Lattice Boltzmann Model for Rarefied Gaseous Mixture Flows in Three-Dimensional Porous Media Including Knudsen Diffusion
Abstract
1. Introduction
2. Description of the Model
2.1. Individual Species-Based LB Model for Gaseous Mixtures
2.2. Transport Coefficients and Rarefaction Rate
2.3. Effective Viscosity Calculation with a Ray-Tracing Approach
2.4. Knudsen Diffusion
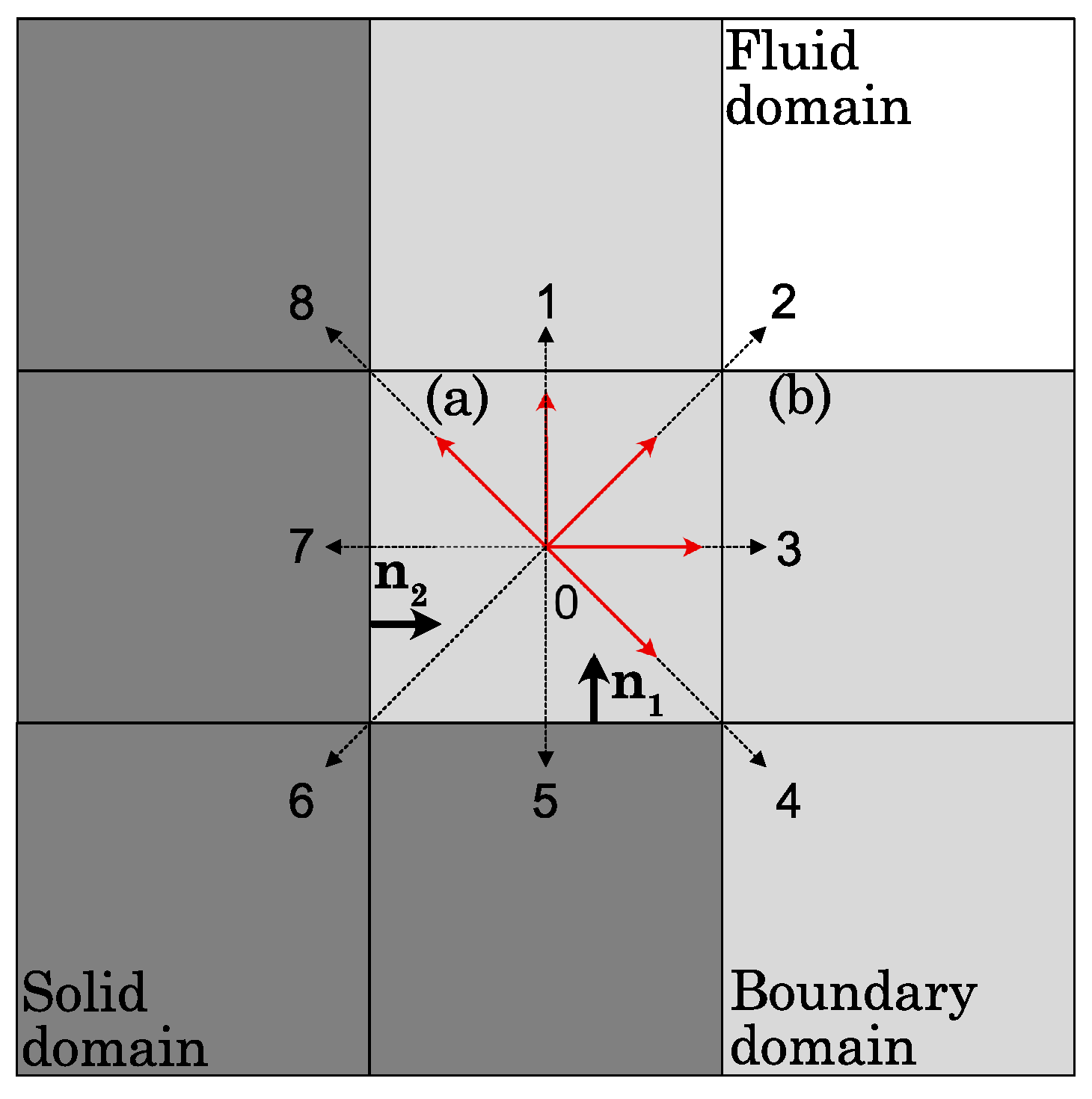
2.5. Slip Boundary Conditions for Arbitrary Geometries
- The distribution functions pointing in the opposite direction to each solid wall are unknown and undergo CBBSR treatment given by Equation (33);
- In the case where a fluid node has only a solid node in the diagonal direction, e.g., the “(b)” boundary fluid node (labeled in the top left corner in Figure 2), the unknown distribution function is updated with bounce-back treatment;
- The unknown distribution functions that have been calculated several times because of the CBBSR boundary condition, e.g., for the “(a)” boundary fluid node, are averaged by the number of times it has been updated.
3. Numerical Results
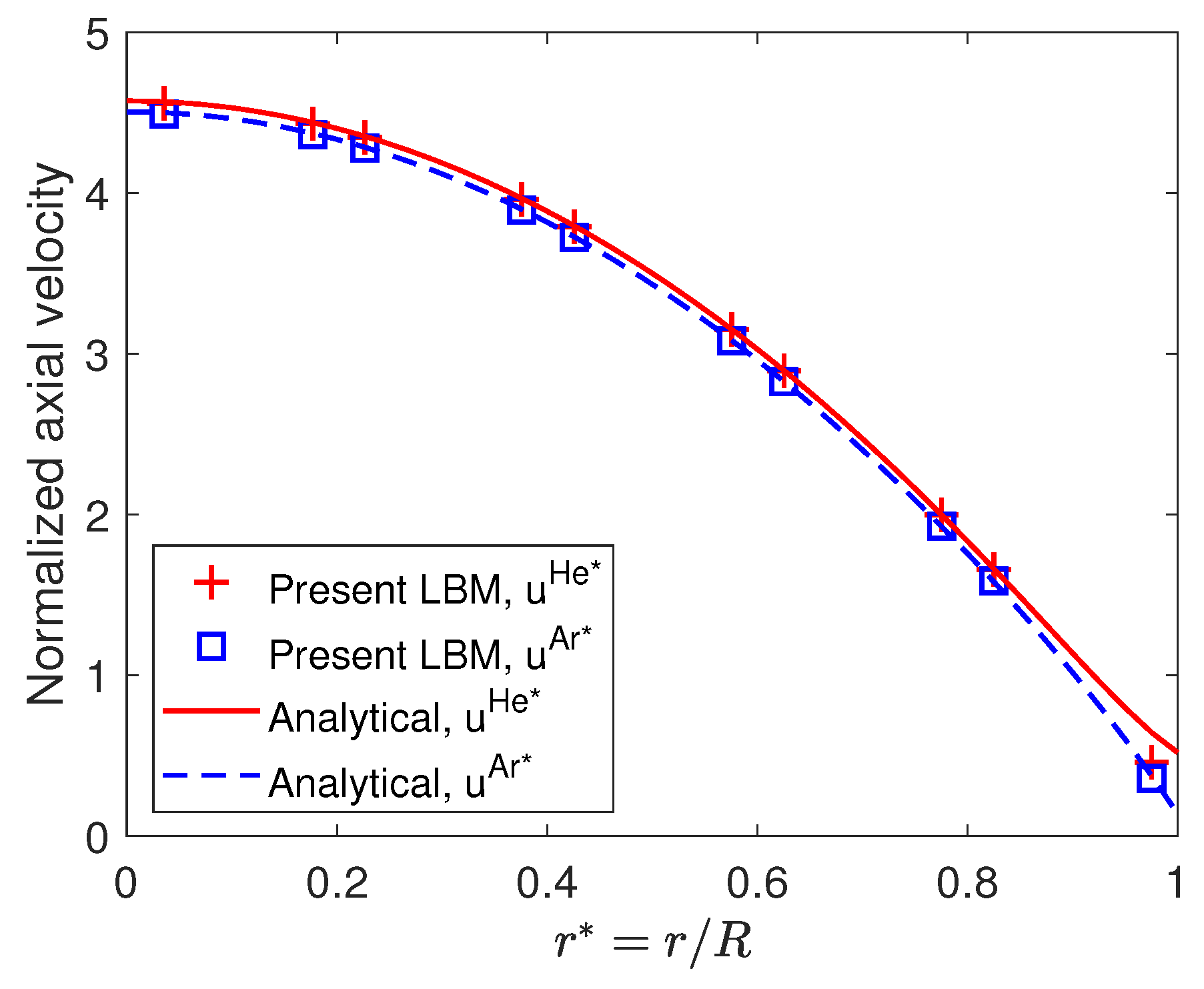
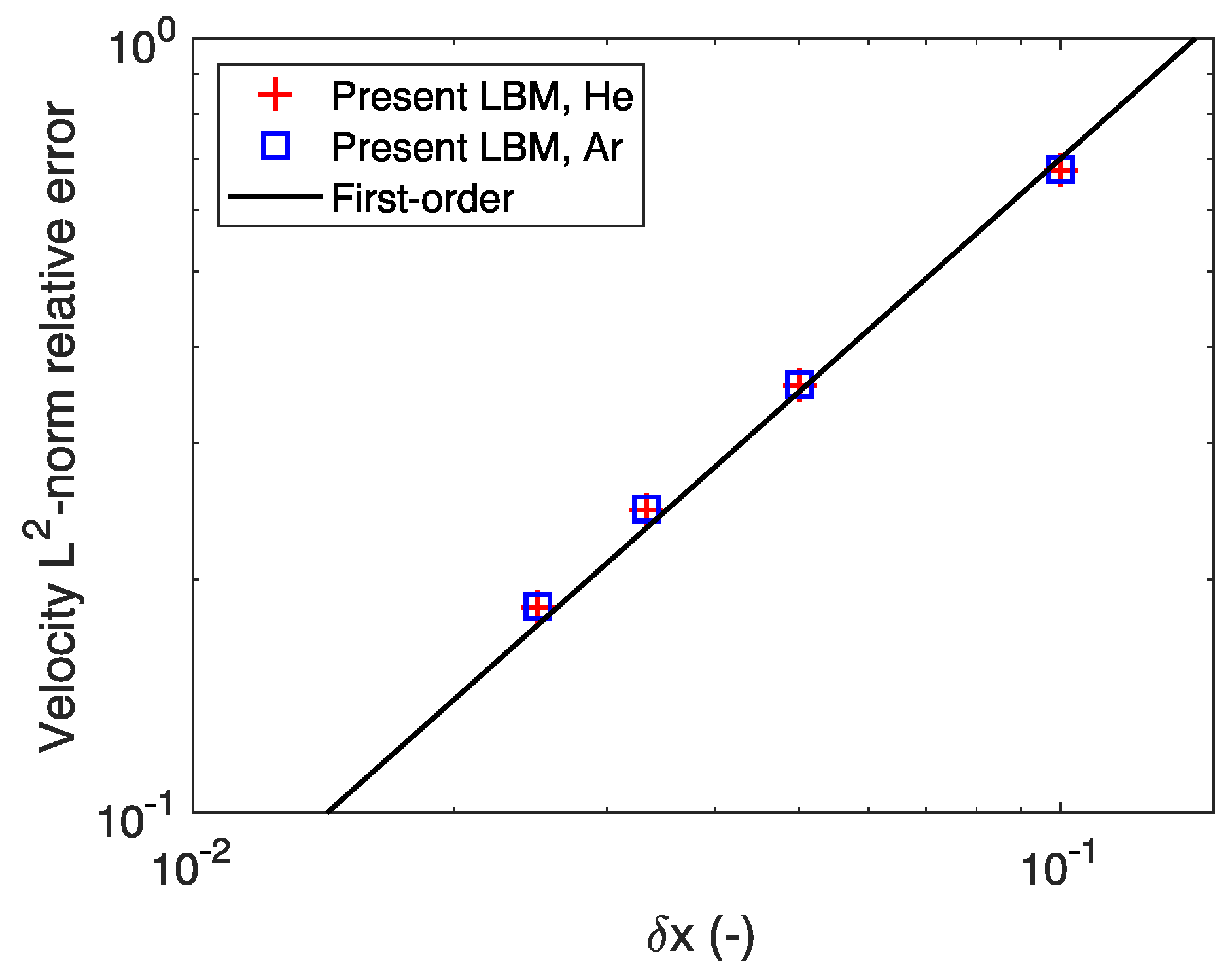
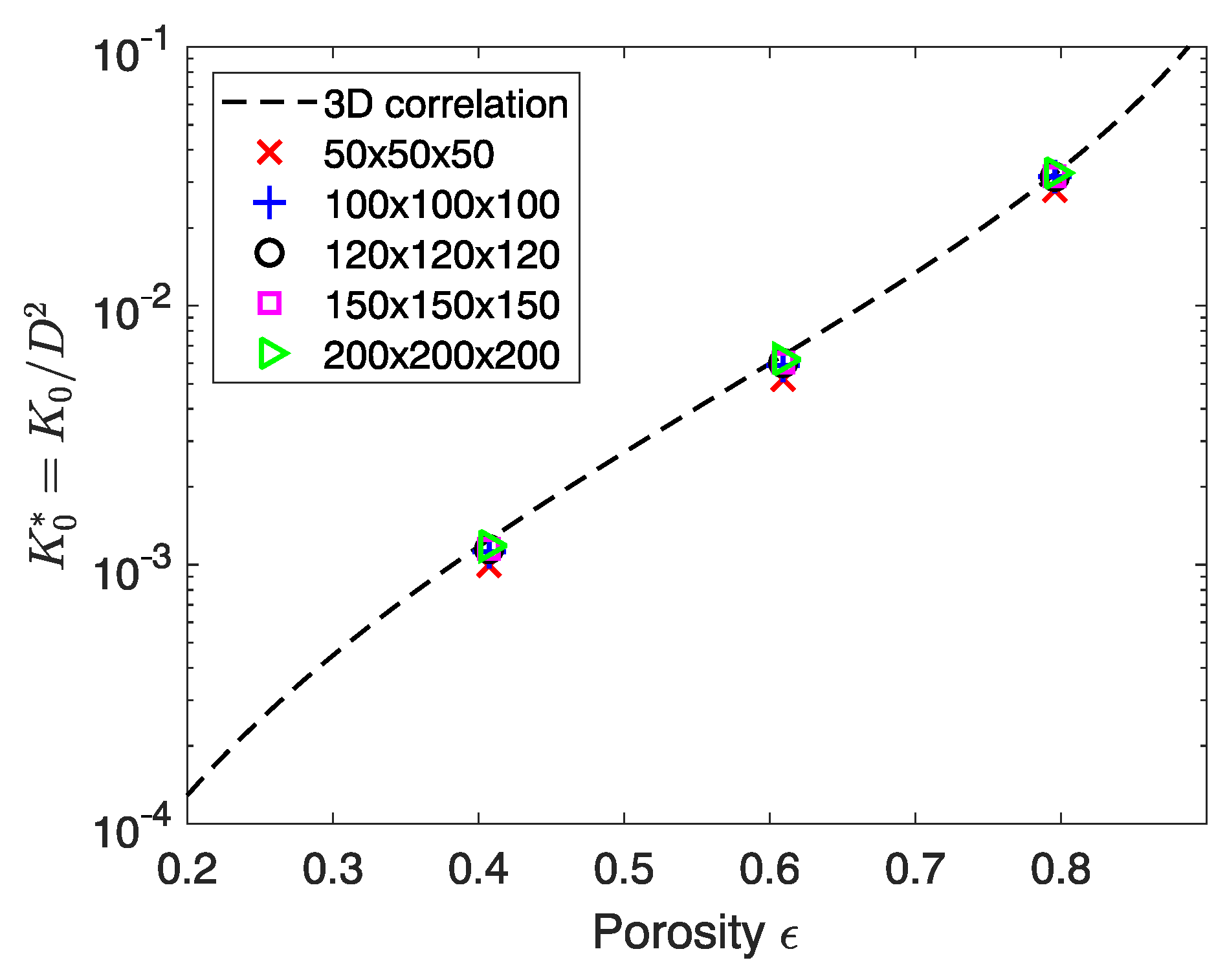
3.1. Model Verification
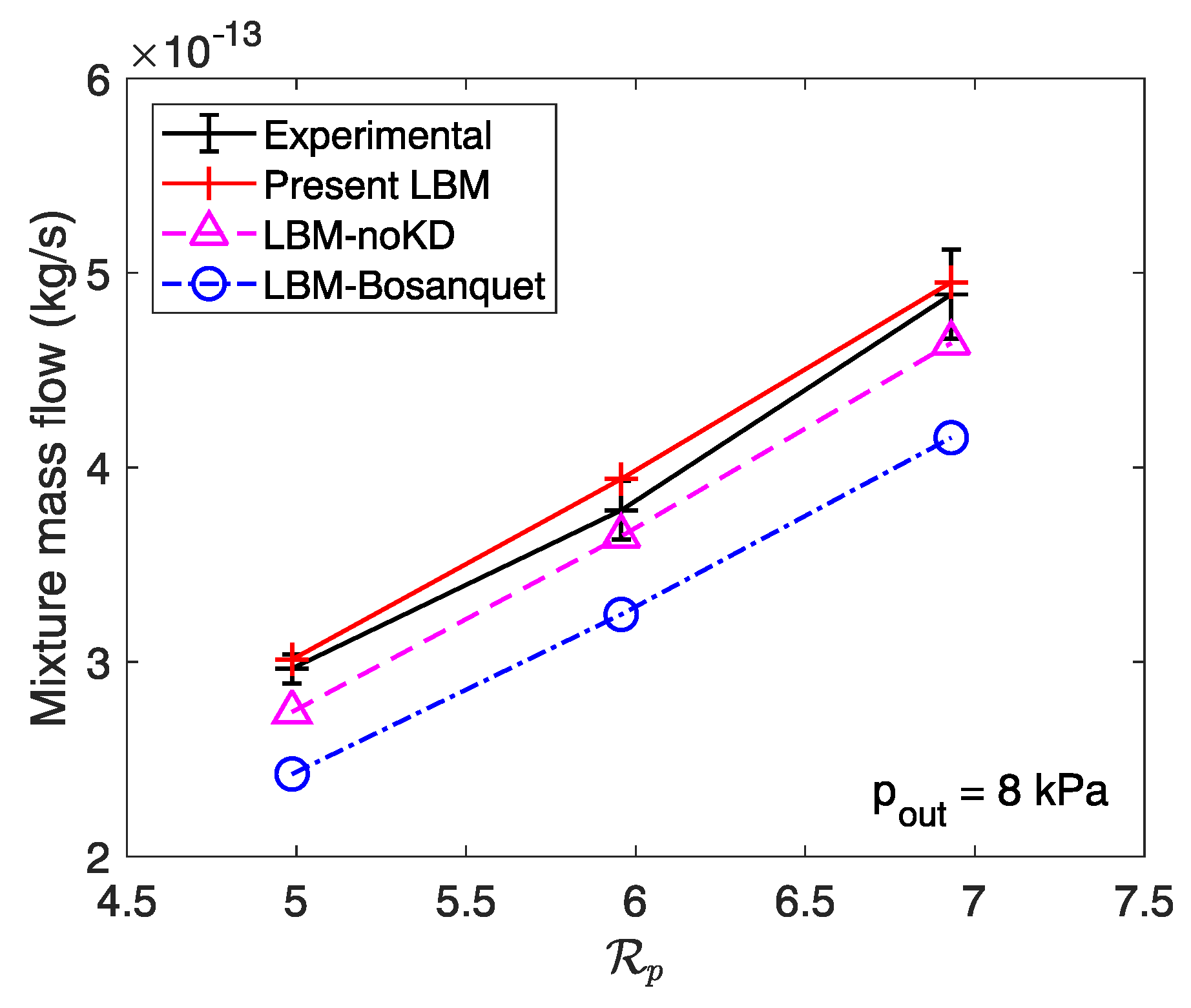
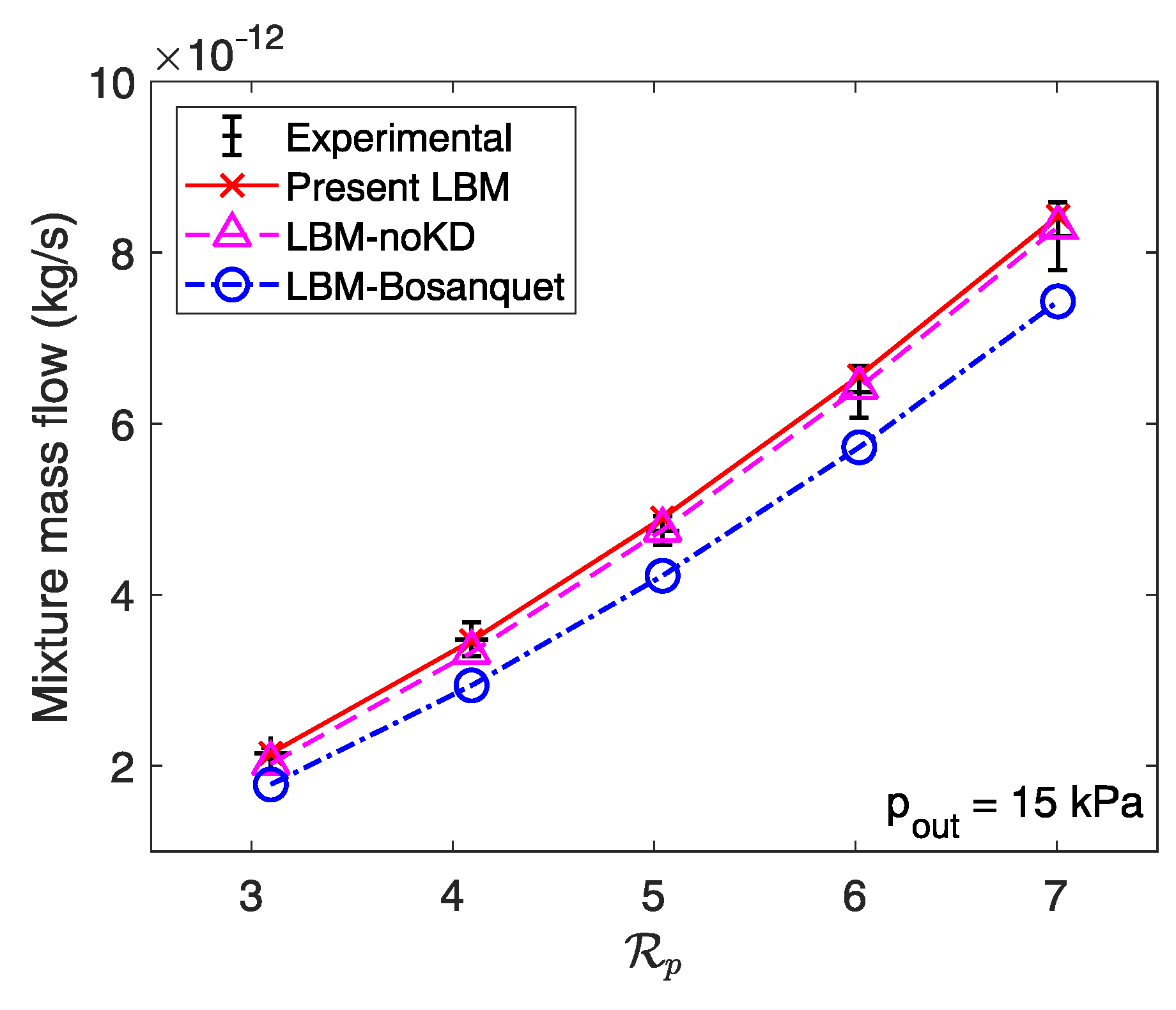
3.2. Mixture Mass Flow Rate Calculation
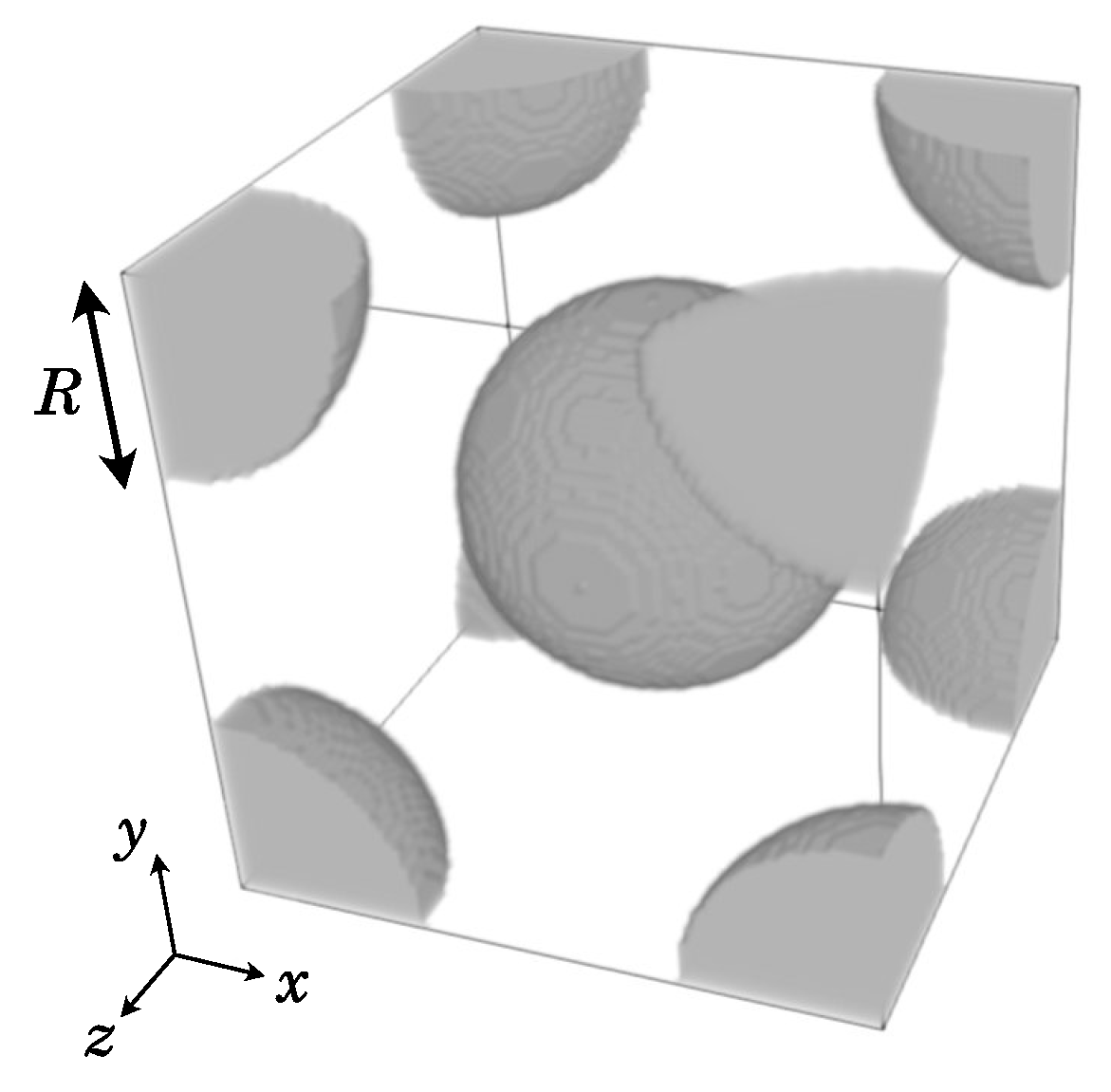
3.3. Rarefied Multicomponent Flow in Porous Media
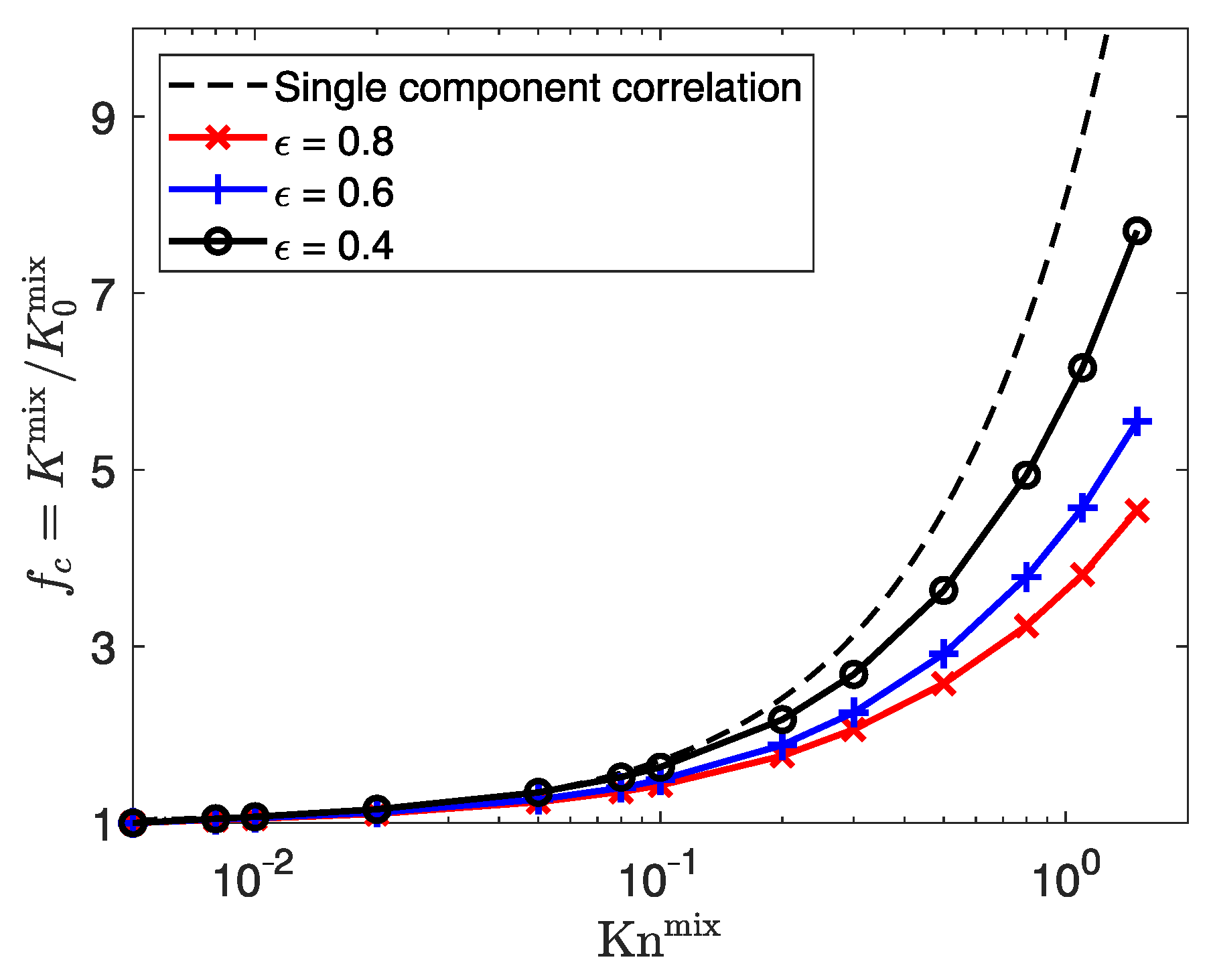
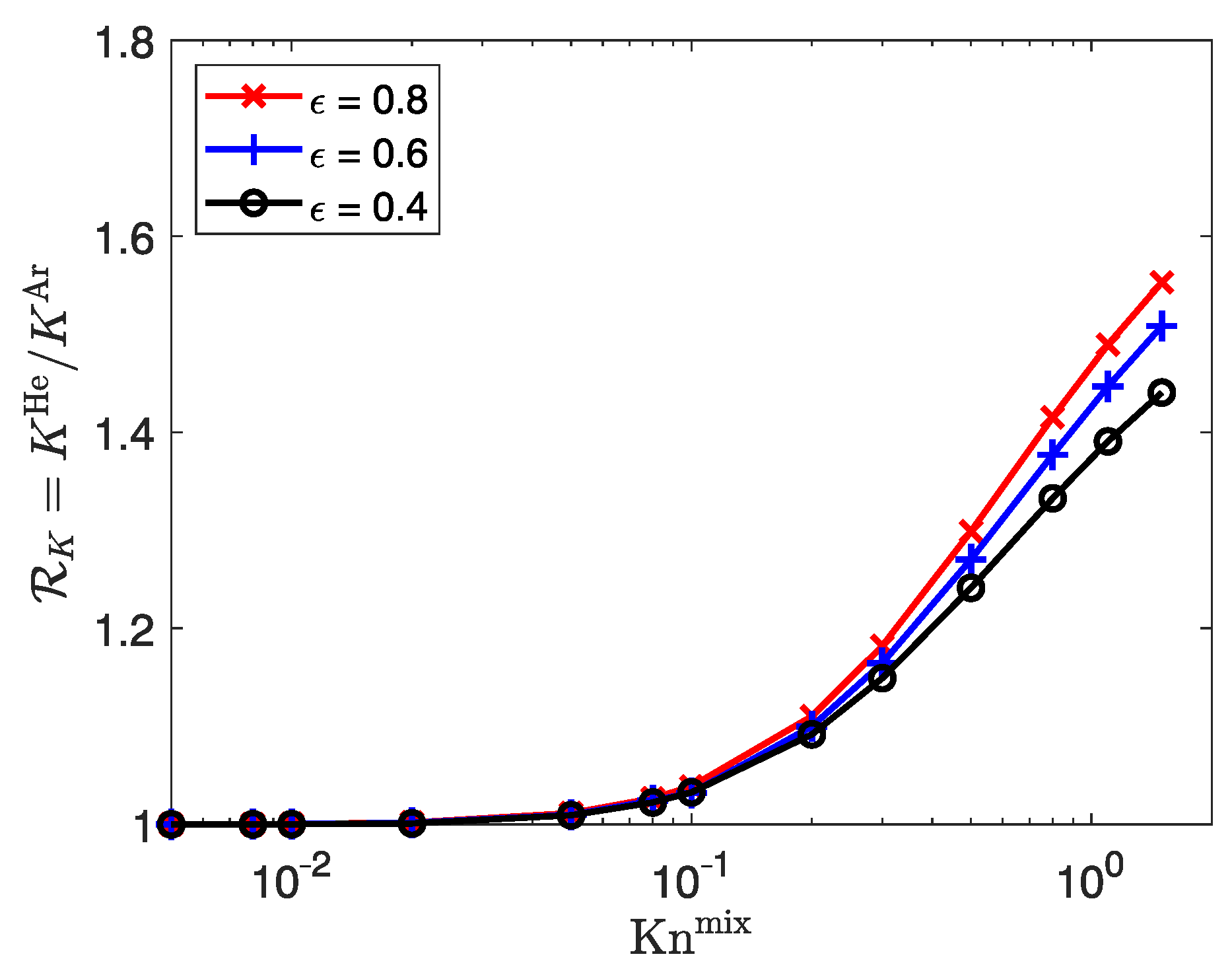
3.3.1. Influence of Porosity
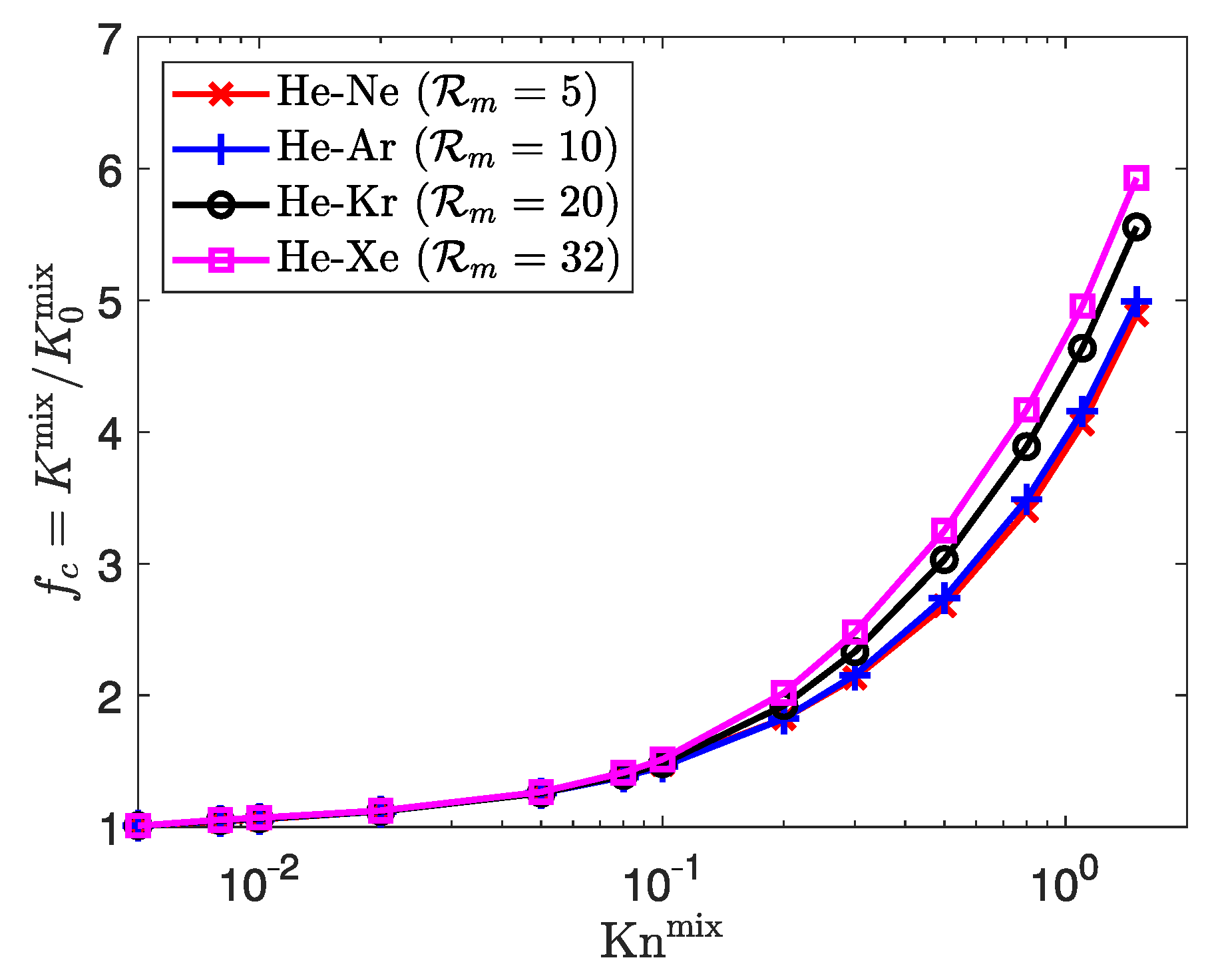
3.3.2. Influence of Mixture Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reed, B.; Dang, L. Experimental evaluation of cold flow micronozzles. In Proceedings of the 37th Joint Propulsion Conference and Exhibit, Salt Lake City, UT, USA, 8–11 July 2001; p. 3521. [Google Scholar]
- Kovvali, A.S.; Sirkar, K. Dendrimer liquid membranes: CO2 separation from gas mixtures. Ind. Eng. Chem. Res. 2001, 40, 2502–2511. [Google Scholar] [CrossRef]
- Takahashi, Y.; Okajima, J.; Iga, Y.; Komiya, A.; Fu, W.S.; Maruyama, S. Study of supersonic micro-channel for cooling electronic devices. In Proceedings of the International Conference on Nanochannels, Microchannels, and Minichannels, Sapporo, Japan, 16–19 June 2013; American Society of Mechanical Engineers: New York, NY, USA, 2013; Volume 55591. [Google Scholar]
- Hossein-Babaei, F.; Paknahad, M.; Ghafarinia, V. A miniature gas analyzer made by integrating a chemoresistor with a microchannel. Lab Chip 2012, 12, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Görke, O.; Pfeifer, P.; Schubert, K. Highly selective methanation by the use of a microchannel reactor. Catal. Today 2005, 110, 132–139. [Google Scholar] [CrossRef]
- Ho, C.M.; Tai, Y.C. Micro-electro-mechanical-systems (MEMS) and fluid flows. Annu. Rev. Fluid Mech. 1998, 30, 579–612. [Google Scholar] [CrossRef]
- Nakaye, S.; Sugimoto, H. Demonstration of a gas separator composed of Knudsen pumps. Vacuum 2016, 125, 154–164. [Google Scholar] [CrossRef]
