Circulating MicroRNAs in Patients with Vulvar Squamous Cell Carcinoma and Its Precursors
Abstract
1. Introduction
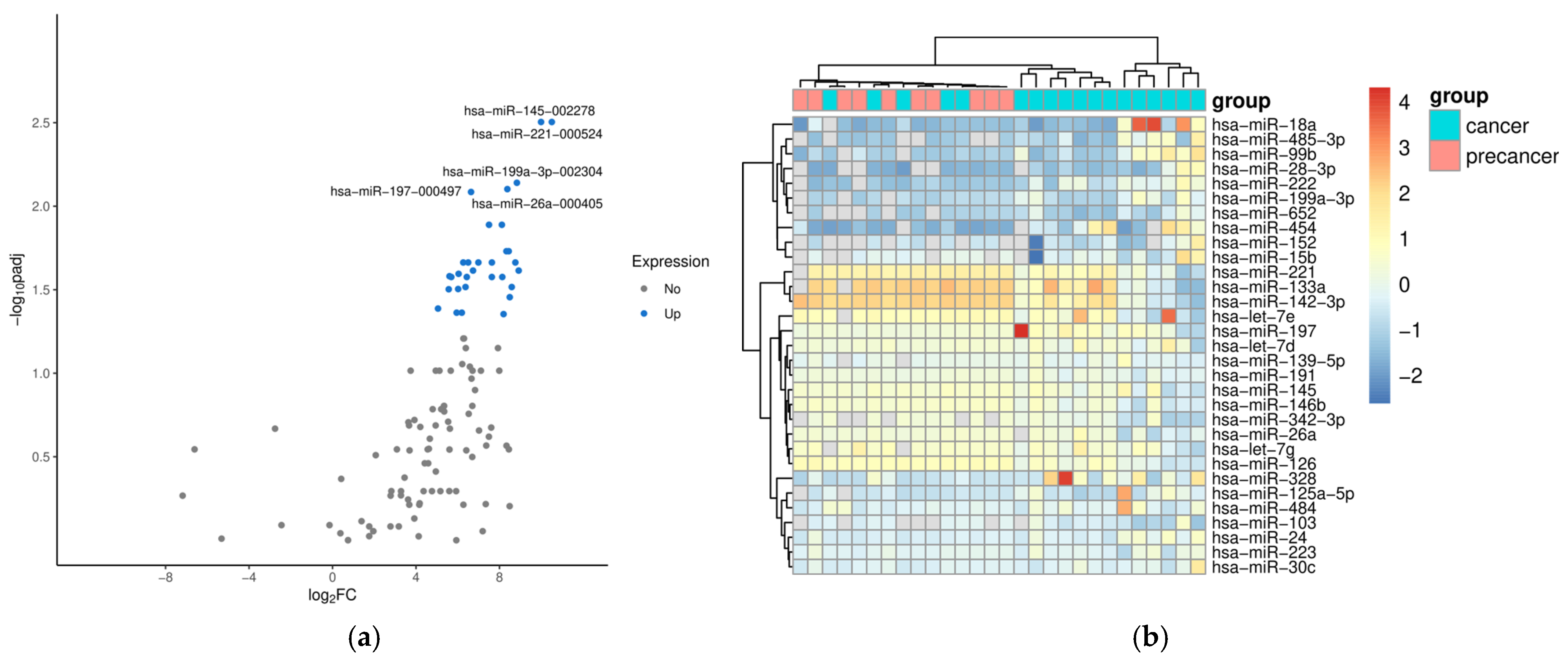
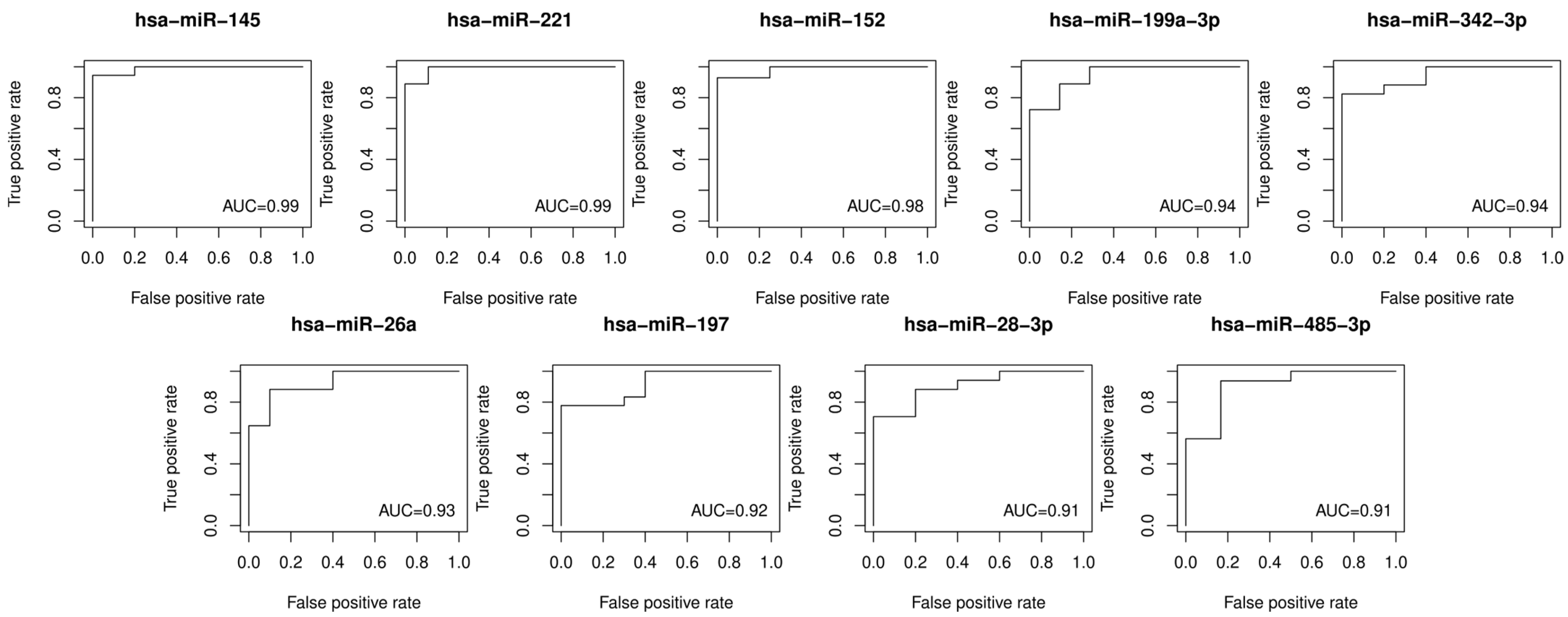
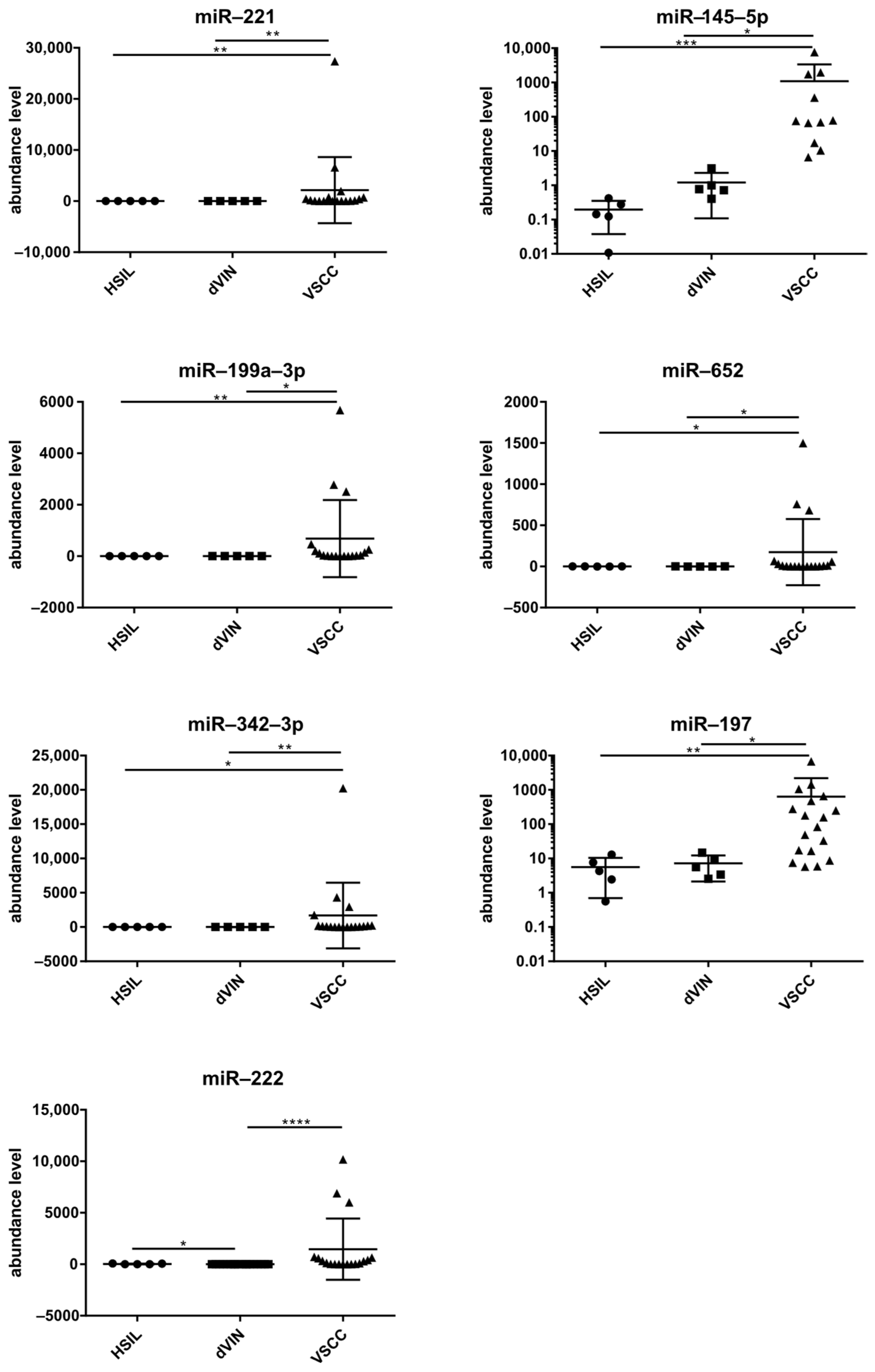
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Populations
4.2. HPV Genotyping
4.3. RNA Isolation from Plasma and qPCR
4.4. Data Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Female genital tumours. In WHO Classification of Tumours Series, 5th ed.; World Health Organization: Geneva, Switzerland, 2020; Volume 4, Available online: https://tumourclassification.iarc.who.int/chapters/34 (accessed on 1 January 2025).
- Yang, H.; Almadani, N.; Thompson, E.F.; Tessier-Cloutier, B.; Chen, J.; Ho, J.; Senz, J.; McConechy, M.K.; Chow, C.; Ta, M.; et al. Classification of Vulvar Squamous Cell Carcinoma and Precursor Lesions by p16 and p53 Immunohistochemistry: Considerations, Caveats, and an Algorithmic Approach. Mod. Pathol. 2023, 36, 100145. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, K.; Proctor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Clavero, O.; Alemany, L.; Saco, A.; Quirós, B.; Lloveras, B.; Alejo, M.; Pawlita, M.; Quint, W.; Del Pino, M.; et al. Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: A study of 1,594 cases. Int. J. Cancer 2017, 141, 2517–2527. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cotler, J.; Cuello, M.A.; Bhatla, N.; Okamoto, A.; Wilailak, S.; Purandare, C.N.; Lindeque, G.; Berek, J.S.; Kehoe, S. FIGO staging for carcinoma of the vulva: 2021 revision. Int. J. Gynaecol. Obstet. 2021, 155, 43–47. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Bosse, T.; Focchi, G. Carcinoma of the Vulva Histopathology Reporting Guide, 2nd ed.; International Collaboration on Cancer Reporting: Sydney, Australia, 2023. [Google Scholar]
- Chehade, R.; Jerzak, K.J.; Tavanger, F.; Plotkin, A.; Gien, L.T.; Leung, E.; Mackay, H. Advances in Vulvar Cancer Biology and Management. J. Clin. Oncol. 2025, 43, 89–100. [Google Scholar] [CrossRef] [PubMed]
