Effect of Functional Surfaces with Gradient Mixed Wettability on Flow Boiling in a High Aspect Ratio Microchannel
Abstract
1. Introduction
2. Experimental Setup, Procedure, and Data Reduction
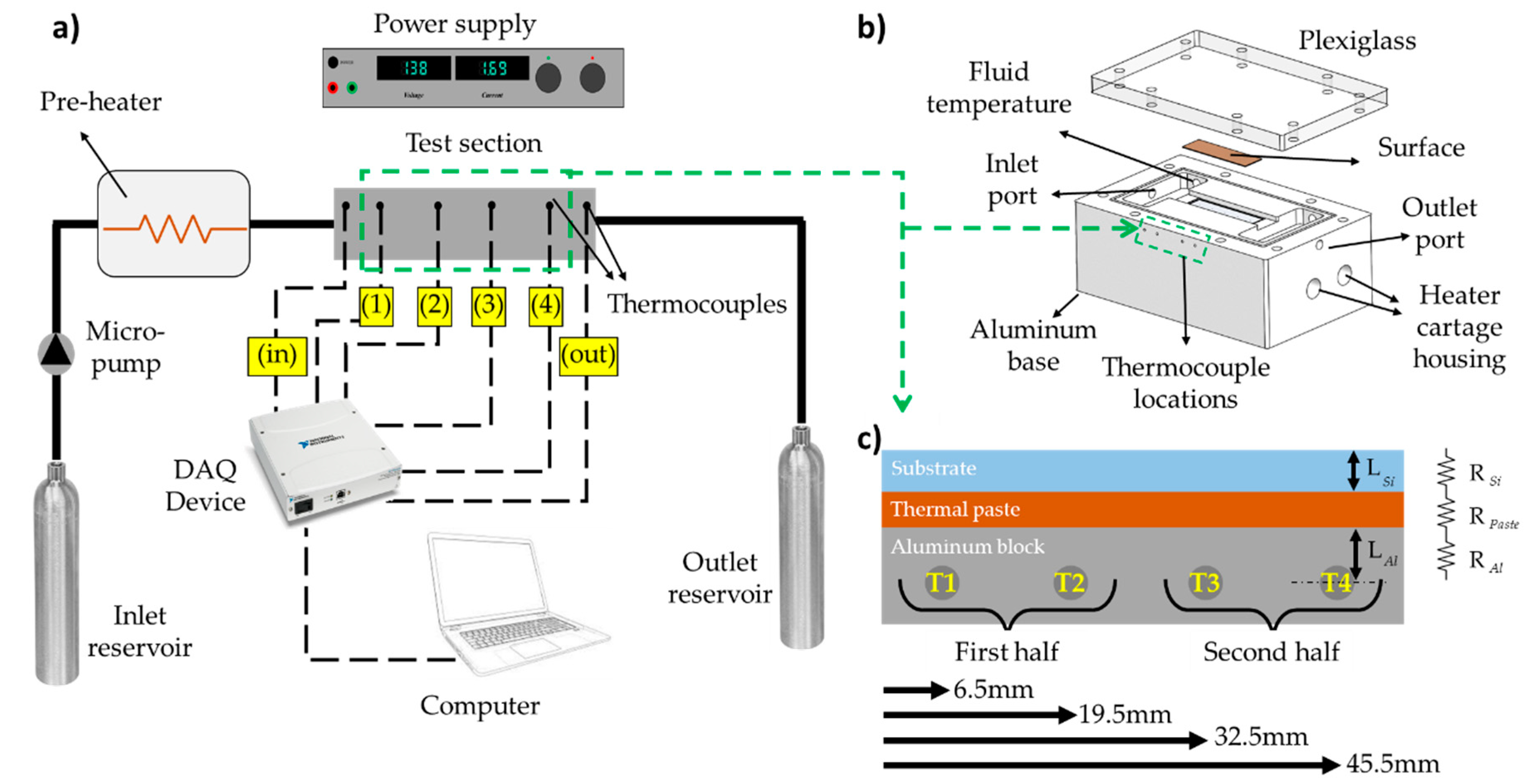
