Chromatin Structure Around Long Non-Coding RNA (lncRNA) Genes in Schistosoma mansoni Gonads
Abstract
1. Introduction
2. Results
2.1. 500 bp Is the Optimal Bin Size for ChromstaR Analysis
2.2. There Are Sex-Specific Differences in Chromatin Profiles Around lncRNA Genes in Gonads of S. mansoni
3. Discussion
4. Materials and Methods
4.1. Life Cycle Maintenance of S. mansoni and Ethics Statement
4.2. Organ Extraction and N-ChIP
4.3. Sequencing and Quality Control
4.4. Alignment and ChromstaR Optimization
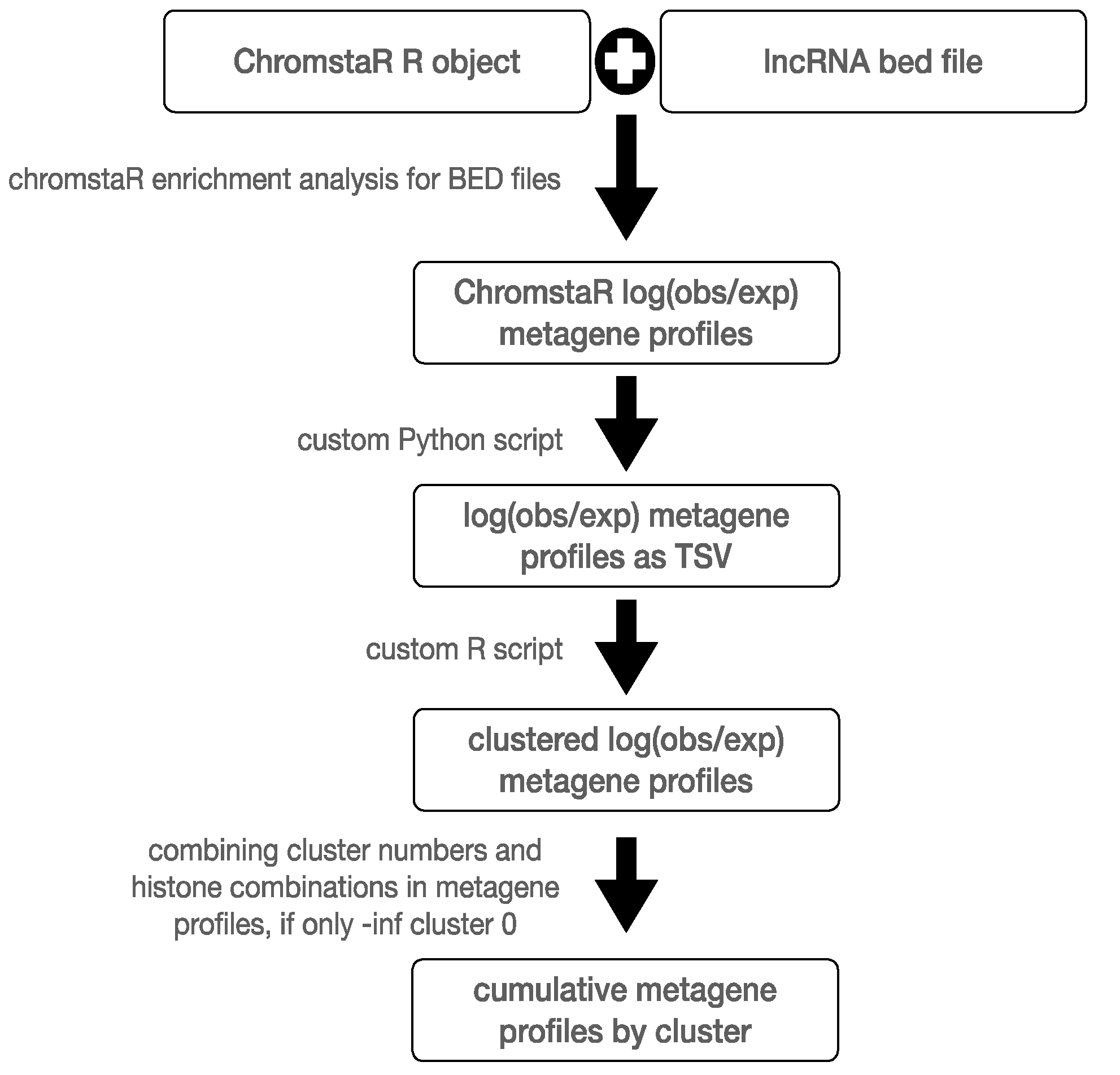
4.5. Metagene Analysis of lncRNA Genes and Clustering by Color
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primer 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Grevelding, C.G. Schistosoma. Curr. Biol. 2004, 14, R545. [Google Scholar] [CrossRef][Green Version]
- Kunz, W. Schistosome Male–Female Interaction: Induction of Germ-Cell Differentiation. Trends Parasitol. 2001, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- LoVerde, P.T.; Chen, L. Schistosome Female Reproductive Development. Parasitol. Today 1991, 7, 303–308. [Google Scholar] [CrossRef]
- Popiel, I.; Basch, P.F. Reproductive Development of Female Schistosoma mansoni (Digenea: Schistosomatidae) Following Bisexual Pairing of Worms and Worm Segments. J. Exp. Zool. 1984, 232, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.E.D.; Erasmus, D.A. Schistosoma mansoni: Male Stimulation and DNA Synthesis by the Female. Parasitology 1985, 91, 449–457. [Google Scholar] [CrossRef]
- Fitzpatrick, J.M.; Hoffmann, K.F. Dioecious Schistosoma mansoni Express Divergent Gene Repertoires Regulated by Pairing. Int. J. Parasitol. 2006, 36, 1081–1089. [Google Scholar] [CrossRef]
- Lu, Z.; Sessler, F.; Holroyd, N.; Hahnel, S.; Quack, T.; Berriman, M.; Grevelding, C.G. Schistosome Sex Matters: A Deep View into Gonad-Specific and Pairing-Dependent Transcriptomes Reveals a Complex Gender Interplay. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Lu, Z.; Spänig, S.; Weth, O.; Grevelding, C.G. Males, the Wrongly Neglected Partners of the Biologically Unprecedented Male–Female Interaction of Schistosomes. Front. Genet. 2019, 10, 796. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Cheng, S.; You, Y.; Wang, X.; Yi, C.; Zhang, W.; Xie, Y.; Xiu, L.; Luo, F.; Lu, Y.; Wang, J.; et al. Dynamic Profiles of lncRNAs Reveal a Functional Natural Antisense RNA That Regulates the Development of Schistosoma Japonicum. PLoS Pathog. 2024, 20, e1011949. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.J.R.; Mesel, V.C.; daSilva, L.F.; Pires, D.S.; Lavezzo, G.M.; Pereira, A.S.A.; Amaral, M.S.; Verjovski-Almeida, S. Atlas of Schistosoma mansoni Long Non-Coding RNAs and Their Expression Correlation to Protein-Coding Genes. Database 2018, 2018, bay068. [Google Scholar] [CrossRef]
