1. Introduction
Medical advancements have enhanced the 5-year survival rates among cancer patients recently; however, clinicians still encounter considerable obstacles in planning effective treatment approaches to transport anti-cancer medications to tumors while alleviating the toxicity associated with drug accumulation in healthy organs and tissue. Drug delivery to solid tumors usually involves intravenous injection. The drug payload is often low using this approach, with less than 5% of the systemically injected drug eventually reaching the targeted tumor, while the majority of the drug is either cleared by the liver, spleen, or kidneys or deposited in other healthy tissue regions [
1]. In addition, this approach is not suitable for poorly perfused and large-sized tumors. Some tumors at the late growth stages often form a necrotic/hypoxic core at the center, relying on the minimal supply of oxygen to survive [
2]. Further, the systemic delivery of drugs may not be effective in brain tumor treatment because of the blood–brain barrier (BBB) [
3]. To overcome the challenges faced by systemic delivery due to the presence of the BBB or limited blood perfusion, intratumoral infusion via convection-enhanced delivery (CED) has been proposed by means of single-port or multiple-port needles. This method has been widely used to deliver a variety of large therapeutic agents in tumors by the continuous injection of a drug-carrying nanofluid under a pressure difference [
4,
5,
6,
7]. With CED, therapeutic agents may achieve tissue penetration of a few centimeters, unlike systemic delivery, which can only achieve a depth of micrometers from the capillary pores [
8,
9,
10].
Several limitations have been noted in the past few decades when using CED. Among these are backflow along the needle, air bubbles, edema, and drug concentration heterogeneity. Backflow, also known as reflux, occurs when a catheter disrupts tissue sufficiently to create a void along its insertion tract, leading to a fluid-filled gap between the needle and tissue. This allows infusates to flow back along the catheter, rather than into the targeted tumor tissue [
10]. Clinical studies have reported severe reflex pain experienced by patients with liver tumors when ethanol was injected directly into the tumor and leaked to the healthy tissue [
11].
Previous studies have shed light on various factors that influence backflow to improve the drug delivery efficacy. Orozco et al. [
12,
13] used a three-dimensional finite element model to evaluate the infusion parameters of backflow during CED in the brain. Their findings underscore the relevance of the distance between the infusion cannula and the ventricles in limiting backflow, with long distances resulting in comparable backflow lengths independently of the ventricular pressure [
12]. They also found that greater insertion speeds provided a larger pre-stress field, reducing the backflow duration over a range of flow rates. The study’s experimental validation in agarose gel phantoms confirmed the predictions, with a significant decrease in the backflow length as the insertion speeds were increased [
13]. Ayers and Smith [
14] used a unique biphasic fluid–structure interaction (FSI) model to mimic infusion processes in agarose gel. Their model correctly recreated the experimental backflow lengths and maximum fluid pressures [
14]. A major discovery indicates that small catheters, which require greater infusion pressures to achieve the appropriate medication distribution, often lead to intensive backflow. Theoretical studies by our group [
15] showed that higher infusion rates, larger needle diameters, and lower elastic moduli yield longer backflow lengths and cause more irregular spreading shapes in the nanofluid. Together, these investigations have added greatly to the understanding of backflow in CED and provided vital insights into techniques for the optimization of medication delivery to tumors, thus leading to enhanced treatment outcomes [
12,
13,
14,
15].
