Abstract
We investigate the rheological behavior of aqueous solutions containing animal gelatin, sugars and polyols. The aim is to study how the gelation kinetics, transition temperatures and gel strengths of an aqueous gelatin solution can be affected by the progressive addition of co-solutes. Aqueous solutions with a fixed mass percentage of gelatin of wt% were prepared at various concentrations of sugars and polyols. Through Dynamic Temperature Ramp tests, performed at various ramp rates, and Dynamic Time Sweep and Dynamic Frequency Sweep tests, carried out at different temperatures, it was possible both to evaluate the transition temperatures and to monitor the gelation kinetics of the samples. It was found that the contribution of co-solutes positively affects both the gelation process and the thermal stability of the aqueous gelatin solution by reducing the gelation time and improving the mechanical properties of the gel in terms of network elasticity.
1. Introduction
Gelatin is a high-molecular-weight bio-polymer that derives from the partial hydrolysis of collagen [1,2]. The latter can be found in animal connective tissue and in bones [3]. The main source to produce gelatin is pig and bovine skin, although gelatins derived from fish exist too [4,5].
Gelatin is mainly composed of proteins (85–92%), mineral salts, and water [6]. The exact chemical composition and structure of gelatin cannot be defined due to the dependence on the raw material used (source and age of the animal), and on the type of treated collagen. In this respect, Ricard-Blum identified 28 different types of collagen [7] that contain at least one triple-helical domain.
Gelatin is easily soluble in water at temperatures above 30 °C. By decreasing the temperature of the solutions, a thermoreversible physical gel is formed [8]. The physical network is composed of triple helices that link together to form a three-dimensional structure [9,10,11,12].
Gelatin gels, in a given concentration range, have the unique feature to be in the sol state at (producing the so-called “melt in the mouth” effect), and in the gel state at [13].
Due to its firm and elastic nature, animal gelatin is one of the most versatile biopolymers with numerous applications in food, confectionery, pharmaceutical, cosmetic and packaging fields [14], mainly at concentrations above 5 wt% [15]. In particular, confectionery is a relevant segment of the food industry manufacturing, and one of its flagship products is candy. Among candies, jellies are a top selling segment. Jellies are a category of sugar confectionery products, mainly composed by co-solutes such as sugars, polyols, glucose syrup and a gelling agent [16]. Animal gelatin is the most common gelling agent. Others are gellan gum, pectin and modified starch [15,17]. Each hydrocolloid imparts its own unique texture and organoleptic properties to the finished product [18].
The jelly matrix is a gel-like network, where co-solutes are structured with the gelling agent [19]. El-Nawai and Heikel studied the non-monotonic effect of PH on jellies’ strength with various amounts of sugars [20]. The physico-chemical and mechanical properties of jellies depend on the formation of physical junctions between the gelling agents and water and sugars in the gel matrix [21]. Sugars are also able to enhance the sweetening power of the final product [22].
Despite the sugar co-solutes not being involved in the polymer network, they can contribute to the formation of confectionery gels [23]. Some works have reported the possibility of affecting the thermal stability as well as the gel strength by adding sugars and polyols to aqueous gelatin solutions [24,25,26,27]. Shimizu and coauthors [27,28] found, through statistical thermodynamics performed on gelatin and k-carrageenan solutions, that co-solvents are excluded from the sol state more than from the gel state. Wang and Hartel [21] proposed an effective mechanism to explain the gelatin gel enhancement by sugars and polyols: (i) sugars/polyols modify the hydrogen-bonding structure of water, thus destabilizing the sol phase and promoting the gel formation; (ii) the strongly hydrated sugars/polyols reduce the available water molecules, thus increasing the gelatin concentration; (iii) the exclusion of sugars and polyols from biopolymer surfaces leads to an enhancement of the gelatin molecule aggregation.
Polysaccharide gels display a similar behavior compared to gelatin gels, up to sugar concentrations of about [29]. For higher concentrations, a non-motonic behavior of the elastic modulus is observed for gelatin and polysaccharide gels. Similar results have been found by Doyle et al. on cryogels of locust bean gum with fructose, sucrose and glucose [30]. Yang et al. [31] report the effect of sucrose, up to 30 wt%, on aqueous -carrageenan solutions, showing that both transition temperatures and gel strength increase with sugar content. The increase in the melting temperature can be ascribed to an increase in OH-groups present on sugars, which leads to a change in the water structure or to a different interaction between OH groups and k-carrageenan [32].
As reported by Miyoshi et al. [33], upon increasing the sugar concentration up to in aqueous gellan solutions, higher values of the viscoelastic moduli are observed. This result demonstrates the positive effect of the glucose on the mechanical properties of gellan gels. Moreover, a similar trend was also reported for pectin gels by Kastner et al. [34].
The order of dissolution of sugars and hydrocolloids in solution also plays a key role. In a recent work, Yang et al. [35] showed that, depending on the order of addition of sugar and hydrocolloid (agar in the specific case) to the solution, a different behavior of the mechanical properties is found. The authors experimentally demonstrated that for solutions prepared by adding the bio-polymer to a sugar solution at 40%, the resulting gel shows lower mechanical strength and high opacity. This behavior results from a lower homogeneity of the microstructure, although such a molecular architecture leads to better sugar release.
Despite the great number of studies on this topic, which involve various hydrocolloids and numerous, even chemically modified [36,37], co-solutes, detailed rheological characterizations and a possible microstructural picture on the gelation kinetics of gelatin gels in the presence of a large amount of co-solutes are rare in the literature. More specifically, this work focuses on a thermo-rheological investigation of multi-component aqueous gelatin solutions. To carry out the analysis, a fixed-weight percentage ( wt%) of gelatin in solution was used, while the co-solute mass percentage as well as the number of co-solutes were varied. The co-solutes were added in increasing number, from one to four. The gelation kinetics of the multi-component gelatin solutions was studied under non-isothermal and isothermal conditions.
2. Materials and Methods
2.1. Chemicals
Pig skin gelatin with 275 Bloom grade, sucrose, dextrose, and sorbitol were kindly supplied by Perfetti Van Melle, Italy. All reagents were food grade and used as received. Bi-distilled water was used to prepare solutions.
2.2. Sample Preparation
Multi-component gelatin solutions were prepared by dissolving each component in bi-distilled water using a magnetic stirrer at 360 rpm and 60 °C for 2 h, to guarantee complete dissolution. Then, each solution was transferred to a glass bottle and stored at room temperature. For each sample, the measured pH value was approximately 5, and no pH adjustment was performed, in view of the weak dependence of the rheological properties on pH [38]. Each multi-component solution was prepared by keeping fixed the gelatin concentration at wt%, as shown in Table 1. The number close to the term “Sol.” in Table 1 stands for the number of components in solution, and the letter S or D defines the sugar (sucrose or dextrose, respectively). Before each rheological test, solutions were kept at 60 °C for 15 min in order to erase any thermal history of the samples.

Table 1.
Composition of multi-component gelatin solutions.
2.3. Rheological Measurements
Dynamic rheological measurements were carried out in a rotational stress-controlled rheometer (Discovery Hybrid Rheometer 2, TA Instruments, New Castle, DE, USA) equipped with a Peltier unit for temperature control and 40 mm-diameter sandblasted parallel plates. During non-isothermal tests, to account for metal thermal expansion, a coefficient of 0.957 m/°C was used. All tests were performed by using a gap of 1 mm and a solvent trap to minimize sample evaporation at high temperature.
Dynamic Temperature Ramp Tests (DTRTs) were conducted by imposing a frequency of 10 rad/s and a deformation of 5%, to remain within the linear viscoelastic regime, as proven by previous strain sweep tests (not reported). Each DTRT was performed in a temperature range between 60 °C and −5 °C, by imposing specific cooling/heating rates (1 °C/min, 3 °C/min and 5 °C/min), in order to detect the temperature dependence of the viscoelastic moduli. Solutions were loaded at 60 °C, cooled down to −5 °C and, after a soaking time of 300 s, heated up again to 60 °C. Such a test allowed us to monitor the evolution of viscoelastic moduli over the temperature and to evaluate the transition temperatures. The latter were defined as the minimum of the derivative of with respect to temperature [39,40,41]. The transition temperature during a cooling ramp was indicated as , whereas the transition temperature during a heating ramp was .