- Zhao, R.; Tang, X.; Lin, H.; Xing, C.; Xu, N.; Dai, B.; Wang, P.; Shao, W.; Liu, M.; Shen, J.; et al. Knocking Down Gm16685 Decreases Liver Granuloma in Murine Schistosomiasis Japonica. Microorganisms 2023, 11, 796. [Google Scholar] [CrossRef]
- Silveira, G.O.; Coelho, H.S.; Pereira, A.S.A.; Miyasato, P.A.; Santos, D.W.; Maciel, L.F.; Olberg, G.G.G.; Tahira, A.C.; Nakano, E.; Oliveira, M.L.S.; et al. Long Non-Coding RNAs Are Essential for Schistosoma mansoni Pairing-Dependent Adult Worm Homeostasis and Fertility. PLoS Pathog. 2023, 19, e1011369. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.A.; Oliveira, V.F.; Castro-Borges, W.; Cabral, F.J.; Guerra-Sá, R. Profiles of LncRNAs Expression in Schistosoma mansoni during Intra- Mammalian Development. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; Zhu, Y.; Chen, J.; Dong, C.; Tao, Y.; He, A.; Liu, J.; Wu, Z. Identification of Long Noncoding RNAs in Schistosoma mansoni and Schistosoma Japonicum. Exp. Parasitol. 2018, 191, 82–87. [Google Scholar] [CrossRef]
- Morales-Montor, J.; Rio-Araiza, V.H.D.; Segovia-Mendoza, M.; Ostoa-Saloma, P.; de Leon, C.T.G. Environmental Parasitology and Its Impact on the Host Nueroimmunoendocrine Network. Front. Biosci.-Landmark 2020, 26, 431–443. [Google Scholar] [CrossRef]
- Amaral, M.S.; Maciel, L.F.; Silveira, G.O.; Olberg, G.G.O.; Leite, J.V.P.; Imamura, L.K.; Pereira, A.S.A.; Miyasato, P.A.; Nakano, E.; Verjovski-Almeida, S. Long Non-Coding RNA Levels Can Be Modulated by 5-Azacytidine in Schistosoma mansoni. Sci. Rep. 2020, 10, 21565. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Taudt, A.; Nguyen, M.A.; Heinig, M.; Johannes, F.; Colome-Tatche, M. ChromstaR: Tracking Combinatorial Chromatin State Dynamics in Space and Time. bioRxiv 2016. [Google Scholar] [CrossRef]
- Hahnel, S.; Lu, Z.; Wilson, R.A.; Grevelding, C.G.; Quack, T. Whole-Organ Isolation Approach as a Basis for Tissue-Specific Analyses in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2013, 7, e2336. [Google Scholar] [CrossRef]
- Maciel, L.F.; Morales-Vicente, D.A.; Silveira, G.O.; Ribeiro, R.O.; Olberg, G.G.O.; Pires, D.S.; Amaral, M.S.; Verjovski-Almeida, S. Weighted Gene Co-Expression Analyses Point to Long Non-Coding RNA Hub Genes at Different Schistosoma mansoni Life-Cycle Stages. Front. Genet. 2019, 10, 823. [Google Scholar] [CrossRef]
- Jardim Poli, P.; Fischer-Carvalho, A.; Tahira, A.C.; Chan, J.D.; Verjovski-Almeida, S.; Sena Amaral, M. Long Non-Coding RNA Levels Are Modulated in Schistosoma mansoni Following In Vivo Praziquantel Exposure. Non-Coding RNA 2024, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Utzinger, J.; Keiser, J. Controlling Schistosomiasis with Praziquantel: How Much Longer without a Viable Alternative? Infect. Dis. Poverty 2017, 6, 74. [Google Scholar] [CrossRef]