2.1. Experimental Setup and Test Section
2.2. Experimental Procedure
2.3. Data Reduction
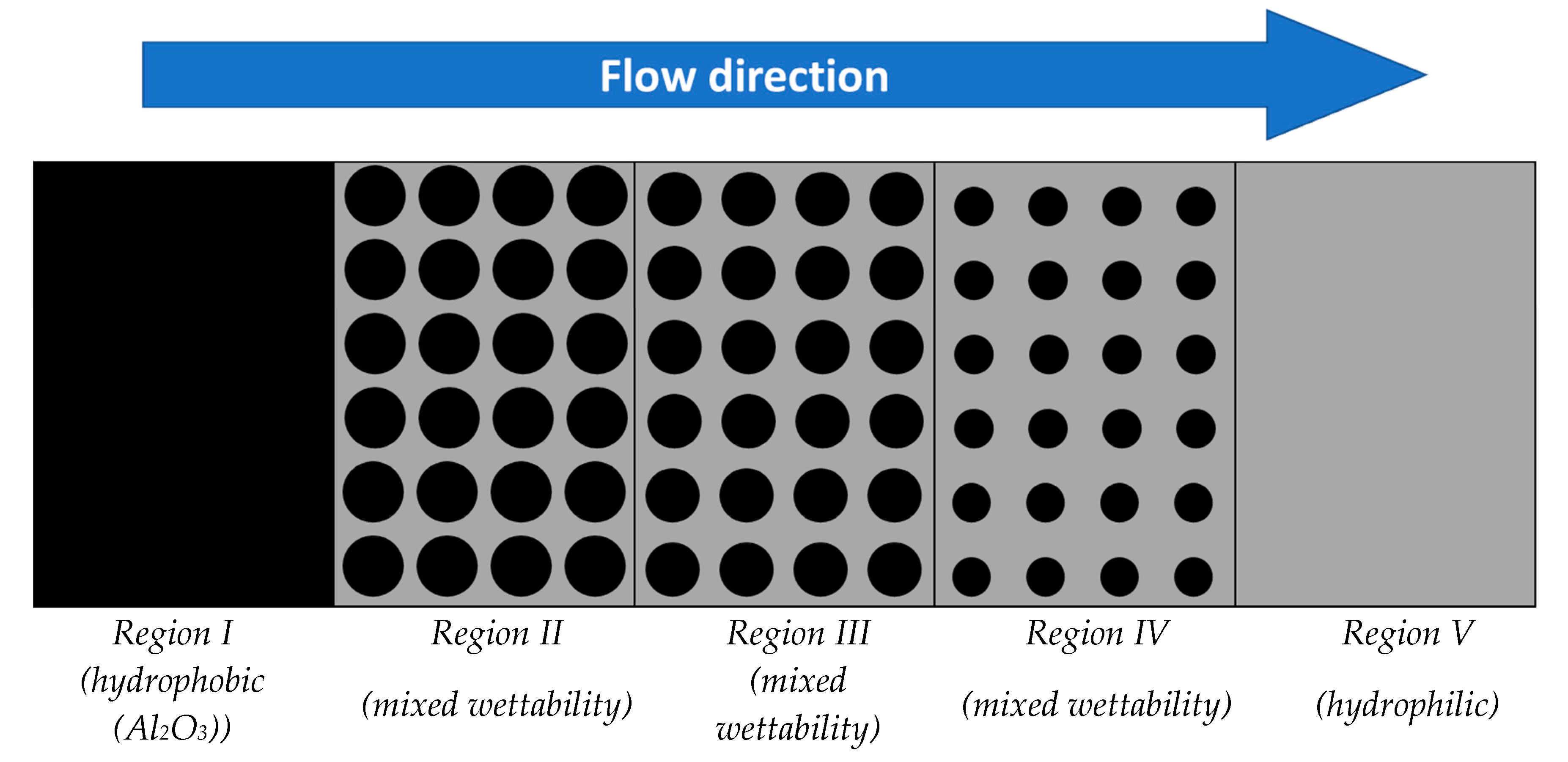
3. Sample Preparation and Characterization
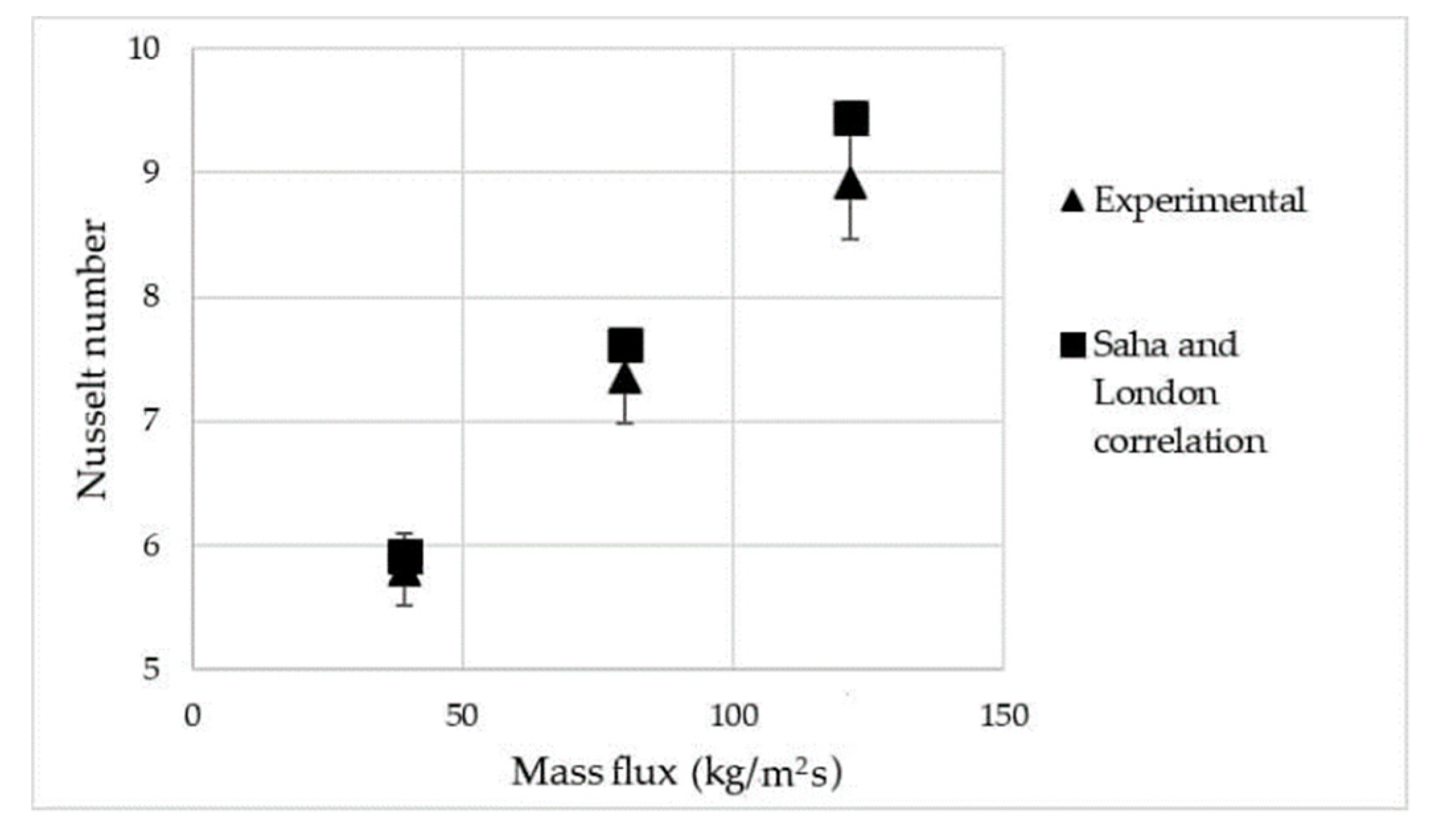