- Gerlach, T. Pumping gases by a silicon micro pump with dynamic passive valves. In Proceedings of the International Solid State Sensors and Actuators Conference (Transducers’ 97), Chicago, IL, USA, 19 June 1997; Volume 1, pp. 357–360. [Google Scholar]
- Wang, X.; Su, T.; Zhang, W.; Zhang, Z.; Zhang, S. Knudsen pumps: A review. Microsyst. Nanoeng. 2020, 6, 1–28. [Google Scholar] [CrossRef]
- Chai, D.; Li, X. Rarefied gas transport in heterogeneous shale matrix using a practical apparent permeability model and fuzzy statistical method. J. Pet. Sci. Eng. 2021, 206, 109029. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Kang, Q.; Rahman, S.S. The lattice Boltzmann method for isothermal micro-gaseous flow and its application in shale gas flow: A review. Int. J. Heat Mass Transf. 2016, 95, 94–108. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Kang, Q.; Rahman, S.S. Apparent permeability prediction of organic shale with generalized lattice Boltzmann model considering surface diffusion effect. Fuel 2016, 181, 478–490. [Google Scholar] [CrossRef]
- Panerai, F.; Cochell, T.; Martin, A.; White, J.D. Experimental measurements of the high-temperature oxidation of carbon fibers. Int. J. Heat Mass Transf. 2019, 136, 972–986. [Google Scholar] [CrossRef]
- Lachaud, J.; Cozmuta, I.; Mansour, N.N. Multiscale approach to ablation modeling of phenolic impregnated carbon ablators. J. Spacecr. Rocket. 2010, 47, 910–921. [Google Scholar] [CrossRef]
- Panerai, F.; Ferguson, J.C.; Lachaud, J.; Martin, A.; Gasch, M.J.; Mansour, N.N. Micro-tomography based analysis of thermal conductivity, diffusivity and oxidation behavior of rigid and flexible fibrous insulators. Int. J. Heat Mass Transf. 2017, 108, 801–811. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Panerai, F.; Lachaud, J.; Martin, A.; Bailey, S.C.; Mansour, N.N. Modeling the oxidation of low-density carbon fiber material based on micro-tomography. Carbon 2016, 96, 57–65. [Google Scholar] [CrossRef]
- Poovathingal, S.; Stern, E.C.; Nompelis, I.; Schwartzentruber, T.E.; Candler, G.V. Nonequilibrium flow through porous thermal protection materials, Part II: Oxidation and pyrolysis. J. Comput. Phys. 2019, 380, 427–441. [Google Scholar] [CrossRef]
- Gad-el Hak, M. Comments on “critical view on new results in micro-fluid mechanics”. Int. J. Heat Mass Transf. 2003, 46, 3941–3945. [Google Scholar] [CrossRef]
- Maxwell, J.C. VII. On stresses in rarified gases arising from inequalities of temperature. Philos. Trans. R. Soc. Lond. 1879, 231–256. [Google Scholar]
- Zhang, W.M.; Meng, G.; Wei, X. A review on slip models for gas microflows. Microfluid. Nanofluid. 2012, 13, 845–882. [Google Scholar] [CrossRef]
- Beskok, A.; Karniadakis, G.E. Report: A model for flows in channels, pipes, and ducts at micro and nano scales. Microscale Thermophys. Eng. 1999, 3, 43–77. [Google Scholar]
- Colin, S.; Lalonde, P.; Caen, R. Validation of a second-order slip flow model in rectangular microchannels. Heat Transf. Eng. 2004, 25, 23–30. [Google Scholar] [CrossRef]