- Grevelding, C.G. The Female-Specific W1 Sequence of the Puerto Rican Strain of Schistosoma mansoni Occurs in Both Genders of a Liberian Strain. Mol. Biochem. Parasitol. 1995, 71, 269–272. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Augusto, R.; Roquis, D.; Al Picard, M.; Chaparro, C.; Cosseau, C.; Grunau, C. Measuring Histone Modifications in the Human Parasite Schistosoma mansoni. In Schistosoma mansoni; Timson, D.J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2151, pp. 93–107. [Google Scholar] [CrossRef]
- de Carvalho Augusto, R.; Tetreau, G.; Chan, P.; Walet-Balieu, M.-L.; Mello-Silva, C.C.; Santos, C.P.; Grunau, C. Double Impact: Natural Molluscicide for Schistosomiasis Vector Control Also Impedes Development of Schistosoma mansoni Cercariae into Adult Parasites. PLoS Negl. Trop. Dis. 2017, 11, e0005789. [Google Scholar] [CrossRef]

| Cluster | sT | bT | sO | bO |
|---|---|---|---|---|
| 0 (flatline) | 19 | 58 | 45 | 37 |
| 1 | 57 | 160 | 163 | 140 |
| 2 | 162 | 13 | 41 | 68 |
| 3 | 6 | 18 | 2 | 5 |
| 4 | 2 | 1 | 1 | 1 |
| 5 | 6 | 5 | 1 | 1 |
| 6 | 4 | 1 | 1 | 2 |
| Part of Gene | sT | bT | sO | bO |
|---|---|---|---|---|
| TSS | Cluster A | Cluster A | Cluster I | |
| Gene body | Cluster B, C | Cluster B, C | Cluster II | Cluster II |
| TES and downstream | Cluster D, E | Cluster D | ||
| Absent in gene body | Cluster III | Cluster III |
| Antibody | Supplier | Catalog Number |
|---|---|---|
| H3K4me3 | Diagenode | c15410003 |
| H3K27me3 | Diagenode | c15410069 |
| H3K9me3 | Abcam | ab8898 |
| H3K9ac | Milipore | 07-352 |
| H4K20me1 | Abcam | ab9051 |
| H4K12ac | Abcam | ab46983 |
| H3K36me3 | Abcam | ab9050 |
| H3K23ac | Milipore | 07-355 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, R.C.; Quack, T.; Grevelding, C.G.; Grunau, C. Chromatin Structure Around Long Non-Coding RNA (lncRNA) Genes in Schistosoma mansoni Gonads. Non-Coding RNA 2025, 11, 25. https://doi.org/10.3390/ncrna11020025
Augusto RC, Quack T, Grevelding CG, Grunau C. Chromatin Structure Around Long Non-Coding RNA (lncRNA) Genes in Schistosoma mansoni Gonads. Non-Coding RNA. 2025; 11(2):25. https://doi.org/10.3390/ncrna11020025
Chicago/Turabian StyleAugusto, Ronaldo C., Thomas Quack, Christoph G. Grevelding, and Christoph Grunau. 2025. "Chromatin Structure Around Long Non-Coding RNA (lncRNA) Genes in Schistosoma mansoni Gonads" Non-Coding RNA 11, no. 2: 25. https://doi.org/10.3390/ncrna11020025
APA StyleAugusto, R. C., Quack, T., Grevelding, C. G., & Grunau, C. (2025). Chromatin Structure Around Long Non-Coding RNA (lncRNA) Genes in Schistosoma mansoni Gonads. Non-Coding RNA, 11(2), 25. https://doi.org/10.3390/ncrna11020025