4. Validation
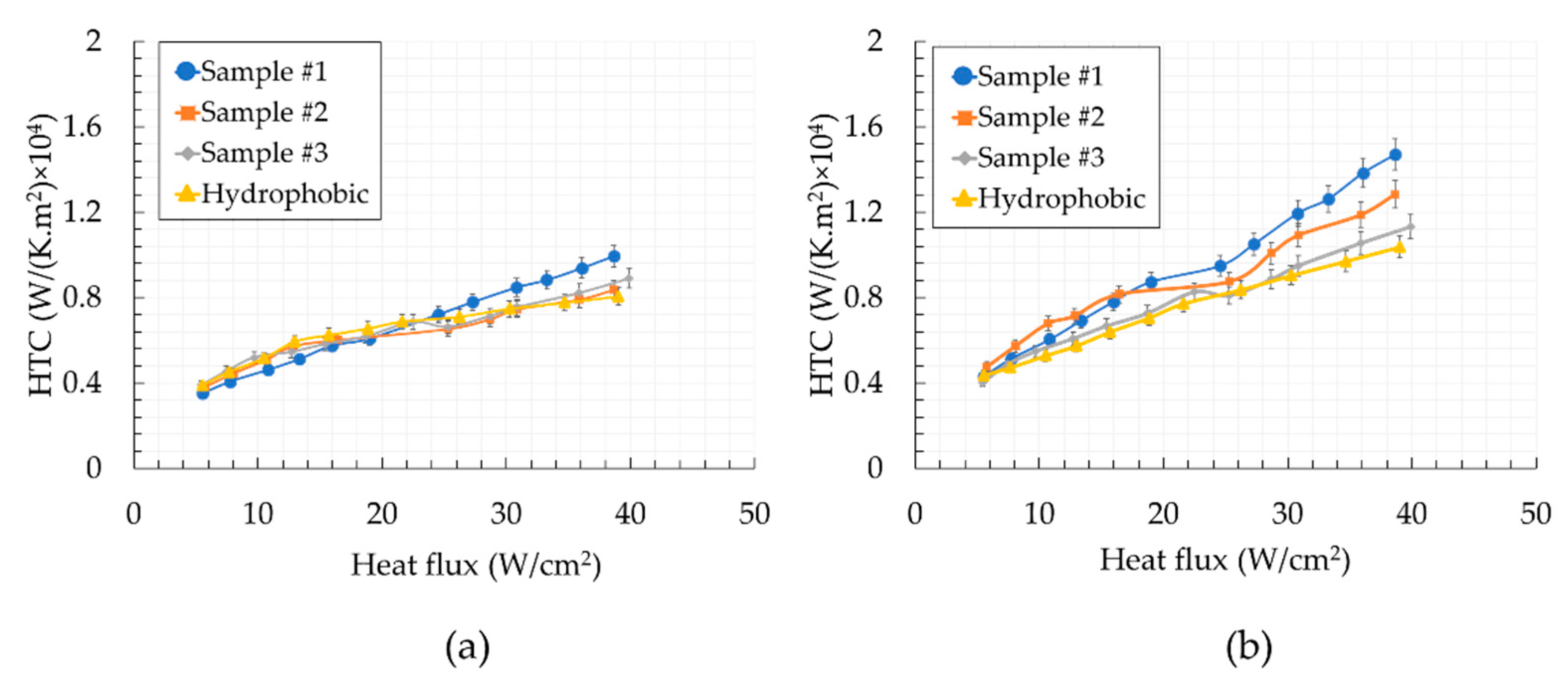
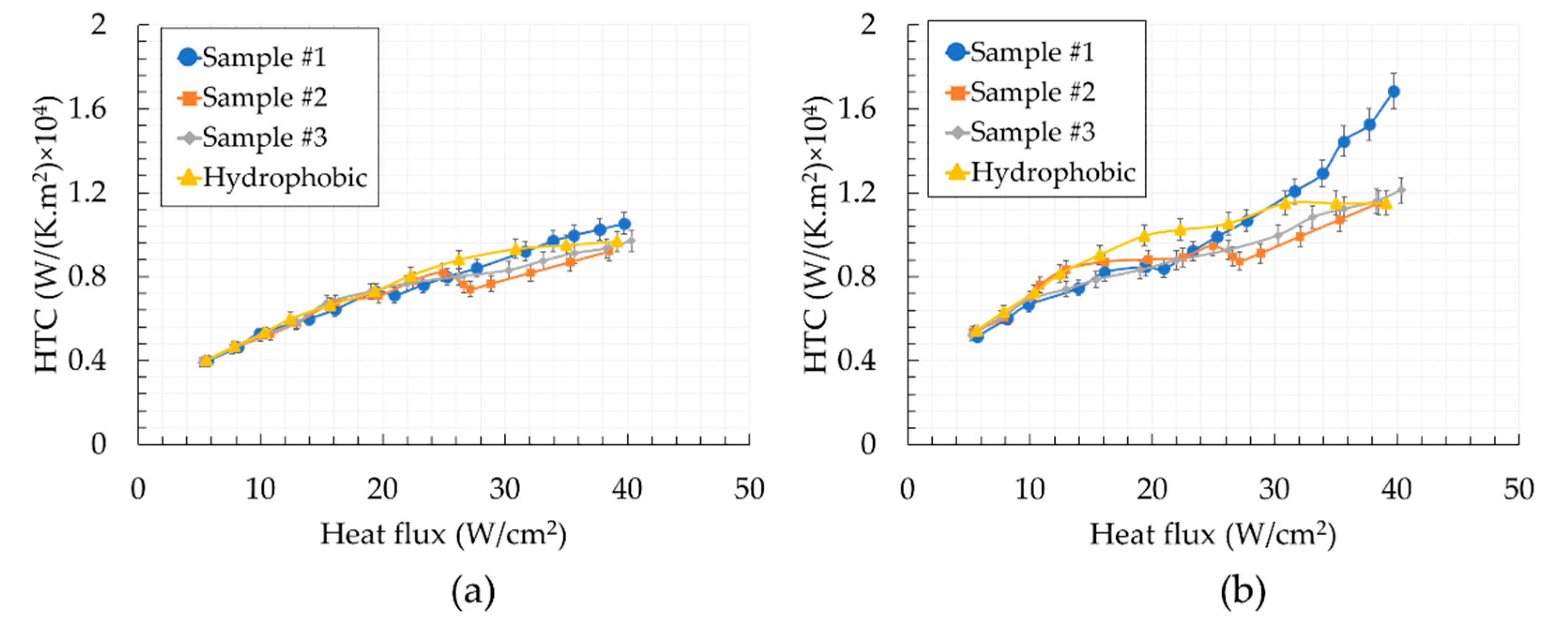
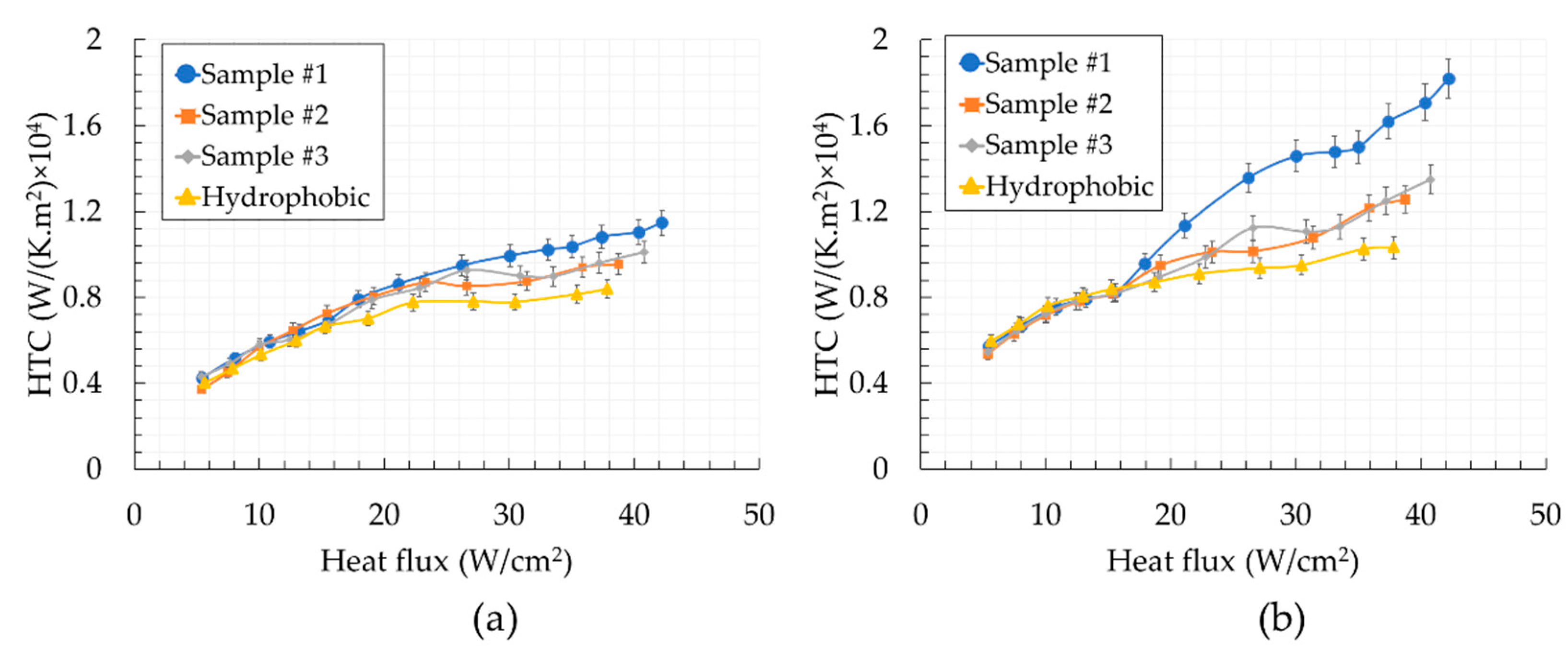
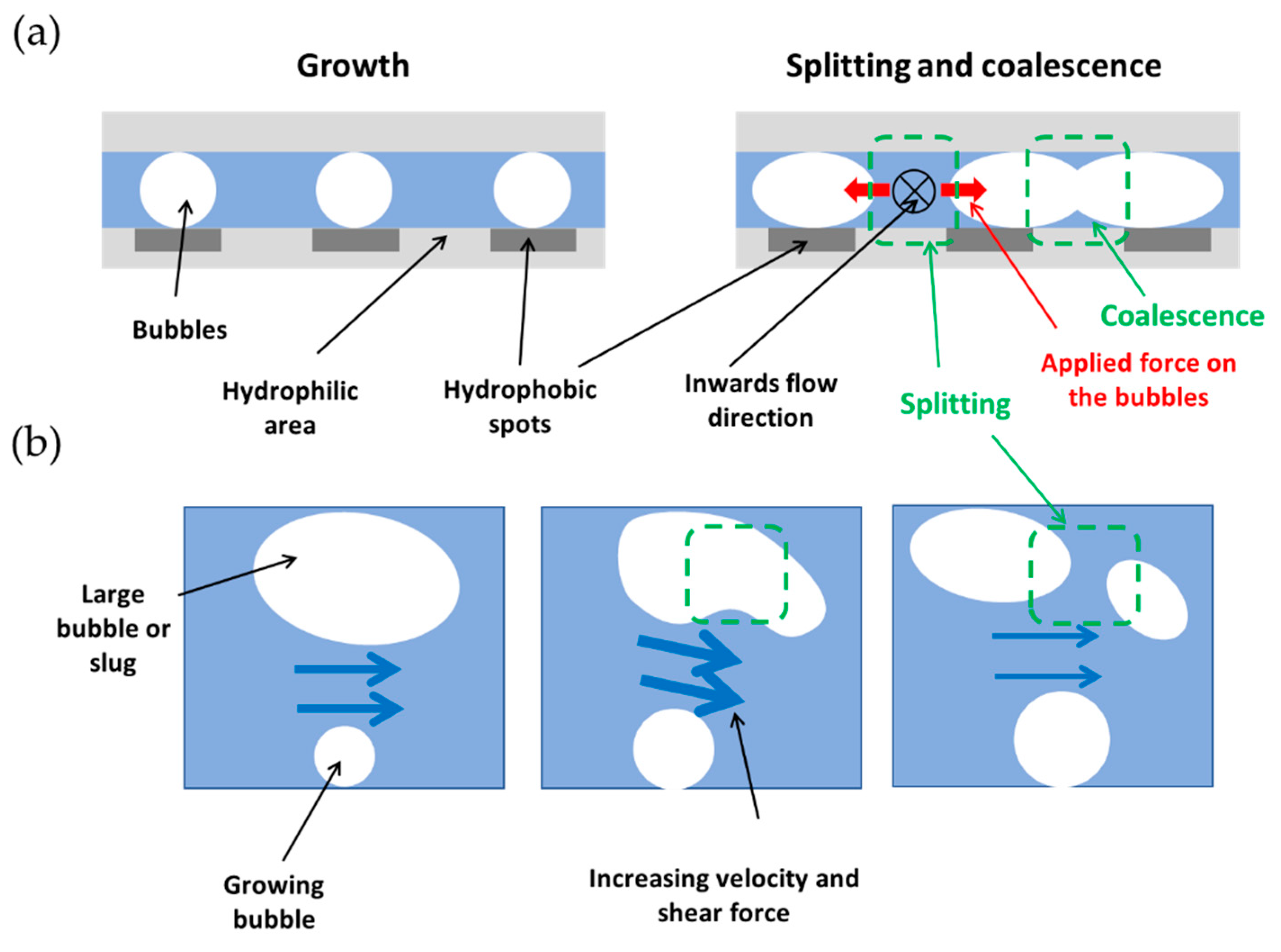