Soft and thin catheters, as well as new “step-design” catheters, have been shown to reduce backflow [
16,
17,
18]. Several improvements in cannula design have implemented flexible cannulas, resulting in increased infusion rates, reduced backflow, and increased fluid volumes through the cannula-induced track. Some experimental results have demonstrated that CED using catheters with larger diameters is more likely to lead to reflux [
19,
20,
21]. This observation resulted in a step-down catheter design called a reflex-preventing catheter [
22]. Multiport catheters were originally designed for hydrocephalus. Later, they were adapted for CED due to their potential for better volume distribution [
6]. However, achieving predictable flows from all ports proved challenging, often resulting in infusates flowing only through the most proximal port. The proposed solutions included using porous materials to spread the infusion area, developing catheters with separate lumens, or using catheters with controllable portholes. A breakthrough came with Twin Star Medical’s development of a hollow-fiber catheter [
23], which contains millions of tiny openings (0.45 μm) along its wall surface [
23]. This design has successfully increased infusate transfer up to three-fold, improved the uniformity of distribution, and reduced backflow, therefore making it a promising advancement in CED technology [
23]. The balloon-tipped catheter approach uses a balloon near the catheter tip to fill the resection cavity, pushing the infusate into the cavity and limiting reflux [
22]. Studies in canine models have shown the extensive delivery of infusates [
22].
The company Rex Medical (Conshohocken, PA) recently developed an infusion catheter (Quadra Fuse ST) consisting of three retractable injection tines. The tines are hidden inside a hosting catheter initially. At the end of the hollow catheter, 10 mm hollow tines with sharp tips are attached for the piercing of tissue. After the catheter is inserted into the targeted tissue, the three tines can be ejected in three directions and therapeutic fluid then flows from their tips. This device also allows repeated infusion. After the first round of infusion, the tines are retracted back to the hosting catheter; one then rotates the hosting catheter by 60°, resulting in three new ejection directions of the tines. The original design of this device aims at using the tines for infusion improvement, and the retracting ability of the tines allows more infusing sites to enlarge the infused tissue volume. Clinical studies have demonstrated the efficiency of this device in delivering ethanol to liver tumors [
11,
24]. They found that, with the traditional single-needle infusion of ethanol to liver tumors, patients often expressed severe pain, possibly due to the leakage of the ethanol through the backflow channel to the surrounding healthy tissue. After they employed the retractable-tine catheter, less pain was experienced by the patients, implying less leakage of ethanol to healthy tissue with sensory nerves [