Depending on the nature of the solution, Dynamic Time Sweep Tests (DTSTs) were performed in isothermal conditions in a selected temperature range between 21 °C and 30 °C. The sample was loaded at 60 °C and cooled down to the test temperature by a cooling ramp of 10 °C/min, a frequency of 10 rad/s and a deformation of 5%. When the sample reached the reference temperature, the test was started and the viscoelastic moduli were measured as a function of time. The gel time, , was defined as the time at which the viscoelastic moduli are equal [42,43].
Dynamic Frequency Sweep Tests (DFSTs) were performed at 5 °C with a linear strain of 5% and in a frequency range between 100 and 0.1 rad/s. Before the test, each solution was loaded at 60 °C, rapidly cooled to 5 °C by a cooling rate of 10 °C/min and kept at 5 °C for 1 h.
3. Experimental Results and Discussion
3.1. DTRTs on Multi-Component Aqueous Gelatin Solutions
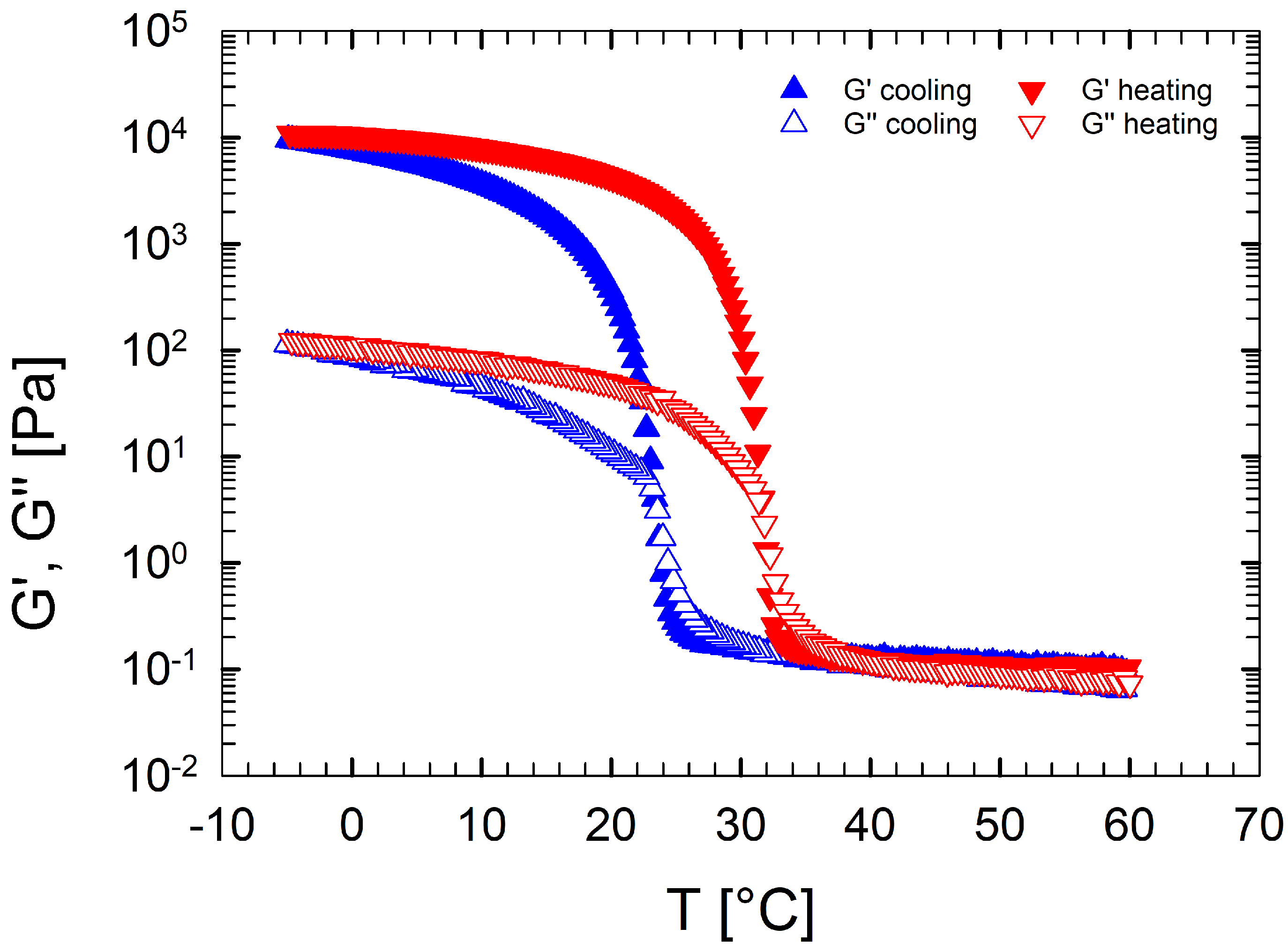
Figure 1 reports the evolution of the elastic and viscous moduli as a function of temperature for an aqueous gelatin solution (Sol. 2) during a DTRT performed at 1 °C/min both in cooling and in heating. As reported elsewhere [44], at high temperatures, the sample is characterized by very low viscoelastic moduli due to the high chain mobility in solution. In such conditions, the sample behaves as a viscous liquid. During cooling, the gelation process takes place and a three-dimensional network is built up [45]. An onset temperature close to 24 °C is evidenced by the abrupt increase in moduli, highlighting the incoming gelation. At low temperatures, a solid-like structure is observed, characterized by an elastic modulus that exceeds the viscous modulus by two orders of magnitude. In such conditions, the microstructure is formed by triple helices connected to each other through hydrogen bonds [46].

Figure 1.
Storage modulus and loss modulus as functions of the temperature for an aqueous gelatin solution (Sol. 2) at 1 °C/min. Up-triangles indicate the cooling phase, down-triangles the heating phase.
During the heating ramp, the process is reversed: melting takes place, characterized by a steep decrease in the moduli. Finally, at high temperatures, and return to their initial values, proving that gelation is thermoreversible.
Figure 1 clearly shows the existence of an hysteresis between the cooling and the heating ramps whose broadness depends on the imposed rate. This hysteretic loop has been previously explained by a different energy barrier required to form (during cooling) and break down (during melting) the triple helices between gelatin strands [47].