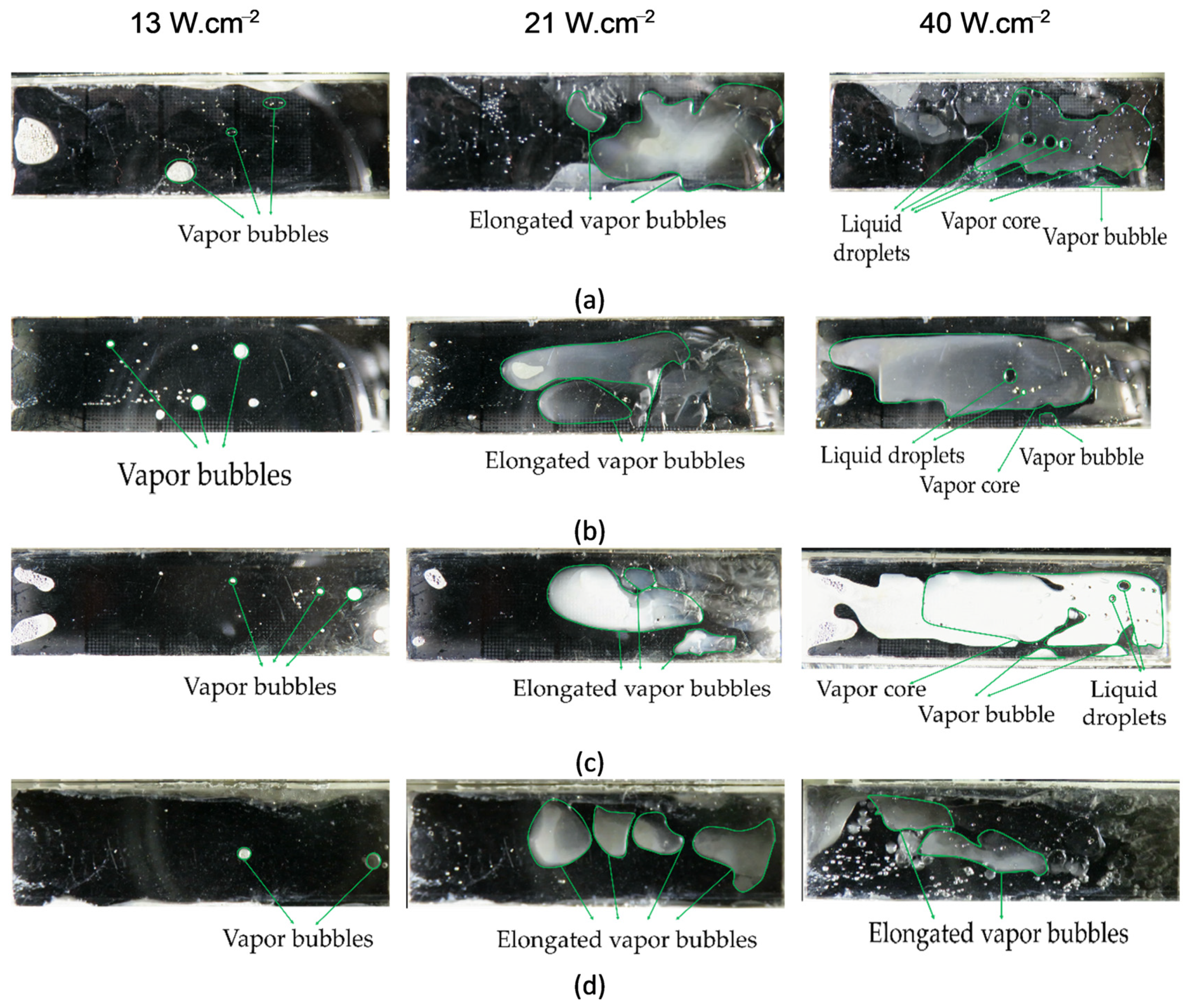
5. Results and Discussion
6. Conclusions
- Biphilic surfaces offered a better performance than the reference wholly hydrophobic surface (enhancements up to 56.7%).
- Vapor breakup on biphilic surfaces is one of the major mechanisms enhancing flow boiling heat transfer.
- All of the biphilic surfaces reduced the length of bubbly flow regime and extended the slug flow regime.
- The exponential decay in the hydrophobic surface area ratio in the first half of the channel (i.e., sample #1) has a more enhancing effect on heat transfer compared to the exponential decay in the second half of the channel (i.e., sample #3).