- Chambre, P.A.; Schaaf, S.A. Flow of Rarefied Gases; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Zhang, T.; Jia, L.; Wang, Z. Validation of Navier–Stokes equations for slip flow analysis within transition region. Int. J. Heat Mass Transf. 2008, 51, 6323–6327. [Google Scholar] [CrossRef]
- Deissler, R. An analysis of second-order slip flow and temperature-jump boundary conditions for rarefied gases. Int. J. Heat Mass Transf. 1964, 7, 681–694. [Google Scholar] [CrossRef]
- Yudistiawan, W.P.; Ansumali, S.; Karlin, I.V. Hydrodynamics beyond Navier-Stokes: The slip flow model. Phys. Rev. E 2008, 78, 016705. [Google Scholar] [CrossRef]
- Le, N.T.; White, C.; Reese, J.M.; Myong, R.S. Langmuir–Maxwell and Langmuir–Smoluchowski boundary conditions for thermal gas flow simulations in hypersonic aerodynamics. Int. J. Heat Mass Transf. 2012, 55, 5032–5043. [Google Scholar] [CrossRef]
- Singh, S.; Karchani, A.; Chourushi, T.; Myong, R.S. A three-dimensional modal discontinuous Galerkin method for the second-order Boltzmann-Curtiss-based constitutive model of rarefied and microscale gas flows. J. Comput. Phys. 2022, 457, 111052. [Google Scholar] [CrossRef]
- Cercignani, C. Mathematical Methods in Kinetic Theory; Springer: Berlin/Heidelberg, Germany, 1969; Volume 1. [Google Scholar]
- Guo, Z.; Zheng, C.; Shi, B. Lattice Boltzmann equation with multiple effective relaxation times for gaseous microscale flow. Phys. Rev. E 2008, 77, 036707. [Google Scholar] [CrossRef] [PubMed]
- Tucny, J.M.; Vidal, D.; Leclaire, S.; Bertrand, F. Comparison of existing and extended boundary conditions for the simulation of rarefied gas flows using the Lattice Boltzmann method. Int. J. Mod. Phys. C 2020, 31, 2050070. [Google Scholar] [CrossRef]
- Tucny, J.M.; Vidal, D.; Leclaire, S.; Bertrand, F. Kinetic Slip Boundary Condition for Isothermal Rarefied Gas Flows Through Static Non-Planar Geometries Based on the Regularized Lattice-Boltzmann Method. Commun. Comput. Phys. 2022, 31, 816–868. [Google Scholar] [CrossRef]
- Verhaeghe, F.; Luo, L.S.; Blanpain, B. Lattice Boltzmann modeling of microchannel flow in slip flow regime. J. Comput. Phys. 2009, 228, 147–157. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zhang, L.; Shan, B. A basic model of unconventional gas microscale flow based on the lattice Boltzmann method. Pet. Explor. Dev. 2021, 48, 179–189. [Google Scholar] [CrossRef]
- Kalarakis, A.; Michalis, V.; Skouras, E.; Burganos, V. Mesoscopic simulation of rarefied flow in narrow channels and porous media. Transp. Porous Media 2012, 94, 385–398. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Tang, G.; Tao, W. Lattice Boltzmann modeling of microchannel flows in the transition flow regime. Microfluid. Nanofluid. 2011, 10, 607–618. [Google Scholar] [CrossRef]