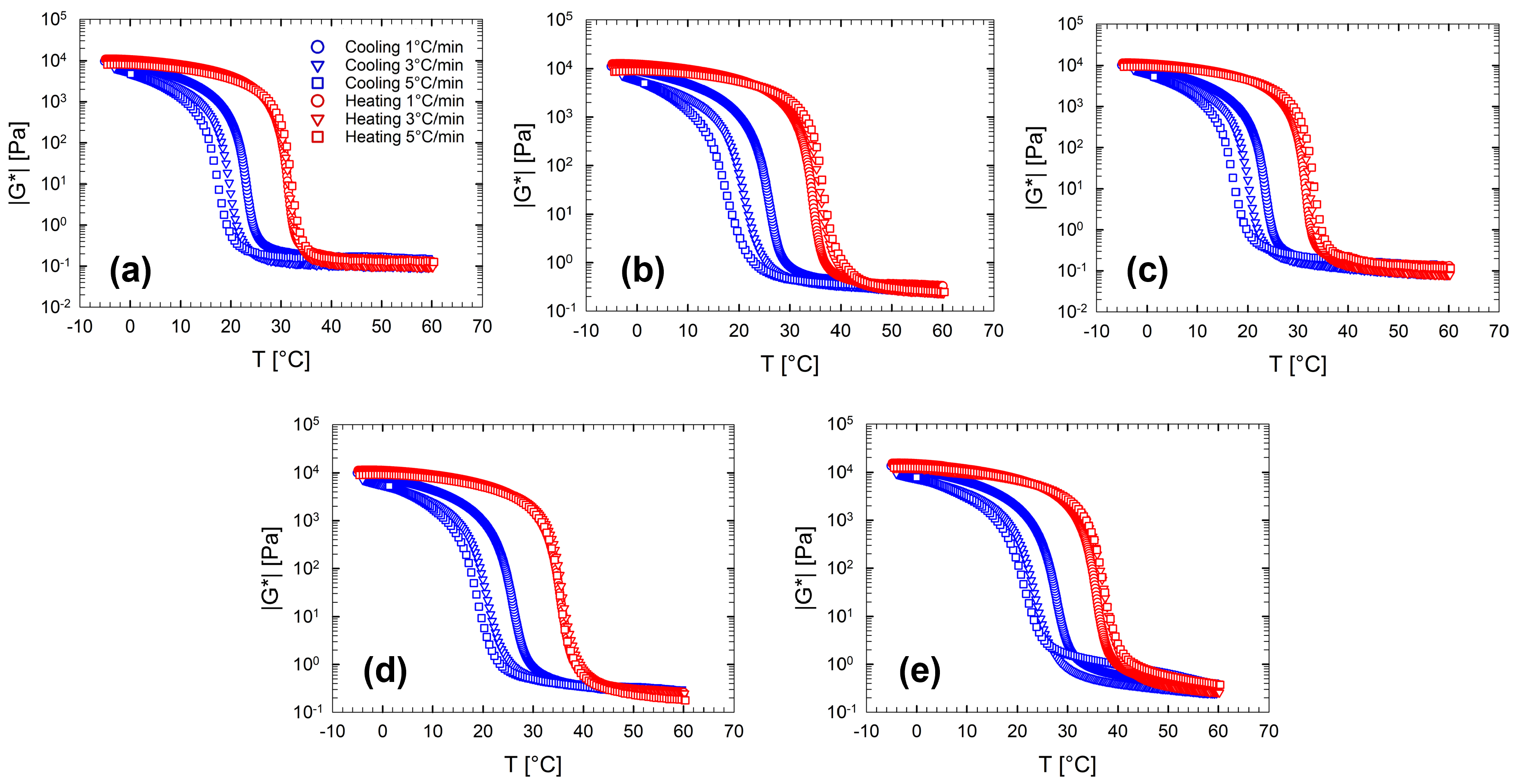
Figure 2 displays the complex modulus as a function of the temperature at different cooling/heating rates. Each panel in Figure 2 shows the temperature dependence for each multi-component aqueous gelatin solution investigated. In spite of the addition of sugars and polyols, the complex modulus trend, shown in Figure 2, shows the same features observed for the pure gelatin solutions (Sol.2) and previously discussed. Among the various features that characterize the sol-gel-sol transitions of gelatin gels, the presence of a marked difference between the cooling and heating ramps is, surely, the most apparent. Figure 2 shows that the hysteresis broadness increases as the imposed cooling/heating rate increases.
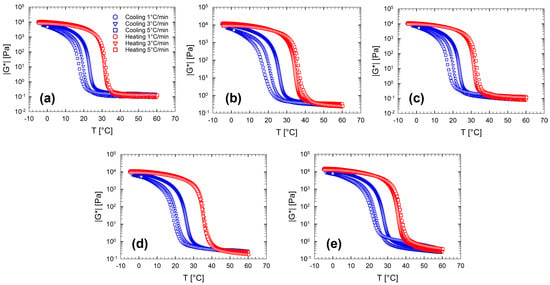
Figure 2.
Complex modulus, , as a function of the temperature at different cooling/heating rates for multi-component aqueous gelatin solutions. (a) Sol. 2; (b) Sol. 3S; (c) Sol. 3D; (d) Sol. 4; (e) Sol. 5. The legend in Figure (a) is valid for all figures.
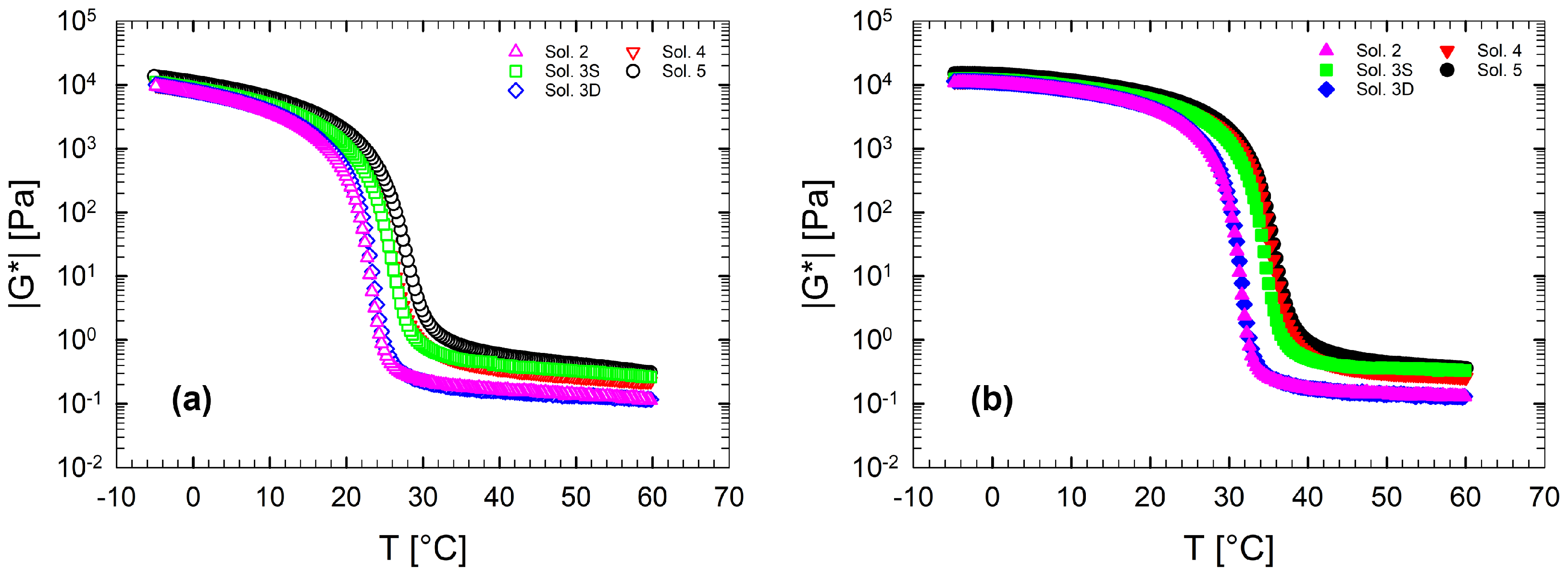
Figure 3 shows a direct comparison of the complex modulus as a function of the temperature for the entire set of multi-component solutions at a fixed ramp rate of 1 °C/min both in cooling (Figure 3a) and in heating (Figure 3b). In both cases, the curves, and consequently the transitions, move to higher temperatures. Since the gelatin content in the solution is fixed, it is clear that the thermal behavior of the complex modulus is purely affected by the number of co-solutes in the solution, with the exception of dextrose that seems not to affect the viscoelastic behaviour, at least at this concentration. On the other hand, the presence of the co-solutes affects the sol state at high temperatures by increasing the viscosity of the binary solution according to the amount and type of sugars and polyols.

Figure 3.
Complex modulus, , as a function of the temperature at 1 °C/min for multi-component aqueous gelatin solutions: (a) Cooling ramps and (b) heating ramps.