- de Melo Maia, B.; Lavorato-Rocha, A.M.; Rodrigues, L.S.; Coutinho-Camillo, C.M.; Baiocchi, G.; Stiepcich, M.M.; Puga, R.; de, A.L.L.; Soares, F.A.; Rocha, R.M. microRNA portraits in human vulvar carcinoma. Cancer Prev. Res. 2013, 6, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- de Melo Maia, B.; Ling, H.; Monroig, P.; Ciccone, M.; Soares, F.A.; Calin, G.A.; Rocha, R.M. Design of a miRNA sponge for the miR-17 miRNA family as a therapeutic strategy against vulvar carcinoma. Mol. Cell. Probes 2015, 29, 420–426. [Google Scholar] [CrossRef]
- Borghi, A.; D’Abundo, L.; Bassi, C.; Lupini, L.; Tagliatti, V.; Zedde, P.; Lanza, G.; Gafa, R.; Negrini, M.; Corazza, M. A microRNA signature to predict risk progression of vulvar lichen sclerosus to squamous cell carcinoma. Br. J. Dermatol. 2023, 188, 680–681. [Google Scholar] [CrossRef]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating microRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef]
- Gayosso-Gómez, L.V.; Ortiz-Quintero, B. Circulating MicroRNAs in Blood and Other Body Fluids as Biomarkers for Diagnosis, Prognosis, and Therapy Response in Lung Cancer. Diagnostics 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Bujko, M.; Zalewski, K.; Szczyrek, M.; Kowalik, A.; Boresowicz, J.; Dlugosz, A.; Goryca, K.; Gozdz, S.; Kowalewska, M. Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions. Diagnostics 2021, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Grillone, K.; Ascrizzi, S.; Carida, G.; Fiorillo, L.; Ciliberto, D.; Staropoli, N.; Tagliaferri, P.; Tassone, P.; Di Martino, M.T. LNA-i-miR-221 activity in colorectal cancer: A reverse translational investigation. Mol. Ther. Nucleic Acids 2024, 35, 102221. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Di Martino, M.T.; Arbitrio, M.; Fiorillo, L.; Staropoli, N.; Ciliberto, D.; Cordua, A.; Scionti, F.; Bertucci, B.; Salvino, A.; et al. Safety and activity of the first-in-class locked nucleic acid (LNA) miR-221 selective inhibitor in refractory advanced cancer patients: A first-in-human, phase 1, open-label, dose-escalation study. J. Hematol. Oncol. 2023, 16, 68. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Arbitrio, M.; Caracciolo, D.; Cordua, A.; Cuomo, O.; Grillone, K.; Riillo, C.; Carida, G.; Scionti, F.; Labanca, C.; et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Mol. Ther. Nucleic Acids 2022, 27, 1191–1224. [Google Scholar] [CrossRef] [PubMed]
- Seeley, J.J.; Baker, R.G.; Mohamed, G.; Bruns, T.; Hayden, M.S.; Deshmukh, S.D.; Freedberg, D.E.; Ghosh, S. Induction of innate immune memory via microRNA targeting of chromatin remodelling factors. Nature 2018, 559, 114–119. [Google Scholar] [CrossRef]
- Xu, W.X.; Liu, Z.; Deng, F.; Wang, D.D.; Li, X.W.; Tian, T.; Zhang, J.; Tang, J.H. MiR-145: A potential biomarker of cancer migration and invasion. Am. J. Transl. Res. 2019, 11, 6739–6753. [Google Scholar]
- Rahman, M.S.; Ghorai, S.; Panda, K.; Santiago, M.J.; Aggarwal, S.; Wang, T.; Rahman, I.; Chinnapaiyan, S.; Unwalla, H.J. Dr. Jekyll or Mr. Hyde: The multifaceted roles of miR-145-5p in human health and disease. Non-Coding RNA Res. 2025, 11, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, J.Y.; Park, H.; Jeong, J.Y.; Kwon, A.Y.; Heo, J.H.; Kang, H.; Kim, G.; An, H.J. miR-145, targeting high-mobility group A2, is a powerful predictor of patient outcome in ovarian carcinoma. Cancer Lett. 2015, 356, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Fatalska, A.; Rusetska, N.; Bakula-Zalewska, E.; Kowalik, A.; Zieba, S.; Wroblewska, A.; Zalewski, K.; Goryca, K.; Domanski, D.; Kowalewska, M. Inflammatory Proteins HMGA2 and PRTN3 as Drivers of Vulvar Squamous Cell Carcinoma Progression. Cancers 2020, 13, 27. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, N.; Yadav, S.K.; Bhatt, M.L.B.; Pandey, A.; Yadav, D.K.; Yadav, S. Expression of miR-145 and miR-18b in Peripheral Blood Samples of Head and Neck Cancer Patients. Indian. J. Clin. Biochem. 2023, 38, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Suvanasuthi, R.; Therasakvichya, S.; Kanchanapiboon, P.; Chamras Promptmas, C.; Chimnaronk, S. Analysis of precancerous lesion-related microRNAs for early diagnosis of cervical cancer in the Thai population. Sci. Rep. 2025, 15, 142. [Google Scholar] [CrossRef]
- Peronace, C.; Cione, E.; Marisol Abrego-Guandique, D.; De Fazio, M.; Panduri, G.; Caroleo, M.C.; Cannataro, R.; Minchella, P. FAM19A4 and hsa-miR124-2 Double Methylation as Screening for ASC-H- and CIN1 HPV-Positive Women. Pathogens 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.N.; Snir, O.; Hindberg, K.D.; Hveem, K.; Brækkan, S.K.; Hansen, J.-B. High microRNA-145 plasma levels are associated with decreased risk of future incident venous thromboembolism: The HUNT study. Blood 2024, 143, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Zieba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piascik, A.; Misiek, M.; Bakula-Zalewska, E.; Kopczynski, J.; Kowalski, K.; et al. Somatic mutation profiling of vulvar cancer: Exploring therapeutic targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef] [PubMed]
| Patients (n) | Median Age (Years) (Range) | FIGO | G | hrHPV Status | |
|---|---|---|---|---|---|
| HSIL | 5 | 64.1 (47.7–83.4) | N/A | ||
| dVIN | 5 | 59.7 (41.1–79.5) | N/A | ||
| VSCC | 27 | 68.6 (46.1–83.4) | I n = 18 III n = 10 | G1 n = 10 G2 n = 12 G3 n = 5 | negative n = 13 positive n = 14 |
| miRNA | log2FC | padj | AUC |
|---|---|---|---|
| hsa-miR-145-5p | 9.99 | 0.0031 | 0.99 |
| hsa-miR-221-3p | 10.51 | 0.0031 | 0.99 |
| hsa-miR-152 | 8.44 | 0.0186 | 0.98 |
| hsa-miR-199a-3p | 8.83 | 0.0072 | 0.94 |
| hsa-miR-342-3p | 8.33 | 0.0186 | 0.94 |
| hsa-miR-26a | 8.38 | 0.0079 | 0.93 |
| hsa-miR-197 | 6.64 | 0.0082 | 0.92 |
| hsa-miR-485-3p | 7.64 | 0.0217 | 0.91 |