11,
24].
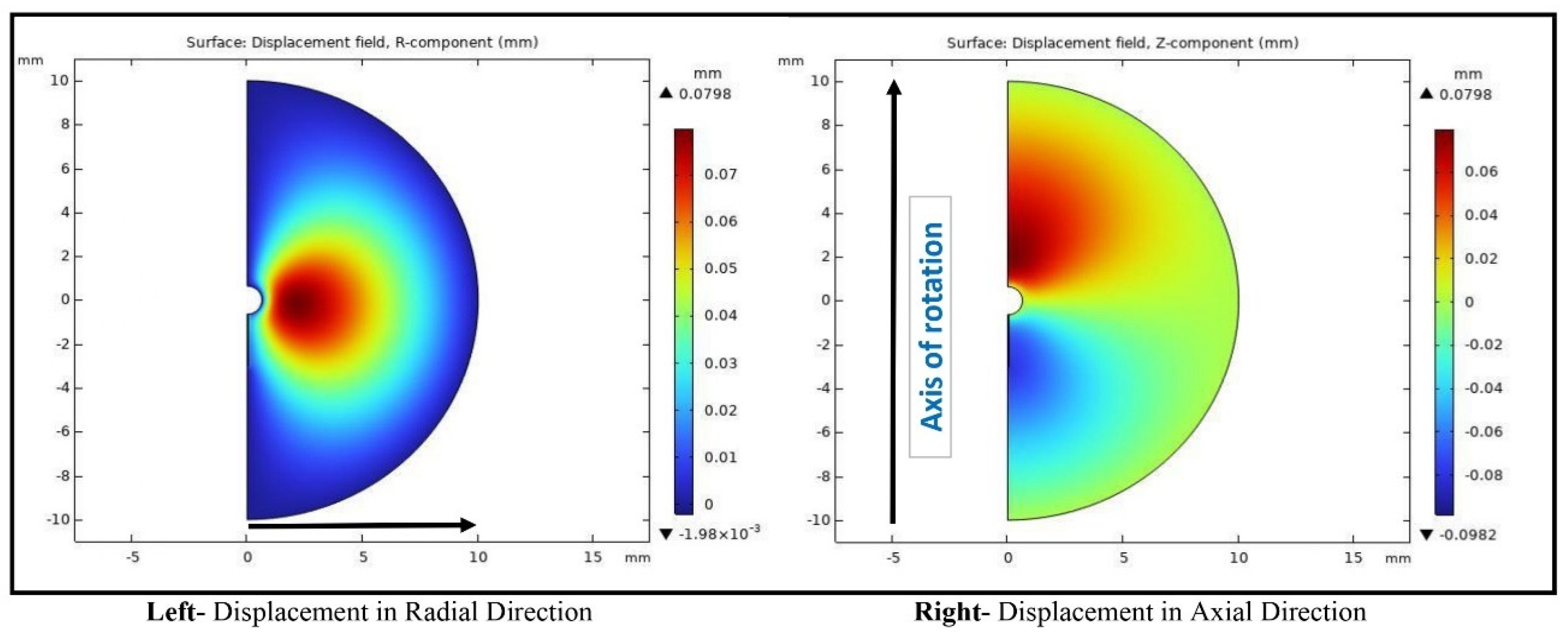
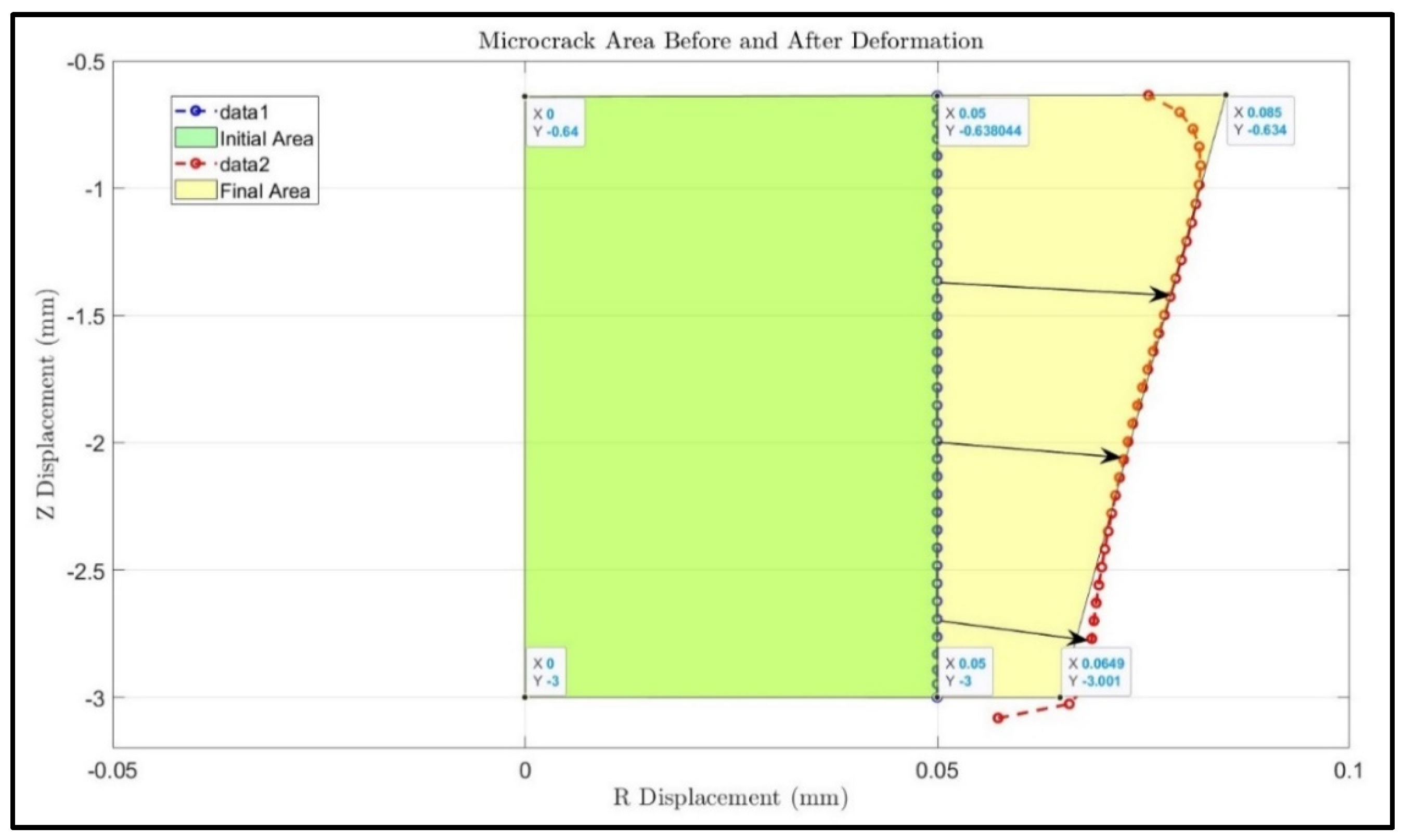
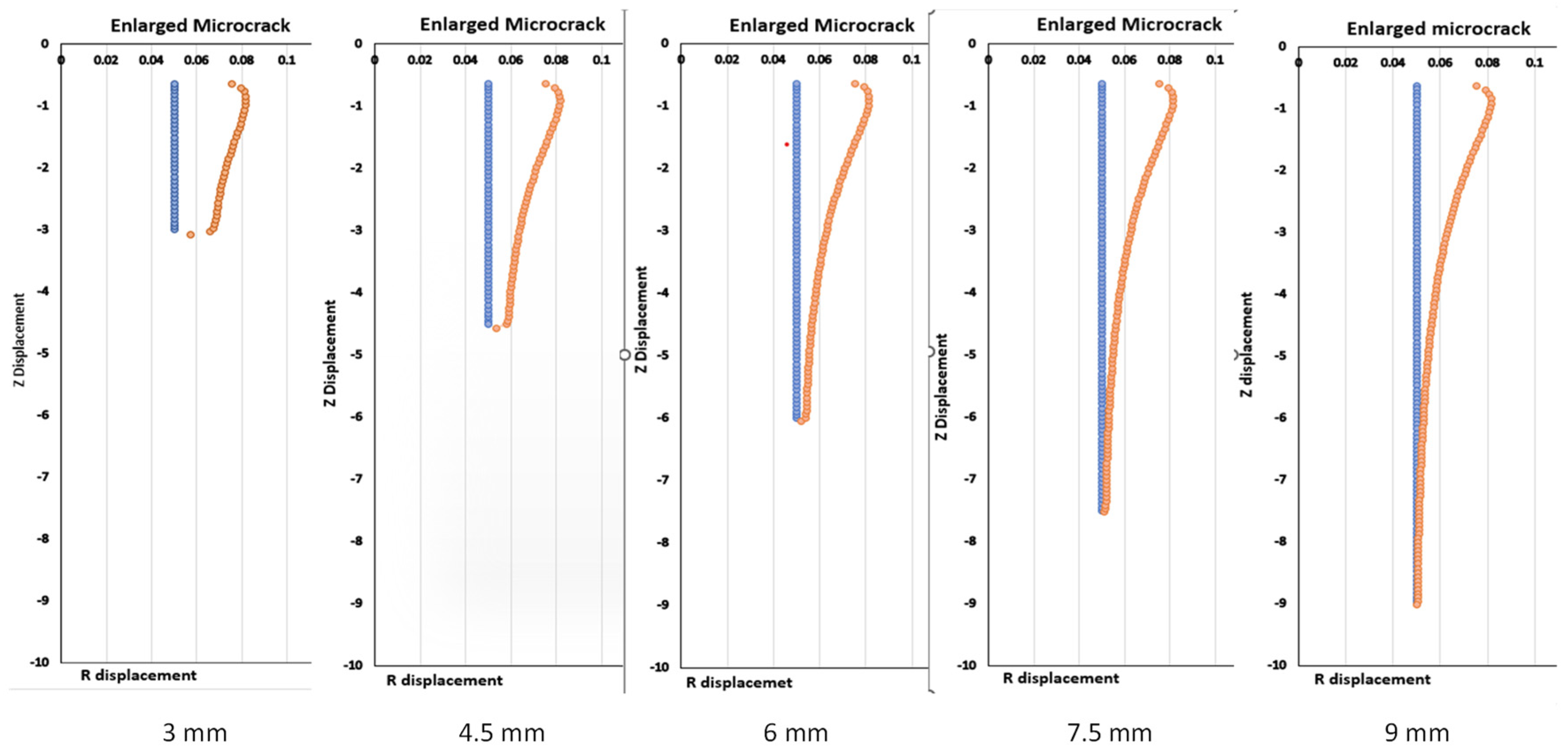
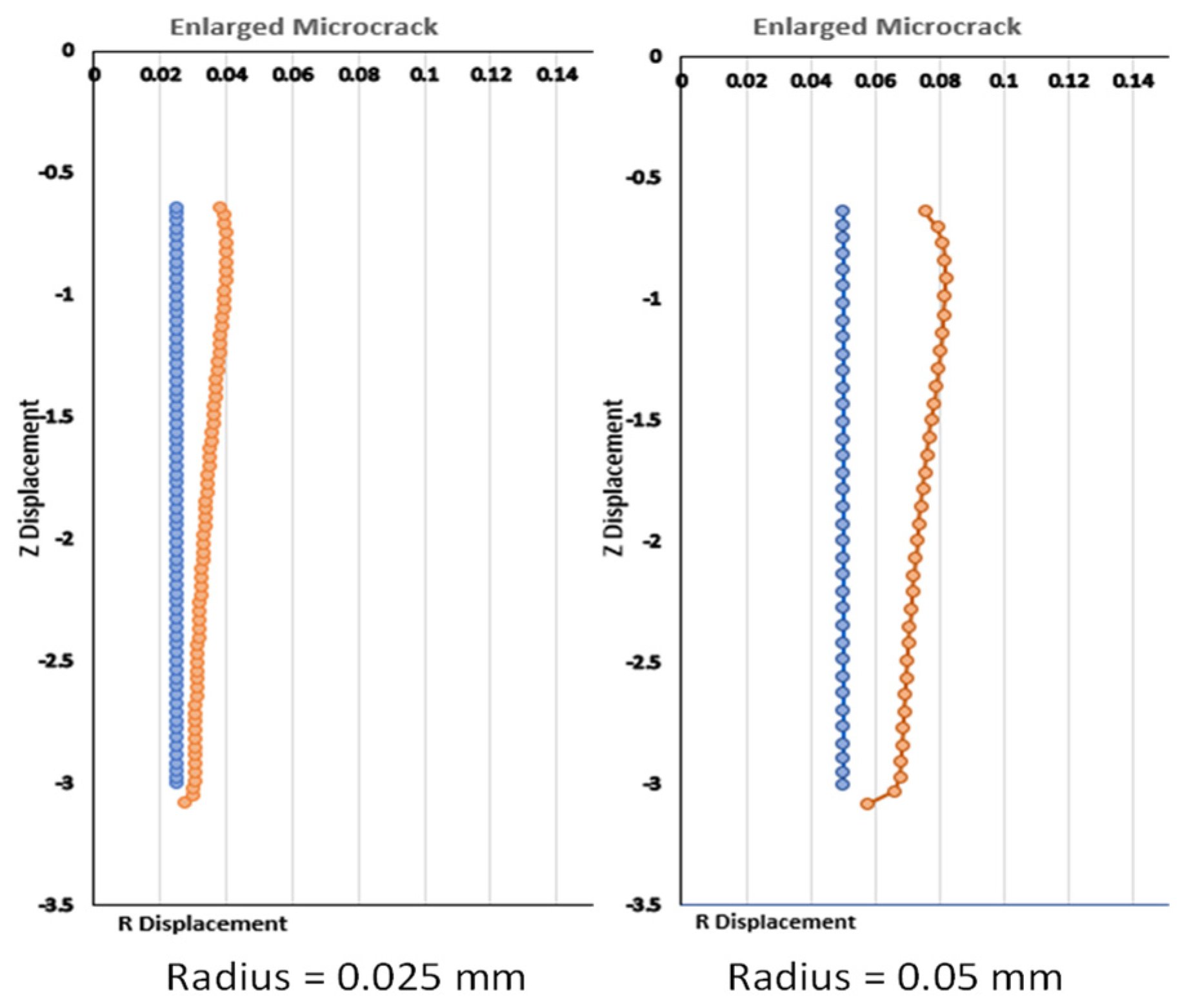
Backflow, in principle, is largely due to tissue deformation. There have been several studies that have evaluated the enlargement of the generated gap between the infusion catheter and the tissue [
14,
15]. In principle, any methods to make the flow easier in the tumor would minimize backflow. None of the previous studies have evaluated a CED system with a retractable tine, especially the effects of the retractable tines on the generation of microcracks in tumors and the resulting change in flow resistance in tumors during direct fluid infusion. In this study, we aim to develop theoretical models to evaluate how the introduction of a microcrack in the tissue reduces the overall flow resistance in a porous tumor, as well as further decreasing the flow resistance as this microcrack enlarges in a direct infusion process using CED. Both Darcy’s law and the theory of poroelasticity are used in the simulation to understand the fluid transport in porous tumors with or without microcrack introduction and/or enlargement. We expect that the study will provide quantitative measures to evaluate whether this approach is effective to enhance nanofluid transport in dense porous tumors.
4. Discussion