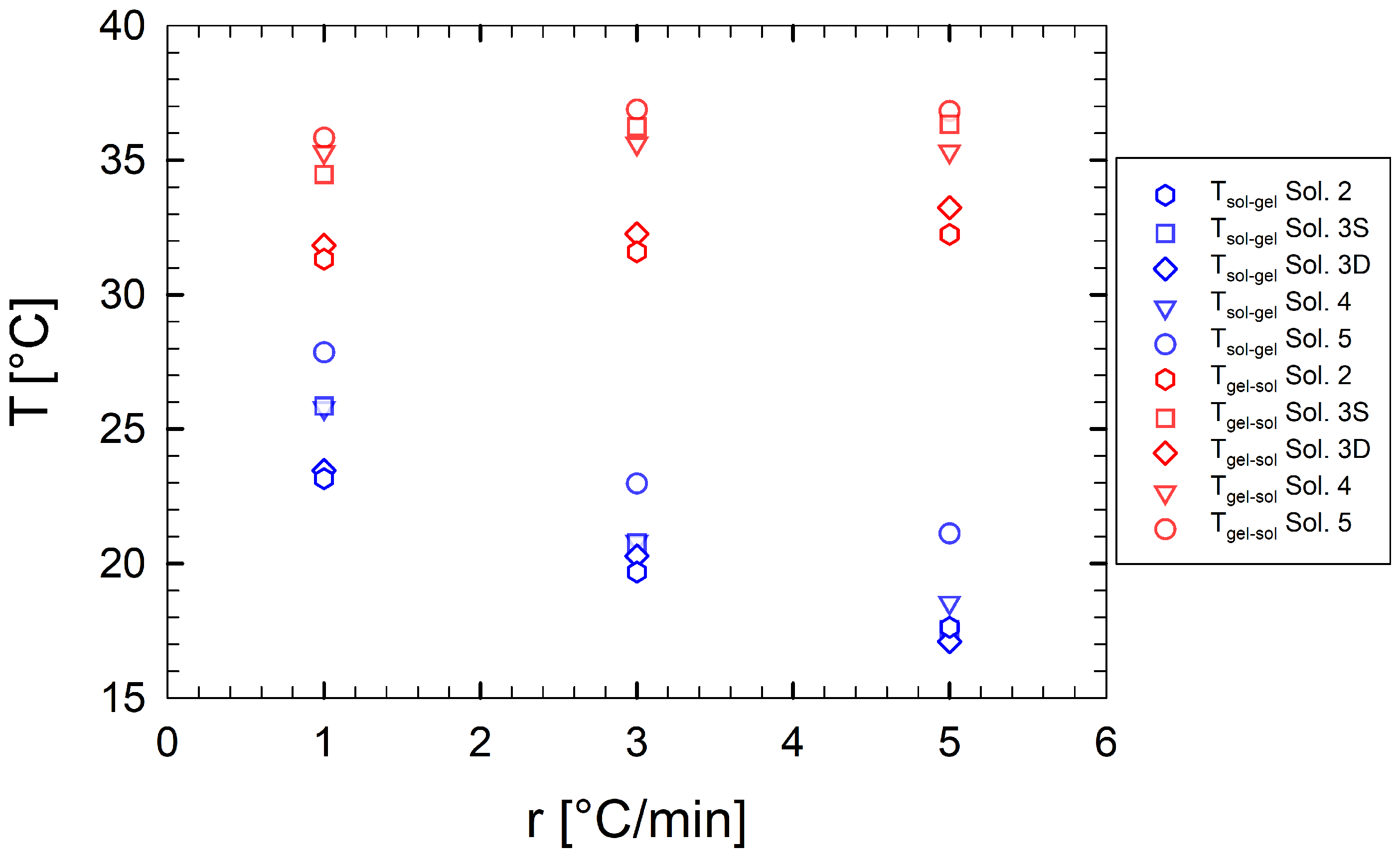
Figure 4 depicts the transition temperatures as a function of the cooling/heating ramp rate for multi-component gelatin samples. For the binary solution, the dependence of the transition temperatures on the cooling/heating ramps has been widely studied and reported in recent works [44,48,49]. Gelatins are nonequilibrium systems, for which a unique sol-gel transition temperature is not expected, even at a vanishing ramp rate [48].
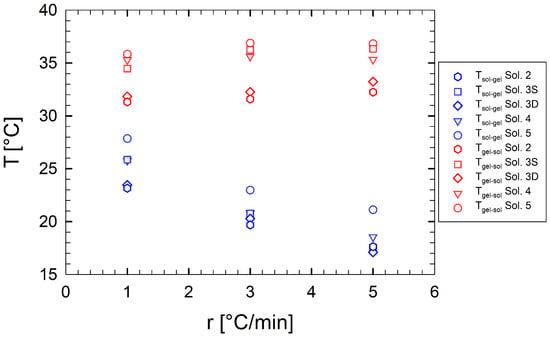
Figure 4.
(blue symbols) and (red symbols) as a function of cooling/heating ramp rates for multi-component aqueous gelatin solutions. Each symbol refers to a different sample.
Figure 4 shows that, as sucrose and sorbitol are added to the binary solution, the thermal stability of the resulting gels increases, that is, the resistance of the macroscopic gel to melt. In other words, the gel structure melts at higher temperatures.
Fixing the sample formulation, we observe that as the ramp rate increases, decreases and increases. In particular, is strongly influenced by the ramp rate. As an example, sample Sol. 5 shows a of approximately 21 and 28 °C at ramp rates of 1 and 5 °C/min, respectively. For a direct comparison between solutions, we report in Table 2 their characteristic transition temperatures as a function of the applied ramp rate.

Table 2.
Characteristic transition temperatures of the investigated solutions at different ramp rates.
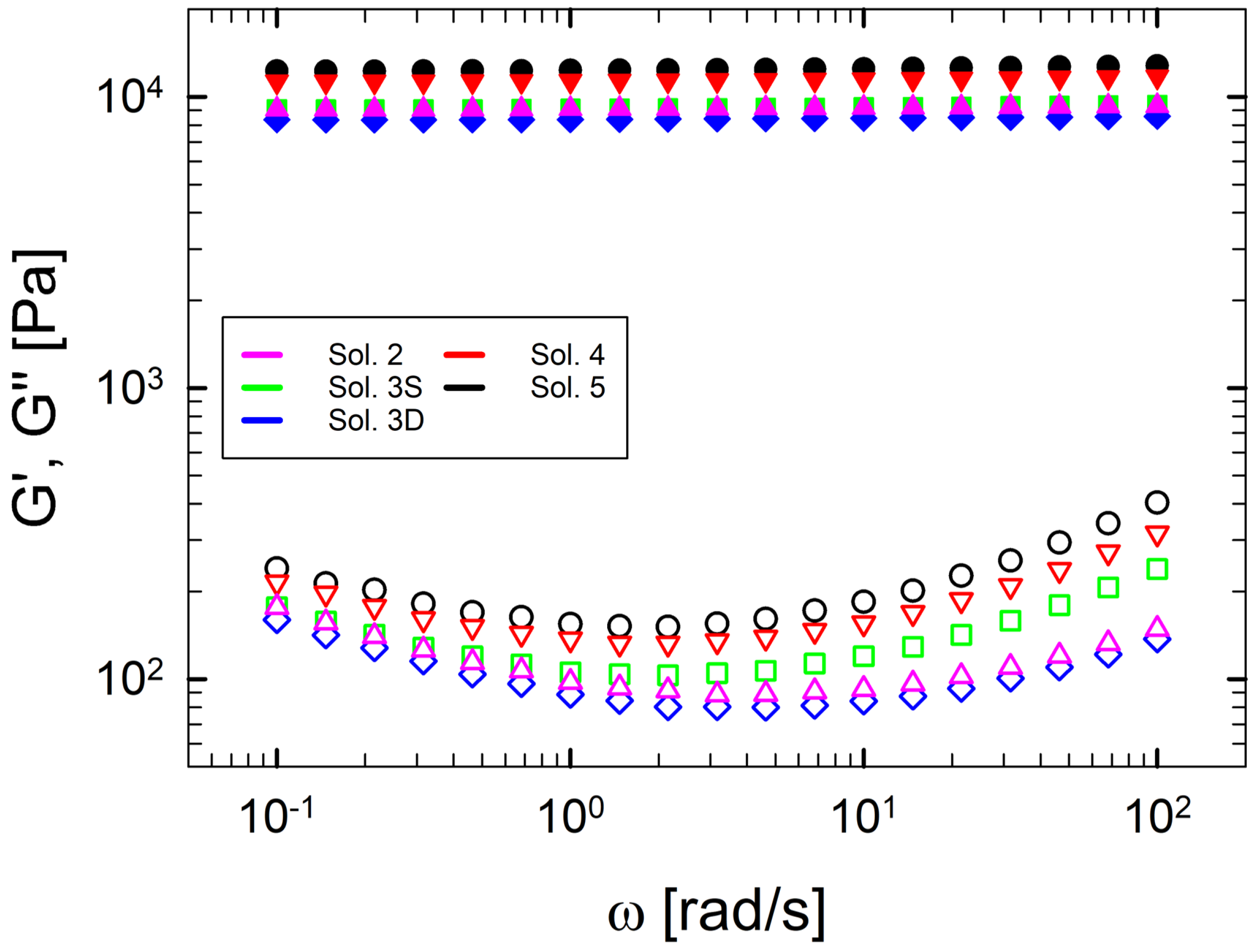
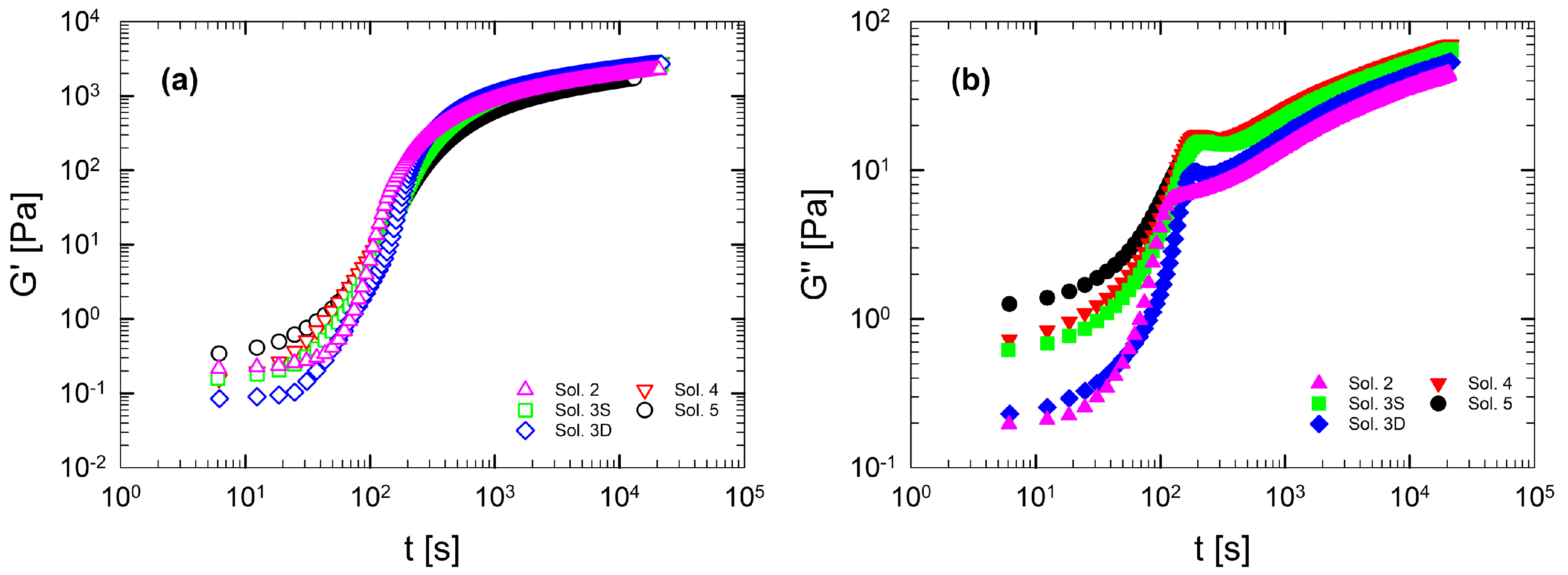
Figure 5 shows the linear viscoelastic behavior by reporting and as a function of the angular frequency at 5 °C. The frequency sweeps have been measured after the time sweep at 5 °C for 1 h in order to ensure complete gelation in the samples. Figure 5 shows the viscoelastic response for each multi-component solution characterized by a well-defined solid network, with a constant elasticity in the entire frequency