- Tang, G.; Tao, W.; He, Y. Lattice Boltzmann method for gaseous microflows using kinetic theory boundary conditions. Phys. Fluids 2005, 17, 058101. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, R.; Emerson, D.R. Lattice Boltzmann simulation of rarefied gas flows in microchannels. Phys. Rev. E 2005, 71, 047702. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shi, B.; Zhao, T.; Zheng, C. Discrete effects on boundary conditions for the lattice Boltzmann equation in simulating microscale gas flows. Phys. Rev. E 2007, 76, 056704. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.L.; Gross, E.P.; Krook, M. A model for collision processes in gases. I. Small amplitude processes in charged and neutral one-component systems. Phys. Rev. 1954, 94, 511. [Google Scholar] [CrossRef]
- Chai, Z.; Shi, B.; Guo, Z.; Lu, J. Gas flow through square arrays of circular cylinders with Klinkenberg effect: A lattice Boltzmann study. Commun. Comput. Phys. 2010, 8, 1052. [Google Scholar]
- Landry, C.J.; Prodanović, M.; Eichhubl, P. Direct simulation of supercritical gas flow in complex nanoporous media and prediction of apparent permeability. Int. J. Coal Geol. 2016, 159, 120–134. [Google Scholar] [CrossRef]
- Ansumali, S.; Karlin, I.V. Kinetic boundary conditions in the lattice Boltzmann method. Phys. Rev. E 2002, 66, 026311. [Google Scholar] [CrossRef]
- Succi, S. Mesoscopic modeling of slip motion at fluid-solid interfaces with heterogeneous catalysis. Phys. Rev. Lett. 2002, 89, 064502. [Google Scholar] [CrossRef]
- Yuan, Y.; Rahman, S. Extended application of lattice Boltzmann method to rarefied gas flow in micro-channels. Phys. A Stat. Mech. Its Appl. 2016, 463, 25–36. [Google Scholar] [CrossRef]
- Ho, M.; Pérez, J.G.; Reggio, M.; Trépanier, J.Y. Permeability calculation of rarefied gas flows through 2D porous structures using the lattice Boltzmann method. Phys. Chem. Earth Parts A/B/C 2019, 113, 43–49. [Google Scholar] [CrossRef]
- Arlemark, E.J.; Dadzie, S.K.; Reese, J.M. An extension to the Navier–Stokes equations to incorporate gas molecular collisions with boundaries. J. Heat Transf. 2010, 132, 041006. [Google Scholar] [CrossRef]
- Dongari, N.; Zhang, Y.; Reese, J.M. Modeling of Knudsen layer effects in micro/nanoscale gas flows. J. Fluids Eng. 2011, 133, 071101. [Google Scholar] [CrossRef]
- Michalis, V.K.; Kalarakis, A.N.; Skouras, E.D.; Burganos, V.N. Rarefaction effects on gas viscosity in the Knudsen transition regime. Microfluid. Nanofluid. 2010, 9, 847–853. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, Q.; Guo, P.; Peng, S.; Wang, Z.; Du, J. Pore-scale lattice Boltzmann simulation of two-component shale gas flow. J. Nat. Gas Sci. Eng. 2019, 61, 46–70. [Google Scholar] [CrossRef]
- Klinkenberg, L. The permeability of porous media to liquids and gases. Drill. Prod. Pract. 1941, 2, 200–213. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Kang, Q.; Viswanathan, H.S.; Yao, J.; Tao, W. Nanoscale simulation of shale transport properties using the lattice Boltzmann method: Permeability and diffusivity. Sci. Rep. 2015, 5, 8089. [Google Scholar] [CrossRef]
- Zhao, Y.l.; Wang, Z.m. Prediction of apparent permeability of porous media based on a modified lattice Boltzmann method. J. Pet. Sci. Eng. 2019, 174, 1261–1268. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, H.; Li, X.; Ning, Z.; Wang, Q.; Zhao, W.; Zhang, J. Pore scale characteristics of gas flow in shale matrix determined by the regularized lattice Boltzmann method. Chem. Eng. Sci. 2018, 187, 245–255. [Google Scholar] [CrossRef]
