA-Acart | regulates preadipocyte differentiation and proliferation decreases cellular apoptosis | PPARγ | [129] |
| PU.1 AS lncRNA | promotes adipogenesis through the formation of a sense-antisense RNA duplex with PU.1 mRNA | PPARγ, FASN | [130] | |
| lncRNA-SRA | enhances adipogenic differentiation increases glucose uptake and phosphorylation of AKT and FOXO1 in response to insulin stimulates IGF-1 signaling and inhibits phosphorylation of MAPK and JNK during early differentiation stages | AKT, FOXO1 | [131] | |
| lncRNA-lncIMF2 | promotes proliferation and adipogenic differentiation acts as a molecular sponge for miR-217 regulates the expression of specific adipogenic marker genes | PPARγ, ATGl | [132] | |
| lncRNA-MIR31HG | favors adipocyte lineage commitment in vitro and in vivo regulates the expression of active histone markers H3K4me3 and AcH3 in the promoter region of FABP4 suppresses WNT/β-catenin pathway | FABP4 | [133] | |
| lncRNA-slincRAD | promotes early adipogenesis by allowing the commitment of growth-arrested cells into the cell cycle through hormone induction guides epigenetic factors to mediate the methylation of cyclin-dependent kinase inhibitor p21 promoter | DNMT1 | [134] | |
| lncRNA-ADINR | promotes adipogenic differentiation by modulating transcription of C/EBPα in cis and recruiting MLL3/4 histone methyltransferase complex increases H3K4me3 and decreases H3K27me3 histone modification in the C/EBPα locus | PA1 | [135] | |
| Negative regulators of white adipogenesis | lncRNA-H19 | inhibits adipogenic commitment of cells through epigenetic modulation of histone deacetylases forms a complex with miR-675 | CTCF | [36] |
| adipoQ (adiponectin) AS lncRNA | inhibits white adipose tissue formation through its transfer from the nucleus to the cytoplasm forms a complex with adipoQ mRNA and suppresses its translation | adipoQ mRNA | [136] | |
| lncRNA-CAAlnc1 | impairs adipogenesis blocks the binding of HuR to adipogenic transcription factor mRNAs and decreases the expression of these factors | HuR | [137] | |
| lncRNA-ADNCR | inhibits adipocyte differentiation functions as a competing endogenous RNA (ceRNA) for miR-204 promotes SIRT1 upregulation (gene implicated in inhibiting adipogenic gene expression by targeting PPARγ activity) | miR-204 | [138] | |
| LncRNA-Bmncr | impairs white adipogenesis by serving as a scaffold to allow the interaction of TAZ and ABL facilitates TAZ-RUNX2/PPARγ transcriptional complex assembly | PPARγ | [139] | |
| Positive regulator of brown adipogenesis | lncRNA-XIST | regulates brown adipocyte differentiation controls metabolic disorders by preventing high-fat diet-induced obesity | C/EBPα | [140] |
| lncRNA-BATE1 | promotes brown adipogenesis by binding to the heterogeneous ribonucleoprotein U plays a significant function in thermogenesis regulates the expression of a set of genes related to brown adipogenesis and mitochondrial biogenesis and function | PPARγ, C/EBPα, C/EBPβ | [141] | |
| lncRNA-BATE10 | favors full brown fat differentiation and development plays a role in the browning of white fat downregulates respiratory electron transport at the genome level decreases significantly the expression of selective-BAT marker genes upon its knockdown competes with PGC1α mRNA for the binding of CELF1 during BAT differentiation | PPARγ, C/EBPα, FABP4, PGC1α | [142] | |
| lncRNA-Blnc1 | promotes brown adipogenesis by stimulating thermogenic gene expression acts by forming a ribonucleoprotein complex with hnRNPU and EBF2 | EBF2 | [143] | |
| lncRNA-AK079912 | promotes brown tissue adipogenesis and WAT browning upregulates the expression of genes implicated in thermogenesis regulates lipid accumulation, mitochondrial copy number, and levels of mitochondrial ETC | PPARγ | [144] | |
| Negative regulator of brown adipogenesis | lncRNA-uc.417 | impairs brown adipogenesis and attenuates the thermogenic program suppresses moderately p38MAPK signaling pathway decreases significantly the expression of BAT- and mitochondrial-related marker genes attenuates mitochondrial respiration rate | PPARγ2, C/EBPβ | [145] |
| circRNA | Function(s) | Target miRNA/Gene(s) | Reference | |
|---|---|---|---|---|
| Positive regulators of white adipogenesis | circRNA-CDR1as | regulates positively adipogenic differentiation acts via the miRNA-7-5p-/WNT5B pathway | miRNA-7-5p, WNT5b | [178] |
| circRNA-ATXN2 | promotes adipogenic differentiation increases the expression of PPARγ and C/EBPα favors lipid droplet synthesis and accumulation inhibits proliferation and promotes apoptosis | PPARγ, C/EBPα | [179] | |
| Negative regulators of white adipogenesis | circRNA-FUT10 | inhibits adipocyte differentiation by sponging miRNA-let-7 promotes adipocyte proliferation | let-7c/let-e, PGC1β | [180] |
| circRNA-INSR | impairs adipogenic differentiation acts via miR-152/MEOX2 pathway | miR-152 | [181] | |
| Positive regulator of brown adipogenesis | circRNA-0001017 | regulates brown adipogenesis by interacting with miR-503 | miR-503 | [182] |
| Negative regulator of brown adipogenesis | circRNA-Ogdh | impairs BAT differentiation promotes lipolysis by upregulating the expression of ATGL (key lipolysis protein) suppresses lipid droplet accumulation downregulates the expression of C/EBPα, C/EBPβ, PPARγ, and RXRA | miR-34a-5p, ATGL | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleiman, L.; Dinescu, S. Role of Non-Coding RNAs in White and Brown Adipose Tissue Differentiation and Development. Non-Coding RNA 2025, 11, 30. https://doi.org/10.3390/ncrna11030030
Sleiman L, Dinescu S. Role of Non-Coding RNAs in White and Brown Adipose Tissue Differentiation and Development. Non-Coding RNA. 2025; 11(3):30. https://doi.org/10.3390/ncrna11030030
Chicago/Turabian StyleSleiman, Lea, and Sorina Dinescu. 2025. "Role of Non-Coding RNAs in White and Brown Adipose Tissue Differentiation and Development" Non-Coding RNA 11, no. 3: 30. https://doi.org/10.3390/ncrna11030030
APA StyleSleiman, L., & Dinescu, S. (2025). Role of Non-Coding RNAs in White and Brown Adipose Tissue Differentiation and Development. Non-Coding RNA, 11(3), 30. https://doi.org/10.3390/ncrna11030030