range. Figure 5 emphasizes that, as the number of components in a solution increases, the properties of the resulting gel are enhanced. This can be seen from the fact that the elastic modulus, at a fixed frequency, increases consistently with the number of co-solutes in solution. A similar trend is also found for . Thus, co-solutes act on both the elastic and the dissipative component of the network.

Figure 5.
Linear viscoelasticity as a function of frequency at 5 °C for each multi-component gelatin solution. is represented with filled symbols, with empty symbols. Data are obtained after a soaking time of 1 h at 5 °C.
The most popular theory that justifies the gel enhancement in the presence of sugar is reported by Shimizu and Matubayasi [27]. They demonstrate that the increase in elasticity is due to the exclusion of co-solutes from the three-dimensional gelatin network. In this microstructural approach, the available water for gelatin coils to gelify is reduced, thus producing a more concentrated gel network. In this way, when gelation takes place, the helices arrange closer together, increasing the degree of packing, and are surrounded by a bulk of water and sugars (and/or polyols).
3.2. Isothermal Gelation of Multi-Component Aqueous Gelatin Solutions
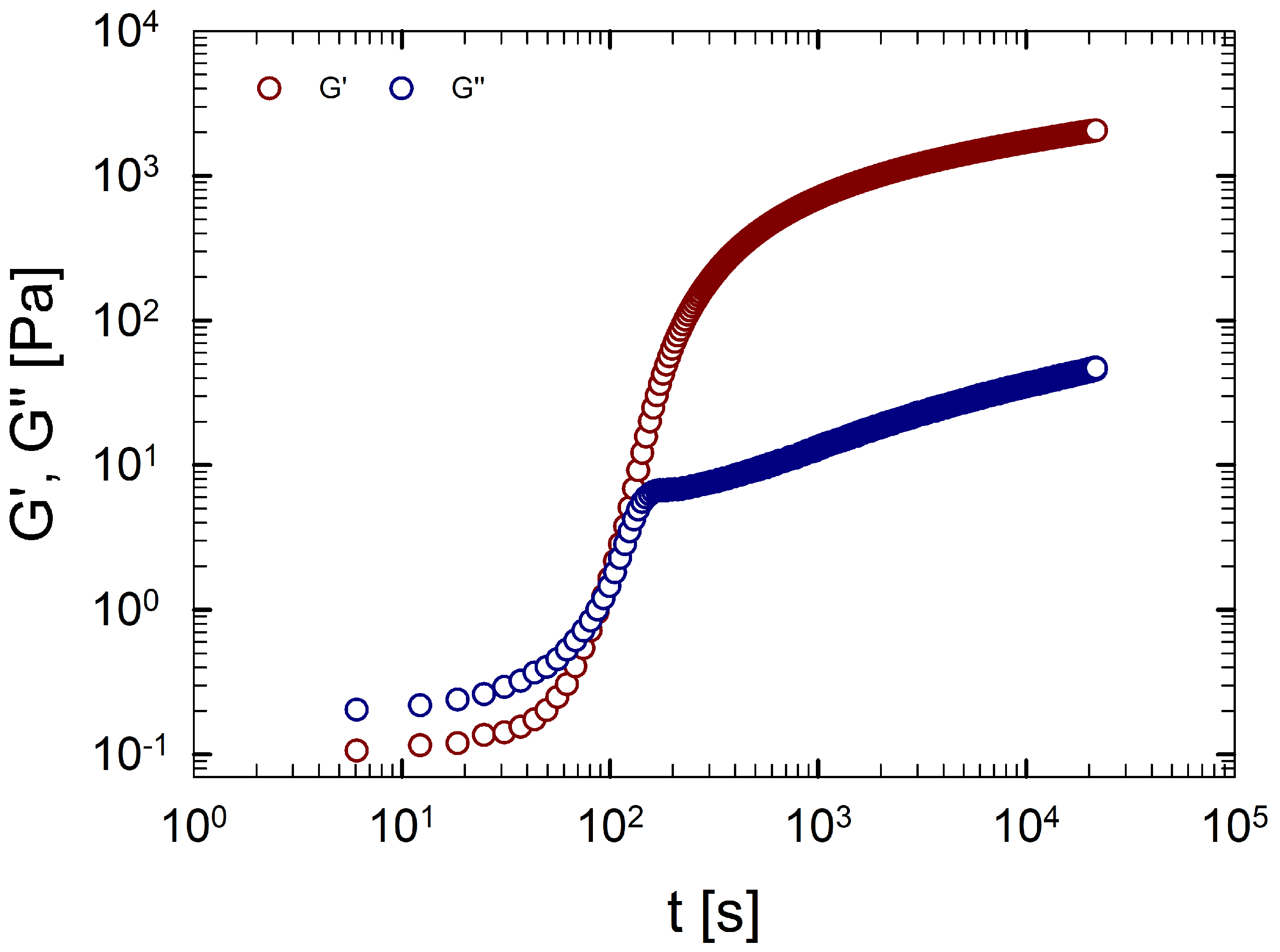
Figure 6 shows a DTST performed on an aqueous gelatin solution (Sol. 2), where the storage and loss moduli are plotted as a function of time, at a fixed temperature of 22 °C. At short time scales, the prevalence of over indicates a liquid-like behavior. As time increases, the viscoelastic moduli grow, and at a characteristic time, known as gel time ([43]), and are equal. Then, the microstructure changes rapidly, the number of triple helices increases, and finally a 3D network is formed [50,51,52]. At long time scales, we can observe the prevalence of the elastic modulus over the viscous one, which is a typical behavior of a solid-like system [53].