This study contributes to the field of tumor treatment by providing a quantitative assessment of a novel method for the convection-enhanced delivery of drug-carrying nanofluids. This method is innovative as it introduces a microcrack and expands the microcrack due to tissue deformation. The results suggest that this is a viable option for the minimization of backflow and the delivery of drugs to the entire tumor. Up to 18% of the flow resistance reduction is predicted by an enlarged microcrack using the parameters in this study. It is anticipated that introducing multiple microcracks, as shown using retractable tines, would provide a significant resistance reduction. Therefore, it will decrease the pressure in the vicinity of the infusion catheter to minimize the deformation there and attenuate the backflow of the nanofluid. With minimal backflow through the catheter track, more drug-carrying nanostructures would be delivered to cover the entire tumor.
One limitation of this study is the inability to properly integrate Darcy’s pressure field with the tissue deformation in the numerical models. A two-way coupling system that allows the pressure field and tissue deformation to impact each other would be ideal in gaining thorough knowledge of the fluid dynamics and tissue response in the tumor environment. However, due to the limitations of the computational tools utilized in this investigation, the module used could only deal with small material deformation, leading to one-way coupling only. This means that, while the pressure field can affect tissue deformation, the updated tissue geometry cannot change the pressure field. To address this shortcoming, a third model is created, which includes an enlarged microcrack based on the geometry produced from the second model’s poroelastic simulation of tissue deformation. The current work provides a workaround; it does not fully depict the dynamic interaction of the fluid pressure and tissue deformation that happens in vivo. For example, tissue deformation would change the porosity of the tumor, which could influence the pressure and velocity fields in Darcy’s law. We contacted COMSOL’s technical support, and, after several rounds of discussion, we were informed that extra COMSOL modules would need to be added to our license. We are aware that, with the updated modules, two-way coupling modeling using COMSOL is possible, as shown by other research groups [
37]. This is a limitation of our study, since we were not able to explore the coupling feature due to the lack of research resources at our institution. Other options to implement two-way coupling may include utilizing other commercial software packages. For example, FEBio, ABACUS, and ANSYS are some of the finite element method simulation packages to explore. The shortcoming of the current study highlight the need for more advanced computational tools or software advancements that can handle fully coupled simulations, allowing for the more realistic simulation of the intricate interactions occurring within the tumor microenvironment during convection-enhanced drug delivery.