- Surfaces having linear and exponential decay transitions in the second half of the channel have similar heat transfer performances.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Lee, J.S. Numerical study on the effects of inertia and wettability on subcooled flow boiling in microchannels. Appl. Therm. Eng. 2019, 152, 175–183. [Google Scholar] [CrossRef]
- Frey, S.W., Jr.; Herson, M.I. Natural Convection Cooling System for Electronic Components. Allen Bradley Co LLC. US Patent US4535386A, 13 August 1985. [Google Scholar]
- Ahmadi, V.E.; Erden, H.S. Investigation of CRAH Bypass for Air-Cooled Data Centers using Computational Fluid Dynamics. In Proceedings of the 2018 IEEE International Telecommunications Energy Conference (INTELEC), Turin, Italy, 7–11 October 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Ahmadi, V.E.; Erden, H.S. A parametric CFD study of computer room air handling bypass in air-cooled data centers. Appl. Therm. Eng. 2020, 166. [Google Scholar] [CrossRef]
- Choi, C.; Shin, J.S.; Yu, D.I.; Kim, M.H. Flow boiling behaviors in hydrophilic and hydrophobic microchannels. Exp. Therm. Fluid Sci. 2011, 35, 816–824. [Google Scholar] [CrossRef]
- Bottini, J.L.; Kumar, V.; Hammouti, S.; Ruzic, D.; Brooks, C.S. Influence of wettability due to laser-texturing on critical heat flux in vertical flow boiling. Int. J. Heat Mass Transf. 2018, 127, 806–817. [Google Scholar] [CrossRef]
- Sadaghiani, A.K.; Koşar, A. Numerical and experimental investigation on the effects of diameter and length on high mass flux subcooled flow boiling in horizontal microtubes. Int. J. Heat Mass Transf. 2016, 92, 824–837. [Google Scholar] [CrossRef]
- Sadaghiani, A.K.; Saadi, N.S.; Parapari, S.S.; Karabacak, T.; Keskinoz, M.; Koşar, A. Boiling heat transfer performance enhancement using micro and nano structured surfaces for high heat flux electronics cooling systems. Appl. Therm. Eng. 2017, 127, 484–498. [Google Scholar] [CrossRef]
- Kaya, A.; Özdemir, M.R.; Koşar, A. High mass flux flow boiling and critical heat flux in microscale. Int. J. Therm. Sci. 2013, 65, 70–78. [Google Scholar] [CrossRef]
- Lin, S.; Sefiane, K.; Christy, J.R.E. Prospects of confined flow boiling in thermal management of microsystems. Appl. Therm. Eng. 2002, 22, 825–837. [Google Scholar] [CrossRef]
- Fang, X.; Wang, R.; Chen, W.; Zhang, H.; Ma, C. A review of flow boiling heat transfer of nanofluids. Appl. Therm. Eng. 2015, 91, 1003–1017. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of channel flow boiling enhancement by surface modification, and instability suppression schemes. Int. J. Heat Mass Transf. 2020, 146, 118864. [Google Scholar] [CrossRef]
- Şişman, Y.; Sadaghiani, A.K.; Khedir, K.R.; Brozak, M.; Karabacak, T.; Koşar, A. Subcooled flow boiling over microstructured plates in rectangular minichannels. Nanoscale Microscale Thermophys. Eng. 2016, 20, 173–190. [Google Scholar] [CrossRef]
- Sadaghiani, A.K.; Motezakker, A.R.; Özpınar, A.V.; İnce, G.Ö.; Koşar, A. Pool boiling heat transfer characteristics of inclined pHEMA-coated surfaces. J. Heat Transfer 2017, 139, 111501. [Google Scholar] [CrossRef]
- Mathew, J.; Lee, P.-S.; Wu, T.; Yap, C.R. Experimental study of flow boiling in a hybrid microchannel-microgap heat sink. Int. J. Heat Mass Transf. 2019, 135, 1167–1191. [Google Scholar] [CrossRef]
- Harirchian, T.; Garimella, S.V. Effects of channel dimension, heat flux, and mass flux on flow boiling regimes in microchannels. Int. J. Multiph. Flow 2009, 35, 349–362. [Google Scholar] [CrossRef]
- Tibirica, C.B.; Ribatski, G. Flow patterns and bubble departure fundamental characteristics during flow boiling in microscale channels. Exp. Therm. Fluid Sci. 2014, 59, 152–165. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, P. An experimental study of flow boiling instability in a single microchannel. Int. Commun. Heat Mass Transf. 2008, 35, 1229–1234. [Google Scholar] [CrossRef]
- Yin, L.; Jia, L. Confined bubble growth and heat transfer characteristics during flow boiling in microchannel. Int. J. Heat Mass Transf. 2016, 98, 114–123. [Google Scholar] [CrossRef]
- Alam, T.; Li, W.; Yang, F.; Chang, W.; Li, J.; Wang, Z.; Khan, J.; Li, C. Force analysis and bubble dynamics during flow boiling in silicon nanowire microchannels. Int. J. Heat Mass Transf. 2016, 101, 915–926. [Google Scholar] [CrossRef]
- Frost, W.; Kippenhan, C.J. Bubble growth and heat-transfer mechanisms in the forced convection boiling of water containing a surface active agent. Int. J. Heat Mass Transf. 1967, 10, 931–949. [Google Scholar] [CrossRef]
- Kandlikar, S.G. Scale effects on flow boiling heat transfer in microchannels: A fundamental perspective. Int. J. Therm. Sci. 2010, 49, 1073–1085. [Google Scholar] [CrossRef]