Figure 6.
Storage modulus (red circles) and loss modulus (blue circles) as a function of the time for an aqueous gelatin solution (Sol. 2) at 22 °C.
Figure 6 clearly shows that the viscoelastic moduli, at large time scales, display a tendency to saturate without reaching a plateau value due to the non-equilibrium nature of gelatin gels [12,48,54].
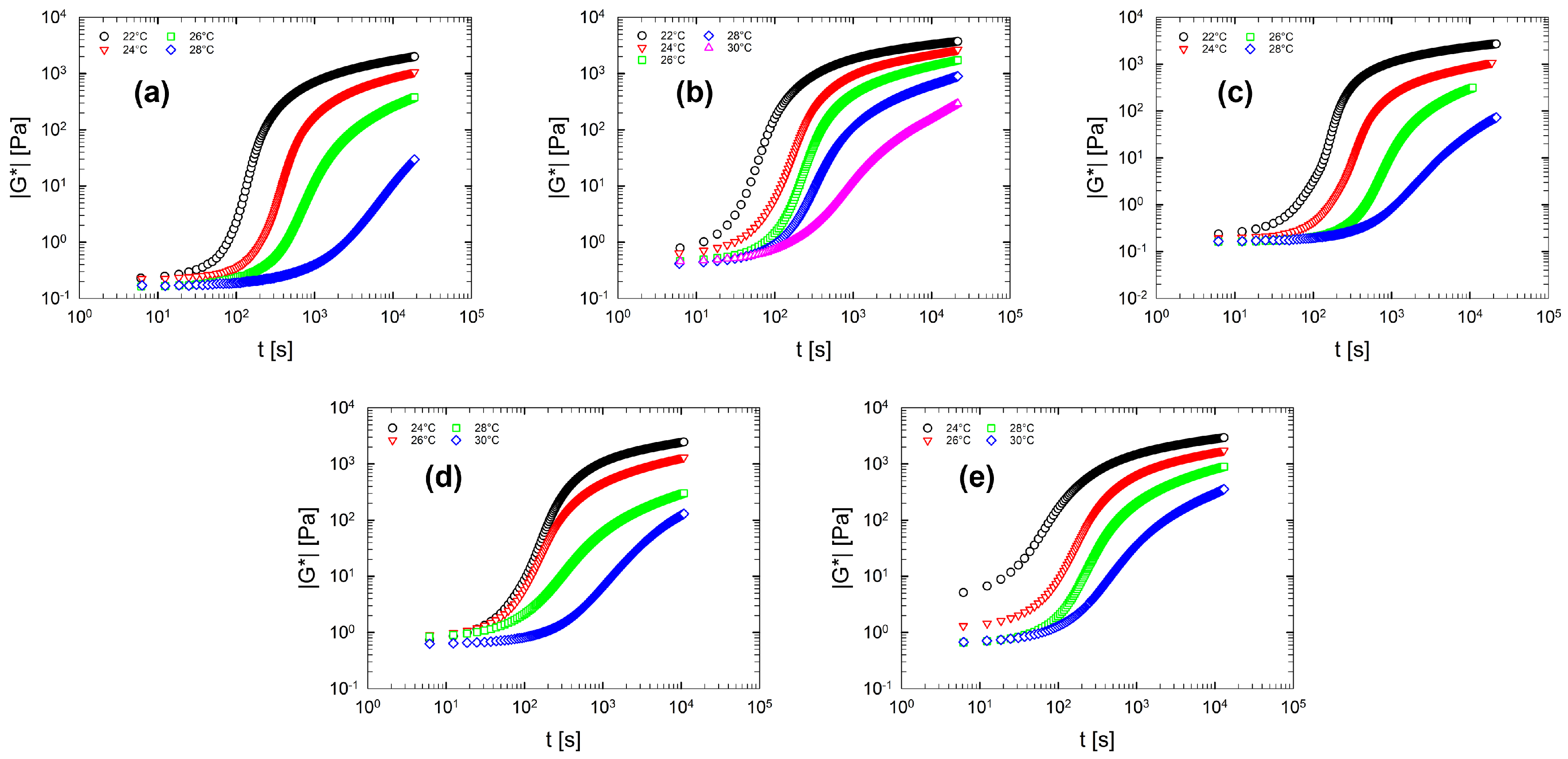
Figure 7 displays the gelation kinetics for the multi-component solutions. As expected, the multi-component solutions (panel (b–e) in Figure 7) show similar gelation features compared to the gelation kinetics of binary solution (Figure 7a). From Figure 7 it can be seen that, as the temperature increases, the curves shift towards larger time values. This means that the gelation process slows down as the temperature increases. In addition, also for multi-component solutions, the elasticity does not reach a plateau value (at least within the experimental time limits).
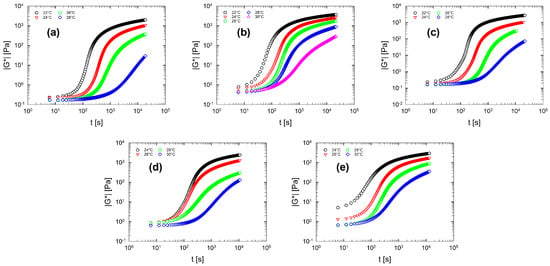
Figure 7.
Gelation kinetics for multi-component aqueous gelatin solutions. For each solution the complex modulus as a function of the time in a selected temperature range is reported. (a) Sol. 2; (b) Sol. 3S; (c) Sol. 3D; (d) Sol. 4; (e) Sol. 5.