| hsa-miR-28-3p | 8.14 | 0.0265 | 0.91 |
| hsa-miR-15b | 8.92 | 0.0243 | 0.90 |
| hsa-miR-133a | 8.11 | 0.0129 | 0.90 |
| hsa-miR-191 | 7.50 | 0.0129 | 0.89 |
| hsa-miR-652 | 8.59 | 0.0305 | 0.89 |
| hsa-miR-103 | 8.49 | 0.0350 | 0.89 |
| hsa-miR-139-5p | 6.50 | 0.0217 | 0.87 |
| hsa-let-7e | 8.76 | 0.0217 | 0.86 |
| hsa-miR-126 | 6.99 | 0.0217 | 0.86 |
| hsa-miR-30c | 6.26 | 0.0217 | 0.86 |
| hsa-let-7d | 6.73 | 0.0243 | 0.84 |
| hsa-let-7g | 5.60 | 0.0263 | 0.84 |
| hsa-miR-484 | 6.04 | 0.0254 | 0.84 |
| hsa-miR-222 | 6.37 | 0.0305 | 0.83 |
| hsa-miR-99b | 5.57 | 0.0314 | 0.83 |
| hsa-miR-146b | 7.63 | 0.0265 | 0.83 |
| hsa-miR-223 | 6.43 | 0.0265 | 0.83 |
| hsa-miR-328 | 5.69 | 0.0265 | 0.83 |
| hsa-miR-24 | 6.02 | 0.0313 | 0.82 |
| hsa-miR-454 | 5.05 | 0.0410 | 0.81 |
| hsa-miR-125a-5p | 6.19 | 0.0434 | 0.81 |
| hsa-miR-18a | 5.95 | 0.0434 | 0.80 |
| hsa-miR-142-3p | 8.20 | 0.0443 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rymuza, J.; Długosz, A.; Zalewski, K.; Kowalik, A.; Bujko, M.; Kowalewska, M. Circulating MicroRNAs in Patients with Vulvar Squamous Cell Carcinoma and Its Precursors. Non-Coding RNA 2025, 11, 13. https://doi.org/10.3390/ncrna11010013
Rymuza J, Długosz A, Zalewski K, Kowalik A, Bujko M, Kowalewska M. Circulating MicroRNAs in Patients with Vulvar Squamous Cell Carcinoma and Its Precursors. Non-Coding RNA. 2025; 11(1):13. https://doi.org/10.3390/ncrna11010013
Chicago/Turabian StyleRymuza, Julia, Angelika Długosz, Kamil Zalewski, Artur Kowalik, Mateusz Bujko, and Magdalena Kowalewska. 2025. "Circulating MicroRNAs in Patients with Vulvar Squamous Cell Carcinoma and Its Precursors" Non-Coding RNA 11, no. 1: 13. https://doi.org/10.3390/ncrna11010013
APA StyleRymuza, J., Długosz, A., Zalewski, K., Kowalik, A., Bujko, M., & Kowalewska, M. (2025). Circulating MicroRNAs in Patients with Vulvar Squamous Cell Carcinoma and Its Precursors. Non-Coding RNA, 11(1), 13. https://doi.org/10.3390/ncrna11010013