- Ho, M.; Leclaire, S.; Reggio, M.; Trépanier, J.Y. Stochastic effects of 2D random arrays of cylinders on rarefied gas permeability using the lattice boltzmann method. Transp. Porous Media 2021, 136, 607–637. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, J.; Zhang, M.; Zhang, L.; Yang, Y.; Sun, H.; An, S.; Li, A. Study of gas flow characteristics in tight porous media with a microscale lattice Boltzmann model. Sci. Rep. 2016, 6, 32393. [Google Scholar] [CrossRef]
- Sirovich, L. Kinetic modeling of gas mixtures. Phys. Fluids 1962, 5, 908–918. [Google Scholar] [CrossRef]
- Hamel, B.B. Kinetic model for binary gas mixtures. Phys. Fluids 1965, 8, 418–425. [Google Scholar] [CrossRef]
- Kerkhof, P.J.; Geboers, M.A. Analysis and extension of the theory of multicomponent fluid diffusion. Chem. Eng. Sci. 2005, 60, 3129–3167. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wang, S.; Pan, Z.; Zhang, J.; Yang, Z.; Wang, Y.; Wu, Y.S.; Li, X.; Lukyanov, A. On the Klinkenberg effect of multicomponent gases. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2017; OnePetro: Richardson, TX, USA, 2017. [Google Scholar]
- Wang, S.; Lukyanov, A.A.; Wu, Y.S. Second-order gas slippage model for the Klinkenberg effect of multicomponent gas at finite Knudsen numbers up to 1. Fuel 2019, 235, 1275–1286. [Google Scholar] [CrossRef]
- Sun, F.; Yao, Y.; Li, G.; Dong, M. Transport behaviors of real gas mixture through nanopores of shale reservoir. J. Pet. Sci. Eng. 2019, 177, 1134–1141. [Google Scholar] [CrossRef]
- Joshi, A.S.; Peracchio, A.A.; Grew, K.N.; Chiu, W.K. Lattice Boltzmann method for multi-component, non-continuum mass diffusion. J. Phys. D Appl. Phys. 2007, 40, 7593. [Google Scholar] [CrossRef]
- Paradis, H.; Sundén, B. Evaluation of lattice Boltzmann method for reaction-diffusion process in a porous SOFC anode microstructure. In Proceedings of the International Conference on Nanochannels, Microchannels, and Minichannels, Rio Grande, Puerto Rico, 8–12 July 2012; American Society of Mechanical Engineers: New York, NY, USA, 2012; Volume 44793, pp. 163–171. [Google Scholar]
- Guo, Y.; He, X.; Huang, W.; Wang, M. Microstructure effects on effective gas diffusion coefficient of nanoporous materials. Transp. Porous Media 2019, 126, 431–453. [Google Scholar] [CrossRef]
- Zheng, W.; Kim, S.H. The effects of catalyst layer microstructure and water saturation on the effective diffusivity in PEMFC. J. Electrochem. Soc. 2018, 165, F468. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z. Lattice Boltzmann simulation of multicomponent noncontinuum diffusion in fractal porous structures. Phys. Rev. E 2015, 92, 013025. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, C.; Choi, C.; Zhang, W.; Machida, H.; Norinaga, K. Lattice Boltzmann simulation of multicomponent reaction-diffusion and coke formation in a catalyst with hierarchical pore structure for dry reforming of methane. Chem. Eng. Sci. 2021, 229, 116105. [Google Scholar] [CrossRef]