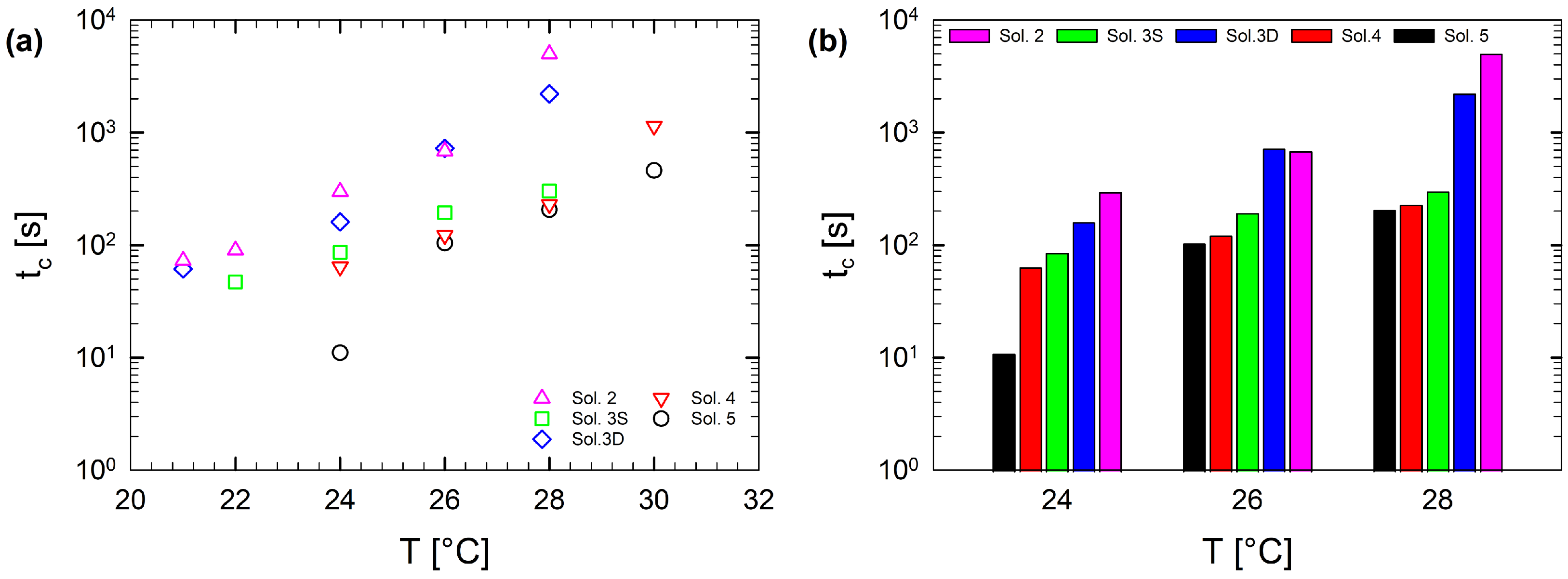
Figure 8 shows the temperature dependence of the gel time, , for the multi-component gelatin solutions. The co-solute addition strongly affects the gel time. Upon increasing temperature, the gel time increases with a non-linear dependence for all multi-component solutions. Figure 8a also shows that, as co-solutes are added to the binary solution, the curves shift down, indicating that the addition of co-solutes enhances the gelation process, since the transition occurs on shorter time scales. This behavior is clearly shown by the bar chart in Figure 8b, which shows the evolution of the gel time for three selected temperatures. A possible explanation is that as the amount of co-solutes decreases, the probability of intermolecular collisions between gelatin chains is reduced (due to an “actual” lower concentration of gelatin in water), and this results in an increase in the gel time under isothermal conditions [29]. Intermolecular collisions are a prerequisite for the coil-to-helix transition, which is crucial for the gelation phenomenon [29,51,52,55].

Figure 8.
Temperature dependence of the gel time, : (a) For the entire temperature range explored and (b) only for three selected temperatures.
In a way, to compare the isothermal gelation of the different solutions, we tried to find a characteristic temperature for each formulation at which the gelation kinetics could show similar trends. By taking as a “reference” the complete formulation (Sol.5: 26 °C), we found that it is actually possible to obtain a similar gelation behaviour if one takes some relative temperatures (Sol.2: 21 °C; Sol.3S: 24 °C; Sol.3D: 21 °C; Sol.4: 24 °C). Figure 9a displays the elastic modulus as a function of time at the previously indicated temperatures for all solutions studied in the current work. It can be seen that all curves overlay on each other irrespective of the co-solutes present in solution. This suggests that the incoming network microstructure and the resulting elasticity are built up only by the content of gelatin, which does not change in solution. On the other hand, Figure 9b shows the trend of with time that seems to be dependent on the co-solute content. The behaviour shown in Figure 9b suggests that co-solutes strongly affects the bulk aqueous phase that surrounds the gelatin chains without interfering with the physical associations that lead to the three-dimensional gelatin network. Based on our rheological data, we can deduce that a complex network is then defined, formed by an aqueous phase rich in sugars and polyols that affects only the dissipative behaviour of the system on which gelatin chains build up junction zones that confer elasticity to the resulting gel. This microstructure insight is in agreement with predictions shown by Shimizu et al. [27].

Figure 9.
(a) Elastic and (b) viscous moduli as a function of time for the entire set of multi component gelatin solutions. The relative temperatures are Sol.2: 21 °C; Sol.3S: 24 °C; Sol.3D: 21 °C; Sol.4: 24 °C; Sol.5: 26 °C.
From a closer look to the thermal behaviour of the solutions, we actually realized that the different temperatures that allow the superposition of the gelation kinetics presented in Figure 9 are effective because at the same distance from the relative sol-gel temperature (the latter evaluated at 1 °C/min for each solution):
In other words, Equation (1) reveals that Figure 9 compares the solutions at the same distance from the relative , which in this case is −2 °C.
This approach reminds us of a similar procedure performed on polymer melts with different molecular weights and, consequently, different glass transition temperatures. Their rheological curves are usually shifted by considering the same distance from the glass transition temperature. In such a way, it is possible to realize the so-called iso-frictional conditions, which allow us to, for example, evaluate the terminal relaxation of polymer chains, irrespective of the temperature at which the glass transition occurs [56,57,58].
4. Conclusions
In this work, the effect of sugars and polyols on the gelation of gelatin solutions was investigated by performing a rheological characterization of some multi-component gelatin solutions. It is worth noting that for these solutions the mass percentage of gelatin was kept fixed, while different co-solutes were added as a replacement for water.
Dynamic temperature ramp tests were performed at different ramp rates, which allowed us to evaluate the transition temperatures. Through DTRTs, it was possible to notice that upon increasing the number of co-solutes, both thermal stability and gel strength improved.
Isothermal dynamic time sweep tests provided a measure of the gel time, showing that as the amount of co-solutes decreases, the gelation process occurs at larger time scales. Thus, the addition of co-solutes enhances the gelation phenomenon.
Finally, an arbitrary normalization of gelation isothermal curves at the same distance of was carried out. In such conditions, it was found that the addition of sugars and polyols affected only the viscous behaviour (sol state) without changing the elasticity of the resulting gel. The main reason was that co-solutes were completely dissolved in the bulk aqueous phase without modifying the gelatin chains aggregation, which instead controlled the network elasticity.
Author Contributions
Conceptualization and methodology, P.R.A., R.P. and N.G.; formal analysis, R.P.; investigation, P.R.A. and M.R.; resources A.S. and M.D.; data curation R.P. and N.G.; writing—original draft preparation P.R.A. and R.P.; writing—review and editing P.R.A., R.P. and N.G; supervision R.P. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
R.P. acknowledges TA Instruments for awarding the rheometer used in this study as part of the “Distinguished Young Rheologist” program.
Conflicts of Interest
The authors declare no conflict of interest. A.S., M.D. and M.R. are employees of Perfetti Van Melle. All of them state that in this paper, there is nothing that may be considered as a conflict of interest.