- Kandlikar, S.G. A theoretical model to predict pool boiling CHF incorporating effects of contact angle and orientation. J. Heat Transf. 2001, 123, 1071–1079. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chen, P.-H. Surface wettability effects on critical heat flux of boiling heat transfer using nanoparticle coatings. Int. J. Heat Mass Transf. 2012, 55, 3713–3719. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Liu, Z.-H.; Wang, G.-S. A predictive model of nucleate pool boiling on heated hydrophilic surfaces. Int. J. Heat Mass Transf. 2013, 65, 789–797. [Google Scholar] [CrossRef]
- Zhou, K.; Coyle, C.; Li, J.; Buongiorno, J.; Li, W. Flow boiling in vertical narrow microchannels of different surface wettability characteristics. Int. J. Heat Mass Transf. 2017, 109, 103–114. [Google Scholar] [CrossRef]
- Searle, M.; Emerson, P.; Crockett, J.; Maynes, D. Influence of microstructure geometry on pool boiling at superhydrophobic surfaces. Int. J. Heat Mass Transf. 2018, 127, 772–783. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Kim, C.J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Motezakker, A.R.; Sadaghiani, A.K.; Çelik, S.; Larsen, T.; Villanueva, L.G.; Koşar, A. Optimum ratio of hydrophobic to hydrophilic areas of biphilic surfaces in thermal fluid systems involving boiling. Int. J. Heat Mass Transf. 2019, 135, 164–174. [Google Scholar] [CrossRef]
- Yamada, M.; Shen, B.; Imamura, T.; Hidaka, S.; Kohno, M.; Takahashi, K.; Takata, Y. Enhancement of boiling heat transfer under sub-atmospheric pressures using biphilic surfaces. Int. J. Heat Mass Transf. 2017, 115, 753–762. [Google Scholar] [CrossRef]
- Zupančič, M.; Steinbücher, M.; Gregorčič, P.; Golobič, I. Enhanced pool-boiling heat transfer on laser-made hydrophobic/superhydrophilic polydimethylsiloxane-silica patterned surfaces. Appl. Therm. Eng. 2015, 91, 288–297. [Google Scholar] [CrossRef]
- Aboubakri, A.; Yanik, C.; Akkuş, Y.; Koşar, A.; Sadaghiani, A.K. Numerical and Experimental Investigation on Evaporation of Water Droplet on Surfaces with Mixed Wettability. In Proceedings of the ASME 2020 18th International Conference on Nanochannels, Microchannels, and Minichannels Collocated with the ASME 2020 Heat Transfer Summer Conference and the ASME 2020 Fluids Engineering Division Summer Meeting, 13–15 July 2020; American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2020. [Google Scholar]
- Gong, S.; Cheng, P. Numerical simulation of pool boiling heat transfer on smooth surfaces with mixed wettability by lattice Boltzmann method. Int. J. Heat Mass Transf. 2015, 80, 206–216. [Google Scholar] [CrossRef]
- Coleman, H.W.; Steele, W.G. Experimentation, Validation, and Uncertainty Analysis for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Shah, R.K.; London, A.L. Laminar Flow Forced Convection in Ducts: A Source Book for Compact Heat Exchanger Analytical Data; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Sun, L.; Mo, Z.; Zhao, L.; Liu, H.; Guo, X.; Ju, X.; Bao, J. Characteristics and mechanism of bubble breakup in a bubble generator developed for a small TMSR. Ann. Nucl. Energy 2017, 109, 69–81. [Google Scholar] [CrossRef]
- Sadaghiani, A.K.; Altay, R.; Noh, H.; Kwak, H.J.; Şendur, K.; Mısırlıoğlu, B.; Park, H.S.; Koşar, A. Effects of bubble coalescence on pool boiling heat transfer and critical heat flux—A parametric study based on artificial cavity geometry and surface wettability. Int. J. Heat Mass Transf. 2020, 147, 1–15. [Google Scholar] [CrossRef]


| Parameters | Uncertainty |
|---|---|
| Voltage | ±1 V |
| Current | ±0.01 A |
| Wall temperature | ±0.3 K |
| Mass flow rate | ±2–5% |
| Channel dimensions | ±15 μm |
| Heat transfer coefficient | ±4.5–7.5% |
| Surface | |||
|---|---|---|---|
| Sample #1 | 75%-500 µm | 67%-475 µm | 50%-400 µm |
| Sample #2 | 75%-500 µm | 50%-400 µm | 25%-300 µm |
| Sample #3 | 50%-400 µm | 25%-300 µm | 12%-200 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, V.E.; Aboubakri, A.; Sadaghiani, A.K.; Sefiane, K.; Koşar, A. Effect of Functional Surfaces with Gradient Mixed Wettability on Flow Boiling in a High Aspect Ratio Microchannel. Fluids 2020, 5, 239. https://doi.org/10.3390/fluids5040239
Ahmadi VE, Aboubakri A, Sadaghiani AK, Sefiane K, Koşar A. Effect of Functional Surfaces with Gradient Mixed Wettability on Flow Boiling in a High Aspect Ratio Microchannel. Fluids. 2020; 5(4):239. https://doi.org/10.3390/fluids5040239
Chicago/Turabian StyleAhmadi, Vahid Ebrahimpour, Akam Aboubakri, Abdolali Khalili Sadaghiani, Khellil Sefiane, and Ali Koşar. 2020. "Effect of Functional Surfaces with Gradient Mixed Wettability on Flow Boiling in a High Aspect Ratio Microchannel" Fluids 5, no. 4: 239. https://doi.org/10.3390/fluids5040239
APA StyleAhmadi, V. E., Aboubakri, A., Sadaghiani, A. K., Sefiane, K., & Koşar, A. (2020). Effect of Functional Surfaces with Gradient Mixed Wettability on Flow Boiling in a High Aspect Ratio Microchannel. Fluids, 5(4), 239. https://doi.org/10.3390/fluids5040239