- Asinari, P.; Luo, L.S. A consistent lattice Boltzmann equation with baroclinic coupling for mixtures. J. Comput. Phys. 2008, 227, 3878–3895. [Google Scholar] [CrossRef]
- d’Humières, D. Generalized lattice-Boltzmann equations. In Rarefied Gas Dynamics; Springer US: New York, NY, USA, 1992. [Google Scholar]
- Luo, L.S.; Girimaji, S.S. Lattice Boltzmann model for binary mixtures. Phys. Rev. E 2002, 66, 035301. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, S.; Karlin, I.; Mantzaras, J.; Frouzakis, C. Lattice Boltzmann model for the simulation of multicomponent mixtures. Phys. Rev. E 2007, 76, 046703. [Google Scholar] [CrossRef] [PubMed]
- Asinari, P. Viscous coupling based lattice Boltzmann model for binary mixtures. Phys. Fluids 2005, 17, 067102. [Google Scholar] [CrossRef]
- Asinari, P. Multiple-relaxation-time lattice Boltzmann scheme for homogeneous mixture flows with external force. Phys. Rev. E 2008, 77, 056706. [Google Scholar] [CrossRef]
- Guo, Z.; Asinari, P.; Zheng, C. Lattice Boltzmann equation for microscale gas flows of binary mixtures. Phys. Rev. E 2009, 79, 026702. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Guo, Z. Lattice Boltzmann simulation of separation phenomenon in a binary gaseous flow through a microchannel. J. Appl. Phys. 2016, 120, 134306. [Google Scholar] [CrossRef]
- Ho, M.; Ammar, S.; Leclaire, S.; Reggio, M.; Trépanier, J.Y. Lattice Boltzmann Modeling of Miscible Multicomponent Gas Mixtures in the Rarefied Regime. Commun. Comput. Phys. 2022, 32, 1179–1216. [Google Scholar]
- Kerkhof, P.J.; Geboers, M.A. Toward a unified theory of isotropic molecular transport phenomena. AIChE J. 2005, 51, 79–121. [Google Scholar] [CrossRef]
- Vienne, L.; Marié, S.; Grasso, F. Lattice Boltzmann method for miscible gases: A forcing-term approach. Phys. Rev. E 2019, 100, 023309. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, J.; Zhu, Y.; Wang, F.; Wu, H. Multiscale transport mechanism of shale gas in micro/nano-pores. Int. J. Heat Mass Transf. 2017, 111, 1172–1180. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Q.; Pawar, R.; He, Y.L.; Tao, W.Q. Pore-scale prediction of transport properties in reconstructed nanostructures of organic matter in shales. Fuel 2015, 158, 650–658. [Google Scholar] [CrossRef]
- Engel, T.; Reid, P.J. Thermodynamics, Statistical Thermodynamics, and Kinetics; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Dongari, N.; Sharma, A.; Durst, F. Pressure-driven diffusive gas flows in micro-channels: From the Knudsen to the continuum regimes. Microfluid. Nanofluid. 2009, 6, 679–692. [Google Scholar] [CrossRef]
- Tucny, J.M.; Leclaire, S.; Vidal, D.; Bertrand, F. Computation of effective viscosities for rarefied gas flows using Ray-tracing. Int. J. Appl. Comput. Math. 2023, 9, 110. [Google Scholar] [CrossRef]
- Tucny, J.M. Modélisation des écoulements de gaz raréfiés au travers de filtres fibreux par la méthode de Boltzmann sur réseau. Ph.D. Thesis, Polytechnique Montréal, Montreal, QC, Canada, 2020. [Google Scholar]