References
- Poppe, J. Gelatin. In Thickening and Gelling Agents for Food; Imeson, A., Ed.; Springer: Boston, MA, USA, 1992; pp. 98–123. [Google Scholar]
- Netter, A.B.; Goudoulas, T.B.; Germann, N. Effects of Bloom number on phase transition of gelatin determined by means of rheological characterization. LWT 2020, 132, 109813. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Alipal, J.; Pu’ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Veis, A. The physical chemistry of gelatin. Int. Rev. Connect. Tissue Res. 1965, 3, 113–200. [Google Scholar] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djabourov, M.; Leblond, J.; Papon, P. Gelation of aqueous gelatin solutions. II. Rheology of the sol-gel transition. J. Phys. 1988, 49, 333–343. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Guo, L.; Colby, R.H.; Lusignan, C.P.; Howe, A.M. Physical gelation of gelatin studied with rheo-optics. Macromolecules 2003, 36, 10009–10020. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All gelatin networks: 1. Biodiversity and physical chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Djabourov, M. Architecture of gelatin gels. Contemp. Phys. 1988, 29, 273–297. [Google Scholar] [CrossRef]
- Dille, M.; Haug, I.; Draget, K. Chapter 34–Gelatin and collagen. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2021; pp. 1073–1097. [Google Scholar]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wu, Y.; Woshnak, L.L.; Mitmesser, S.H. Effects of hydrocolloids, acids and nutrients on gelatin network in gummies. Food Hydrocoll. 2021, 113, 106549. [Google Scholar] [CrossRef]
- Hartel, R.W.; Joachim, H.; Hofberger, R. Confectionery Science and Technology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Nazir, A.; Asghar, A.; Aslam Maan, A. Chapter 13–Food Gels: Gelling Process and New Applications. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2017; pp. 335–353. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burey, P.; Bhandari, B.; Rutgers, R.; Halley, P.; Torley, P. Confectionery Gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef] [Green Version]
- El-Nawawi, S.; Heikel, Y. Factors affecting gelation of high-ester citrus pectin. Process Biochem. 1997, 32, 381–385. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery gels: Gelling behavior and gel properties of gelatin in concentrated sugar solutions. Food Hydrocoll. 2022, 124, 107132. [Google Scholar] [CrossRef]
- Jackson, E.B. Sugar Confectionery Manufacture; Blackie: New York, NY, USA, 1990. [Google Scholar]
- Burey, P.; Bhandari, B.; Howes, T.; Gidley, M. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Stabilization of gelatin gels by sugars and polyols. Food Hydrocoll. 1986, 1, 163–175. [Google Scholar] [CrossRef]
- Tau, T.; Gunasekaran, S. Thermorheological evaluation of gelation of gelatin with sugar substitutes. LWT-Food Sci. Technol. 2016, 69, 570–578. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Du, J.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Zhang, Y. Effect of interaction between sorbitol and gelatin on gelatin properties and its mechanism under different citric acid concentrations. Food Hydrocoll. 2020, 101, 105557. [Google Scholar] [CrossRef]
- Shimizu, S.; Matubayasi, N. Gelation: The role of sugars and polyols on gelatin and agarose. J. Phys. Chem. B 2014, 118, 13210–13216. [Google Scholar] [CrossRef] [PubMed]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Kasapis, S.; Al-Marhoobi, I.M.; Deszczynski, M.; Mitchell, J.R.; Abeysekera, R. Gelatin vs. polysaccharide in mixture with sugar. Biomacromolecules 2003, 4, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.; Giannouli, P.; Martin, E.; Brooks, M.; Morris, E. Effect of sugars, galactose content and chainlength on freeze–thaw gelation of galactomannans. Carbohydr. Polym. 2006, 64, 391–401. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M. Effects of sugars and polyols on the gel-sol transition of kappa-carrageenan gels. Thermochim. Acta 1992, 206, 149–162. [Google Scholar] [CrossRef]
- Miyoshi, E.; Nishinari, K. Effects of sugar on the sol-gel transition in gellan gum aqueous solutions. In Physical Chemistry and Industrial Application of Gellan Gum; Springer: Berlin/Heidelberg, Germany, 1999; pp. 83–91. [Google Scholar]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels–Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wang, Z.; Brenner, T.; Kikuzaki, H.; Fang, Y.; Nishinari, K. Sucrose release from agar gels: Effects of dissolution order and the network inhomogeneity. Food Hydrocoll. 2015, 43, 100–106. [Google Scholar] [CrossRef]
- Chen, L.; Revel, S.; Morris, K.; Spiller, D.G.; Serpell, L.C.; Adams, D.J. Low molecular weight gelator–dextran composites. Chem. Commun. 2010, 46, 6738–6740. [Google Scholar] [CrossRef]
- Bianco, S.; Panja, S.; Adams, D.J. Using Rheology to Understand Transient and Dynamic Gels. Gels 2022, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Acierno, S.; Pasquino, R.; Grizzuti, N. Rheological techniques for the determination of the crystallization kinetics of a polypropylene–EPR copolymer. J. Therm. Anal. Calorim. 2009, 98, 639–644. [Google Scholar] [CrossRef]
- Venezia, V.; Avallone, P.R.; Vitiello, G.; Silvestri, B.; Grizzuti, N.; Pasquino, R.; Luciani, G. Adding Humic Acids to Gelatin Hydrogels: A Way to Tune Gelation. Biomacromolecules 2022, 23, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Li, L.; Hu, X.; Zhao, X. Sol-gel transition of methylcellulose in phosphate buffer saline solutions. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1849–1860. [Google Scholar] [CrossRef]
- Te Nijenhuis, K.; Winter, H.H. Mechanical properties at the gel point of a crystallizing poly (vinyl chloride) solution. Macromolecules 1989, 22, 411–414. [Google Scholar] [CrossRef]
- Ross-Murphy, S. Incipient behaviour of gelatin gels. Rheol. Acta 1991, 30, 401–411. [Google Scholar] [CrossRef]
- Avallone, P.R.; Raccone, E.; Costanzo, S.; Delmonte, M.; Sarrica, A.; Pasquino, R.; Grizzuti, N. Gelation kinetics of aqueous gelatin solutions in isothermal conditions via rheological tools. Food Hydrocoll. 2021, 111, 106248. [Google Scholar] [CrossRef]
- Ahmed, J. Chapter 15–Rheological Properties of Gelatin and Advances in Measurement. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2017; pp. 377–404. [Google Scholar]
- Bello, J.; Bello, H.R.; Vinograd, J.R. The mechanism of gelation of gelatin the influence of pH, concentration, time and dilute electrolyte on the gelation of gelatin and modified gelatins. Biochim. Biophys. Acta 1962, 57, 214–221. [Google Scholar] [CrossRef]
- Michon, C.; Cuvelier, G.; Launay, B. Concentration dependence of the critical viscoelastic properties of gelatin at the gel point. Rheol. Acta 1993, 32, 94–103. [Google Scholar] [CrossRef]
- Avallone, P.R.; Pasquino, R.; Costanzo, S.; Sarrica, A.; Delmonte, M.; Greco, F.; Grizzuti, N. On the inverse quenching technique applied to gelatin solutions. J. Rheol. 2021, 65, 1081–1088. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Li, L. Thermoreversible gelation and viscoelasticity of κ-carrageenan hydrogels. J. Rheol. 2016, 60, 203–214. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Ajdari, A.; Djabourov, M. All gelatin networks: 2. The master curve for elasticity. Langmuir 2002, 18, 7158–7166. [Google Scholar] [CrossRef]
- Tanaka, F. Thermoreversible gelation driven by coil-to-helix transition of polymers. Macromolecules 2003, 36, 5392–5405. [Google Scholar] [CrossRef]
- Gornall, J.L.; Terentjev, E.M. Helix–coil transition of gelatin: Helical morphology and stability. Soft Matter 2008, 4, 544–549. [Google Scholar] [CrossRef]
- Normand, V.; Muller, S.; Ravey, J.C.; Parker, A. Gelation kinetics of gelatin: A master curve and network modeling. Macromolecules 2000, 33, 1063–1071. [Google Scholar] [CrossRef]
- Parker, A.; Normand, V. Glassy dynamics of gelatin gels. Soft Matter 2010, 6, 4916–4919. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Radha, A. Molecular architectures and functional properties of gellan gum and related polysaccharides. Trends Food Sci. Technol. 1995, 6, 143–148. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Majeste, J.C.; Montfort, J.P.; Allal, A.; Marin, G. Viscoelasticity of low molecular weight polymers and the transition to the entangled regime. Rheol. Acta 1998, 37, 486–499. [Google Scholar] [CrossRef]
- Pasquino, R.; Zhang, B.; Sigel, R.; Yu, H.; Ottiger, M.; Bertran, O.; Aleman, C.; Schlüter, A.; Vlassopoulos, D. Linear viscoelastic response of dendronized polymers. Macromolecules 2012, 45, 8813–8823. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).