In this study, our focus is on the overall resistance reduction induced by the microcrack, without including a backflow channel in the vicinity of an infusion catheter. Without a microcrack, backflow along an infusion catheter occurs, and it is significant. There is strong evidence that adding a microcrack would cause some fluid to flow through the enlarged microcrack, therefore alleviating the backflow along the infusion catheter. However, the extent of this is unknown. Therefore, incorporating an infusion catheter with tissue deformation into the current model would allow the thorough evaluation of whether the inclusion of microcracks effectively reduces backflow and improves therapeutic agent targeting. One could also explore the minimal number of microcracks necessary to significantly decrease backflow. One previous study implemented an infusion catheter with three retractable tines (Rex Medical) in patients [
24]. They found that, using the retractable tines to infuse ethanol to liver tumors, most patients did not experience the reflex pain typically experienced when ethanol leaks through the backflow channel. This indicates that the three microcracks facilitated the flow in the tumors, resulting in smaller backflow along the infusion catheter. In addition, theoretical simulations could be developed to achieve the more realistic representation of CED devices and their effects on the backflow. This will entail not only fine-tuning the computational models but also undertaking experimental validation to guarantee that the theoretical insights are transferable to real applications in medication delivery.
Another noteworthy constraint of this study is its dependence on theoretical models with uniform transport and mechanical properties, as opposed to integrating empirical data on authentic tumor specimens. While the simulations quantify the possible influences of microcrack introduction on medication delivery in tumors, the lack of experimental validation with actual tumor tissue restricts the capacity to corroborate the findings’ relevance in a real-world setting. The simplified model may fail to reflect the intricacies and heterogeneities of real tumors, reducing the efficacy of convection-enhanced delivery (CED) in clinical situations. In future studies, one could model the transport process based on reconstructed realistic tumor models from imaging scans. Different layers/regions with various transport properties could be included in the model. The modeling of the pressure and velocity fields in realistic tumors with complexity and heterogeneity would enhance the prediction capabilities of theoretical simulations.
One future study could be focused on nanostructure transport in tumors. In the current model, we only simulate the fluid pressure field and velocity field. The current simulation can be extended to simulate drug-carrying nanoparticle diffusion and convection in the tumor. With the simulated fluid field, the transient process of nanoparticle spreading in the tumor can be evaluated to understand the infusion duration and other strategies for nanoparticle deposition in tumors.
Finally, although the use of microcracks has shown promise in improving therapeutic drug delivery to tumors, the dynamic interaction between the microcrack and its surrounding tissue is unknown. The introduction of microcracks may impair the structural and functional integrity of the tumor’s extracellular matrix, resulting in unexpected consequences such as inflammation, edema, or even neuronal injury. Furthermore, the long-term stability of these microcracks, as well as their propensity to mend or spread further, requires future investigation, seeking to employ an effective yet safe delivery method for the treatment of tumors. Moreover, experimental studies are warranted to evaluate the performance of the proposed approach in tissue-equivalent gels, as well as in biological tissue. Transparent gels would be very useful to observe progressing crack enlargement and fluid flow pathways. In vivo or in vitro experiments on animal tissue are also critical to understand the interaction between the microcrack and the surrounding tissue. Experimental validation is necessary to not only provide the extracted transport properties needed for theoretical simulations, but also to help evaluate the assumptions and simplifications made in the theoretical models. By comparing the simulation results to experimental results, researchers may detect differences and improve the theoretical models to better reflect the behavior of therapeutic drugs in actual tumors. A future comparison study would give useful inputs on the accuracy and relevance of simulation-based predictions, resulting in accurate and successful tumor treatment.