- Leclaire, S.; Parmigiani, A.; Malaspinas, O.; Chopard, B.; Latt, J. Generalized three-dimensional lattice Boltzmann color-gradient method for immiscible two-phase pore-scale imbibition and drainage in porous media. Phys. Rev. E 2017, 95, 033306. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shu, C. Lattice Boltzmann Method and Its Application in Engineering; World Scientific: Singapore, 2013; Volume 3. [Google Scholar]
- Ammar, S.; Pernaudat, G.; Trépanier, J.Y. A multiphase three-dimensional multi-relaxation time (MRT) lattice Boltzmann model with surface tension adjustment. J. Comput. Phys. 2017, 343, 73–91. [Google Scholar] [CrossRef]
- d’Humières, D. Multiple–relaxation–time lattice Boltzmann models in three dimensions. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2002, 360, 437–451. [Google Scholar] [CrossRef]
- Yu, H.; Luo, L.S.; Girimaji, S.S. LES of turbulent square jet flow using an MRT lattice Boltzmann model. Comput. Fluids 2006, 35, 957–965. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, C.; Shi, B. Discrete lattice effects on the forcing term in the lattice Boltzmann method. Phys. Rev. E 2002, 65, 046308. [Google Scholar] [CrossRef]
- Leclaire, S.; El-Hachem, M.; Trépanier, J.Y.; Reggio, M. High order spatial generalization of 2D and 3D isotropic discrete gradient operators with fast evaluation on GPUs. J. Sci. Comput. 2014, 59, 545–573. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1964. [Google Scholar]
- Vienne, L. Simulation of Multi-Component Flows by the Lattice Boltzmann Method and Application to the Viscous Fingering Instability. Ph.D. Thesis, Conservatoire National des Arts et Metiers-CNAM, Paris, France, 2019. [Google Scholar]
- Stops, D. The mean free path of gas molecules in the transition regime. J. Phys. D Appl. Phys. 1970, 3, 685. [Google Scholar] [CrossRef]
- Dongari, N.; Zhang, Y.; Reese, J.M. Molecular free path distribution in rarefied gases. J. Phys. D Appl. Phys. 2011, 44, 125502. [Google Scholar] [CrossRef]
- Guo, Z.; Shi, B.; Zheng, C.G. An extended Navier-Stokes formulation for gas flows in the Knudsen layer near a wall. EPL (Europhys. Lett.) 2007, 80, 24001. [Google Scholar] [CrossRef]
- Abramov, R.V. Gas near a wall: Shortened mean free path, reduced viscosity, and the manifestation of the Knudsen layer in the Navier–Stokes solution of a shear flow. J. Nonlinear Sci. 2018, 28, 833–845. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, C. Analysis of lattice Boltzmann equation for microscale gas flows: Relaxation times, boundary conditions and the Knudsen layer. Int. J. Comput. Fluid Dyn. 2008, 22, 465–473. [Google Scholar] [CrossRef]
- Ivchenko, I.; Loyalka, S.; Tompson, R. Slip coefficients for binary gas mixtures. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 1997, 15, 2375–2381. [Google Scholar] [CrossRef]
- Chapman, S.; Cowling, T.G. The Mathematical Theory of Non-Uniform Gases: An Account of the Kinetic Theory of Viscosity, Thermal Conduction and Diffusion in Gases; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Zou, Q.; He, X. On pressure and velocity boundary conditions for the lattice Boltzmann BGK model. Phys. Fluids 1997, 9, 1591–1598. [Google Scholar] [CrossRef]
- Latt, J.; Chopard, B. Lattice Boltzmann method with regularized pre-collision distribution functions. Math. Comput. Simul. 2006, 72, 165–168. [Google Scholar] [CrossRef]
- Pitakarnnop, J.; Varoutis, S.; Valougeorgis, D.; Geoffroy, S.; Baldas, L.; Colin, S. A novel experimental setup for gas microflows. Microfluid. Nanofluid. 2010, 8, 57–72. [Google Scholar] [CrossRef]
- Szalmas, L.; Colin, S.; Valougeorgis, D. Flow rate measurements of binary gas mixtures through long trapezoidal microchannels. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2012; Volume 362, p. 012003. [Google Scholar]
- Jeong, N.; Choi, D.H.; Lin, C.L. Prediction of Darcy–Forchheimer drag for micro-porous structures of complex geometry using the lattice Boltzmann method. J. Micromech. Microeng. 2006, 16, 2240. [Google Scholar] [CrossRef]
- Yang, G.; Weigand, B. Investigation of the Klinkenberg effect in a micro/nanoporous medium by direct simulation Monte Carlo method. Phys. Rev. Fluids 2018, 3, 044201. [Google Scholar] [CrossRef]
- Valougeorgis, D.; Vargas, M.; Naris, S. Analysis of gas separation, conductance and equivalent single gas approach for binary gas mixture flow expansion through tubes of various lengths into vacuum. Vacuum 2016, 128, 1–8. [Google Scholar] [CrossRef]
- Szalmas, L.; Pitakarnnop, J.; Geoffroy, S.; Colin, S.; Valougeorgis, D. Comparative study between computational and experimental results for binary rarefied gas flows through long microchannels. Microfluid. Nanofluid. 2010, 9, 1103–1114. [Google Scholar] [CrossRef]
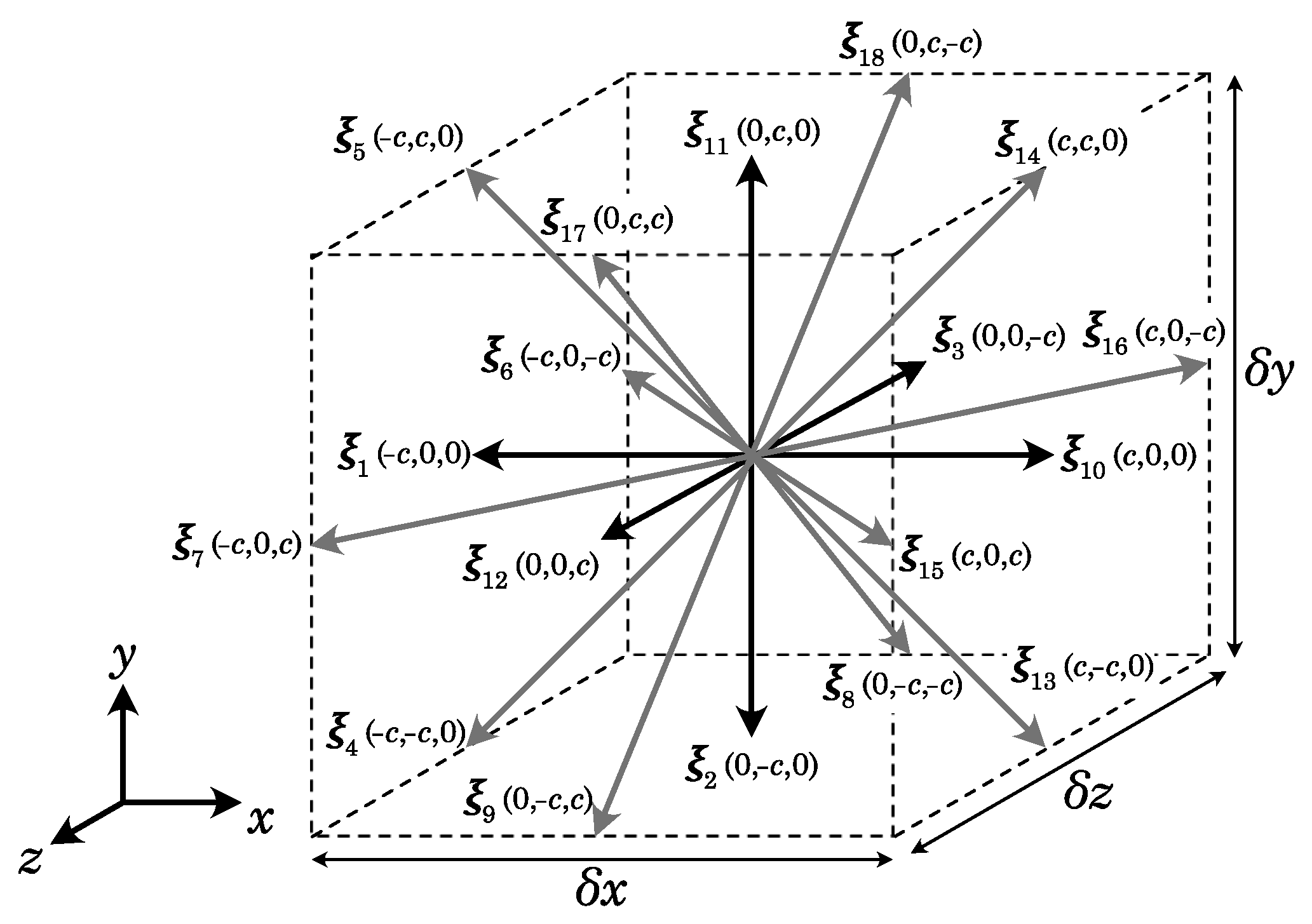


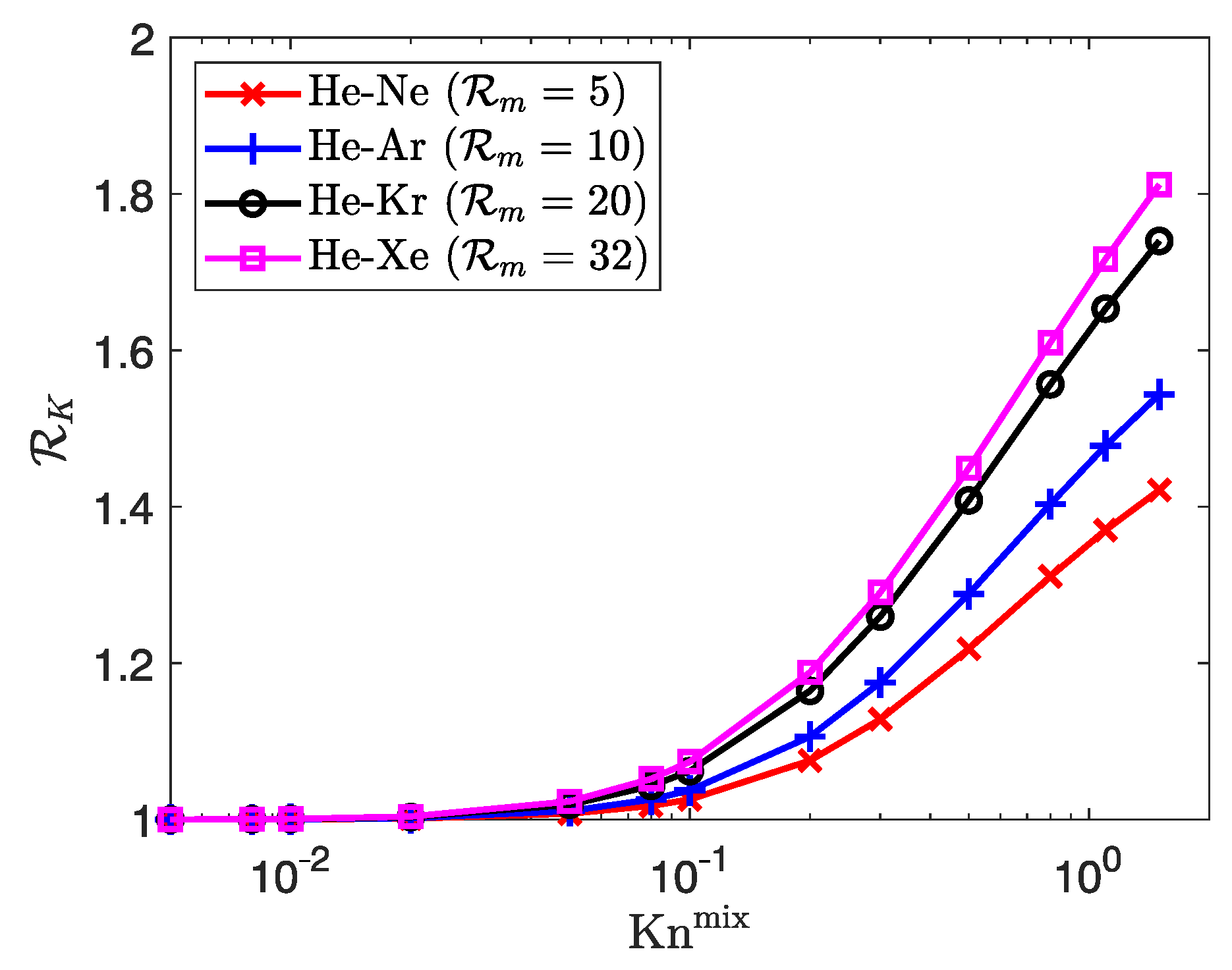
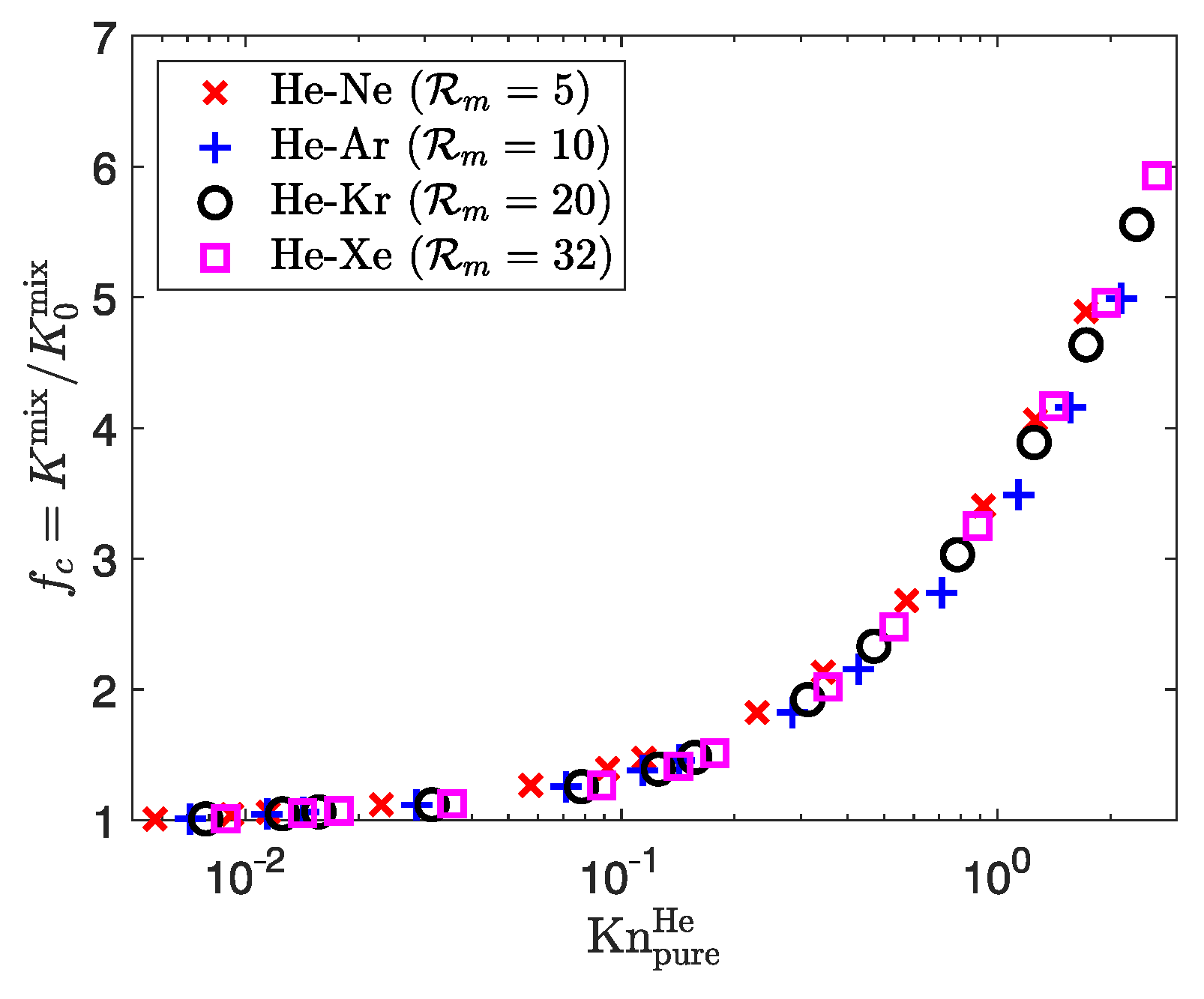
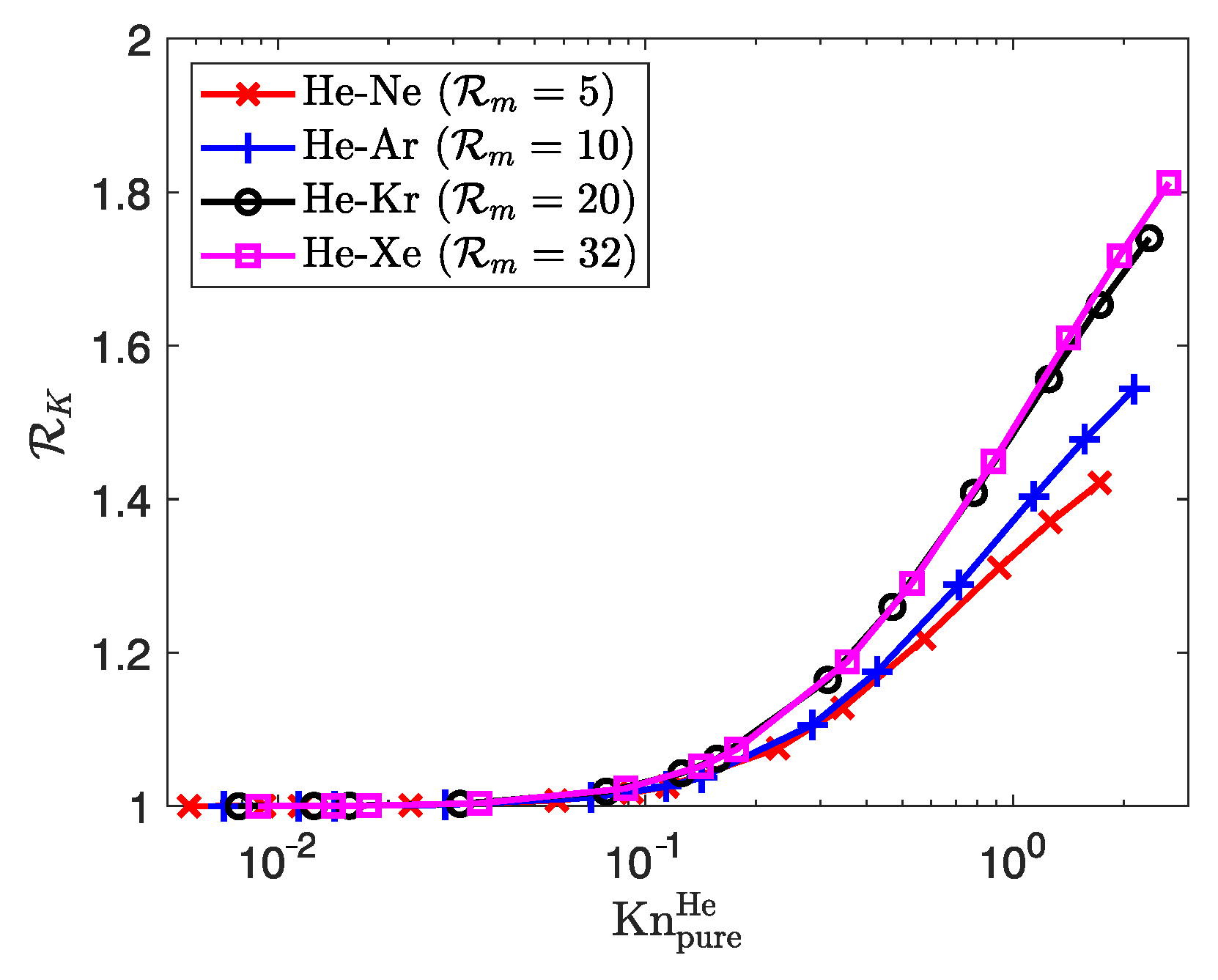
| Property | Unit | He | Ne | Ar | Kr | Xe |
|---|---|---|---|---|---|---|
| Mass | [g/mol] | 4.003 | 20.18 | 39.94 | 83.80 | 131.3 |
| Diameter | [m] | 2.745 | 2.602 | 3.659 | 4.199 | 4.939 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, M.; Tucny, J.-M.; Ammar, S.; Leclaire, S.; Reggio, M.; Trépanier, J.-Y. Lattice Boltzmann Model for Rarefied Gaseous Mixture Flows in Three-Dimensional Porous Media Including Knudsen Diffusion. Fluids 2024, 9, 237. https://doi.org/10.3390/fluids9100237
Ho M, Tucny J-M, Ammar S, Leclaire S, Reggio M, Trépanier J-Y. Lattice Boltzmann Model for Rarefied Gaseous Mixture Flows in Three-Dimensional Porous Media Including Knudsen Diffusion. Fluids. 2024; 9(10):237. https://doi.org/10.3390/fluids9100237
Chicago/Turabian StyleHo, Michel, Jean-Michel Tucny, Sami Ammar, Sébastien Leclaire, Marcelo Reggio, and Jean-Yves Trépanier. 2024. "Lattice Boltzmann Model for Rarefied Gaseous Mixture Flows in Three-Dimensional Porous Media Including Knudsen Diffusion" Fluids 9, no. 10: 237. https://doi.org/10.3390/fluids9100237
APA StyleHo, M., Tucny, J.-M., Ammar, S., Leclaire, S., Reggio, M., & Trépanier, J.-Y. (2024). Lattice Boltzmann Model for Rarefied Gaseous Mixture Flows in Three-Dimensional Porous Media Including Knudsen Diffusion. Fluids, 9(10), 237. https://doi.org/10.3390/fluids9100237